Abstract
Single nucleotide polymorphisms (SNPs) in miRNA biosynthesis genes DROSHA and DGCR8 were indicated to be correlated with cancer risk. We comprehensively reviewed and analyzed the effect of DROSHA and DGCR8 polymorphisms on cancer risk. Eligible articles were selected according to a series of inclusion and exclusion criteria. Consequently, ten case–control studies (from nine citations) with 4265 cancer cases and 4349 controls were involved in a meta-analysis of seven most prevalent SNPs (rs10719 T/C, rs6877842 G/C, rs2291109 A/T, rs642321 C/T, rs3757 G/A, rs417309 G/A, rs1640299 T/G). Our findings demonstrated that the rs417309 SNP in DGCR8 was significantly associated with an elevated risk of overall cancer in every genetic model. In stratified analysis, correlations of DROSHA rs10719 and rs6877842 SNPs were observed in Asian and laryngeal cancer subgroups, respectively. Moreover, associations of the rs417309 SNP could also be found in numerous subgroups including: Asian and Caucasian population subgroups; laryngeal and breast cancer subgroups; population-based (PB) and hospital-based (HB) subgroups. In conclusion, the DROSHA rs10719, rs6877842 SNPs, and DGCR8 rs417309 SNP play pivotal roles in cancerogenesis and may be potential biomarkers for cancer-forewarning.
Keywords: cancer, DROSHA, DGCR8, miRNA, risk, single nucleotide polymorphisms
Introduction
miRNAs are a type of small non-coding RNAs that play roles at post-transcriptional level by sequence-specific binding to the 3′-UTRs of target mRNAs [1]. During the miRNA maturing processing, primary miRNAs (pri-miRNAs) are first synthesized by RNA II polymerase in nucleus. And then, they are converted into precursor miRNAs (pre-miRNAs) by a Drosha–DGCR8 microprocessor complex which is constituted by DROSHA, an RNase III superfamily member and its cofactor DGCR8 [2]. Next, the pre-miRNAs are exported to cytoplasm and converted into mature miRNAs by DICER. miRNA genes are deemed to function as both oncogenes and tumor suppressors and their expressions have been confirmed to be associated with varieties of cancers [3–5]. Hence, imparied miRNA processing caused by the aberrant expression of miRNA biosynthesis genes DROSHA or DGCR8 can noticeably promote the tumorigenesis [6].
As the most prevalent genetic variation, single nucleotide polymorphisms (SNPs) in DROSHA and DGCR8 genes can affect their structure or expression, resulting in incomplete miRNA processing and in turn influence the expression of target genes, thereby acting as risk factor for diseases such as cancer. Thus far, accumulating studies have been concerned with the association between DROSHA and DGCR8 SNPs and the susceptibility to cancer. However, the findings were inconsistent and there was no systematic analysis for DROSHA and DGCR8 SNPs and cancer risk. In the present study, we comprehensively reviewed the eligible studies and analyzed all available data. Our aim is to explore the association of DROSHA and DGCR8 SNPs with cancer risk, supplying clues to researchers for screening novel cancer biomarkers.
Methods
Retrieval strategy
A detailed literature retrieval was performed by two independent investigators (J.W. and Z.L.) for publications regarding the association between DROSHA and DGCR8 polymorphisms and cancer risk. Relevant publications were selected from PubMed and Web of Science using a combination of the following keywords: ‘DROSHA/drosha ribonuclease III/RNase III/DGCR8/Digeorge syndrome critical region gene 8/Pasha’; ‘SNP/polymorphism/variation/variant’; and ‘tumor/cancer/carcinoma/neoplasm’, up to 1 January 2018.
Inclusion and exclusion criteria
Eligible publications were selected by the following inclusion criteria: (i) a case–control designed study; (ii) regarding the correlation between DROSHA or DGCR8 polymorphisms and cancer risk. Articles meeting the following criteria were excluded: (i) reviews, letters, or editorials; (ii) duplicate records; (iii) unrelated to cancer or DROSHA and DGCR8 polymorphisms; (iv) no available data to extract.
Data extraction
Data extraction was completed by two independent investigators (J.W. and Z.L.). Basic features obtained from each eligible article were as follows: first author’s name, publication year (unpublished collected study year), country, ethnicity, type of cancer, gene, polymorphisms, sample size of cases and controls, genotype distribution, Hardy–Weinberg equilibrium (HWE) in controls, source of control groups (population-based (PB) or hospital-based (HB)), genotyping method, adjusted factors, and quality score. When the article covered multiple stages, data were extracted individually. When the data in eligible articles were unavailable, we tried our best to contact the corresponding authors for original data.
Methodology quality assessment
Quality of the selected studies was assessed by two independent reviewers (H.D. and X.F.) according to a study regarding the method for assigning quality scores, which was mentioned in prior meta-analyses [7,8]. Six items were evaluated in the quality assessment scale: (i) the representativeness of the cases; (ii) the source of controls; (iii) the ascertainment of relevant cancers; (iv) the sample size; (v) the quality control of the genotyping methods; (vi) HWE in controls. The quality scores of eligible studies ranged from 0 to 10. Studies with scores less than 5 and HWE disequilibrium were removed from the subsequent analyses.
Statistical analysis
All statistical analyses in the present study were performed by STATA software, version 11.0 (STATA Corp, College Station, TX, U.S.A.). All statistical tests presented were two-tailed and the P-values<0.05 were regarded as statistically significant, unless highlighted otherwise. And the Bonferroni correction was conducted to justify P-values [40]. The HWE for the genotype frequencies of DROSHA and DGCR8 polymorphisms in controls was computed by χ2 test. The intensity of the correlations between the DROSHA and DGCR8 polymorphisms and the risk of cancer was estimated by odds ratios (ORs) with its corresponding 95% confidence intervals (95% CIs). Between-study heterogeneity was computed by a χ2-based Cochran’s Qtest (significance at P<0.10 and I2 > 50%). We summarized the results by using fixed effect models [9] when the interstudy heterogeneity was absent, otherwise random effect models [10]. Begg’s test and Egger’s linear regression analysis were performed to estimate the publication bias statistically [11,12]. P<0.10 was regarded as statistically significant in both Egger’s and Begg’s test [8,28]. What is more, sensitivity analysis was shown to inspect whether the pooled results were steady after we excluded the outlying studies.
Results
Characteristics of the included studies
As presented in Figure 1, a total of 148 publications were collected through database search after eliminating the duplicate studies. We eliminated 83 records after browsing the titles and abstracts (40 were functional studies; 12 were reviews or meta-analysis; 9 were not case–control studies; 52 were unrelated to DROSHA or DGCR8 SNPs; 8 were unrelated to cancer; 12 were not correlated with cancer risks). What was more, six studies were excluded by calculating (one for the unavailable data; five for the limited study number of DROSHA or DGCR8 polymorphisms). Moreover, the removal of two records from the subsequent analyses was due to the inconformity of their genotype distributions to HWE (PHWE<0.05). Hence, in total, ten case–control studies (from nine citations) containing 4265 cancer cases and 4349 cancer-free controls were involved in our meta-analyses, which were accorded with our inclusion criteria and the evaluation of methodology quality. The characteristics of these involved articles were presented in Table 1 and the frequency distributions of DROSHA and DGCR8 polymorphisms genotype were shown in Table 2. In summary obtained from ten eligible case–control studies, seven SNPs of DROSHA or DGCR8 genes were investigated in the eventual analysis. According to the SNPs selection criteria mentioned in eligible studies [2,14], we found that none of these seven SNPs were in strong linkage disequilibrium (r2 ≥ 0.8). In DROSHA, the analyzed SNPs were rs10719 T/C, rs6877842 G/C, rs2291109 A/T, rs642321 C/T; in DGCR8, the analyzed SNPs are rs3757 G/A, rs417309 G/A, rs1640299 T/G (Figure 2).
Figure 1. The flow chart of identification for studies included in the meta-analysis.
