Abstract
The α-proteobacterium Caulobacter crescentus possesses a developmental cell cycle that restricts chromosome replication to a stalked cell type. The proposed C.crescentus chromosome replication origin (Cori) lies between hemE and RP001, an unusual intergenic region not previously associated with bacterial replication origins, although a similar genomic arrangement is also present at the putative replication origin in the related bacterium Rickettsia prowazekii. The cloned Cori supports autonomous plasmid replication selectively in the stalked cell type implying that replication of the entire chromosome also initiates between hemE and RP001. To confirm this location, we applied the 2-D (N/N) agarose gel electrophoresis technique to resolve and identify chromosome replication intermediates throughout a 30 kb region spanning Cori. Replication initiation in Cori was uniquely characterized by an ‘origin bubble and Y-arc’ pattern and this observation was supported by simple replication fork ‘Y-arc’ patterns that characterized the regions flanking Cori. These replication forks originated bi-directionally from within Cori as determined by the fork direction assay. Therefore, chromosomal replication initiates from the unusual hemE/RP001 intergenic region that we propose represents a new class of replication origins.
INTRODUCTION
Prokaryotic replication origins are identified primarily by the autonomous plasmid replication activity of cloned DNA. Fragments that uniquely support replication are often termed autonomous replicating sequence (ARS) elements. In prokaryotes, ARS elements contain conspicuous DNA sequence motifs such as DnaA boxes and AT-rich regions (1,2). In addition, genes flanking the ARS element are often conserved. For example, the gidAB and dnaA genes are generally adjacent to replication origins (3).
However, ARS assays and sequence analysis only provide indirect evidence that replication initiates from these sequences on the actual chromosome. Thus, it is important to directly demonstrate that chromosomal replication initiates within the ARS elements in vivo. The two-dimensional (2-D) DNA neutral/neutral (N/N) agarose gel assay detects DNA structures (4) and can map replication origins (5). This assay detects unique DNA structures characteristic of certain replication intermediates such as replication forks (Y-arcs) and origin bubbles, which indicate a replication origin within the area of interest. In eukaryotes, 2-D gel analysis demonstrated that not all ARS elements are active replication origins on the chromosome (6).
In Bacillus subtilis (7), this 2-D gel assay confirmed the identified ARS element as the chromosome replication origin. However, 2-D gel assay analysis of an ARS element identified in Coxiella burnetii proved otherwise, since in vivo chromosomal replication initiation was not detected within the fragment (8).
In vivo chromosomal replication has been successfully mapped by 2-D gel analysis in a wide variety of eukaryotes (9–14), but relatively few prokaryotes (15–17). Prokaryotes are often poor subjects for 2-D gel analysis as the majority are difficult to culture with synchronized chromosomal DNA replication.
Caulobacter crescentus chromosomal replication is easily synchronized and studied as a model developmental control problem. This Gram-negative aquatic bacterium divides asymmetrically to produce two distinct cell types (swarmer and stalked cells) (18) that differ in both their morphology and developmental programs (19) (Fig. 1A). The progeny stalked cell possesses a tubular appendage (the stalk), and has the ability to initiate chromosomal replication immediately after asymmetric division. The progeny swarmer cell, however, is equipped with a polar flagellum and chemosensory system used to actively seek out a suitable environment for growth, and its chromosomal replication is delayed until it differentiates into a stalked cell. The swarmer cells can be isolated via a density gradient (20), and synchronous chromosome replication can be studied as the swarmer cells transit into stalked cells.
Figure 1.
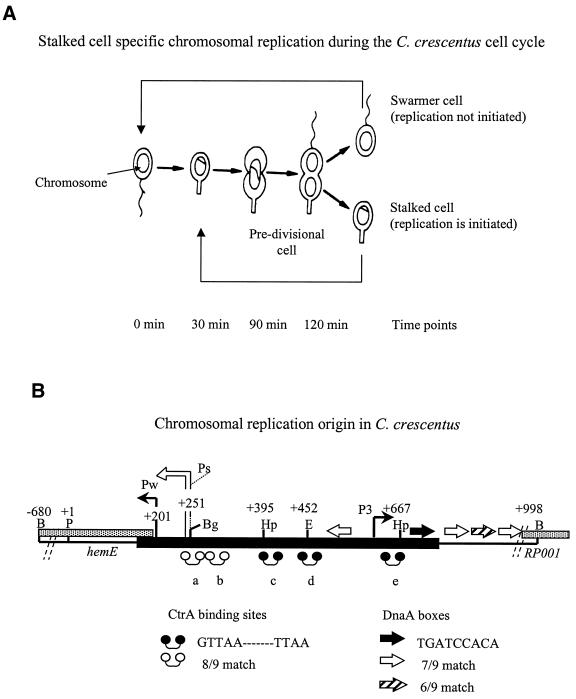
(A) The C.crescentus life cycle. The flagellated swarmer cell differentiates into the stalked cell where replication is initiated and asymmetric cell division proceeds. (B) The chromosome replication origin (Cori). The thick line represents minimal ∼500 bp DNA required for autonomous Cori-plasmid replication. Arrows are proposed DnaA boxes: the filled arrow is a 8/9 bp match to the E.coli consensus sequence, TTATCCACA; the open and diagonally filled arrows are weaker 7/9 bp and 6/9 bp matches to consensus sequence, respectively. Dumbbells are CtrA binding sites: filled dumbbells are CtrA consensus sequences GTTAA-N7-TTAA; open dumbbells are 8/9 matches to this consensus sequence. Restriction enzymes: B = BamHI; Bg = BglII; E = EcoRI; Hp = HpaI; P = PstI. The strong (Ps) and weak (Pw) promoters are located upstream of the 5′ termini of the hemE homolog that overlaps one end of Cori. The third promoter (P3) is approximately located just before CtrA binding site ‘e’ and upstream of the RP001 homolog.
Caulobacter crescentus has apparently one chromosomal origin of bi-directional replication based on low resolution pulsed-field gel electrophoresis (PFGE) (21), early 32P pulse-labeling and ARS assays (22). Sequence analysis of the ∼500 bp ARS Caulobacter origin of replication initiation (Cori) shares features with the Escherichia coli origin (oriC), namely DnaA boxes and an AT-rich region (22) (Fig. 1B). However, several features are unique to Cori (Fig. 1B). Two promoters are located in the AT-rich region of Cori: a strong promoter (Ps) and a weak promoter (Pw) (23). Ps produces transcripts that are not translated, and it has been speculated that these transcripts are involved in the initiation of chromosomal replication. Pw directs transcription of the heme biosynthetic (hemE) operon of which the 5′ terminus overlap ARS Cori (23). An unassigned but conserved open reading frame, homologous to Rickettsia prowazekii RP001, lies on the other side of Cori (24). Additional unique Cori features include five 9mer (GTTAA-N7-TTAA) motifs, designated ‘a’ to ‘e’, which are binding sites for a global response regulator, cell cycle transcription activator (CtrA) (25,26). CtrA plays a major role in the regulation of chromosomal replication by binding these motifs (27,28). A third promoter (P3) is located upstream of CtrA binding site ‘e’ and the RP001 homolog (Fig. 1B).
