Abstract
Fracture nonunion, a serious bone fracture complication, remains a challenge in clinical practice. Although the molecular pathogenesis of nonunion remains unclear, a better understanding may provide better approaches for its prevention, diagnosis and treatment at the molecular level. This review tries to summarise the progress made in studies of the pathogenesis of fracture nonunion. We discuss the evidence supporting the concept that the development of nonunion is related to genetic factors. The importance of several cytokines that regulate fracture healing in the pathogenesis of nonunion, such as tumour necrosis factor-α, interleukin-6, bone morphogenetic proteins, insulin-like growth factors, matrix metalloproteinases and vascular endothelial growth factor, has been proven in vitro, in animals and in humans. Nitric oxide and the Wnt signalling pathway also play important roles in the development of nonunion. We present potential strategies for the prevention, diagnosis and treatment of nonunion, and the interaction between genetic alteration and abnormal cytokine expression warrants further investigation.
The translational potential of this article
A better understanding of nonunion molecular pathogenesis may provide better approaches for its prevention, diagnosis and treatment in clinical practice.
Keywords: Cytokine, Gene, Molecular pathogenesis, Nonunion
Introduction
Fracture healing is a complex but well-orchestrated process that results in the regeneration and functional restoration of bones. The US Food and Drug Administration has defined fracture nonunion as the absence of radiographic healing over 9 months with no visible healing progression in the last 3 months. As a serious fracture complication, nonunion has a significant effect on the quality of life and financial situation of patients and may be associated with severe functional and psychological impairments. The latest research has demonstrated that the average risk of nonunion per fracture was 1.9%, and the rate of nonunion was up to 9% in specific fracture types (tibial and clavicular fractures) and in old patients [1]. According to a 2007 study, the cost of nonunion treatment is as high as US$ 25,000 per patient, more than twice that for normal fracture healing [2].
Many factors, including systemic, local and treatment factors, impair fracture healing and eventually result in nonunion. Systemic factors mainly include age, nutrition and systemic diseases, the most common of which are diabetes mellitus, anaemia and endocrine disorders. Local factors, such as the type of fracture, blood supply and infection, may also influence fracture healing. In addition, some evidence indicates that smoking and certain medications, including nonsteroidal antiinflammatory drugs (NSAIDs) and corticosteroids, might have side-effects that affect bone healing [3], [4].
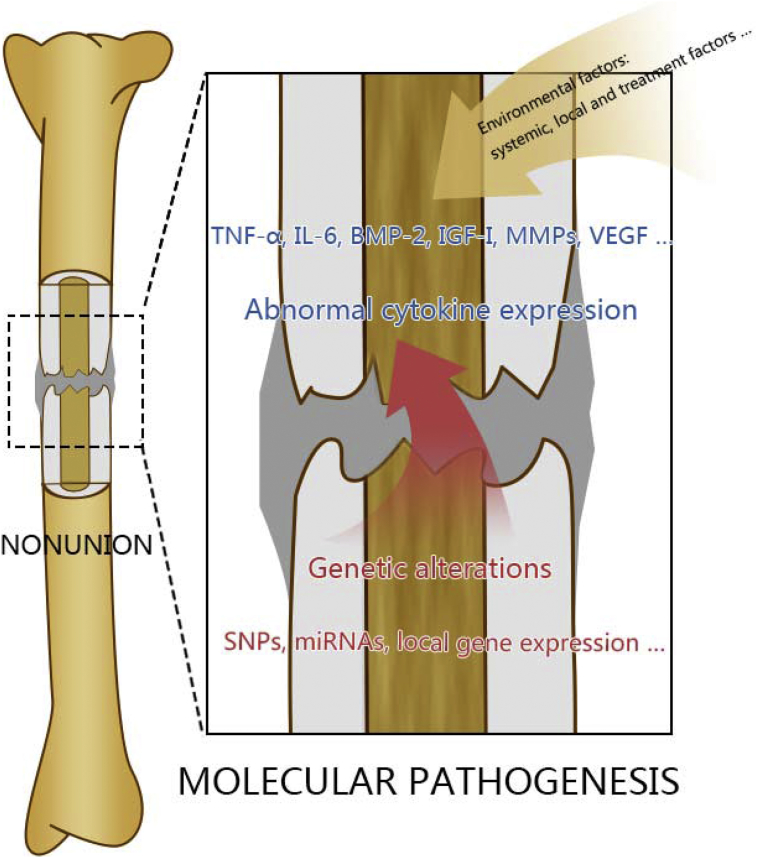
Although these environmental causes of nonunion at the individual (macro) level have been extensively studied, the specific signal pathways involved remain unclear. Under the influence of external risk factors, the systemic or local expression of specific cytokines and growth factors might be disturbed. In addition, some patients present a predisposition towards nonunion development because of genetic defects. For example, young patients without any systemic disease develop nonunion at every fracture site after multiple fractures, even when undergoing proper treatments. Genetic factors also lead to abnormal expression of cytokines and result in nonunion. Alterations of genes and cytokines, at the molecular level, are discussed in this review (Figure 1).
Figure 1.
The pathogenesis of nonunion at molecular level. Both environmental risk factors and genetic factors lead to the abnormal expression of cytokines, which is the key point for nonunion development. Genetic factors and abnormal cytokine expression, at the molecular level, are discussed in this review. BMP = bone morphogenetic protein; IGF = insulin-like growth factor; IL = interleukin; MMP = matrix metalloproteinase; SNP = single nucleotide polymorphism; TNF-α = tumour necrosis factor-α; VEGF = vascular endothelial growth factor.
Three aspects involved in the molecular mechanism of nonunion development are discussed in this review: genetic factors, abnormal cytokine expression and other small molecules. A clear understanding of the molecular pathogenesis of nonunion can improve our knowledge of this complication and provide different approaches for its prevention, diagnosis and treatment at the molecular level.
Genetic factors
From a clinical perspective, it remains unknown why some patients without systemic or local risk factors present a predisposition towards nonunion development. Much evidence indicates that specific genetic variants and abnormal gene expression are the inherent causes of many diseases, which may also be true for fracture nonunion. Indeed, recent studies in this field have yielded valuable findings. The genetic factors related to nonunion that have been investigated to date are described below, and details of the related experiments are summarised in Table 1.
Table 1.