Table 1. The main features of enrolled studies.
| Ref. no. | Year | Country | Ethnicity | Sample size | Source of controls | Genotyping method | Adjusted factors | Quality score | Citation | |
|---|---|---|---|---|---|---|---|---|---|---|
| Case | Control | |||||||||
| 1 | 2010 | Korean | Asian | 93 | 93 | HB | MS | NM | 5.5 | [15] |
| 2 | 2013 | China | Asian | 878 | 900 | PB | Taqman | Age and residential area | 7.5 | [2] |
| 3 | 2013 | China | Asian | 914 | 967 | PB | Taqman | Age and residential area | 7.5 | [2] |
| 4 | 2013 | China | Asian | 685 | 730 | HB | Taqman | Age, sex, and smoking status | 7 | [16] |
| 5 | 2013 | America | Caucasian | 277 | 278 | PB | SNPlex technology | Age, sex, ethnicity, and county of residence | 7.5 | [14] |
| 6 | 2015 | Korean | Asian | 408 | 400 | HB | PCR-RFLP | Age, gender, hypertension, diabetes mellitus | 7 | [24] |
| 7 | 2015 | Polish | Caucasian | 135 | 170 | HB | Taqman | NM | 7.5 | [25] |
| 8 | 2016 | Polish | Caucasian | 100 | 100 | NM | Taqman | NM | 6 | [13] |
| 9 | 2016 | Korean | Asian | 147 | 209 | HB | PCR-RFLP | Age, gender, hypertension, diabetes mellitus, drinking status, and smoking | 7 | [26] |
| 10 | 2017 | China | Asian | 628 | 502 | HB | HRM | Age, sex, region, smoking status, and drinking status | 7 | [27] |
Abbreviations: HRM, high-resolution melting; MS, sequenome MS-based genotyping assay; PCR-RFLP, polymerase chain reaction-restriction fragment length polymorphism; NM, not mentioned.
Table 2. Genotype frequency distributions of DROSHA and DGCR8 SNPs in included studies.
| Ref. No. | Year | Cancer type | Gene | SNPs1 | Sample size | Case | Control | PHWE | MAF in controls (Global MAF4) | Included in meta-analysis | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Control | Homozygote wild | Heterozygote | Homozygote variant | Homozygote wild | Heterozygote | Homozygote variant | ||||||||
| 1 | 2010 | Lung cancer | DROSHA | rs6877842 | 93 | 93 | 81 | 11 | 1 | 84 | 8 | 1 | 0.136 | 0.054 (0.138) | Yes |
| (G > C) | |||||||||||||||
| Lung cancer | DROSHA | rs10719 | 97 | 97 | 59 | 29 | 9 | 52 | 38 | 7 | 0.987 | 0.268 (0.483) | Yes | ||
| (T > C) | |||||||||||||||
| Lung cancer | DGCR8 | rs3757 | 94 | 90 | 60 | 27 | 7 | 60 | 24 | 6 | 0.114 | 0.200 (0.182) | Yes | ||
| (G > A) | |||||||||||||||
| Lung cancer | DGCR8 | rs417309 | 98 | 97 | 90 | 8 | 0 | 88 | 9 | 0 | 0.632 | 0.046 (0.043) | Yes | ||
| (G > A) | |||||||||||||||
| Lung cancer | DGCR8 | rs1640299 | 98 | 97 | 58 | 33 | 7 | 52 | 40 | 5 | 0.444 | 0.258 (0.381) | Yes | ||
| (T > G) | |||||||||||||||
| 2 | 2013 | Breast cancer | DROSHA | rs10719 | 847 | 878 | 433 | 346 | 68 | 463 | 353 | 62 | 0.635 | 0.272 (0.483) | Yes |
| (T > C) | |||||||||||||||
| Breast cancer | DROSHA | rs17409893 | 849 | 885 | 527 | 287 | 35 | 575 | 276 | 34 | 0.902 | 0.194 (0.222) | No3 | ||
| (A > G) | |||||||||||||||
| Breast cancer | DROSHA | rs2291109 | 858 | 886 | 552 | 273 | 33 | 535 | 306 | 45 | 0.884 | 0.223 (0.061) | Yes | ||
| (A > T) | |||||||||||||||
| Breast cancer | DROSHA | rs642321 | 854 | 883 | 212 | 423 | 219 | 231 | 433 | 219 | 0.571 | 0.493 (0.322) | Yes | ||
| (C > T) | |||||||||||||||
| Breast cancer | DGCR8 | rs1640299 | 849 | 891 | 465 | 330 | 54 | 476 | 357 | 58 | 0.412 | 0.265 (0.381) | Yes | ||
| (T > G) | |||||||||||||||
| Breast cancer | DGCR8 | rs417309 | 860 | 893 | 771 | 89 | 0 | 826 | 67 | 0 | 0.244 | 0.038 (0.043) | Yes | ||
| (G > A) | |||||||||||||||
| Breast cancer | DGCR8 | rs720012 | 867 | 891 | 225 | 425 | 217 | 240 | 451 | 200 | 0.668 | 0.478 (0.221) | No3 | ||
| (G > A) | |||||||||||||||
| Breast cancer | DGCR8 | rs720014 | 836 | 880 | 542 | 264 | 30 | 555 | 287 | 38 | 0.907 | 0.206 (0.183) | No3 | ||
| (T > C) | |||||||||||||||
| 3 | 2013 | Breast cancer | DROSHA | rs2291109 | 899 | 957 | 563 | 296 | 40 | 625 | 298 | 34 | 0.835 | 0.191 (0.061) | Yes |
| (A > T) | |||||||||||||||
| Breast cancer | DGCR8 | rs417309 | 901 | 960 | 830 | 68 | 3 | 910 | 49 | 1 | 0.687 | 0.027 (0.043) | Yes | ||
| (G > A) | |||||||||||||||
| 4 | 2013 | Bladder cancer | DROSHA | rs2291109 | 685 | 730 | 421 | 228 | 36 | 419 | 280 | 31 | 0.062 | 0.234 (0.061) | Yes |
| (A > T) | |||||||||||||||
| Bladder cancer | DROSHA | rs10719 | 684 | 727 | 352 | 278 | 54 | 413 | 275 | 39 | 0.437 | 0.243 (0.483) | Yes | ||
| (T > C) | |||||||||||||||
| Bladder cancer | DROSHA | rs642321 | 685 | 730 | 197 | 326 | 162 | 176 | 371 | 183 | 0.655 | 0.505 (0.322) | Yes | ||
| (C > T) | |||||||||||||||
| 5 | 2013 | Renal cell carcinoma | DROSHA | rs10719 | 252 | 246 | 161 | 75 | 16 | 155 | 76 | 15 | 0.177 | 0.215 (0.483) | Yes |
| (T > C) | |||||||||||||||
| Renal cell carcinoma | DROSHA | rs6877842 | 275 | 278 | 200 | 65 | 10 | 204 | 65 | 9 | 0.185 | 0.149 (0.138) | Yes | ||
| (G > C) | |||||||||||||||
| Renal cell carcinoma | DGCR8 | rs3757 | 276 | 278 | 163 | 102 | 11 | 162 | 102 | 14 | 0.688 | 0.234 (0.182) | Yes | ||
| (G > A) | |||||||||||||||
| Renal cell carcinoma | DGCR8 | rs417309 | 277 | 278 | 243 | 30 | 4 | 243 | 34 | 1 | 0.87 | 0.065 (0.043) | Yes | ||
| (G > A) | |||||||||||||||
| Renal cell carcinoma | DGCR8 | rs1640299 | 277 | 278 | 61 | 151 | 65 | 75 | 136 | 67 | 0.729 | 0.486 (0.381) | Yes | ||
| (T > G) | |||||||||||||||
| 6 | 2015 | Colorectal cancer | DROSHA | rs10719 | 408 | 400 | 224 | 154 | 30 | 211 | 168 | 21 | 0.09 | 0.263 (0.483) | Yes |
| (T > C) | |||||||||||||||
| 7 | 2015 | Laryngeal cancer | DROSHA | rs6877842 | 128 | 170 | 73 | 49 | 6 | 76 | 79 | 15 | 0.384 | 0.321 (0.138) | Yes |
| (G > C) | |||||||||||||||
| Laryngeal cancer | DGCR8 | rs417309 | 112 | 170 | 67 | 32 | 13 | 116 | 46 | 8 | 0.227 | 0.182 (0.043) | Yes | ||
| (G > A) | |||||||||||||||
| Laryngeal cancer | DGCR8 | rs1640299 | 113 | 170 | 60 | 47 | 6 | 61 | 93 | 16 | 0.021 | 0.368 (0.381) | No2 | ||
| (T > G) | |||||||||||||||
| Laryngeal cancer | DGCR8 | rs3757 | 122 | 170 | 29 | 89 | 4 | 36 | 119 | 15 | <0.001 | 0.438 (0.182) | No2 | ||
| (G > A) | |||||||||||||||
| 8 | 2016 | Laryngeal cancer | DROSHA | rs6877842 | 100 | 100 | 60 | 35 | 5 | 44 | 47 | 9 | 0.476 | 0.325 (0.138) | Yes |
| (G > C) | |||||||||||||||
| Laryngeal cancer | DGCR8 | rs417309 | 100 | 100 | 60 | 28 | 12 | 69 | 27 | 4 | 0.516 | 0.175 (0.043) | Yes | ||
| (G > A) | |||||||||||||||
| Laryngeal cancer | DGCR8 | rs1640299 | 100 | 100 | 52 | 42 | 6 | 36 | 55 | 9 | 0.062 | 0.365 (0.381) | Yes | ||
| (T > G) | |||||||||||||||
| 9 | 2016 | Hepatocellular carcinoma | DROSHA | rs10719 | 147 | 209 | 81 | 53 | 13 | 110 | 88 | 11 | 0.215 | 0.263 (0.483) | Yes |
| (T > C) | |||||||||||||||
| Hepatocellular carcinoma | DROSHA | rs6877842 | 147 | 209 | 138 | 9 | 0 | 200 | 9 | 0 | 0.75 | 0.022 (0.138) | Yes | ||
| (G > C) | |||||||||||||||
| 10 | 2017 | Gastirc cancer | DROSHA | rs10719 | 628 | 502 | 314 | 257 | 57 | 248 | 205 | 49 | 0.487 | 0.302 (0.483) | Yes |
| (T > C) | |||||||||||||||
The results were in bold if P<0.05. Abbreviations: MAF, minor allele frequency; PHWE, the P-value for HWE in control groups.