Therefore, ARS Cori has unusual features and it is located within an unusual intergenic hemE/RP001 region that has not been previously observed to be associated with replication origins. Based on genome-wide GC strand bias, a putative replication origin has been assigned to R.prowazekii, which also shares this intergenic hemE/RP001 region (24). In addition, a CtrA homolog has been found in R.prowazekii as well as in other members of the α-proteobacteria (29). These molecular as well as genetic differences, in comparison with E.coli oriC as well as other bacterial origins, suggest an alternative class of replication origins unique to α-proteobacteria, and we therefore sought direct evidence that chromosomal replication initiates within Cori.
In this study, we performed 2-D gel assays with chromosomal DNA prepared from wild-type and conditional DNA synthesis mutant strains of C.crescentus. We mapped the movements of the replication forks emerging from Cori within an ∼30 kb region of earliest replicating DNA. These studies confirm that Cori is a bidirectional replication origin.
MATERIALS AND METHODS
Bacterial strains, plasmids and growth conditions
All strains and plasmids used in this study are listed in Table 1. Caulobacter crescentus cells were grown at 30 and 28°C for strains NA1000 and PC2179, respectively, in PYE medium or in M2G (synthetic 0.2% glucose) medium (30) supplemented with antibiotics, 20 µg/ml nalidixic acid (post-conjugation) and 5 µg/ml gentamycin (plasmid selection), when appropriate. Escherichia coli host strains were grown at 37°C in LB supplemented with antibiotics, ampicillin 100 µg/ml and tetracycline 15 µg/ml, when appropriate.
Table 1. Bacterial strains and plasmid used in this work.
| Strains |
|
Genotype or description |
Reference or source |
|
C.crescentus |
NA1000 |
Synchronizable derivative of CB15 |
20 |
| |
PC2179 |
CB15N dnaC303 temperature sensitive |
33 |
|
E.coli |
S17-1 |
294::RP4-2 (Tc::Mu) (Km::Tn7); conjugation strain |
40 |
| |
DH10B |
F– mcrA Δ(mrr-hsdRMS-mcrBC) φ80dlacZΔM15 ΔlacX74 endA1 recA1 deoR Δ(ara,leu)7697 araD139 galU galK nupG rpsL |
Gibco BRL |
| |
DH5α |
F′ /endA1 hsdR17(rk–mk+) supE44 thi-1 recA1 gyrA (NaIr) relA1 Δ(lacIZYA-argF)U169 deoR [φ80dlacΔ(lacZ)M15] |
Dr Michael S. DuBow |
| Plasmids |
pBluescriptII |
ApR; SK(+) or KS(+) cloning sites |
Stratagene |
| |
pGM1497 |
DH10B BluescriptII pSK(+) BamHI fragment C2 (includes dnaA) |
This study |
| |
pGM1499 |
DH10B BluescriptII pSK(+) BamHI fragment D |
22 |
| |
pGM1500 |
DH10B BluescriptII pSK(+) BamHI fragment E (includes Cori) |
22 |
| |
pGM1728 |
DH10B BluescriptII pSK(+) BamHI fragment C1 |
This study |
| |
pGM1519 |
DH10B BluescriptII pKS(+) HindIII fragment B |
This study |
| |
pGM1542 |
DH10B BluescriptII pKS(+) EcoRI fragment A |
This study |
| |
PGM948 |
DH10B BluescriptII pSK(+) 810 bp XbaI to SalI C.crescentus dnaA |
35 |
| pGM2526 | DH5α BluescriptII pKS (+) 2.2 kb HincII/SacI fragment (containing Cori) of pGM1500 | This study |
Synchrony and preparation of chromosomal DNA samples
Synchronized swarmer cells (NA1000 and dnaC303) were prepared from cultures grown to OD660 = 0.8–1.0 in M2G. Swarmer cells (judged >95% pure by light microscopy) were isolated by the Ludox (DuPont) gradient method (20), and released in fresh M2G media. Synchronized culture dnaC303 was initially incubated at the non-permissive temperature 37°C and down-shifted to the permissive temperature 28°C at the 60 min time-point, whereas the synchronized culture NA1000 remained at 30°C throughout the time course.
For the hemi-methylation assay, at each time-point in the synchrony, 1 ml of culture was taken and centrifuged in an eppendorf tube and resuspended in 100 µl of GT solution (50 mM glucose, 25 mM Tris pH 7.5). Immediately before quick freezing in –80°C ethanol, 10 µl of 0.5 M EDTA pH 8.0 was added, and stored at –80°C overnight. After thawing at 37°C, 400 µl of digestion buffer was added (20 mM Tris pH 7.5, 0.5% Triton X-100, 40 mM EDTA pH 8.0, 1 mg/ml lysozyme) and incubated at 37°C for 8 h. Proteinase K (5 µl of 20 mg/ml stock) was added and incubated at 37°C overnight. Ammonium acetate (50 µl of 10 M stock) was added, gently mixed, 600 µl of phenol:chloroform:isoamyl alcohol was added, vortexed, and centrifuged at 10 000 r.p.m. (IEC MicroMax) for 10 min at room temperature. The aqueous layer and back extraction with 100 µl TE-1 buffer (10 mM Tris pH 7.5, 1 mM EDTA pH 8.0) was re-extracted and mixed with 2 vol of absolute ethanol, and centrifuged at 10 000 r.p.m. for 30 min at 4°C. The DNA pellet was washed in 70% ethanol, air-dried, re-suspended in 50 µl TE-1 buffer with RNaseA (20 µg/ml) and stored at 4°C. To digest, 10 µl of each DNA sample was cut with HincII (10 U) in standard restriction endonuclease digest reactions including 100 µg/ml bovine serum albumin (BSA). The samples were run on a 1.2% 1× TBE with 0.2 µg/ml ethidium bromide (EtBr) agarose gel at 6 V/cm.
For 2-D gel analysis samples, at each time-point, 5 ml of culture was taken and mixed with 1 ml of cold (0°C) 1% sodium azide and 1 M sodium phosphate, centrifuged at 7000 r.p.m. for 5 min at 4°C, and resuspended in 100 µl of TS buffer (10 mM Tris pH 7.5 and 1 M NaCl). The sample was mixed with 100 µl molten 2% low-melt agarose, pipetted into gel brick molds (BioRad) and allowed to harden at 4°C. The gel bricks were incubated at 37°C overnight in 5 ml of EC lysis buffer (6 mM Tris pH 7.5, 1 M NaCl, 100 mM EDTA, 0.5% N-Lauryl Sarcosine Sulfate, 20 µg/ml RNaseA and 1 mg/ml lysozyme). The EC lysis buffer was removed and replaced with 5 ml of ESP buffer (0.5 M EDTA, 1% N-Lauryl Sarcosine Sulfate and 2 mg/ml proteinase K) and incubated at 37°C overnight. The gel bricks were rinsed with TE-1 buffer for 1 h at 4°C and repeated twice more. The gel bricks were stored in TE-2 buffer (10 mM Tris pH 7.5, 0.1 mM EDTA pH 8.0) at 4°C.