Summary of human studies that have investigated genes related to nonunion.
| Author and date | Groups | Exclusion criteria | Methods | Genes related to nonunion |
|---|---|---|---|---|
| Dimitriou et al., 2011 | 62 patients with atrophic nonunion 47 patients with normal fracture healing |
None | SNP analysis | Risk factors of nonunion: G/G genotype of the rs1372857 SNP located within NOGGIN, T/T genotype of the rs2053423 SNP located within SMAD6 |
| Zeckey et al., 2011 | 50 patients with femoral and tibial nonunion 44 patients with normal fracture healing |
Smoking, diabetes, bilateral fractures, use of corticoids and septic nonunion | SNP analysis | Risk factors of nonunion: A PDGF haplotype (rs1800814, rs62433334, rs13309625; CCG) |
| Grzegorz Szczęsny et al., 2011 | 151 patients with nonunion 144 patients with normal fracture healing |
Open fractures, massive contusion of soft tissues covering the fracture gap, trophic lesions of soft tissues, chronic inflammatory foci and diseases requiring medication with immunosuppressive drugs. | Mutation frequency found using gene analysis | Risk factors of nonunion: T/T and C/T genotype of TGF-β gene codon 10, mutated TLR4 gene W/1 |
| Sathyendra et al., 2014 | 33 patients with atrophic nonunion 29 patients with normal fracture healing |
None | SNP analysis | Risk factors of nonunion: (OR>1): C/T or T/T genotype at SNP rs2853550 within the IL1B gene, the C/T or T/T genotype at rs2297514 and the A/G or G/G genotype at rs2248814 within the NOS2 gene Protective factors (OR<1): G/G at rs3819089 within the MMP13 gene, G/G at rs270393 within the BMP6 gene |
| Sabir Ali et al., 2015 | 250 patients with nonunion 250 patients with normal fracture healing |
Children and patients with a known systemic inflammatory disease, osteoporosis and other metabolic bone diseases, pathological fractures and subsequent nonunion and hypertrophic and infected nonunion | SNP analysis | Risk factors of nonunion: T/G genotype at SNP rs3753793 within the CYR61 gene |
| João Matheus Guimarães et al., 2013 | 66 patients with nonunion 101 patients with normal fracture healing |
Patients presenting with pathological fractures, osteoporosis, other bone diseases that could interfere with calcium or phosphorus metabolism, hypertrophic and infected nonunion, pregnancy, and aged younger than 18 years | SNP analysis | Risk factors of nonunion: A BMP4 haplotype (rs2761884, rs17563, rs2071047, rs762642; GTAA); C allele at rs13317 within the FGFR1 gene Protective factors: G/T and G/G genotype at rs1342913 within the FAM5C gene |
| G. Zimmermann et al., 2012 | 8 patients with nonunion 7 patients with normal fracture healing |
Renal insufficiency, liver disease, malignant tumours, collagenosis, inflammatory bowel disease, haematological diseases, long-term treatment with steroidal or nonsteroidal antiphlogistic drugs or other immunosuppressive agents, thromboprophylactic agents, fluoroquinolones and tetracyclines, hormone substitution and smoking | cDNA array analysis | Eight genes are overexpressed in the nonunion tissue: CDO1, COMP, FMOD, FN1, CLU, TCS22, ACTA2 and PDE4DIP |
SNP = single nucleotide polymorphism.
The first clinical study on the genetic predisposition towards nonunion was published in 2011 by Dimitriou et al. Fifteen single nucleotide polymorphisms (SNPs, which are a variation, such as a substitution, deletion or insertion, in a single nucleotide at a specific gene position) in four genes of the bone morphogenetic protein (BMP) pathway (BMP-2, BMP-7, Noggin and Smad6) were examined in 109 patients retrospectively. Two specific SNPs in Noggin and Smad6, both inhibitors of BMPs, appear to be responsible for the development of atrophic nonunion. It should be noted that this study did not exclude patients with other environmental risk factors, such as the type of fracture, smoking and NSAIDs use, because no significant difference in these factors was found between groups; however, the conclusion is weak because of the small size of the specific samples [5]. Thus, the correlation between these genes and nonunion needs to be further investigated. After excluding patients with other environmental factors, Zeckey et al. analysed SNPs in several cytokine genes that regulate fracture healing in patients with aseptic nonunion after femoral and tibial shaft fractures. Based on a comparison of the findings with those for patients with normal fracture healing, a platelet-derived growth factor (PDGF) haplotype was reported to be associated with aseptic nonunion [6]. In a study assessing the mutation frequency of genes that are crucial for the recognition and elimination of pathogens and fracture healing, the T and C/T alleles of the transforming growth factor-β (TGF-β) gene codon 10 and the mutated TLR4 gene W/1 were identified as possible risk factors for impaired recognition and elimination of bacteria, increasing the susceptibility of a fracture patient to develop septic nonunion [7].
Several recent studies have also suggested that genetic alterations significantly contribute as an etiological factor to the development of nonunion. Sathyendra et al. found that patients carrying five SNPs in four genes showed a significant association with atrophic nonunion [8]. Another study demonstrated that a T/G genotype at SNP rs3753793 in the CYR61 gene, which encodes an important signalling molecule that participates in many signalling pathways, may contribute to the development of nonunion [9]. The haplotype GTAA in BMP4 and C allele at rs13317 in FGFR1 are also associated with nonunion [10].
Some researchers have attempted to analyse the local gene expression at the fracture site and investigate different gene expression patterns between normal and impaired fracture healing. For instance, expression of eight genes in nonunion tissue was significantly increased compared with fresh callus tissue based on a cDNA array. Among these genes, CDO1, COMP, FMOD and FN1 are important for the assembly and stabilisation of the extracellular matrix, CLU and TCS22 induce cell differentiation and proliferation and ACTA2 and PDE4DIP gene products, such as actin, participate in cytoskeletal organisation and maintenance. Moreover, overexpression of these genes in fracture tissue may impair the structure and function of bone healing–related cells, eventually leading to nonunion [11].
MicroRNAs (miRNAs) regulate gene expression related to many biological processes, such as cell proliferation, differentiation and organ development. Increasing evidence suggests that miRNAs play a key role in fracture healing and development of nonunion by regulating bone formation, resorption and remodelling. A comparison between fracture tissues with normal healing and those with impaired healing in mice showed that five miRNAs, miR-140-3p, miR-140-5p, miR-181a-5p, miR-181d-5p and miR-451a, are significantly upregulated in normally healing tissues [12]. Interestingly, a similar study in mice also reported that five different miRNAs, miR-31a-3p, miR-31a-5p, miR-146a-5p, miR-146b-5p and miR-223-3p, are highly expressed in nonunion tissues [13], suggesting that miRNAs may contribute to the development of nonunion at the molecular level. Recently, investigators designed a new scoring system to assess the miRNA contribution to impaired fracture healing, and 11 miRNAs were identified to impairing fracture healing in aged female mice [14]. The expression of five miRNAs (miR-140-3p, miR-140-5p, miR-181a-1-3p, miR-210-3p and miR-222-3p) is altered in diabetic mice with impaired fracture healing when compared to normal mice [15]. As SPC3649, the first miRNA-targeting drug for the treatment of hepatitis C virus infection, is being investigated in clinical trials [16], we speculate that promoting or inhibiting miRNA function is a potential therapy for nonunion. Human studies need to be conducted to investigate the exact role of miRNAs in nonunion development.
This type of nonunion, which is caused by genetic alterations and abnormal gene expression, may account for refractory nonunion and cases of undetermined aetiology in clinical practice. New methods (e.g., genetic testing) can quickly assess the risk of developing nonunion for fracture patients, enabling prevention and timely intervention. If fracture patients with nonsurgical indications present a genetic predisposition for developing nonunion, then they may need to undergo a more aggressive treatment strategy, such as surgical treatment or adjuvant therapy, including electromagnetic field and ultrasound therapies. Additionally, if fracture patients with surgical indications present a genetic predisposition towards nonunion development, then bone-grafting or mesenchymal stem cells (MSCs) transplantation or the use of BMPs may be advised during the first surgical procedure to prevent nonunion. MSCs transplantation is a representative approach of cell therapy for promoting fracture healing [17], [18]. Therapy selection for nonunion can also benefit from knowledge of genes related to nonunion. For example, the use of BMPs can prevent patients with genetic defects in the BMP pathway from developing nonunion and might be more effective for nonunion treatment in such patients than in those without such genetic defects. Because many studies have shown that the delivery of BMP genes as well as other genes, including vascular endothelial growth factor (VEGF), PDGF, fibroblast growth factor (FGF), Osterix, Nell-1 and Runx-2, can enhance the generation of bone both in vivo and ex vivo [19], [20], [21], [22], [23], gene therapy might become applicable for nonunion in the foreseeable future and may represent the most useful treatment for this serious condition.