1, The ancestral alleles were referenced in the NCBI database.
2, Excluded due to the SNP not being in accordance with HWE.
3, Excluded due to the limited number for this locus;
4, The global MAFs were referenced in the NCBI database.
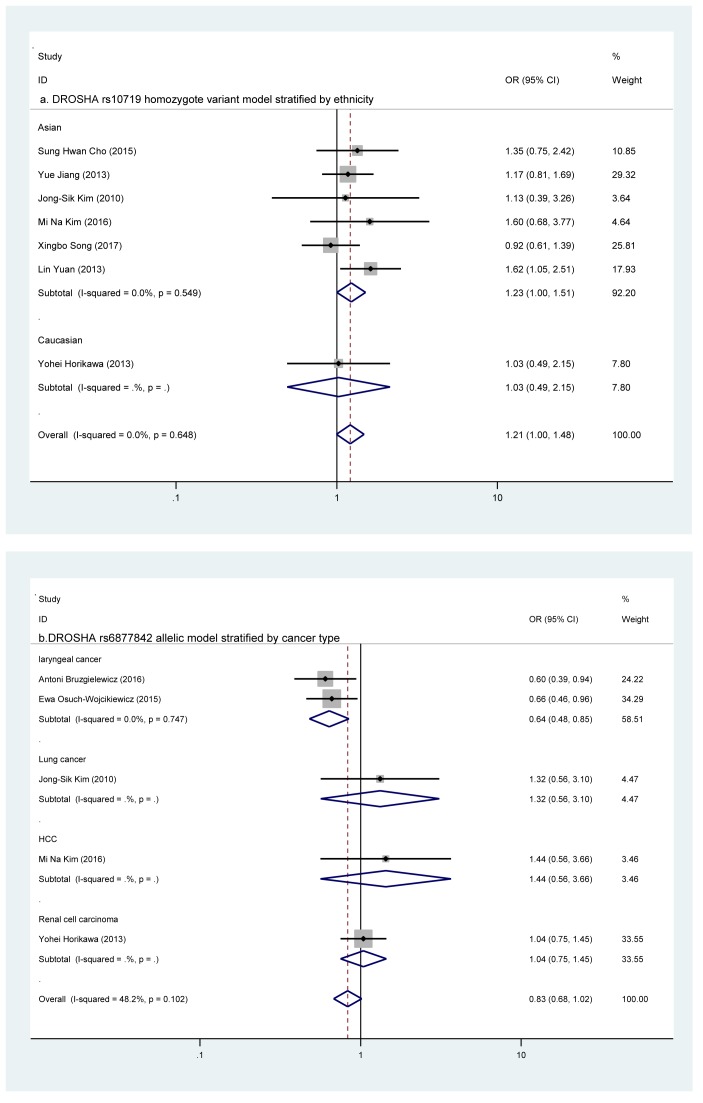
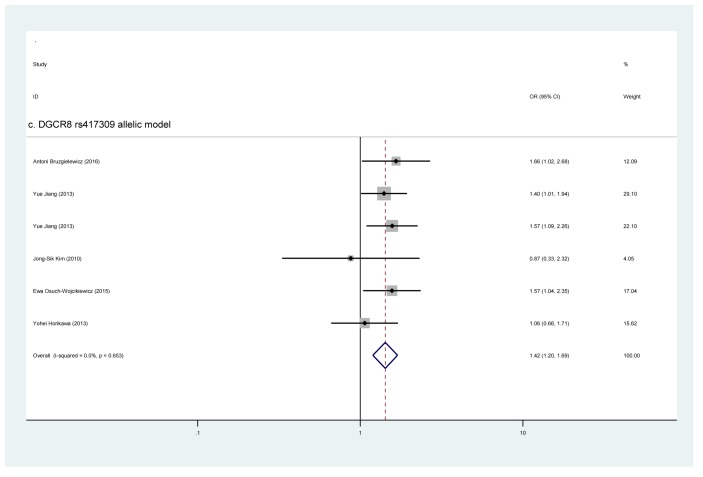
Figure 2. A forest plot of the DROSHA and DGCR8 SNPs associated with cancer risk.
((a) DROSHA rs10719 in the ethnicity subgroup analysis; (b) DROSHA rs6877842 in the cancer type subgroups; (c) DGCR8 rs417309 under allelic model: A compared with G).
Quantitative data synthesis of seven SNPs in DROSHA and DGCR8 genes
Four SNPs in DROSHA
First, all eligible articles were summarized to evaluate the correlation strength of each DROSHA SNP with the risk of overall cancer. However, these four SNPs (rs10719 T/C, rs6877842 G/C, rs642321 C/T, and rs2291109 A/T) did not manifest any significant associations with cancer risk in any genetic models (Table 3). Due to the existence of interstudy heterogeneity, stratified analyses were performed.
Table 3. Meta-analysis of the association between DROSHA and DGCR8 polymorphisms and cancer risk.