For 2-D gel digest, approximately half of the agarose gel brick was used for each digest assuming the agarose slice has a density equal to water (1 g/ml) to account for volume in the restriction endonuclease digest. The half gel brick was rinsed twice in distilled water for 15 min each on ice, and repeated for 1× restriction endonuclease buffer. The standard restriction digest included 100 µg/ml BSA. For the fork direction assay, the first dimension lane was excised and placed in a multi-pipette reservoir. The gel slice was rinsed with TE-2 buffer two times for 1 h each at room temperature. This was repeated with 1× restriction endonuclease buffer. After draining and removal of excess buffer, a clean razor blade was used to make a lengthwise cut of ∼0.5 mm depth along one of the cut surfaces of the gel slice. SacI or SacII (∼300–400 U) was applied evenly in the cut. The reservoir containing the gel slice was placed in a closed container with a little water for humidity at 37°C. After overnight incubation, the gel slice was briefly rinsed with TE-2 buffer and placed in a gel tray for preparation of the second dimension agarose gel.
2-D N/N DNA agarose gel electrophoresis
The procedure for 2-D gel electrophoresis is as outlined in Brewer and Fangman (5) and Friedmann and Brewer (31) with a few modifications. The dimensions of the gel tray used were 12 × 14 cm. Loading of the gel brick in to the well (12-well comb) was accomplished with 1% molten low-melt agarose. For fragments of ≥2 kb in size, the first dimension, 0.7% 1× TBE agarose gel, was run at 1.5 V/cm and stained in 1× TBE/EtBr for 1 h, for photographic record and orientation. The second dimension, 1.3% 1× TBE/EtBr agarose gel, was run at 9 V/cm. For fragments <2 kb in size, agarose concentration was 0.4% for the first dimension and 1.6% for the second dimension.
Southern blotting, probe preparation and hybridization
Agarose gels were alkaline Southern blotted with Hybond N+ blotting paper (Amersham), and the blots UV crosslinked (32). Probes were prepared by cleaving fragments of interest from standard alkali plasmid mini-preps (32) with appropriate restriction endonucleases in standard digest reactions. The desired fragments were isolated from 1% 1× TBE/EtBr agarose gels and purified with GeneClean (Bio101 Inc.). Probe DNA (∼30 ng) was labeled with [32P]dCTP (6000 Ci/mmol; Amersham) by standard random priming reactions (32). Blots were hybridized with appropriate probes at 65°C and washed by standard procedure (32). Blots were exposed to BioMax MR film (Kodak) at –80°C overnight to 7 days or exposed to PhosphorImager screens (Molecular Dynamics) and analyzed with ImageQuant software (Molecular Dynamics).
RESULTS
Synchrony and hemi-methylation assay
Our 2-D gel analyses require synchronous cultures. The synchronizable CB15N wild-type C.crescentus (NA1000) is the obvious choice to attain chromosomal DNA samples containing replication intermediates for 2-D gel analysis. However, as the replication fork rate is estimated to be 21 kb/min (21), it is difficult to obtain samples at the precise moment replication occurs in the 30 kb region surrounding Cori. Therefore, we also used the temperature-sensitive dnaC303 mutant strain (33,34), and we demonstrate replication forks halt within the 30 kb Cori region at the non-permissive temperature. This replication block is reversible and movement of replication forks resume when cells are downshifted to the permissive temperature. Therefore, in the experiments, both strains were used for comparison and control.
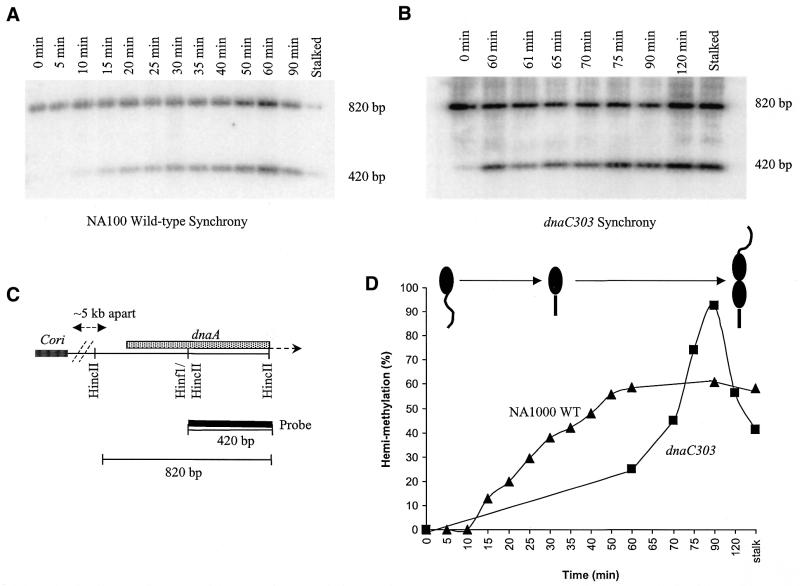
The NA1000 synchrony commenced when isolated swarmer cells were resuspended in fresh medium. The dnaC303 synchrony commenced with the holding of the resuspended swarmer cells at the non-permissive temperature (37°C) for 60 min before the downshift to the permissive temperature (28°C). As the swarmer cells transitioned into the stalked cells, chromosomal DNA samples were taken at the indicated time-points (Fig. 2). The chromosomal DNA was prepared in agarose plugs to preserve the fragile replication intermediate structures. DNA hemi-methylation assays were used to determine which time-points have abundant replication intermediates. A CcrM methylation site (GANTC) is present at dnaA, located ∼5 kb from Cori, and this site overlaps the HincII restriction site (GTPyPuAC) (Fig. 2C) (35,36). Therefore, fully methylated DNA (i.e. unreplicated DNA) cannot be cleaved by HincII at this site due to interference by the methylated adenine residue, and cleavage requires hemi-methylated DNA produced by DNA replication (35,36). Accordingly, for both NA1000 and dnaC303 strains, the DNA samples were digested with HincII, Southern-blotted and probed with a 32P-labeled HincII/HincII 420 bp fragment (Fig. 2C). Fully methylated DNA is indicated by the 820 bp band and hemi-methylated DNA is indicated by the 420 bp band (Fig. 2A and B). The bands were quantified and the percent ratio of hemi-methylated DNA to total DNA is shown at each time-point (Fig. 2D). The NA1000 synchrony clearly demonstrates the increase in hemi-methylation as the synchrony progressed and the percentile values of hemi-methylation at steady-state does not exceed 60%. The 60 min non-permissive temperature period clearly has an effect on hemi-methylation in the dnaC303 synchrony which remains at ∼20% before the downshift. After the downshift to the permissive temperature, replication rapidly proceeds and hemi-methylation percentile values increase to almost 100% at 90 min before dropping to ∼50% at 120 min. The high level of hemi-methylation of dnaC303 chromosomal DNA at 90 min indicates that the dnaC303 synchrony is of superior quality in comparison to the NA1000 synchrony.