However, more studies with larger sample sizes and stricter screening criteria should be carried out to investigate more genes that encode cytokines involved in the fracture healing cascade and their expression patterns in the fracture region. More genes associated with nonunion remain to be found, thus offering greater possibilities and more alternatives to gene therapy for this type of nonunion. Notably, no study has shown an interaction or coordination between genetic factors and environmental risk factors, which both cause abnormal expression of cytokines and are equally essential for the development of nonunion. Therefore, these correlations await further study.
Abnormal cytokine expression
Fracture healing comprises four overlapping processes (the inflammatory phase, the cartilage formation and mineralisation phase, the cartilage resorption phase and the remodelling phase), each of which is regulated by expression of cytokines. The multiple cytokines that regulate the fracture healing cascade can be grouped into three categories: (1) proinflammatory cytokines, (2) members of the TGF-β superfamily and other growth factors and (3) metalloproteinases and angiogenic factors [24]. Under the influence of environmental risk factors, such as hyperglycaemia and using NSAIDs, the local and systemic expression of cytokines may be easily altered. Disruption of any of these cytokines can impair fracture healing and result in nonunion (Table 2). Based on the classification of the cytokines, we discuss three categories of cytokines which are related to fracture nonunion. Importantly, molecular targeted therapy is an effective, accurate and feasible approach for this type of nonunion.
Table 2.
Abnormal cytokines related to nonunion at the stage of fracture healing.
| Stage of fracture healing | Biological process | Correlation between abnormal cytokine expression and nonunion |
|---|---|---|
| Inflammation | Haematoma Hypoxia Inflammation Recruitment of MSCs |
Interference of hypoxia increases ROS production, impairing BMP-2 expression |
| Cartilage formation and mineralisation | Chondrogenesis Angiogenesis Cartilage mineralisation Initiation of primary bone formation |
Alteration of IL-6/sIL-6Ra inhibits differentiation of MSCs to the osteogenic lineage Decreased BMP-2a impairs MSC differentiation and delays cartilage mineralisation (elevated MMP-7/MMP-12 degrades BMP-2; hypophosphate and NSAIDs impairs the BMP-2 pathway) IGF-Ia deficiency impairs cartilage mineralisation Disturbance of Wnt pathway impairs cartilage formation (caused by alcohol) |
| Cartilage resorption | Hypertrophic chondrocytes apoptosis Cartilage resorption by osteoclasts Angiogenesis Secondary bone formation |
Elevated TNF-αa (caused by hyperglycaemia) accelerates cartilage resorption, decreased TNF-α (caused by smoking) delays cartilage resorption Deficiency of MMP-13 and MMP-9 impairs cartilage resorption by osteoclasts |
| Remodelling | Mineralised bone matrix resorption by osteoclasts Bone marrow established |
Deficiency in MMP-2 impairs the remodelling of new bone in the callus Disturbance of NO and related amino acidsa can impair the remodelling phase |
BMP = bone morphogenetic protein; IGF = insulin-like growth factor; MMP = matrix metalloproteinase; MSC = mesenchymal stem cell; NO = nitric oxide; NSAID = nonsteroidal antiinflammatory drug; ROS = reactive oxygen species; TNF-α = tumour necrosis factor-α.
Altered cytokine expression has been observed in patients with nonunion.
Proinflammatory cytokines
Proinflammatory cytokines, including tumour necrosis factor-α (TNF-α) and, interleukin-6 (IL-6), help initiate the fracture healing cascade, and they may also play key roles in the remodelling phase [25], [26].
Tumour necrosis factor-α
Local application of low-dose TNF-α within 24 h of injury, which corresponds to the inflammatory phase of fracture healing, promotes fracture healing by upregulating the innate immune response [27]. Based on research on the delayed resorption of cartilage during endochondral bone formation in TNF-α receptor–deficient mice, Gerstenfeld et al. concluded that TNF-α regulates cartilage resorption by inducing apoptosis in hypertrophic chondrocytes and recruiting osteoclasts [28]. Smoking impairs fracture healing, and nicotine is the main factor responsible for nonunion development in smokers [29], [30]. Through the cholinergic antiinflammatory pathway, the secretion of TNF-α is inhibited by nicotine and the resorption of cartilage may be delayed [31].
Diabetes mellitus is another common risk factor for nonunion development. Cartilage resorption is accelerated in diabetic mice overexpressing TNF-α, which induces an increase in osteoclast numbers and apoptosis in chondrocytes [32], [33]. Thus, applying TNF-α antagonists in a diabetic mouse model might reverse the accelerated resorption of cartilage caused by elevated TNF-α [33], [34]. In addition, angiogenesis is impaired, and MSCs are significantly reduced in the fracture region in diabetic mice due to the cooperation of TNF-α and high glucose levels, which is also reversed by TNF-α antagonists [35], [36]. Another study found that the serum concentration of TNF-α in 28 fracture patients with type 2 diabetes mellitus was significantly higher than that in 25 fracture patients without the disease [37]. However, in another study comparing 30 diabetic to 20 normoglycemic patients, no significant difference in the serum concentration of TNF-α was observed [38]. Because fracture patients with type 2 diabetes mellitus present a higher risk of developing nonunion, these studies indicate that the coordination between increased concentrations of TNF-α and glucose might alter the specific cell behaviours involved in cartilage resorption and angiogenesis, eventually resulting in nonunion. These findings indicate a potential approach to the prevention or treatment of fracture in patients with diabetes mellitus through TNF-α antagonists. Nonetheless, further studies are needed to prove that accelerated cartilage resorption and impaired angiogenesis are due solely to overexpression of TNF-α in diabetic patient.
Interleukin-6
IL-6, along with its soluble receptor sIL-6R, is another important proinflammatory cytokine that regulates recruitment and proliferation of MSCs and their differentiation to the osteogenic lineage [39], [40]. Moreover, IL-6/sIL-6R can induce peripheral blood monocytes to differentiate into osteoclasts [41]. In IL-6 knockout mice, callus strength decreased and callus mineralisation and remodelling were delayed during early fracture healing because IL-6 increases nuclear factor kappa beta ligand production to promote osteoclastogenesis [42]. As other cytokines, such as macrophage colony-stimulating factor, also promote osteoclastogenesis, no difference in fracture healing was observed between IL-6–knockout and wild-type mice at 4–6 weeks postfracture [43]. It seems that the IL-6 mainly participates in the remodelling phase rather than the inflammatory phase. In another study, the investigators suppressed the activity of IL-6 in inflammatory tissue and found the fracture healing was unexpectedly promoted in fracture mice [44].