| Heterozygote compared with homozygote wild | Homozygote variant compared with homozygote wild | Dominant model | Recessive model | Allelic model | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SNPs | n | P (Pcorr) | OR (95% CI) | I2 (%) | P (Pcorr) | OR (95% CI) | I2 (%) | P (Pcorr) | OR (95% CI) | I2 (%) | P (Pcorr) | OR (95% CI) | I2 (%) | P (Pcorr) | OR (95% CI) | I2 (%) |
| DROSHA rs10719 (T > C) | 7 | 0.934 | 1.004 (0.904–1.117) | 0.2 | 0.055 | 1.214 (0.996–1.480) | 0.0 | 0.502 | 1.035 (0.936–1.145) | 10.5 | 0.053 | 1.210 (0.998–1.467) | 0.0 | 0.179 | 1.056 (0.975–1.145) | 0 |
| Ethnicity | ||||||||||||||||
| Asian | 6 | 0.874 | 1.009 (0.904–1.126) | 15.6 | 0.048 (0.336) | 1.230 (1.001–1.511) | 0.0 | 0.449 | 1.041 (0.938–1.157) | 10.5 | 0.04 (0.336) | 1.223 (1.002–1.494) | 0.0 | 0.154 | 1.062 (0.978–1.154) | 0 |
| Caucasian | 1 | 0.796 | 0.950 (0.645–1.400) | NA | 0.944 | 1.027 (0.491–2.148) | NA | 0.838 | 0.963 (0.668–1.387) | NA | 0.907 | 1.044 (0.504–2.161) | NA | 0.904 | 0.981 (0.725–1.329) | NA |
| Source of controls | ||||||||||||||||
| HB | 5 | 0.905 | 0.992 (0.869–1.132) | 30.1 | 0.071 | 1.257 (0.981–1.610) | 0 | 0.643 | 1.030 (0.908–1.169) | 27.2 | 0.061 | 1.259 (0.989–1.602) | 0 | 0.25 | 1.060 (0.0960–1.172) | 16.7 |
| PB | 2 | 0.767 | 1.027 (0.861–1.225) | 0 | 0.396 | 1.142 (0.822–1.588) | 0.0 | 0.616 | 1.044 (0.883–1.234) | 0 | 0.463 | 1.128 (0.818–1.555) | 0.0 | 0.398 | 1.049 (0.918-1.199) | 0 |
| DROSHA rs6877842 (G > C) | 5 | 0.174 | 0.841 (0.655–1.079) | 39.3 | 0.101 | 0.627 (0.358–1.096) | 0.0 | 0.3581 | 0.839 (0.577–1.220) | 50.7 | 0.218 | 0.706 (0.406–1.228) | 0.0 | 0.073 | 0.832 (0.680–1.017) | 48.2 |
| Ethnicity | ||||||||||||||||
| Asian | 2 | 0.292 | 1.438 (0.732–2.824) | 0 | 0.98 | 1.037 (0.064–16.860) | NA | 0.303 | 1.414 (0.731–2.735) | 0.0 | 1 | 1.000 (0.062–16.230) | NA | 0.326 | 1.372 (0.731–2.576) | 0 |
| Caucasian | 3 | 0.059 | 0.772 (0.590–1.010) | 47.1 | 0.094 | 0.613 (0.346–1.087) | 27.5 | 0.1251 | 0.716 (0.467–1.097) | 61.1 | 0.21 | 0.697 (0.396–1.225) | 0.0 | 0.1231 | 0.762 (0.540–1.076) | 60.1 |
| Source of controls | ||||||||||||||||
| HB | 3 | 0.395 | 0.845 (0.574–1.245) | 44.3 | 0.103 | 0.460 (0.181–1.169) | 0 | 0.881 | 0.953 (0.506–1.793) | 52.5 | 0.194 | 0.545 (0.218–1.361) | 0 | 0.155 | 0.796 (0.581–1.090) | 48.0 |
| PB | 1 | 0.922 | 1.020 (0.687–1.514) | NA | 0.79 | 1.133 (0.451–2.848) | NA | 0.862 | 1.034 (0.710–1.505) | NA | 0.797 | 1.128 (0.451–2.820) | NA | 0.807 | 1.042 (0.750–1.447) | NA |
| NM | 1 | 0.043 | 0.546 (0.304–0.981) | NA | 0.129 | 0.407 (0.128–1.300) | NA | 0.024 | 0.524 (0.299–0.919) | NA | 0.274 | 0.532 (0.172–1.648) | NA | 0.026 | 0.603 (0.387–0.941) | NA |
| Cancer type | ||||||||||||||||
| Laryngeal cancer | 2 | 0.008 (0.56) | 0.604 (0.417–0.875) | 0 | 0.022 (0.154) | 0.413 (0.193–0.881) | 0.0 | 0.002 (0.014) | 0.573 (0.401–0.819) | 0.0 | 0.081 | 0.518 (0.247–1.084) | 0.0 | 0.002 (0.014) | 0.638 (0.481–0.847) | 0.0 |
| Lung cancer | 1 | 0.469 | 1.426 (0.546–3.726) | NA | 0.98 | 1.037 (0.064–16.860) | NA | 0.488 | 1.383 (0.553–3.458) | NA | 1 | 1.000 (0.062–16.230) | NA | 0.52 | 1.323 (0.565–3.096) | NA |
| Hepatocellular carcinoma | 1 | 0.443 | 1.449 (0.561–3.744) | NA | NA | NA | NA | 0.443 | 1.449 (0.561–3.744) | NA | NA | NA | NA | 0.45 | 1.435 (0.563–3.660) | NA |
| Renal cell carcinoma | 1 | 0.922 | 1.020 (0.687–1.514) | NA | 0.79 | 1.133 (0.451–2.848) | NA | 0.862 | 1.034 (0.710–1.505) | NA | 0.797 | 1.128 (0.451–2.820) | NA | 0.807 | 1.042 (0.750–1.447) | NA |
| DROSHA rs642321 (C > T) | 2 | 0.576 | 0.918 (0.682–1.237) | 0.0 | 0.6721 | 0.934 (0.683–1.279) | 60.5 | 0.6031 | 0.923 (0.681–1.250) | 72.2 | 0.911 | 0.991 (0.843–1.165) | 0 | 0.6651 | 0.965 (0.821–1.134) | 62.3 |
| DROSHA rs2291109 (A > T) | 3 | 0.3891 | 0.922 (0.765–1.110) | 58.8 | 0.92 | 1.014 (0.771–1.333) | 44.7 | 0.4691 | 0.932 (0.770–1.128) | 63.8 | 0.746 | 1.046 (0.798–1.371) | 37.4 | 0.6061 | 0.958 (0.815–1.126) | 64.6 |
| Cancer type | ||||||||||||||||
| Breast cancer | 2 | 0.8511 | 0.977 (0.770–1.240) | 64.9 | 0.8991 | 0.962 (0.530–1.747) | 69.2 | 0.8591 | 0.975 (0.738–1.289) | 76.4 | 0.9091 | 0.971 (0.580–1.624) | 59.6 | 0.8711 | 0.978 (0.752–1.272) | 81 |
| Bladder cancer | 1 | 0.062 | 0.810 (0.650–1.011) | NA | 0.57 | 1.156 (0.702–1.903) | NA | 0.12 | 0.845 (0.683–1.045) | NA | 0.373 | 1.251 (0.765–2.046) | NA | 0.332 | 0.917 (0.768–1.093) | NA |
| Source of controls | ||||||||||||||||
| PB | 2 | 0.8511 | 0.977 (0.770–1.240) | 64.9 | 0.8991 | 0.962 (0.530–1.747) | 69.2 | 0.8591 | 0.975 (0.738–1.289) | 76.4 | 0.9091 | 0.971 (0.580–1.624) | 59.6 | 0.8711 | 0.978 (0.752–1.272) | 81 |
| HB | 1 | 0.062 | 0.810 (0.650–1.011) | NA | 0.57 | 1.156 (0.702–1.903) | NA | 0.12 | 0.845 (0.683–1.045) | NA | 0.373 | 1.251 (0.765–2.046) | NA | 0.332 | 0.917 (0.768–1.093) | NA |
| DGCR8 rs3757 (G > A) | 2 | 0.892 | 1.022 (0.750–1.391) | 0.0 | 0.741 | 0.894 (0.460–1.737) | 0 | 0.974 | 1.005 (0.748–1.350) | 0 | 0.715 | 0.885 (0.460–1.704) | 0 | 0.913 | 0.986 (0.772–1.260) | 0.0 |
| DGCR8 rs417309 (G > A) | 6 | 0.012 (0.084) | 1.282 (1.057–1.555) | 0.0 | 0.001 (0.007) | 3.169 (1.634–6.146) | 0 | 0.001 0.007) | 1.365 (1.131–1.647) | 0 | 0.001 (0.007) | 3.026 (1.574–5.817) | 0 | 6.90E-05 (4.83E-04) | 1.423 (1.196–1.693) | 0.0 |
| Ethnicity | ||||||||||||||||
| Asian | 3 | 0.004 (0.028) | 1.420 (1.115–1.809) | 0.0 | 0.303 | 3.289 (0.341–31.682) | NA | 0.003 0.021) | 1.435 (1.129–1.825) | 0 | 0.314 | 3.204 (0.333–30.856) | NA | 0.003 (0.021) | 1.429 (1.131–1.806) | 0.0 |
| Caucasian | 3 | 0.699 | 1.066 (0.772–1.472) | 0.0 | 0.001 (0.007) | 3.157 (1.579–6.310) | 0 | 0.133 | 1.260 (0.932–1.704) | 0 | 0.002 (0.014) | 3.009 (1.520–5.954) | 0 | 0.009 (0.063) | 1.415 (1.092–1.834) | 2.7 |
| Cancer type | ||||||||||||||||
| laryngeal cancer | 2 | 0.387 | 1.199 (0.795–1.810) | 0.0 | 0.003 (0.021) | 3.059 (1.474–6.351) | 0 | 0.05 | 1.460 (1.000–2.131) | 0 | 0.004 (0.028) | 2.895 (1.410–5.945) | 0 | 0.003 (0.021) | 1.604 (1.176–2.188) | 0.0 |