Figure 2.
Methylation state assay for chromosome replication. (A) NA1000 wild-type C.crescentus and (B) dnaC303 mutant C.crescentus synchrony time-point chromosomal DNA samples digested with HincII, alkaline Southern blotted and probed with 32P-labeled 420 bp HincII to HincII C.crescentus dnaA for detection of fully-methylated and hemi-methylated DNA states. As a positive internal control for hemi-methylation, isolated stalked cell chromosomal DNA digested with HincII was included in the gel electrophoresis. (C) Schematic of the location of dnaA relative to Cori and HincII dnaA fragment used as a random-primed 32P-labeled probe. Note the unique overlapped HinfI(GANTC)/HincII restriction site where fully-methylated DNA will block cleavage by HincII resulting in a 820 bp hybridized band, and hemi-methylated DNA will allow cleavage by HincII resulting in a 420 bp hybridized band. (D) Percentage of hemi-methylated DNA in synchrony time-points of NA1000 wild-type (filled triangles) and dnaC303 (filled squares) C.crescentus strains. Note the cartoon of the wild-type C.crescentus cell cycle corresponds to the plotted increase in hemi-methylated DNA.
As dnaA is located proximal to Cori, the optimal chromosomal DNA samples are those that reflect a sharp increase in hemi-methylation percentage from one time-point to the next. This parameter ensures the highest probability of the presence of replication intermediates. For the NA1000 synchrony, the sharp increase in the percentage of hemi-methylation between the 10 and 15 min time-points indicates that the optimal time-point for analysis was at the 15 min time-point (Fig. 2D). For the dnaC303 synchrony, approximately two-thirds of the replication forks stall before dnaA at 37°C, then proceed through dnaA at 28°C (Fig. 2D). Thus, the 60 min period in which the cells were held at non-permissive temperature allowed accumulation of synchronous replication forks within the ∼30 kb region thereby increasing the population of replication intermediates (data not shown). After the downshift to the permissive temperature at 60 min, the 60–75 min time-points were determined to be the period of maximum fork movement through dnaA as demonstrated by the sharp increase in hemi-methylation. For consistency, the 60 min time-point was chosen for dnaC303 2-D gel analyses.
2-D gel N/N analyses of ∼30 kb Cori region
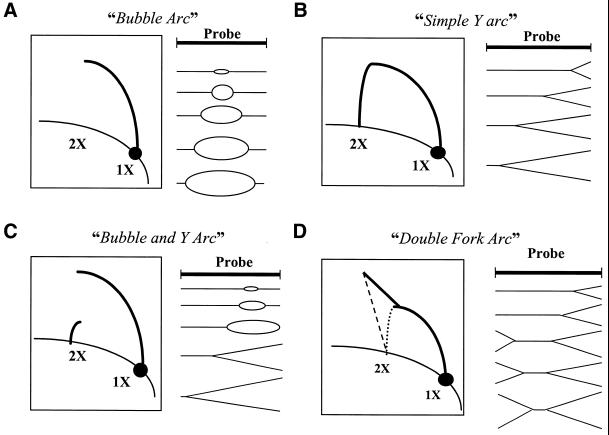
The typical 2-D gel blot hybridization patterns (5,9) are illustrated in Figure 3. Replicative chromosomal DNA is restriction endonuclease-digested, separated on a low-percentage agarose gel (first dimension) on the basis of molecular size, then placed in a second high-percentage agarose gel (second dimension) for separation in the perpendicular direction on the basis of the shape (4,5). Upon Southern blotting and hybridization, different DNA replication intermediate structures can be distinguished. The illustrations represent scenarios of four replication intermediate structures: the ‘bubble arc’, a bi-directional replication origin symmetrically located within a fragment (Fig. 3A); the ‘simple Y-arc’, a replication fork passing through a fragment from one end to the other (Fig. 3B); the ‘bubble and Y-arc’, an asymmetrically placed bi-directional replication origin that opens into a replication fork proceeding to the other end (Fig. 3C); and the ‘double-fork arc’, asymmetric progression of replication forks moving towards one another (Fig. 3D). In Figure 3 the 1X spot represents the non-replicated linear form of the fragment and the 2X spot refers to the almost complete replicated form of the fragment. These scenarios form the basis for interpretation of 2-D gel hybridization patterns obtained with C.crescentus.
Figure 3.
Typical hybridization patterns representative of certain replication intermediate structures as described by Brewer and Fangman (5). (A) Bubble arc, centralized replication origin with symmetric progression of replication forks outwards. (B) Simple Y-arc, progress of a simple replication fork from one side of the probed region. (C) Bubble and Y-arc, asymmetrically placed replication origin. (D) Double-fork arc, asymmetric progression of replication forks approaching one another in a region of interest.
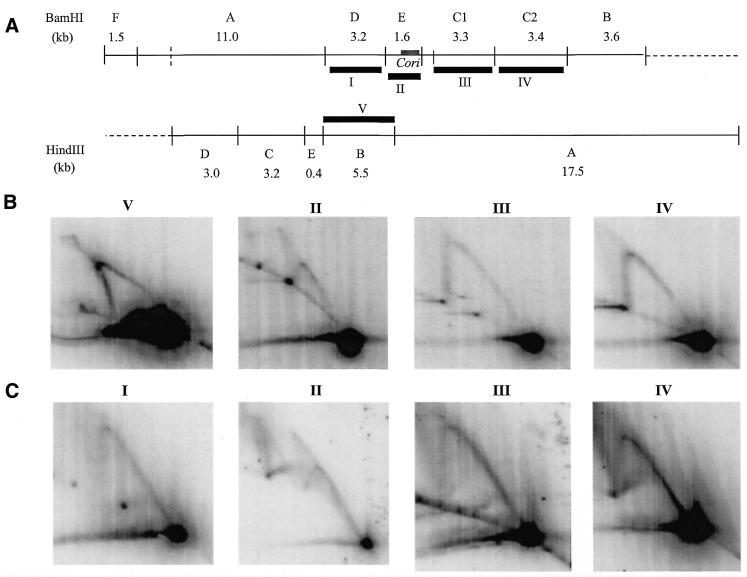
Figure 4A illustrates the BamHI and HindIII restriction site map of the ∼30 kb region, as well as the fragments (marked by roman numerals) utilized as hybridization probes. Figure 4B and C are 2-D gel hybridized blots of chromosomal DNA of NA1000 and dnaC303, respectively. The NA1000 HindIII blot hybridized with probe V (HindIII fragment B) shows a Y-arc indicative of a simple replication fork. The corresponding BamHI dnaC303 blot hybridized with probe I (BamHI fragment D) also clearly shows the Y-arc indicative of a simple replication fork (Fig. 4C). The NA1000 and dnaC303 BamHI blots hybridized with probe III (BamHI fragment C1) demonstrates simple replication fork structure. The same observation was made with NA1000 and dnaC303 BamHI blots hybridized with probe IV (BamHI fragment C2).