An in vitro experiment demonstrated that elevating the concentration of IL-6 alone does not promote MSCs differentiation into the osteogenic lineage because IL-6 and sIL-6R do not synergistically induce differentiation of MSCs under sIL-6R deficiency [45]. In fact, this study proved that low concentrations of sIL-6R inhibit MSCs differentiation. Cho et al. suggested that increasing concentrations of IL-6 have no influence on osteogenesis and can even inhibit the proliferation of primitive mesenchymal cells [46]. Interestingly, a recent study found significantly higher and lower serum concentrations of IL-6 and sIL-6R, respectively, in nonunion patients than in healthy individuals [47]. Thus, we can speculate that the alteration of IL-6/sIL-6R may account for nonunion development in some fracture patients. Indeed, raising the concentration of sIL-6R may promote the differentiation of MSCs and accelerate healing, especially in nonunion patients who have been transplanted with enriched MSCs. More recently however, researchers found the IL-6/sIL-6R, the IL-6 trans-signalling pathway, is not essential for fracture healing. Actually, the IL-6 classic signalling, IL-6 with membrane-anchored IL-6 receptor, plays a vital role in fracture healing [48]. As a result, distinguishing the exact role of the IL-6 trans-signalling pathway and IL-6 classic signalling in fracture healing is necessary to clarify the mechanism of IL-6 signalling for nonunion development.
Members of the TGF-β superfamily and other growth factors
Members of the TGF-β superfamily include BMPs, TGF-β, growth differentiation factors (GDFs), activins, inhibins and Müllerian-inhibiting substance; other important growth factors include insulin-like growth factors (IGFs), PDGF and FGFs [49]. All are important mediators of fracture healing. BMPs and IGFs, two types of cytokines that are related to nonunion, are discussed below.
Bone morphogenetic proteins
BMPs have been used clinically for the treatment of nonunion as an adjuvant therapy, and currently, BMP-2 and BMP-7 are the only commercially available BMPs [50], [51]. However, a prospective study found that no benefit was observed when BMP-2 was added to autogenous bone graft in fracture patients with long bone nonunion [52]. Recently, a more convincing study that aimed to investigate the efficacy of BMP therapy in treating nonunion showed a similar result. The prospective, randomised, controlled cohort study demonstrated that no difference was seen when BMP-2 or BMP-7 was added to autogenous bone grafts in nonunion treatment [53]. The therapeutic efficacy of BMPs is limited in clinical practice—possibly because the use of BMPs locally may induce expression of BMP antagonists. Inhibition of BMPs antagonist seems to enhance the osteoinductive capacity of BMPs. However, this has only been proven in animal models [54]. Moreover, a study showed that the complications associated with the use of BMPs in scaphoid nonunion cannot be ignored [51]. These findings suggest us to re-evaluate the use of BMPs and find novel method of using BMPs in treating nonunion.
The expression levels of BMP-2, BMP-3, BMP-3B, BMP-6 and BMP-7; GDF-5 and GDF-7 and the BMP antagonists noggin, drm, sclerostin, BAMBI and follistatin are significantly lower within fibrous nonunion tissue when compared with a standard healing callus at several time points both in animal and human studies [55], [56], and BMP-4 upregulation in nonunion tissue is proven in a prospective self-control study [56]. BMP-2, BMP-7 and BMP-14 expression levels are also decreased by a larger margin within the cartilage of human nonunion fractures [57], [58]. As a result, the efficacy of other members of the BMP family in the treatment of nonunion need further study. Another study demonstrated no significant difference in circulating BMP levels between patients with nonunion and those with normal fracture healing [59], suggesting that BMP expression at the fracture site is not directly correlated with systemic BMPs. These findings provide evidence for the use of BMPs at fracture and nonunion sites and suggest that detecting the local concentration of BMPs might aid in the early diagnosis of nonunion.
Many environmental factors may lead to disturbance of the BMP pathway. NSAIDs, which are frequently used for postoperative pain control, may be a risk factor to develop nonunion [60]. Most NSAIDs inhibit the activity of cyclooxygenase-1 and cyclooxygenase-2 (COX-2), and thereby decrease the synthesis of prostaglandins. A study conducted by Daluiski et al found that after treating osteoprogenitor cells with NSAIDs to inhibit the activity of COX-2, the response of osteoprogenitor cells to BMP decreases. As a result, the osteogenic potential of the cells is reduced [61]. Another study demonstrated that through upregulating the expression of COX-2, BMP-2 induces the phosphorylation of ATF4 in chondrocytes, which plays a critical role in skeletal development and maintenance [62]. It can therefore be speculated that by inhibiting the activity of COX-2, NSAIDs can impair fracture healing through the BMP pathway. Animal studies and clinical studies are needed to validate that NSAIDs impair fracture healing through the BMP pathway. BMP-2 expression of MSCs depends on hypoxic signals in the fracture tissue, which is mediated by reactive oxygen species. Interference of hypoxia during the early inflammatory phase of fracture healing leads to deficient scavenging and abnormal increase in reactive oxygen species production, thus impairing BMP-2 expression and cartilage ossification during endochondral bone formation, which leads to delayed union or nonunion [63].
Model mice with femoral fracture that received a phosphate-restricted diet exhibited BMP-2 resistance, resulting in impaired fracture healing exactly resembling that is observed in the absence of BMP-2 signalling [64]. Moreover, phosphate restriction increased parathyroid hormone-related peptide expression, further attenuated chondrocyte differentiation and impaired hypertrophic chondrocyte apoptosis via the parathyroid hormone/parathyroid hormone-related peptide receptor [65]. Combined with phosphate, the use of BMP-2 in the treatment of nonunion may be more effective. However, as for acute fracture healing, a double-blind, randomised, controlled phase-II/III trial performed in 2013 on the efficacy and safety of recombinant human BMP-2/calcium phosphate matrix for treating closed tibial diaphyseal fracture suggested that the use of rhBMP-2/CPM did not significantly accelerate fracture healing after patients were treated with reamed intramedullary nailing [66]. Calcium phosphate bone substitute is a suitable alternative to the bone grafts used for clinical nonunion treatment. Regardless, further study is needed to determine whether increasing the local concentration of phosphate or combining BMP-2 with phosphate is effective for treating nonunion.
Insulin-like growth factors
IGFs, the most abundant growth factors in bone, comprise IGF-I and IGF-II. IGF-I is more potent than IGF-II. When bound to the IGF type 1 receptor (IGF1R), IGF-I activates autophosphorylation of the IGF1R cytoplasmic kinase domain, stimulating downstream signalling pathways through its interaction with various docking proteins, including insulin receptor substrate-1 (IRS-1) and Src homology/α-collagen [67]. IGF is a mediator of growth hormone, whereas the function of IGF is regulated by specific binding proteins, including IGF-binding proteins (IGFBs; IGFBP-1 to IGFBP-6) and acid-labile subunit [68].