| Breast cancer | 2 | 0.003 (0.021) | 1.465 (1.141–1.881) | 0.0 | 0.303 | 3.289 (0.341–31.682) | NA | 0.002 (0.014) | 1.481 (1.155–1.898) | 0 | 0.314 | 3.204 (0.333–30.856) | NA | 0.002 (0.014) | 1.473 (1.157–1.876) | 0.0 |
| Lung cancer | 1 | 0.783 | 0.869 (0.321–2.355) | NA | NA | NA | NA | 0.783 | 0.869 (0.321–2.355) | NA | NA | NA | NA | 0.788 | 0.875 (0.330–2.316) | NA |
| Renal cell carcinoma | 1 | 0.638 | 0.882 (0.523–1.487) | NA | 0.217 | 4.000 (0.444–36.046) | NA | 0.91 | 0.971 (0.587–1.609) | NA | 0.212 | 4.059 (0.451–36.544) | NA | 0.797 | 1.064 (0.664–1.705) | NA |
| Source of controls | ||||||||||||||||
| PB | 3 | 0.012 (0.084) | 1.333 (1.065–1.669) | 33.7 | 0.107 | 3.652 (0.755–17.659) | 0 | 0.006 (0.042) | 1.364 (1.093–1.704) | 12.4 | 0.036 (0.252) | 2.659 (1.064–6.643) | NA | 0.059 | 1.434 (0.986–2.085) | 14.7 |
| HB | 2 | 0.648 | 1.117 (0.694–1.799) | 0.0 | 0.029 (0.203) | 2.813 (1.109–7.135) | NA | 0.243 | 1.302 (0.836–2.030) | 0 | 0.108 | 3.635 (0.752–17.564) | 0 | 0.003 (0.021) | 1.377 (1.111–1.707) | 0.0 |
| NM | 1 | 0.585 | 1.193 (0.634–2.243) | NA | 0.04 | 3.450 (1.057–11.265) | NA | 0.184 | 1.484 (0.828–2.658) | NA | 0.047 | 3.273 (1.018–10.523) | NA | 0.04 | 1.656 (1.022–2.684) | NA |
| DGCR8 rs1640299 (T > G) | 4 | 0.5081 | 0.895 (0.645–1.243) | 60.2 | 0.988 | 0.998 (0.751–1.325) | 0 | 0.494 | 0.898 (0.659–1.223) | 59.1 | 0.786 | 0.965 (0.745–1.249) | 0 | 0.48 | 0.959 (0.852–1.078) | 34.6 |
| Ethnicity | ||||||||||||||||
| Asian | 2 | 0.403 | 0.923 (0.766–1.113) | 0.0 | 0.91 | 0.979 (0.674–1.420) | 0 | 0.434 | 0.931 (0.778–1.114) | 0 | 0.952 | 1.011 (0.703–1.455) | 0 | 0.545 | 0.957 (0.829–1.104) | 0.0 |
| Caucasian | 2 | 0.7691 | 0.870 (0.344–2.202) | 85.3 | 0.7261 | 0.853 (0.350–2.076) | 57.3 | 0.7131 | 0.844 (0.342–2.085) | 85.6 | 0.656 | 0.920 (0.638–1.328) | 0 | 0.5761 | 0.864 (0.517–1.443) | 77.9 |
| Source of controls | ||||||||||||||||
| PB | 2 | 0.6431 | 1.086 (0.767–1.537) | 60.2 | 0.788 | 1.043 (0.770–1.412) | 0 | 0.6941 | 1.063 (0.785–1.439) | 53.5 | 0.831 | 0.971 (0.738–1.276) | 0 | 0.974 | 0.998 (0.879–1.133) | 0.0 |
| HB | 1 | 0.32 | 0.740 (0.408–1.339) | NA | 0.712 | 1.255 (0.375–4.197) | NA | 0.433 | 0.797 (0.452–1.405) | NA | 0.565 | 1.415 (0.433–4.623) | NA | 0.682 | 0.908 (0.574-–1.438) | NA |
| NM | 1 | 0.033 | 0.529 (0.295–0.949) | NA | 0.175 | 0.462 (0.151–1.410) | NA | 0.023 | 0.519 (0.295–0.915) | NA | 0.424 | 0.645 (0.221–1.886) | NA | 0.042 | 0.643 (0.421–0.984) | NA |
The results are in bold if P<0.05. Abbreviation: Pcorr, P-values after Bonferroni correction.
1, P was calculated by random model.
In subgroup analyses, rs10719 and rs6877842 SNPs were analyzed in ‘ethnicity’ subgroup; the rs6877842 and rs2291109 SNPs were analyzed in ‘cancer type’ subgroup; rs10719, rs6877842, and rs2291109 SNPs were analyzed in ’source of controls’ subgroup. For rs10719 T/C SNP, its homozygote variant genotype and recessive models were correlated with an elevated cancer risk in Asian (CC compared with TT: OR = 1.230, 95% CI = 1.001–1.511, P=0.048; CC + CT compared with TT: OR = 1.223, 95% CI = 1.002–1.494, P=0.048, Table 3). For rs6877842 G/C SNP, its heterozygote model had strong correlation with a reduced risk of laryngeal cancer (CG compared with GG: OR = 0.413, 95% CI = 0.193–0.881, P=0.022, Table 3) and its homozygote variant genotype, dominant and allelic models had moderate associations with a descending risk of laryngeal cancer (CC compared with GG: OR = 0.604, 95% CI = 0.417–0.875, P=0.008; CC compared with CG + GG: OR = 0.573, 95% CI = 0.401–0.819, P=0.002; C compared with G: OR = 0.638, 95% CI = 0.481–0.847, P=0.002, Table 3). For rs2291109 polymorphism, however, the correlations with cancer risk were not elucidated in any stratified analyses.
Three SNPs in DGCR8
We evaluated the correlation strength of the polymorphisms in DROSHA gene with cancer risk, based on the entire population. The rs417309 G/A SNP was demonstrated to be associated with an increased risk of cancer. Strong associations of rs417309 were found in homozygote variant genotype and recessive models (AA compared with GG: OR = 3.169, 95% CI = 1.634–6.146, P=0.001; AA + AG compared with GG: OR = 3.026, 95% CI = 1.574–5.817, P=0.001). Correlations of rs417309 could also be found in other three models (AG compared with GG: OR = 1.282, 95% CI = 1.057–1.555, P=0.012; AA compared with AG + GG: OR = 1.365, 95% CI = 1.131–1.647, P=0.001; A compared with G: OR = 1.423, 95% CI = 1.196–1.693, P<0.001, Table 3). Associations of the rs3757 G/A and rs1640299 T/G SNPs with cancer risk were not illustrated in primary analyses.
In stratified analyses, rs417309 and rs1640299 SNPs were analyzed in ‘ethnicity’ and ‘source of controls’ subgroups; the rs417309 SNP was also analyzed in ‘cancer type’ subgroup. For rs417309 G/A SNP, its associations were observed in every subgroup (including: Asian population, Caucasian population; laryngeal cancer, breast cancer; PB, HB). Amongst all significant associations in subgroup analyses, only strong associations were reported below. In Caucasian subgroup, strong correlations were indicated in homozygote variant genotype and recessive models (AA compared with GG: OR = 3.169, 95% CI = 1.634–6.146, P=0.001; AA + AG compared with GG: OR = 3.026, 95% CI = 1.574–5.817, P=0.001). In laryngeal cancer subgroup, strong relationships were observed in homozygote variant genotype and recessive models (AA compared with GG: OR = 3.169, 95% CI = 1.634–6.146, P=0.001; AA + AG compared with GG: OR = 3.026, 95% CI = 1.574–5.817, P=0.001), When the control groups were PB, the recessive type (AA + AG) showed a strong relationship with an increased risk of cancer, compared with the wild-type GG (OR = 1.604, 95% CI = 1.176–2.188, P=0.003). When the controls groups were HB, the homozygote variant model of rs417309 presented a strong correlation with cancer risk (AA compared with GG: OR = 2.813, 95% CI = 1.109–7.135, P=0.029, Table 3). For rs1640299 T/G polymorphism, however, no significant relationship was found in any subgroup analyses.