Figure 4.
(A) Restriction endonuclease site map of the ∼30 kb region of earliest replicating DNA which contains Cori (thick grey line). Thick black lines represent fragments (labeled with roman numerals I–VI) used as random-primed 32P-labeled probes for hybridization of 2-D gel blots. (B) NA1000 wild-type C.crescentus 15 min synchrony time point and (C) dnaC303 mutant C.crescentus 60 min time-point chromosomal DNA 2-D gel hybridized blots. Chromosomal DNA was digested with restriction endonuclease corresponding to the probes used for hybridization indicated above each blot.
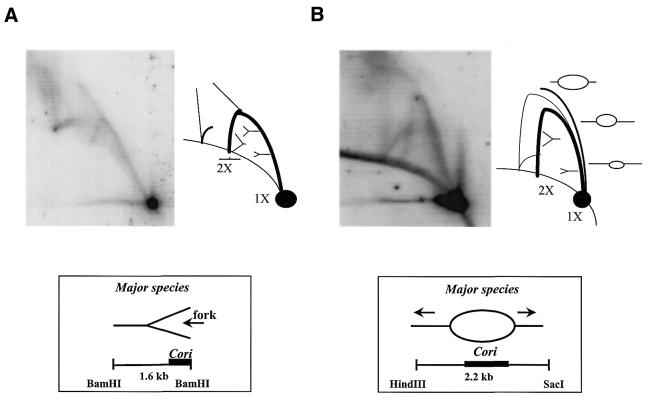
However, the NA1000 BamHI blot hybridized with probe II [BamHI fragment E (Cori)] exhibited a complex pattern, which we refer to as the ‘arc and cone’ (Fig. 4B). This arc and cone pattern is very similar to that exhibited by probe II hybridization of the dnaC303 BamHI blot, with the exception of an additional partial arc located further up from the 2X spot (Fig. 4C). This additional partial arc may be the result of incomplete digestion due to the presence of ssDNA regions within the replicative chromosomal DNA. However, this 2-D gel experiment does not show the anticipated hybridization pattern of a bubble and Y-arc as it would for an asymmetrically placed replication origin within the fragment of interest (Fig. 3C). Instead, the arc and cone pattern was tentatively interpreted as asymmetric replication forks approaching one another, which clearly did not fit the existing model of initiation of bi-directional replication proposed for C.crescentus.
In order to resolve the pattern above the BamHI fragment E (Cori) Y-arc, we increased the size of the Cori probe and changed the electrophoresis conditions. The new 2.2 kb HincII/SacI probe centralizes Cori, allowing the occurrence of the typical bubble arc pattern (Fig. 3A), and the increased agarose gel percentage in the second dimension electrophoresis optimizes resolution of a smaller than average fragment. The modified 2-D gel dnaC303 HincII/SacI blot is shown (Fig. 5B) in comparison to the original dnaC303 BamHI blot from Figure 4C (Fig. 5A). The modified 2-D gel HincII/SacI blot presents a very different hybridization pattern from that observed in the BamHI blot. The accompanying illustration to the modified HincII/SacI blot outlines the replication intermediates interpreted from the hybridization pattern (Fig. 5B). A faint ‘origin bubble arc’ signal is detected in combination with a dominantly intense Y-arc signal. This combination of hybridization signals is indicative of a bubble and Y-arc hybridization pattern that may be due to asymmetric progression of outgoing replication forks from the origin bubble, or perhaps it is due to the asymmetric placement of the replication initiation site within Cori. Upon observation of the bubble and Y-arc in the modified HincII/SacI blot, an alternative interpretation of the 2-D gel hybridization pattern for the BamHI blot is proposed and illustrated in Figure 5B. It is suggested that the replication fork moves towards the other end of the fragment from the near terminal location of Cori, and that the ‘cone’ pattern may be a combination of merged origin bubble and Y-arcs. The additional partial arc is observed in both blots and it is presumed to be due to excess ssDNA and incomplete digestion.
Figure 5.
2-D gel analysis of Cori. (A) dnaC303 mutant C.crescentus 60 min time-point chromosomal DNA digest with BamHI, alkaline blotted and hybridized with 1.6 kb BamHI fragment E. Interpretation of the hybridization pattern and resulting replication intermediate structures are illustrated in the accompanying pictorials. Major species of replication intermediates in the region in which Cori is asymmetrically placed are simple replication forks. (B) dnaC303 mutant C.crescentus 60 min time-point chromosomal DNA digest with BamHI, alkaline blotted and hybridized with 2.2 kb HindIII/SacI fragment. This 2-D gel is run with modified agarose gel electrophoresis conditions. Interpretation of the hybridization pattern and resulting replication intermediate structures are illustrated in the accompanying pictorials. Major species of replication intermediates in the region in which Cori is symmetrically placed are origin bubbles.
Multiplex 2-D gel analyses
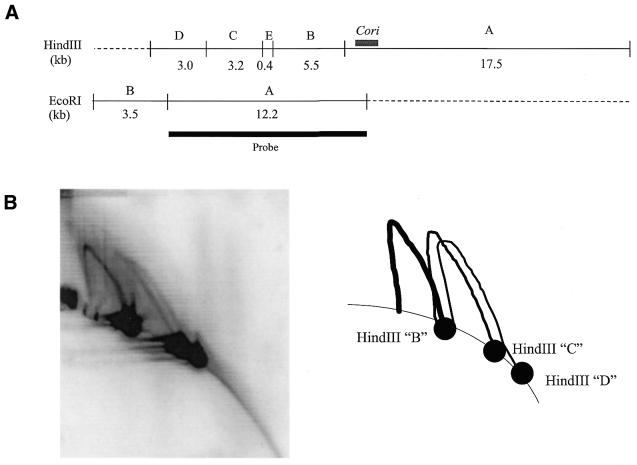
Simple replication forks are observed in the regions flanking Cori, and from Figure 5 we interpret that these replication forks emerge from within Cori. However, the standard 2-D gel cannot discern replication fork direction. To determine the direction of these replication forks, experiments utilizing two modified versions of the 2-D gel technique were performed. The multiplex 2-D gel uses a large probe that overlaps two or three consecutive fragments of differing sizes such that, upon hybridization, multiple hybridization patterns are observed. Using this technique, a 2-D gel dnaC303 HindIII blot was probed with EcoRI fragment A that overlaps three consecutive HindIII fragments flanking Cori (Fig. 6A), resulting in three clear individual hybridization patterns of HindIII fragments B, C and D as depicted in Figure 6B. All three hybridization patterns (i.e. Y-arcs) indicate simple replication forks. The HindIII fragment B Y-arc is the strongest, and Y-arc intensity decreases subsequently in HindIII fragments C and D. This observation is significant as HindIII fragment B is located proximal to Cori with HindIII fragments C and D located more distantly in that order (Fig. 6A). The decrease in intensity in the Y-arcs is not due to lesser amounts of chromosomal DNA since the hybridization signals of the 1X spot remains the same intensity throughout the three hybridization patterns. The observation suggests that replication intermediates first make their appearance in HindIII fragment B bordering the replication origin. A multiplex 2-D gel assay of three consecutive fragments flanking the alternative side of the Cori region also shows progressive replication forks emanating from Cori with the indication of the forks accumulating in the bordering fragment (data not shown). In addition, the observation of replication forks in the regions flanking Cori clearly supports the fact that replication forks stall proximal to dnaA (BamHI fragment C2) and gidA (HindIII C fragment) (24), in which each gene is located ∼5 kb from alternate sides of Cori, at the non-permissive temperature in the dnaC303 C.crescentus mutant strain.