The knockout of IGF system–related genes impairs embryonic development and postnatal development of the skeleton in mice [69], [70], [71], and a recent study using an osteoblast-specific IGF1R conditional knockout mouse model also demonstrated impaired endochondral bone formation [72]. However, knockout of IGF-I in osteocytes of mice augmented the healing of a fracture gap because loss of IGF-I was compensated for by upregulation of BMP-2 and Wnt [73]. Significantly higher gene expression of IGF-I/II and IGFBP-6 has been reported in mice with nonunion, and IGFBP-5 expression in these mice is significantly lower than that in mice with normal fracture healing, thus exhibiting opposite trends [74]. Another prospective study showed that the time course of the serum concentrations of GH (growth hormone)/IGF-I axis components in patients with atrophic nonunion are significantly different from those of patients with normal fracture healing. Acid-labile subunit expression is lower during the early and late stages of fracture healing, while IGFBP-3 expression is lower during the late stage of fracture healing in nonunion [75]. Mathieu et al also proved that the serum concentrations of IGF-I are lower in patients with nonunion [47]. The GH/IGF-I axis is easily affected by endocrine disorders; for example, in diabetic mice with fracture, the concentration of IGF-I is lower in both the serum and the fracture region [76]. Therefore, further studies in vivo are necessary to validate whether changes in the GH/IGF-I axis might initially prompt the development of nonunion before therapeutic targeting of GH/IGF-I axis can be considered.
The broken ends of fractures cannot connect in IRS-1 gene knockout-mice because IRS-1 deficiency cannot be compensated for by other signalling molecules, and transplanting MSCs that express IGF-I into these mice can induce the transplanted MSCs to differentiate into the osteogenic lineage via autocrine and paracrine regenerative effects; as a result, the resulting increased bone volume and callus mineralisation rescue the failed fracture healing [77]. In addition, transplanting MSCs with systemically delivered IGF-I can also enhance fracture healing [78]. From the perspective of promoting fracture healing, IGF-I has shown great benefit in many experiments. Indeed, experiments with several animal models have shown that local administration of IGF-I can accelerate fracture healing [79], [80], [81]. Furthermore, the application of the recombinant human IGF-I/IGF binding protein-3 complex in osteoporotic patients with femoral fracture may be feasible and safe [82]. However, the potential therapeutic effects of IGF-I on nonunion require further study. As the factor that potentially initiates the development of nonunion, the GH/IGF-I axis represents a possible target for the prevention and treatment of the condition. Notably, either transplanting MSCs expressing IGF-I or transplanting MSCs with systemically delivered IGF can fully implement the regenerative capacity of these cells to repair bone, suggesting a novel approach for improving the therapeutic efficacy of MSCs transplantation.
Metalloproteinases and angiogenic factors
Matrix metalloproteinases
During the endochondral ossification and remodelling phases, matrix metalloproteinases (MMPs) degrade the extracellular matrix of cartilage and bone, allowing vascular invasion into the newly generated bone. Imbalance among MMP family members and their regulators, tissue inhibitors of metalloproteinases, may also account for the molecular pathogenesis of nonunion. In MMP-13–knockout mice, chondroclast recruitment to the fracture callus is disturbed, thus slowing cartilage resorption [83]. A similar phenomenon was observed in MMP-9–knockout mice, whereby persistent cartilage hindered vascular penetration into the newly generated bone [84]. In contrast to the MMP-13– and MMP-9–knockout mice, MMP-2–knockout mice exhibit no defects in chondroclast recruitment or cartilage resorption [85]. These findings indicate that MMP-13 and MMP-9 affect both cartilage and bone remodelling, whereas MMP-2 delays only the remodelling of new bone in the callus. A prospective study showed that patients with nonunion had significantly higher serum concentrations of pro-MMP-1 and MMP-8 compared with those with normal fracture healing but that tissue inhibitors of metalloproteinase-1 was lower at several times points after fracture surgery [86]. Because the serum concentrations of enzymes may reflect local enzyme activity, detecting serum MMP levels might represent a novel approach for evaluating the risk of nonunion development in fracture patients. Interestingly, noninvasive examination of the levels of MMP-9 and MMP-13 in urine, which is more accurate than the serum levels, demonstrated that these molecules are potential biomarkers for fracture healing [87]. Moreover, MMP-7 and MMP-12 are significantly elevated in hypertrophic nonunion tissue, and these proteinases may bind to and degrade BMP-2 [88].
Vascular endothelial growth factor
The VEGF-dependent pathway modulates angiogenesis during fracture healing [89]. Fracture healing in rabbits could be delayed by anti-VEGF antibody [90]. During fracture healing, the expression of VEGF and vascularisation was higher in mice with impaired fracture healing when compared to mice with normal fracture healing [91]. Human studies showed a similar result: serum VEGF concentrations in patients with nonunion were higher compared to the patients with normal fracture healing during the 6-month observation after the fracture [92]. However, local application of VEGF can promote bone repair in established nonunion animal models [93], [94]. It seems that hypervascularisation may impede fracture healing especially in the early phase of fracture healing whereas the treatment of an established nonunion can benefit from VEGF administration and vascularisation.
Other molecules
Nitric oxide
As a free radical gas, nitric oxide (NO) is a well-known inflammatory mediator in fracture healing [95], [96]. NO-mediated vasodilation is essential for increasing blood flow into the fracture site in the inflammatory phase [97]. During the remodelling phase, NO mediates vascular reactivity, bone formation and resorption [98], [99]. NO is produced through the conversion of arginine to citrulline, which is catalysed by nitric oxide synthases (NOSs). In another pathway, arginine can be converted to ornithine and urea by arginase as a precursor of collagens.
NOS-knockout mice exhibit disturbed arginine-NO metabolism and impaired fracture healing [100]. Moreover, a study in humans found lower concentrations of arginine, citrulline and ornithine in the fracture callus of patients with atrophic nonunion than in those with normal fracture. Compared to patients with normal fracture healing, arginine was significantly higher and ornithine was lower in the fracture callus in patients with hypertrophic nonunion [101]. We can conclude that amino acids metabolism related to NO is compromised in atrophic nonunion calluses, and the concentration of arginine and NO production increase in hypertrophic nonunion calluses. These findings suggest that NO is involved in the molecular pathogenesis of nonunion. Under stress conditions, such as wound healing and sepsis, arginine levels decrease, and arginine-NO metabolism can be disturbed; these changes may be the initial events in the development of nonunion, especially atrophic nonunion, in some patients [102], [103], [104]. Additionally, animal studies showed that oral l-arginine and oral products which can upregulate NOS expression promote fracture healing [105], [106]. However, whether arginine or NOS supplementation can prevent and treat nonunion need to be investigated.
Wnt signalling
Wnts are a family of secreted glycoproteins involved in the fracture healing process. In the canonical Wnt signalling pathway, Wnts bind to the membrane receptor Fzd and one of the coreceptors, LRP5 and LRP6 (low-density lipoprotein receptor-related protein), and eventually the cytoplasmic level of β-catenin increases to regulate gene expression. The gene expressions involved in Wnt signalling pathway were upregulated during fracture healing in mice [107].
Acute alcohol exposure can disturb the Wnt signalling pathway by deregulating β-catenin expression in mice. Thus, fracture healing is impaired as a result of cartilage formation deficiency [108]. Applying a Wnt pathway activator can increase the level of β-catenin and reverse the impaired fracture healing in mice under alcohol exposure [109]. It is reported that the FOXO transcription factors, antagonists of the Wnt signalling pathway, may also be activated by alcohol [110].