Sensitivity analysis
Sensitivity analyses were conducted to calculate the effect of individual study on the merged findings by evaluating the sensitivity before and after eliminating each study from our meta-analysis (Supplementary Table S1). For rs417309 SNP, it was no longer statistically significant after we removed the study conducted by Jiang et al. (Supplementary Table S1) [2].
Table 4. The results of Begg’s and Egger’s tests for the publication bias.
| Begg’s test | Egger’s test | |||
|---|---|---|---|---|
| Comparison type | Z-value | P-value | t-value | P-value |
| DROSHA rs10719 (T > C) | ||||
| Heterozygote compared with homozygote wild | −2.250 | 0.024 | −3.030 | 0.029 |
| Homozygote variant compared with homozygote wild | 0.450 | 0.652 | 0.300 | 0.774 |
| Dominant model | −1.950 | 0.051 | −2.340 | 0.066 |
| Recessive model | 0.560 | 0.573 | 1.100 | 0.332 |
| Allelic model | −0.750 | 0.453 | −1.330 | 0.241 |
| DROSHA rs6877842 (G > C) | ||||
| Heterozygote compared with homozygote wild | −0.490 | 0.624 | 0.620 | 0.581 |
| Homozygote variant compared with homozygote wild | 0.000 | 1.000 | 0.020 | 0.988 |
| Dominant model | 0.000 | 1.000 | 0.500 | 0.650 |
| Recessive model | 0.000 | 1.000 | 0.030 | 0.976 |
| Allelic model | 0.000 | 1.000 | 0.740 | 0.511 |
| DROSHA rs642321 (C > T) | ||||
| Heterozygote compared with homozygote wild | −1.000 | 0.317 | NA | NA |
| Homozygote variant compared with homozygote wild | −1.000 | 0.317 | NA | NA |
| Dominant model | −1.000 | 0.317 | NA | NA |
| Recessive model | −1.000 | 0.317 | NA | NA |
| Allelic model | −1.000 | 0.317 | NA | NA |
| DROSHA rs2291109 (A > T) | ||||
| Heterozygote compared with homozygote wild | −1.570 | 0.117 | −1.270 | 0.426 |
| Homozygote variant compared with homozygote wild | 0.520 | 0.602 | 0.560 | 0.673 |
| Dominant model | −0.520 | 0.602 | −0.830 | 0.558 |
| Recessive model | 0.520 | 0.602 | 0.870 | 0.545 |
| Allelic model | −0.520 | 0.602 | −0.430 | 0.741 |
| DGCR8 rs3757 (G > A) | ||||
| Heterozygote compared with homozygote wild | 1.000 | 0.317 | NA | NA |
| Homozygote variant compared with homozygote wild | 1.000 | 0.317 | NA | NA |
| Dominant model | 1.000 | 0.317 | NA | NA |
| Recessive model | 1.000 | 0.317 | NA | NA |
| Allelic model | 1.000 | 0.317 | NA | NA |
| DGCR8 rs417309 (G > A) | ||||
| Heterozygote compared with homozygote wild | −0.940 | 0.348 | −2.150 | 0.098 |
| Homozygote variant compared with homozygote wild | 0.680 | 0.497 | 1.460 | 0.282 |
| Dominant model | −1.320 | 0.188 | −1.460 | 0.219 |
| Recessive model | 0.680 | 0.497 | 1.710 | 0.230 |
| Allelic model | −0.190 | 0.851 | −1.320 | 0.258 |
| DGCR8 rs1640299 (T > G) | ||||
| Heterozygote compared with homozygote wild | −0.680 | 0.497 | −0.540 | 0.644 |
| Homozygote variant compared with homozygote wild | 0.000 | 1.000 | −0.560 | 0.635 |
| Dominant model | −0.680 | 0.497 | −0.560 | 0.634 |
| Recessive model | 0.000 | 1.000 | −0.070 | 0.949 |
| Allelic model | −0.680 | 0.497 | −0.880 | 0.471 |
The results are in bold if P<0.1. Abbreviation: NA, not available.
Publication bias
Begg’s and Egger’s tests were performed to evaluate the potential publication bias. The publication bias was revealed in the heterozygote genotype and the dominant models of rs10719 SNP in both Begg’s and Egger’s tests, for P<0.1 (Table 4). This might be due to the language bias, the lack of publications with opposing results, and/or the inflated estimates caused by a deficient methodological design in smaller studies [1].
Discussion
In the present study, total seven SNPs in DROSHA and DGCR8 genes were comprehensively reviewed and analyzed to estimate their associations with the risk of overall cancer. Of these seven SNPs, four (rs6877842, rs642321, rs2291109, rs3757) were analyzed for the first time. Our findings indicated that rs417309 SNP of DGCR8 might facilitate the cancerogenesis. Moreover, correlations with cancer risk could also be observed in stratified analyses of DROSHA rs10719, rs6877842 SNPs and DGCR8 rs417309 SNP. No associations were revealed amongst other studied SNPs.
Polymorphisms in DROSHA
As an RNase III superfamily member, DROSHA initiates miRNA processing by converting pri-miRNA into pre-miRNA. Current studies have indicated the role of DROSHA on the development of several sorts of cancers such as laryngeal, bladder, lung, and so on [13–16]. And mounting studies have focussed on the correlations of DROSHA polymorphisms with cancer risk. Based on our analyses, the significant associations with cancer risk could be observed in rs10719 and rs6877842 SNPs.
Regarding rs10719 T/C polymorphism, we presented significant associations between rs10719 SNP (CC or TC + CC genotypes) and cancer risk in Asian population. Located in the DROSHA 3′-UTR region, the T to C substitution of rs10719 disrupted an hsa-miR-27b binding site, which was identified by luciferase reported gene assays, leading to an overexpression of DROSHA gene at the post-transcriptional level [16]. The overexpression of DROSHA caused by rs10719-C allele was elucidated to facilitate the proliferation and inhibit apoptosis of cancer cells [17–19], which was in-line with our meta-analysis findings. The meta-analysis of rs10719 analyzed six case–control studies, five of which, however, were inconsistent with our study. From our viewpoint, this phenomenon might be owing to the limitation of sample size, diversity of cancer type, and/or complexity of environmental factors. Hence, further investigations that concentrate on rs10719 SNP are extremely needed to obtain more credible results.
As for rs6877842 G/C polymorphism, strong/moderate correlations with the laryngeal cancer could be observed in every genetic model except recessive model and the rs687742-C allele manifested a protective effect on laryngeal cancer. Located in the promoter region of DROSHA, the rs6877842 SNP might influence the expression level of DROSHA by altering the transcription factor binding sites, which was forecasted by a bioinformatics website ‘https://snpinfo.niehs.nih.gov/’, thus inhibiting the laryngeal cancer development. Our study analyzed five case–control studies on different cancers: laryngeal (two), lung (one), hepatocellular (one), and renal cell carcinoma (one). Interestingly, the association of rs6877842 SNP could only be observed in laryngeal cancer subgroup, rather than in the overall cancer analysis. And the between-study heterogeneity was absent after we conducted the ‘cancer type’ subgroup. Thus, it is reasonable to suggest that the effect of rs6877842 SNP on overall cancer susceptibility could be masked by the existence of heterogeneity deriving from different types of cancer. Further investigations on this SNP are in demand to verify our speculation.
Polymorphisms in DGCR8
The Drosha–DGCR8 microprocessor complex could mediate the biogenesis from pri-miRNA to pre-miRNA, whereas neither Drosha or recombinant DGCR8 alone is active in this processing, suggesting that both the proteins are indispensable in miRNA maturing processing [20]. DGCR8 are also referred to as Pasha, stabilizes Drosha by protein–protein, and takes charge of recognizing ssRNA and dsRNA structures [21]. Studies have revealed the up-regulation of DGCR8 expression in various cancers [22,23]. And accumulating researches have focussed on the associations between the DGCR8 polymorphisms and cancer risk.