Figure 6.
Multiplex 2-D gel analysis. (A) Restriction endonuclease map of the ∼30 kb region of earliest replicating DNA containing Cori (thick grey line). The single thick black line represents the EcoRI fragment A used as a random-primed 32P-labeled probe. (B) 2-D gel blot hybridized with 32P-labeled EcoRI fragment A and the three significant hybridization patterns are illustrated in the accompanying schematic. All three hybridization patterns are indicative of simple replication forks.
Fork direction assays
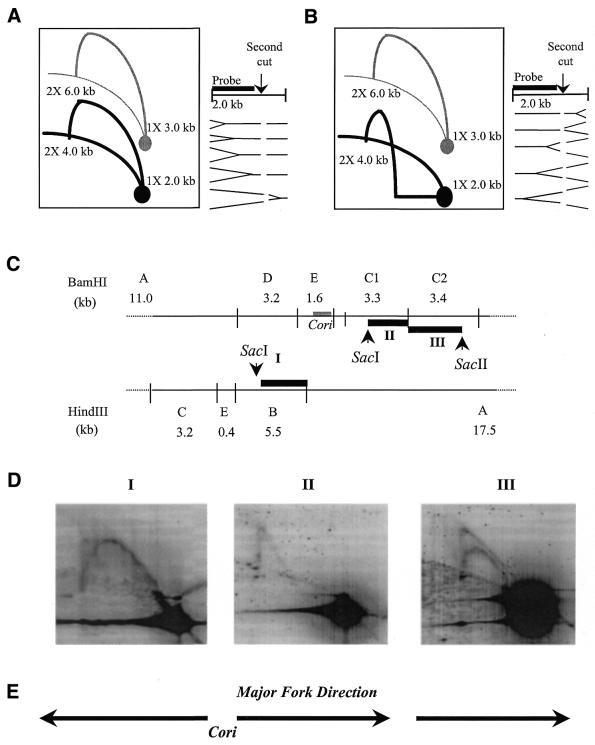
The multiplex 2-D gel analyses gives preliminary evidence of replication forks emerging from Cori. In order to elucidate the direction of the replication forks, the fork direction assay is utilized. In the standard 2-D gel assay, left-ward and right-ward fork directions are indistinguishable, because their structures are symmetric and produce identical Y-arcs. The fork direction assay uses a unique second endonuclease cut following resolution in the first dimension, and asymmetric hybridization, as illustrated in Figure 7A and B (31,37). The 1X and 2X spots of fork direction Y-arc (with second endonuclease digestion) will be lower than those of the original Y-arc (without second endonuclease digestion). In this study the assay was applied to those fragments that have detected replication forks (see above).
Figure 7.
Fork direction assays. Compared with the original hybridization pattern, the resultant hybridization pattern (black) is decreased in linear size due to second unique endonuclease digestion of the first dimensional resolved DNA and interpretation of this pattern is indicated as the simple replication fork (A) moving from left to right and (B) moving from right to left in the region of interest. Based on Friedman and Brewer (31). (C) Restriction endonuclease map of 12.2 kb region with centralized Cori. Thick black lines marked with roman numerals (I–III) represent fragments used as random-primed 32P-labeled probes: probe I is SacI/HindIII segment of HindIII fragment B; probe II is SacI/BamHI segment of BamHI fragment C1; and probe III is BamHI/SacII segment of BamHI fragment C2. (D) NA1000 wild-type C.crescentus 15 min synchrony time-point chromosomal DNA is digested first with BamHI or HindIII, run in first dimension gel electrophoresis, and digested with a second unique endonuclease (SacI or SacII) that cleaves within the BamHI or HindIII fragment before the second dimension gel electrophoresis run. The 2-D gel was then alkaline Southern blotted, and hybridized with 32P-labeled probes [see (C)] marked with corresponding roman numerals above each hybridized blot. (E) Direction of replication fork movement interpreted from the hybridization patterns depicted in the 2-D gel blots.
In Figure 7C, the HindIII/BamHI restriction map of the Cori region is illustrated. Three fragments were analyzed (HindIII fragment B, BamHI fragment C1 and BamHI fragment C2) with their second dimension restriction cut sites depicted by arrowheads. Fragments obtained from digestion with the second endonuclease were used as probes (marked with roman numerals in Fig. 7C) for fork direction assays performed on NA1000 chromosomal DNA (Fig. 7D). The NA1000 blot hybridized with probe I shows the interpreted hybridization pattern of a replication fork moving away from Cori (Fig. 7D, see Fig. 7A for comparison). Whereas the NA1000 blot hybridized with probe II clearly shows the alternate hybridization pattern interpreted of replication fork moving away from Cori (Fig. 7D, see Fig. 7B for comparison). In addition, NA1000 blot hybridized with probe III also shows the hybridization pattern indicative of the replication fork moving away from Cori (Fig. 7D). Note that the second SacII digestion was only ∼50% complete, and therefore two Y-arcs are seen. The first Y-arc originates from the BamHI to BamHI (uncut) 1X spot. The second Y-arc originates from the BamHI to SacII (cut) 1X spot. Similar results were obtained from fork direction 2-D gel assays performed with dnaC303 arrested replication intermediates using identical probes and otherwise comparable conditions (data not shown). We summarized the direction of these replication fork results in Figure 7E. This agrees with our proposal that replication initiates inside Cori.
DISCUSSION
Our 2-D N/N agarose gel electrophoresis experiments agree with earlier ARS experiments and provide evidence that Cori is a chromosomal replication origin. Synchronized chromosomal DNA samples were assessed by the hemi-methylation assay for the optimal time-point of abundant replication intermediates. Simple replication forks were mapped in regions flanking Cori, clearly indicating the absence of any additional replication origins within the ∼30 kb region of earliest replicating DNA. The 2-D gel blot of the 1.6 kb BamHI fragment with Cori asymmetrically placed did not give the expected bubble arc pattern or the expected bubble and Y-arc combination. We propose that inappropriately low percentage agarose gel run in second dimension caused the bubble arc DNA to merge with the Y-arc DNA resulting in the arc and cone hybridization pattern. The 2.2 kb HincII/SacI fragment containing centralized Cori run with higher percentage agarose resolved the origin bubble DNA, and the characteristic origin bubble arc was detected despite the faint intensity of the signal. In addition, it is possible that nicked origin bubbles may have resulted in simple replication fork structures, which may account for dominance of the Y-arc signal over the weak bubble arc signal. Multiplex 2-D analyses confirmed that the replication forks were arrested proximal to dnaA and gidA, and implied that the replication forks emerged bidirectionally from Cori. However, like the standard 2-D gel technique, the multiplex 2-D gel technique cannot aid in detection of the replication fork direction. Thus, the simple replication forks in the regions flanking Cori were subjected to fork direction assays that determined the movement of the forks to be emerging from Cori from both sides. For proper application of this technique, the second digest site should ideally be in a sub-terminal location approximately one-third of the fragment size from the termini. However, incomplete second endonuclease digestion of the DNA is prevalent in this technique and, when hybridized, traces of the original Y-arc hybridization pattern as well as the 1X and 2X spots can still be observed, which can complicate interpretation of the final hybridization pattern. Nevertheless, the direction of the replication forks was unequivocally determined to originate from within Cori. Thus, the multiplex 2-D gel analyses and the fork direction assays clearly support the modified HincII/SacI 2-D gel result that replication initiates in Cori.