The Wnt signalling pathway could be targeted to enhance fracture healing. Sclerostin and Dkk1 can inhibit the Wnt signalling pathway and impede fracture healing by binding to the LRP5/6 and other transmembrane protein. Sclerostin depletion and systemic administration of a sclerostin antibody with or without BMP-2 have shown the effect of promoting bone repair in mice [111], [112], [113]. Dkk1 antibodies also have the ability to enhance fracture healing in mice [114]. However, the therapeutic potential of Wnt antagonists for the nonunion treatment and prevention needs further researches.
Summary
Undoubtedly, some variations in genes, gene expression and cytokines related to fracture healing are associated with the development of fracture nonunion, though some aspects of the molecular pathogenesis remain unclear. A number of new approaches targeting different genes and cytokines might aid in the early identification of nonunion development risk in fracture patients and might be useful in preventing and treating nonunion. However, some cytokine alterations discussed may be caused by genetic factors rather than by external risk factors because both genetic factors and external risk factors contribute to abnormal cytokine expression. The current evidence needs to be validated in animal and human studies, and the interaction and coordination between genetic factors and environmental risk factors, as well as correlations among different cytokines, warrant further investigation.
Conflicts of interest statement
The authors have no conflicts of interest relevant to this article.
Acknowledgements and funding
We acknowledge Dr. Wen-xiang Chu for his help during the publication, and Dr. Wei Zhang for his help too. This work was support by the National Natural Science Foundation of China [30801175], the Western Medicine Guiding Fund of the Science and Technology Commission of Shanghai Municipality [134119a65500], the Clinical Research Program of 9th People's Hospital, Shanghai Jiao Tong University School of Medicine [JYLJ015], the Clinical Research Plan of SHDC [16CR3099B], and the National Key Research and Development Programs of China [2016YFC1102104, 2017YFC1103900].
Contributor Information
Zi-chuan Ding, Email: dingzichuan1994@163.com.
Yi-kai Lin, Email: ilinyikai@163.com.
Yao-kai Gan, Email: ganyk2004@126.com.
Ting-ting Tang, Email: ttt@sjtu.edu.cn.
References
- 1.Mills L.A., Aitken S.A., Simpson A. The risk of non-union per fracture: current myths and revised figures from a population of over 4 million adults. Acta Orthop. 2017;88:434–439. doi: 10.1080/17453674.2017.1321351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antonova E., Le T.K., Burge R., Mershon J. Tibia shaft fractures: costly burden of nonunions. BMC Muscoskel Disord. 2013;14:42. doi: 10.1186/1471-2474-14-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Niikura T., Lee S.Y., Sakai Y., Nishida K., Kuroda R., Kurosaka M. Causative factors of fracture nonunion: the proportions of mechanical, biological, patient-dependent, and patient-independent factors. J Orthop Sci. 2014;19:120–124. doi: 10.1007/s00776-013-0472-4. [DOI] [PubMed] [Google Scholar]
- 4.Gaston M.S., Simpson A.H. Inhibition of fracture healing. J Bone Joint Surg Br. 2007;89:1553–1560. doi: 10.1302/0301-620X.89B12.19671. [DOI] [PubMed] [Google Scholar]
- 5.Dimitriou R., Carr I.M., West R.M., Markham A.F., Giannoudis P.V. Genetic predisposition to fracture non-union: a case control study of a preliminary single nucleotide polymorphisms analysis of the BMP pathway. BMC Muscoskel Disord. 2011;12:44. doi: 10.1186/1471-2474-12-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeckey C., Hildebrand F., Glaubitz L.M., Jurgens S., Ludwig T., Andruszkow H. Are polymorphisms of molecules involved in bone healing correlated to aseptic femoral and tibial shaft non-unions? J Orthop Res. 2011;29:1724–1731. doi: 10.1002/jor.21443. [DOI] [PubMed] [Google Scholar]
- 7.Szczesny G., Olszewski W.L., Zagozda M., Rutkowska J., Czapnik Z., Swoboda-Kopec E. Genetic factors responsible for long bone fractures non-union. Arch Orthop Trauma Surg. 2011;131:275–281. doi: 10.1007/s00402-010-1171-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sathyendra V., Donahue H.J., Vrana K.E., Berg A., Fryzel D., Gandhi J. Single nucleotide polymorphisms in osteogenic genes in atrophic delayed fracture-healing: a preliminary investigation. J Bone Joint Surg Am. 2014;96:1242–1248. doi: 10.2106/JBJS.M.00453. [DOI] [PubMed] [Google Scholar]
- 9.Ali S., Hussain S.R., Singh A., Kumar V., Walliullah S., Rizvi N. Study of cysteine-rich protein 61 genetic polymorphism in predisposition to fracture nonunion: a case control. Genet Res Int. 2015;2015:754872. doi: 10.1155/2015/754872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guimaraes J.M., Guimaraes I.C., Duarte M.E., Vieira T., Vianna V.F., Fernandes M.B. Polymorphisms in BMP4 and FGFR1 genes are associated with fracture non-union. J Orthop Res. 2013;31:1971–1979. doi: 10.1002/jor.22455. [DOI] [PubMed] [Google Scholar]
- 11.Zimmermann G., Schmeckenbecher K.H., Boeuf S., Weiss S., Bock R., Moghaddam A. Differential gene expression analysis in fracture callus of patients with regular and failed bone healing. Injury. 2012;43:347–356. doi: 10.1016/j.injury.2011.10.031. [DOI] [PubMed] [Google Scholar]
- 12.Waki T., Lee S.Y., Niikura T., Iwakura T., Dogaki Y., Okumachi E. Profiling microRNA expression during fracture healing. BMC Muscoskel Disord. 2016;17:83. doi: 10.1186/s12891-016-0931-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waki T., Lee S.Y., Niikura T., Iwakura T., Dogaki Y., Okumachi E. Profiling microRNA expression in fracture nonunions: potential role of microRNAs in nonunion formation studied in a rat model. Bone Joint Lett J. 2015;97:1144–1151. doi: 10.1302/0301-620X.97B8.34966. [DOI] [PubMed] [Google Scholar]
- 14.He B., Zhang Z.K., Liu J., He Y.X., Tang T., Li J. Bioinformatics and microarray analysis of miRNAs in aged female mice model implied new molecular mechanisms for impaired fracture healing. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17081260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takahara S., Lee S.Y., Iwakura T., Oe K., Fukui T., Okumachi E. Altered expression of microRNA during fracture healing in diabetic rats. Bone Joint Res. 2018;7:139–147. doi: 10.1302/2046-3758.72.BJR-2017-0082.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janssen H.L., Kauppinen S., Hodges M.R. HCV infection and miravirsen. N Engl J Med. 2013;369:878. doi: 10.1056/NEJMc1307787. [DOI] [PubMed] [Google Scholar]