The rs417309 G/A polymorphism was the most extensively investigated one amongst DGCR8 SNPs, and the rs417309-A allele was strongly associated with an elevated cancer susceptibility. Based on the bioinformatics website prediction ‘https://snpinfo.niehs.nih.gov/’, rs417909 SNP was located at miRNA-binding sites (miR-106b and miR-579) in 3′-UTR region of DGCR8. The risk allele rs417309-A could elevate DGCR8 expression level, probably through interrupting miRNA binding [2], thus facilitating the cancer development. The meta-analysis of rs417309 SNP involved six case–control studies. Two of them, however, showed no association with cancer risk. Thus, further investigations concerned with rs417309 SNP remain in strong demand for identifying this potential cancer biomarker.
Limitations in our meta-analysis must be recognized. First, only eligible articles published in English were incorporated in our study, which might result in certain publication bias. Second, studies of DROSHA and DGCR8 polymorphisms on cancer predisposition field remain emerging, which resulted in limited number of the relevant investigations. Third, we did not analyze the association of polymorphisms in other miRNA-machinery genes, which were listed in Table 5 including: DICER1, XPO5, RAN, TARBP2, AGO2, HIWI, GEMIN3, and GEMIN4. Because their study number was limited or because they have already been analyzed in other meta-analyses.
Table 5. Reviews of the other miRNA-machinery gene polymorphisms studied in regard to cancer risk.
| Gene | SNP | Position | Cancer type | Citation |
|---|---|---|---|---|
| XPO5 | rs11077 (A > G) | 3′-UTR | EC, BC, CRC, TC, RCC, bladder, and larynx cancer | [2,14,15,25,29–34] |
| RAN | rs14035 (C > T) | 3′-UTR | RCC, LC, CRC, EC, GC, OC, and larynx cancer | [14,15,24,25,29,32–35] |
| rs3803012 (A > G) | 3′-UTR | BC, HNC, CC, and HCC | [2,36–38] | |
| rs3809142 (C > T) | Upstream | BC | [2] | |
| rs7301722 (C > A) | Upstream | BC | [2] | |
| rs7958223 (C > A) | Intron | BC | [31] | |
| rs10848236 (G > A) | Intron | BC | [31] | |
| DICER1 | rs1057035 (T > C) | 3′-UTR | BC | [2,31] |
| rs3742330 (A > G) | 3′UTR | PC, LC, and larynx cancer | [15,25,39] | |
| rs2282265 (A > G) | Intron | BC | [31] | |
| rs13078 (T > A) | 3′-UTR | LC and larynx | [15,25] | |
| TARBP2 | rs784567 (A > G) | 5′-UTR | PC and larynx | [25] |
| rs2280448 (C > T) | 5′-UTR | BC | [31] | |
| AGO1 | rs595055 (G > A) | Intron | BC | [31] |
| rs11263833 (T > G) | Intron | BC | [31] | |
| rs636832 (A > G) | Intron | LC | [15] | |
| rs595961 (G > A) | Intron | LC and RCC | [14,15] | |
| AGO2 | rs4961280 (A > C) | Upstream | PC | [39] |
| rs77216619 (G/T) | Intron | BC | [2] | |
| rs78796470 (C > T) | Intron | BC | [2] | |
| rs2292779 (G > C) | Intron | BC | [31] | |
| rs3864659 (A > C) | Intron | BC | [31] | |
| rs7016981 (T > C) | Intron | BC | [31] | |
| rs7824304 (C > T) | Intron | BC | [31] | |
| rs11786030 (A > G) | 3′-UTR | BC | [31] | |
| HIWI | rs10773771 (T > C) | 3′-UTR | BC | [2] |
| rs4759659 (G > A) | Intron | BC | [31] | |
| rs7963072 (G > A) | Intron | BC | [31] | |
| rs1106042 (G > A) | Exon (K527R) | BC and LC | [15,31] | |
| rs11060845 (G > T) | Intron | BC | [31] | |
| GEMIN3 | rs197414 (C > A) | Exon (S693R) | PC, BC, and LC | [15] |
| rs197388 (T > A) | Upstream | LC | [15] | |
| rs197412 (T > C) | Exon (T636I) | LC, RCC, and OC | [14,15,35] | |
| rs11584657 (C > T) | Upstream | BC | [2] | |
| rs17504173 (A > G) | 3′-UTR | BC | [2] | |
| rs197413 (G > A) | Exon (V642V) | BC | [31] | |
| rs17569368 (A > T) | Intron | BC | [31] | |
| GEMIN4 | rs7813 (C > T) | Exon (C1033R) | PC,BC, LC, and RCC | [14,15,31] |
| rs3744741 (C > T) | Exon (R684Q) | BC and LC | [2,15,31] | |
| rs4968104 (T > A) | Exon (V593E) | BC and LC | [2,15,31] | |
| rs2251689 (G > A) | Upstream | BC | [2] | |
| rs2740348 (C > G) | Exon (E450Q) | BC and LC | [15] | |
| rs910924 (C > T) | Upstream | LC | [15] | |
| rs910925 (G > C) | Exon (G579A) | LC | [15] | |
| rs1062923 (T > C) | Exon (T739I) | LC | [15] | |
| rs2740349 (A > G) | Exon (N929D) | BC | [31] | |
| FMR1 | rs25704 (T > C) | 3′-UTR | BC | [31] |
| rs28900 (A > C) | Intron | BC | [31] | |
| rs971000 (C > T) | Intron | BC | [31] |
Abbreviations: BC, breast cancer; CC, cervical cancer; CRC, colorectal cancer; EC, esophageal cancer; GC, gastric cancer; HCC, hepatocellular carcinoma; HNC, head and neck cancer; LC, lung cancer; OC, oral cancer; PC, prostate cancer; RCC, renal cell carcinoma; TC, thyroid cancer.
In summary, we performed a systematic review on the association between DROSHA and DGCR8 polymorphisms and risk of cancer. Meanwhile, all available data were utilized to achieve a meta-analysis for seven prevalent SNPs. Three of them (DROSHA rs10719, rs6877842, and DGCR8 rs417309) were revealed to be associated with risk of cancer in whole population or some particular subgroups. Our study generalized the status quo of the current studies on cancer-related polymorphisms in DROSHA and DGCR8 genes, supplying investigators with novel clues for identifying new biomarkers with cancer-forewarning function.
Supporting information
Table S1. ORs (95%CIs) of sensitivity analysis.
Abbreviations
- HB
hospital-based
- HWE
Hardy–Weinberg equilibrium
- OR
odds ratio
- PB
population-based
- pre-miRNA
precursor miRNA
- pri-miRNA
primary miRNA
- SNP
single nucleotide polymorphism
- 95% CI
95% confidence interval
Author contribution
M.S. conceived and designed the study. J.W. and Z.L. were responsible for the data extraction. H.D. and X.F. were responsible for the quality assessment. J.W. and M.S. wrote the manuscript, and M.S. revised the manuscript.
Competing interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
The authors confirm that there is no funding to be acknowledged.