Caulobacter crescentus is well suited for replication studies by 2-D gel analysis as the cells are easily synchronized. Ideally, wild-type NA1000 C.crescentus would be the best source of chromosomal DNA for 2-D gel analysis as it does not have mutations that may interfere with replication and otherwise affect replicating DNA structures. However, due to the high replication fork rate, it is very difficult to obtain samples at the precise moment of replication initiation and to isolate early replication intermediates emerging from Cori. Different approaches were utilized to slow down the fork rate; decreased growth temperatures had little effect on the resulting 2-D analysis (data not shown), and minimal concentrations of novobiocin resulted in complete inhibition of replication (data not shown). We therefore employed the mutant dnaC303 C.crescentus strain to impede replication after initiation at the non-permissive temperature and allow accumulation of replication intermediates when held for a period of time. This strain was originally identified as DNA elongation mutant, and the mutation was traced to one open reading frame which is homologous to E.coli holB encoding for the δ′-subunit of DNA polymerase III (33,38). The dnaC303 strain optimizes the collection of replication intermediates for 2-D gel analysis. The DNA structures of replication intermediates from the dnaC303 samples were essentially the same as wild-type NA1000, but with the addition of partial arcs. Such structures may include exposed ssDNA regions that resist cleavage by restriction endonucleases, and these ssDNA regions may hybridize with the parental strands resulting in branched structures. Thus, to exclude artifactual hybridization signals, the wild-type NA1000 was always utilized for comparison. Also note that the horizontal linear hybridization signals emanating from the 1X spots in 2-D gel blots is not particular to the dnaC303 strain as this pattern is seen in NA1000 2-D gel blots as well. This pattern may be due to collapsed replication intermediate structures, and this would also complicate detection of Cori bubble arcs.
Physical isolation of swarmer cells for synchronies allows for control of the stage of the cell cycle, however, it does not allow control of the events of replication initiation. Although a population of isolated cells is deemed to consist of pure swarmer cells, replication initiation within the swarmer cells may be asynchronous. This possibility is considered for the modified 2-D HincII/SacI gel blot that exhibits the origin bubble and Y-arc (Fig. 5B), which may result from a heterogeneous population of origin bubble and fork structures in the time-point sample. Alternatively, asymmetric progression of replication forks of varying rates may account for dominant Y-arc, or perhaps the replication initiation site in Cori may be asymmetrically placed resulting in a bubble and Y-arc. Again, the asynchronous replication initiation event is relevant to the multiplex 2-D gel assay (Fig. 6) as there appears to be replication fork structures present in each of the three consecutive fragments, clearly indicating that replication initiation was not precisely synchronous. Despite the imprecise initiation of chromosomal replication in stalked cells as well as altered replication intermediate structures due to the dnaC303 strain, the harvested DNA samples provided sufficient material for mapping of the replication intermediates.
Additional support for divergent replication forks from within Cori is provided by the alignment of open reading frames in the Cori region (21) that parallel the replication forks emerging from Cori. This parallel replication/transcription pattern was first observed in E.coli (39). It was observed that, in general, E.coli strong promoters are aligned with the direction of the replication forks. Thus, the similar replication/transcription pattern found in C.crescentus supports the location of the bidirectional replication origin to be within Cori.
We conclude that the ARS Cori is the only replication origin present, and Cori clearly exhibits bidirectional replication behavior. This study presents only the third prokaryotic organism to date in which chromosomal replication was successfully characterized by 2-D gel analysis. In addition, with the recent report of the shared identical gene cluster in the replication origin region of C.crescentus and R.prowazekii (24), this study may aid elucidation of the mechanism of replication initiation in R.prowazekii as well.
Acknowledgments
ACKNOWLEDGEMENTS
We are grateful to Dr Peter Dijkwel for his assistance to this work. We thank Dr Bonita Brewer and Dr Joel Huberman for their advice and discussions regarding 2-D gel analysis. We also thank Rania Siam for critical reading of the manuscript. This work was supported by Fonds pour la Formation de Chercheurs et l’Aide à la Recherche (FCAR) Ph.D. Fellowship and F. C. Harrison Fellowship to A.K.C.B., and Medical Research Council of Canada (MRC) Grant MT-13453 and an MRC Scholarship Award SH-50791-AP007403 to G.T.M.
References
- 1.Kornberg A. and Baker,T.A. (1992) DNA Replication, 2nd edn. W.H.Freeman and Company, New York, USA.