- 17.Lin W.P., Xu L.L., Zwingenberger S., Gibon E., Goodman S.B., Li G. Mesenchymal stem cells homing to improvebone healing. J Orthop Translation. 2017;9:19–27. doi: 10.1016/j.jot.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li G. Stem cells and regenerative medicine: the time is right for translation. J Orthop Translation. 2017;9:A1–A2. doi: 10.1016/j.jot.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yue B., Lu B., Dai K.R., Zhang X.L., Yu C.F., Lou J.R. BMP2 gene therapy on the repair of bone defects of aged rats. Calcif Tissue Int. 2005;77:395–403. doi: 10.1007/s00223-005-0180-y. [DOI] [PubMed] [Google Scholar]
- 20.Pensak M.J., Lieberman J.R. Gene therapy for bone regeneration. Curr Pharm Des. 2013;19:3466–3473. doi: 10.2174/1381612811319190012. [DOI] [PubMed] [Google Scholar]
- 21.Evans C. Gene therapy for the regeneration of bone. Injury. 2011;42:599–604. doi: 10.1016/j.injury.2011.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cao H., Sun Z.B., Zhang L., Qian W., Li C.Y., Guo X.P. Adenovirus-mediated bone morphogenetic protein-2 promotes osteogenic differentiation in human mesenchymal stem cells in vitro. Exp Ther Med. 2017;14:377–382. doi: 10.3892/etm.2017.4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Munsell E.V., Kurpad D.S., Freeman T.A., Sullivan M.O. Histone-targeted gene transfer of bone morphogenetic Protein-2 enhances mesenchymal stem cell chondrogenic differentiation. Acta Biomater. 2018;18:S1742–S7061. doi: 10.1016/j.actbio.2018.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerstenfeld L.C., Cullinane D.M., Barnes G.L., Graves D.T., Einhorn T.A. Fracture healing as a post-natal developmental process: molecular, spatial, and temporal aspects of its regulation. J Cell Biochem. 2003;88:873–884. doi: 10.1002/jcb.10435. [DOI] [PubMed] [Google Scholar]
- 25.Gibon E., Lu L.Y., Nathan K., Goodman S.B. Inflammation, ageing, and bone regeneration. J Orthop Translation. 2017;10:28–35. doi: 10.1016/j.jot.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kon T., Cho T.J., Aizawa T., Yamazaki M., Nooh N., Graves D. Expression of osteoprotegerin, receptor activator of NF-kappaB ligand (osteoprotegerin ligand) and related proinflammatory cytokines during fracture healing. J Bone Miner Res. 2001;16:1004–1014. doi: 10.1359/jbmr.2001.16.6.1004. [DOI] [PubMed] [Google Scholar]
- 27.Chan J.K., Glass G.E., Ersek A., Freidin A., Williams G.A., Gowers K. Low-dose TNF augments fracture healing in normal and osteoporotic bone by up-regulating the innate immune response. EMBO Mol Med. 2015;7:547–561. doi: 10.15252/emmm.201404487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gerstenfeld L.C., Cho T.J., Kon T., Aizawa T., Tsay A., Fitch J. Impaired fracture healing in the absence of TNF-alpha signaling: the role of TNF-alpha in endochondral cartilage resorption. J Bone Miner Res. 2003;18:1584–1592. doi: 10.1359/jbmr.2003.18.9.1584. [DOI] [PubMed] [Google Scholar]
- 29.Clement N.N.D., Goudie E.E.B., Brooksbank A.A.J., Chesser T.T.J., Robinson C.C.M. Smoking status and the Disabilities of the Arm Shoulder and Hand score are early predictors of symptomatic nonunion of displaced midshaft fractures of the clavicle. Bone Joint Lett J. 2016;98:125–130. doi: 10.1302/0301-620X.98B1.36260. [DOI] [PubMed] [Google Scholar]
- 30.Scolaro J.J.A., Schenker M.M.L., Yannascoli S., Baldwin K., Mehta S., Ahn J. Cigarette smoking increases complications following fracture: a systematic review. J Bone Joint Surg Am. 2014;96:674–681. doi: 10.2106/JBJS.M.00081. [DOI] [PubMed] [Google Scholar]
- 31.Chen Y., Guo Q., Pan X., Qin L., Zhang P. Smoking and impaired bone healing: will activation of cholinergic anti-inflammatory pathway be the bridge? Int Orthop. 2011;35:1267–1270. doi: 10.1007/s00264-011-1243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kayal R.R.A., Tsatsas D., Bauer M.M.A., Allen B., Al-Sebaei M.M.O., Kakar S. Diminished bone formation during diabetic fracture healing is related to the premature resorption of cartilage associated with increased osteoclast activity. J Bone Miner Res. 2007;22:560–568. doi: 10.1359/jbmr.070115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kayal R.R.A., Siqueira M., Alblowi J., McLean J., Krothapalli N., Faibish D. TNF-alpha mediates diabetes-enhanced chondrocyte apoptosis during fracture healing and stimulates chondrocyte apoptosis through FOXO1. J Bone Miner Res. 2010;25:1604–1615. doi: 10.1002/jbmr.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alblowi J., Kayal R.R.A., Siqueira M., McKenzie E., Krothapalli N., McLean J. High levels of tumor necrosis factor-alpha contribute to accelerated loss of cartilage in diabetic fracture healing. Am J Pathol. 2009;175:1574–1585. doi: 10.2353/ajpath.2009.090148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ko K.K.I., Coimbra L.L.S., Tian C., Alblowi J., Kayal R.R.A., Einhorn T.T.A. Diabetes reduces mesenchymal stem cells in fracture healing through a TNFalpha-mediated mechanism. Diabetologia. 2015;58:633–642. doi: 10.1007/s00125-014-3470-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lim J.J.C., Ko K.K.I., Mattos M., Fang M., Zhang C., Feinberg D. TNFalpha contributes to diabetes impaired angiogenesis in fracture healing. Bone. 2017;99:26–38. doi: 10.1016/j.bone.2017.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun M., Yang J., Wang J., Hao T., Jiang D., Bao G. TNF-alpha is upregulated in T2DM patients with fracture and promotes the apoptosis of osteoblast cells in vitro in the presence of high glucose. Cytokine. 2016;80:35–42. doi: 10.1016/j.cyto.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 38.Liuni F.F.M., Rugiero C., Feola M., Rao C., Pistillo P., Terracciano C. Impaired healing of fragility fractures in type 2 diabetes: clinical and radiographic assessments and serum cytokine levels. Aging Clin Exp Res. 2015;27(Suppl 1):S37–S44. doi: 10.1007/s40520-015-0422-4. [DOI] [PubMed] [Google Scholar]
- 39.Franchimont N., Wertz S., Malaise M. Interleukin-6: an osteotropic factor influencing bone formation? Bone. 2005;37:601–606. doi: 10.1016/j.bone.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 40.Kondo M., Yamaoka K., Sakata K., Sonomoto K., Lin L., Nakano K. Contribution of the interleukin-6/STAT-3 signaling pathway to chondrogenic differentiation of human mesenchymal stem cells. Arthritis Rheumatol. 2015;67:1250–1260. doi: 10.1002/art.39036. [DOI] [PubMed] [Google Scholar]