References
- 1.Xu Q., Liu J.W. and Yuan Y. (2015) Comprehensive assessment of the association between miRNA polymorphisms and gastric cancer risk. Mutat. Res. Rev. Mutat. Res. 763, 148–160 10.1016/j.mrrev.2014.09.004 [DOI] [PubMed] [Google Scholar]
- 2.Jiang Y., et al. (2013) Evaluation of genetic variants in microRNA biosynthesis genes and risk of breast cancer in Chinese women. Int. J. Cancer 133, 2216–2224 10.1002/ijc.28237 [DOI] [PubMed] [Google Scholar]
- 3.He L., et al. (2005) A microRNA polycistron as a potential human oncogene. Nature 435, 828–833 10.1038/nature03552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iorio M.V., et al. (2005) MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 65, 7065–7070 10.1158/0008-5472.CAN-05-1783 [DOI] [PubMed] [Google Scholar]
- 5.Takamizawa J., et al. (2004) Reduced expression of the let-7 microRNAs in human lung cancers in association with shortened postoperative survival. Cancer Res. 64, 3753–3756 10.1158/0008-5472.CAN-04-0637 [DOI] [PubMed] [Google Scholar]
- 6.Kumar M.S., et al. (2007) Impaired microRNA processing enhances cellular transformation and tumorigenesis. Nat. Genet. 39, 673–677 10.1038/ng2003 [DOI] [PubMed] [Google Scholar]
- 7.Li Y., Zhang F. and Yang D. (2017) Comprehensive assessment and meta-analysis of the association between CTNNB1 polymorphisms and cancer risk. Biosci. Rep. 37, 10.1042/BSR20171121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lv Z., Xu Q. and Yuan Y. (2017) A systematic review and meta-analysis of the association between long non-coding RNA polymorphisms and cancer risk. Mutat. Res. 771, 1–14 10.1016/j.mrrev.2016.10.002 [DOI] [PubMed] [Google Scholar]
- 9.Mantel N. and Haenszel W. (1959) Statistical aspects of the analysis of data from retrospective studies of disease. J. Natl. Cancer Inst. 22, 719–748 [PubMed] [Google Scholar]
- 10.DerSimonian R. and Laird N. (1986) Meta-analysis in clinical trials. Control. Clin. Trials 7, 177–188 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 11.Egger M., et al. (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harbord R.M., Egger M. and Sterne J.A. (2006) A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat. Med. 25, 3443–3457 10.1002/sim.2380 [DOI] [PubMed] [Google Scholar]
- 13.Bruzgielewicz A., et al. (2016) Evaluation of polymorphisms in microRNA biosynthesis genes and risk of laryngeal cancer in the Polish population. Pol. J. Pathol. 67, 283–290 10.5114/pjp.2016.63781 [DOI] [PubMed] [Google Scholar]
- 14.Horikawa Y., et al. (2008) Single nucleotide polymorphisms of microRNA machinery genes modify the risk of renal cell carcinoma. Clin. Cancer Res. 14, 7956–7962 10.1158/1078-0432.CCR-08-1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim J.S., et al. (2010) Association of a common AGO1 variant with lung cancer risk: a two-stage case-control study. Mol. Carcinog. 49, 913–921 10.1002/mc.20672 [DOI] [PubMed] [Google Scholar]
- 16.Yuan L., et al. (2013) Genetic variation in DROSHA 3′UTR regulated by hsa-miR-27b is associated with bladder cancer risk. PLoS One 8, e81524 10.1371/journal.pone.0081524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dedes K.J., et al. (2011) Down-regulation of the miRNA master regulators Drosha and Dicer is associated with specific subgroups of breast cancer. Eur. J. Cancer 47, 138–150 10.1016/j.ejca.2010.08.007 [DOI] [PubMed] [Google Scholar]
- 18.Han Y., et al. (2013) Inducing cell proliferation inhibition and apoptosis via silencing Dicer, Drosha, and Exportin 5 in urothelial carcinoma of the bladder. J. Surg. Oncol. 107, 201–205 10.1002/jso.23214 [DOI] [PubMed] [Google Scholar]
- 19.Sugito N., et al. (2006) RNASEN regulates cell proliferation and affects survival in esophageal cancer patients. Clin. Cancer Res. 12, 7322–7328 10.1158/1078-0432.CCR-06-0515 [DOI] [PubMed] [Google Scholar]
- 20.Han J., et al. (2006) Molecular basis for the recognition of primary microRNAs by the Drosha-DGCR8 complex. Cell 125, 887–901 10.1016/j.cell.2006.03.043 [DOI] [PubMed] [Google Scholar]
- 21.Han J., et al. (2009) Posttranscriptional crossregulation between Drosha and DGCR8. Cell 136, 75–84 10.1016/j.cell.2008.10.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim B., et al. (2014) An essential microRNA maturing microprocessor complex component DGCR8 is up-regulated in colorectal carcinomas. Clin. Exp. Med. 14, 331–336 10.1007/s10238-013-0243-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sand M., et al. (2012) Expression levels of the microRNA maturing microprocessor complex component DGCR8 and the RNA-induced silencing complex (RISC) components argonaute-1, argonaute-2, PACT, TARBP1, and TARBP2 in epithelial skin cancer. Mol. Carcinog. 51, 916–922 10.1002/mc.20861 [DOI] [PubMed] [Google Scholar]
- 24.Cho S.H., et al. (2015) 3′-UTR polymorphisms in the MiRNA machinery genes DROSHA, DICER1, RAN, and XPO5 are associated with colorectal cancer risk in a Korean population. PLoS ONE 10, e0131125 10.1371/journal.pone.0131125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Osuch-Wojcikiewicz E., et al. (2015) Association of polymorphic variants of miRNA processing genes with larynx cancer risk in a Polish population. Biomed. Res. Int. 2015, 298378 10.1155/2015/298378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim M.N., et al. (2016) Variation in the Dicer and RAN genes are associated with survival in patients with hepatocellular carcinoma. PLoS ONE 11, e0162279 10.1371/journal.pone.0162279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song X., et al. (2017) Association between SNPs in microRNA machinery genes and gastric cancer susceptibility, invasion, and metastasis in Chinese Han population. Oncotarget 8, 86435–86446 10.18632/oncotarget.21199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang B., et al. (2011) Genetic variants associated with breast-cancer risk: comprehensive research synopsis, meta-analysis, and epidemiological evidence. Lancet Oncol. 12, 477–488 10.1016/S1470-2045(11)70076-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang H., et al. (2008) Evaluation of genetic variants in microRNA-related genes and risk of bladder cancer. Cancer Res. 68, 2530–2537 10.1158/0008-5472.CAN-07-5991 [DOI] [PubMed] [Google Scholar]
- 30.Ye Y., et al. (2008) Genetic variations in microRNA-related genes are novel susceptibility loci for esophageal cancer risk. Cancer Prev. Res. (Phila.) 1, 460–469 10.1158/1940-6207.CAPR-08-0135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sung H., et al. (2011) Common genetic polymorphisms of microRNA biogenesis pathway genes and risk of breast cancer: a case-control study in Korea. Breast Cancer Res. Treat. 130, 939–951 10.1007/s10549-011-1656-2 [DOI] [PubMed] [Google Scholar]
- 32.Xie Y., et al. (2015) Single-nucleotide polymorphisms of microRNA processing machinery genes are associated with risk for gastric cancer. Onco. Targets Ther. 8, 567–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao Y., et al. (2015) Single-nucleotide polymorphisms of microRNA processing machinery genes and risk of colorectal cancer. Onco. Targets Ther. 8, 421–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buas M.F., et al. (2015) MiRNA-related SNPs and risk of esophageal adenocarcinoma and Barrett’s esophagus: post genome-wide association analysis in the BEACON consortium. PLoS ONE 10, e0128617 10.1371/journal.pone.0128617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roy R., et al. (2014) Genetic variations at microRNA and processing genes and risk of oral cancer. Tumour Biol. 35, 3409–3414 10.1007/s13277-013-1450-3 [DOI] [PubMed] [Google Scholar]
- 36.Ma H., et al. (2012) Genetic variations in key microRNA processing genes and risk of head and neck cancer: a case-control study in Chinese population. PLoS ONE 7, e47544 10.1371/journal.pone.0047544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen J., et al. (2013) Genetic variants in RAN, DICER and HIWI of microRNA biogenesis genes and risk of cervical carcinoma in a Chinese population. Chin. J. Cancer Res. 25, 565–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu L., et al. (2013) Potentially functional genetic variants in microRNA processing genes and risk of HBV-related hepatocellular carcinoma. Mol. Carcinog. 52, E148–E154 10.1002/mc.22062 [DOI] [PubMed] [Google Scholar]
- 39.Nikolic Z., et al. (2017) Genetic variants in RNA-induced silencing complex genes and prostate cancer. World J. Urol. 35, 613–624 10.1007/s00345-016-1917-0 [DOI] [PubMed] [Google Scholar]
- 40.Lv Z., et al. (2017) Long non-coding RNA polymorphisms in 6p21.1 are associated with atrophic gastritis risk and gastric cancer prognosis. Oncotarget 8, 95303–95315 10.18632/oncotarget.20115 [DOI] [PMC free article] [PubMed] [Google Scholar]