- 2.Marczynski G.T. and Shapiro,L. (1993) Bacterial chromosomes of replication. Curr. Opin. Genet. Dev., 3, 775–782. [DOI] [PubMed]
- 3.Richter S., Hess,W.R., Krause,M. and Messer,W. (1998) Unique organization of the dnaA region from Prochlorococcus marinus CCMP1375, a marine cyanobacterium. Mol. Gen. Genet., 257, 534–541. [DOI] [PubMed] [Google Scholar]
- 4.Bell L. and Byers,B. (1983) Separation of branched from linear DNA by two-dimensional gel electrophoresis. Anal. Biochem., 130, 527–535. [DOI] [PubMed] [Google Scholar]
- 5.Brewer B.J. and Fangman,W.L. (1987) The localization of replication origins on ARS plasmids in S. cerevisiae. Cell, 51, 463–471. [DOI] [PubMed] [Google Scholar]
- 6.Fangman W.L. and Brewer,B.J. (1992) A question of time: replication origins of eukaryotic chromosomes. Cell, 71, 363–366. [DOI] [PubMed] [Google Scholar]
- 7.Moriya S., Firshein,W., Yoshikawa,H. and Ogasawara,N. (1994) Replication of a Bacillus subtilis oriC plasmid in vitro. Mol. Microbiol., 12, 469–478. [DOI] [PubMed] [Google Scholar]
- 8.Suhan M., Chen,S.-Y., Thompson,H.A., Hoover,T.A., Hill,A. and Williams,J.C. (1994) Cloning and characterization of an autonomous replication sequence from Coxiella burnetii. J. Bacteriol., 176, 5233–5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brewer B.J. and Fangman,W.L. (1991) Mapping replication origins in yeast chromosomes. Bioessays, 13, 317–322. [DOI] [PubMed] [Google Scholar]
- 10.Bénard M., Lagnel,C., Pallotta,D. and Pierron,G. (1996) Mapping of a replication origin within the promoter region of two unlinked, abundantly transcribed actin gens of Physarum polycephalum. Mol. Cell. Biol., 16, 968–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reischmann K.P., Zhang,Z. and Kaper,G.M. (1999) Long range cooperative interactions regulate the initiation of replication in the Tetrahymena thermophila rDNA minichromosome. Nucleic Acids Res., 15, 3079–3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caddle M.S. and Calos,M. (1992) Analysis of the autonomous replication behavior in human cells of the dihydrofolate reductase putative chromosomal origin of replication. Nucleic Acids Res., 20, 5971–5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu Z., Kunnimalaiyaan,M. and Nielsen,B.L. (1996) Characterization of replication origins flanking the 23S rRNA gene in tobacco chloroplast DNA. Plant Mol. Biol., 32, 693–706. [DOI] [PubMed] [Google Scholar]
- 14.Han Z. and Stachow,C. (1994) Analysis of Schizosaccharomyces pombe mitochondrial DNA replication by two dimensional gel electrophoresis. Chromosoma, 103, 162–170. [DOI] [PubMed] [Google Scholar]
- 15.Moriya S. and Ogasawara,N. (1996) Mapping of the replication origin of the Bacillus subtilis chromosome by the two-dimensional gel method. Gene, 176, 81–84. [DOI] [PubMed] [Google Scholar]
- 16.Miyata M., Wang,L. and Fukumura,T. (1993) Mapping of replication initiation site in Mycoplasma capricolum genome by two-dimensional gel-electrophoretic analysis. Nucleic Acids Res., 21, 4816–4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyata M. and Fukumura,T. (1997) Asymmetrical progression of replication forks just after initiation on Mycoplasma capricolum chromosome revealed by two-dimensional gel-electrophoresis. Gene, 193, 39–47. [DOI] [PubMed] [Google Scholar]
- 18.Poindexter J.S. (1964) Biological properties and classification of the Caulobacter group. Bacteriol. Rev., 28, 231–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brun Y.V., Marczynski,G. and Shapiro,L. (1994) The expression of asymmetry during Caulobacter cell differentiation. Annu. Rev. Biochem., 63, 419–450. [DOI] [PubMed] [Google Scholar]
- 20.Evinger M. and Agabian,N. (1977) Envelope-associated nucleoid from Caulobacter crescentus stalked and swarmer cells. J. Bacteriol., 172, 1262–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dingwall A. and Shapiro,L. (1989) Rate, origin, and bidirectionality of Caulobacter chromosome replication as determined by pulse-field gel electrophoresis. Proc. Natl Acad. Sci. USA, 86, 119–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marczynski G.T. and Shapiro,L. (1992) Cell-cycle control of a cloned chromosomal origin of replication from Caulobacter crescentus. J. Mol. Biol., 226, 959–977. [DOI] [PubMed] [Google Scholar]
- 23.Marczynski G.T., Lentine,K. and Shapiro,L. (1995) A developmentally regulated chromosomal origin of replication uses essential transcription elements. Genes Dev., 9, 1543–1557. [DOI] [PubMed] [Google Scholar]
- 24.Brassinga A.K.C., Siam,R. and Marczynski,G.T. (2001) Conserved gene cluster at replication origins of α-proteobacteria Caulobacter crescentus and Rickettsia prowazekii. J. Bacteriol., 183, 1824–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quon K.C., Marczynski,G.T. and Shapiro,L. (1996) Cell cycle control by an essential bacterial two-component signal transduction protein. Cell, 84, 83–93. [DOI] [PubMed] [Google Scholar]
- 26.Domian I.J., Quon,K.C. and Shapiro,L. (1997) Cell type-specific phosphorylation and proteolysis of a transcriptional regulator controls the G1-to-S transition in a bacterial cell cycle. Cell, 90, 415–424. [DOI] [PubMed] [Google Scholar]
- 27.Quon K.C., Yang,B., Domian,I., Shapiro,L. and Marczynski,G.T. (1998) Negative control of bacterial DNA replication by a cell cycle regulatory protein that binds at the chromosome origin. Proc. Natl Acad. Sci. USA, 95, 120–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siam R. and Marczynski,G.T. (2000) Cell cycle regulator phosphorylation stimulates two distinct modes of binding at a chromosome replication origin. EMBO J., 19, 1138–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barnett M.J., Hung,D.Y., Reisenauer,A., Shapiro,L. and Long,S.R. (2001) A homolog of the CtrA cell cycle regulator is present and essential in Sinorhizobium meliloti. J. Bacteriol., 183, 915–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ely B. (1991) Genetics of Caulobacter crescentus. Methods Enzymol., 204, 372–384. [DOI] [PubMed] [Google Scholar]
- 31.Friedman K.L. and Brewer,B.J. (1995) Analysis of replication intermediates by two-dimensional agarose gel electrophoresis. Methods Enzymol., 262, 613–627. [DOI] [PubMed] [Google Scholar]
- 32.Maniatis T., Fritsch,E.F. and Sambrook,J. (1982) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 33.Osley M.A. and Newton,A. (1977) Mutation analysis of developmental control in Caulobacter crescentus. Proc. Natl Acad. Sci. USA, 74, 124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ohta N., Masurekar,M. and Newton,A. (1990) Cloning and cell cycle dependent expression of DNA replication gene dnaC from Caulobacter crescentus. J. Bacteriol., 172, 7027–7034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zweiger G., Marczynski,G. and Shapiro,L. (1994) A Caulobacter DNA methyltransferase that functions only in the predivisional cell. J. Mol. Biol., 235, 472–485. [DOI] [PubMed] [Google Scholar]
- 36.Marczynski G.T. (1999) Chromosome methylation and measurement of faithful, once and only once per cell cycle chromosome replication in Caulobacter crescentus. J. Bacteriol., 181, 1984–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Brabant A.J., Hunt,S.Y., Fangman,W.L. and Brewer,B. (1998) Identifying sites of replication initiation in yeast chromosomes: Looking for origins in all the right places. Electrophoresis, 19, 1239–1246. [DOI] [PubMed] [Google Scholar]
- 38.Ohta N. and Newton,A. (1999) Promoters and transcription analysis of the temporally regulated thymidylate kinase (tmk) operon in Caulobacter. ASM 99th General Meeting, Chicago, USA. American Society for Microbiology, Washington D.C., USA.
- 39.Brewer ,B.J. (1988) When polymerases collide: replication and the transcriptional organization of the E. coli chromosome. Cell, 53, 679–686. [DOI] [PubMed] [Google Scholar]
- 40.Simon R., Priefer,U. and Puler,A. (1983) A broad host range mobilization system for in vivo genetic engineering. Biotechnology, 1, 784–791. [Google Scholar]