- 41.Kudo O., Sabokbar A., Pocock A., Itonaga I., Fujikawa Y., Athanasou N.N.A. Interleukin-6 and interleukin-11 support human osteoclast formation by a RANKL-independent mechanism. Bone. 2003;32:1–7. doi: 10.1016/s8756-3282(02)00915-8. [DOI] [PubMed] [Google Scholar]
- 42.Yang X., Ricciardi B.B.F., Hernandez-Soria A., Shi Y., Pleshko Camacho N., Bostrom M.M.P. Callus mineralization and maturation are delayed during fracture healing in interleukin-6 knockout mice. Bone. 2007;41:928–936. doi: 10.1016/j.bone.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wallace A., Cooney T.T.E., Englund R., Lubahn J.J.D. Effects of interleukin-6 ablation on fracture healing in mice. J Orthop Res. 2011;29:1437–1442. doi: 10.1002/jor.21367. [DOI] [PubMed] [Google Scholar]
- 44.Huang L., Liu S., Song T., Zhang W., Fan J., Liu Y. Blockade of interleukin 6 by rat anti-mouse interleukin 6 receptor antibody promotes fracture healing. Biochemistry (Mosc) 2017;82:1193–1199. doi: 10.1134/S0006297917100121. [DOI] [PubMed] [Google Scholar]
- 45.Erices A., Conget P., Rojas C., Minguell J.J.J. Gp130 activation by soluble interleukin-6 receptor/interleukin-6 enhances osteoblastic differentiation of human bone marrow-derived mesenchymal stem cells. Exp Cell Res. 2002;280:24–32. doi: 10.1006/excr.2002.5627. [DOI] [PubMed] [Google Scholar]
- 46.Cho T.T.J., Kim J.J.A., Chung C.C.Y., Yoo W.W.J., Gerstenfeld L.L.C., Einhorn T.T.A. Expression and role of interleukin-6 in distraction osteogenesis. Calcif Tissue Int. 2007;80:192–200. doi: 10.1007/s00223-006-0240-y. [DOI] [PubMed] [Google Scholar]
- 47.Mathieu M., Rigutto S., Ingels A., Spruyt D., Stricwant N., Kharroubi I. Decreased pool of mesenchymal stem cells is associated with altered chemokines serum levels in atrophic nonunion fractures. Bone. 2013;53:391–398. doi: 10.1016/j.bone.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 48.Prystaz K., Kaiser K., Kovtun A., Haffner-Luntzer M., Fischer V., Rapp A.A.E. Distinct effects of IL-6 classic and trans-signaling in bone fracture healing. Am J Pathol. 2018;188:474–490. doi: 10.1016/j.ajpath.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 49.Xu X., Zheng L.L.W., Yuan Q., Zhen G.G.H., Crane J.J.L., Zhou X.X.D. Transforming growth factor-β in stem cells and tissue homeostasis. Bone Res. 2018;6:2. doi: 10.1038/s41413-017-0005-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Papanagiotou M., Dailiana Z.Z.H., Karachalios T., Varitimidis S., Vlychou M., Hantes M. RhBMP-7 for the treatment of nonunion of fractures of long bones. Bone Joint Lett J. 2015;97:997–1003. doi: 10.1302/0301-620X.97B7.35089. [DOI] [PubMed] [Google Scholar]
- 51.Brannan P.P.S., Gaston R.R.G., Loeffler B.B.J., Lewis D.D.R. Complications with the use of BMP-2 in scaphoid nonunion surgery. J Hand Surg Am. 2016;41:602–608. doi: 10.1016/j.jhsa.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 52.Takemoto R., Forman J., Taormina D.D.P., Egol K.K.A. No advantage to rhBMP-2 in addition to autogenous graft for fracture nonunion. Orthopedics. 2014;37:e525–e530. doi: 10.3928/01477447-20140528-51. [DOI] [PubMed] [Google Scholar]
- 53.von Ruden C., Morgenstern M., Hierholzer C., Hackl S., Gradinger F.F.L., Woltmann A. The missing effect of human recombinant Bone Morphogenetic Proteins BMP-2 and BMP-7 in surgical treatment of aseptic forearm nonunion. Injury. 2016;47:919–924. doi: 10.1016/j.injury.2015.11.038. [DOI] [PubMed] [Google Scholar]
- 54.Sebald H.H.J., Klenke F.F.M., Siegrist M., Albers C.C.E., Sebald W., Hofstetter W. Inhibition of endogenous antagonists with an engineered BMP-2 variant increases BMP-2 efficacy in rat femoral defect healing. Acta Biomater. 2012;8:3816–3820. doi: 10.1016/j.actbio.2012.06.036. [DOI] [PubMed] [Google Scholar]
- 55.Niikura T., Hak D.D.J., Reddi A.A.H. Global gene profiling reveals a downregulation of BMP gene expression in experimental atrophic nonunions compared to standard healing fractures. J Orthop Res. 2006;24:1463–1471. doi: 10.1002/jor.20182. [DOI] [PubMed] [Google Scholar]
- 56.Fajardo M., Liu C.C.J., Egol K. Levels of expression for BMP-7 and several BMP antagonists may play an integral role in a fracture nonunion: a pilot study. Clin Orthop Relat Res. 2009;467:3071–3078. doi: 10.1007/s11999-009-0981-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kloen P., Lauzier D., Hamdy R.R.C. Co-expression of BMPs and BMP-inhibitors in human fractures and non-unions. Bone. 2012;51:59–68. doi: 10.1016/j.bone.2012.03.032. [DOI] [PubMed] [Google Scholar]
- 58.Kwong F.F.N., Hoyland J.J.A., Freemont A.A.J., Evans C.C.H. Altered relative expression of BMPs and BMP inhibitors in cartilaginous areas of human fractures progressing towards nonunion. J Orthop Res. 2009;27:752–757. doi: 10.1002/jor.20794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van Baardewijk L.L.J., van der Ende J., Lissenberg-Thunnissen S., Romijn L.L.M., Hawinkels L.L.J., Sier C.C.F. Circulating bone morphogenetic protein levels and delayed fracture healing. Int Orthop. 2013;37:523–527. doi: 10.1007/s00264-012-1750-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Richards C.C.J., Graf K.K.W., Jr., Mashru R.R.P. The effect of opioids, alcohol, and nonsteroidal anti-inflammatory drugs on fracture union. Orthop Clin North Am. 2017;48:433–443. doi: 10.1016/j.ocl.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 61.Daluiski A., Ramsey K.K.E., Shi Y., Bostrom M.M.P., Nestor B.B.J., Martin G. Cyclooxygenase-2 inhibitors in human skeletal fracture healing. Orthopedics. 2006;29:259–261. doi: 10.3928/01477447-20060301-02. [DOI] [PubMed] [Google Scholar]
- 62.Li T.T.F., Yukata K., Yin G., Sheu T., Maruyama T., Jonason J.J.H. BMP-2 induces ATF4 phosphorylation in chondrocytes through a COX-2/PGE2 dependent signaling pathway. Osteoarthritis Cartilage. 2014;22:481–489. doi: 10.1016/j.joca.2013.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Muinos-Lopez E., Ripalda-Cemborain P., Lopez-Martinez T., Gonzalez-Gil A.A.B., Lamo-Espinosa J.J.M., Valenti A. Hypoxia and reactive oxygen species homeostasis in mesenchymal progenitor cells define a molecular mechanism for fracture nonunion. Stem Cell. 2016;34:2342–2353. doi: 10.1002/stem.2399. [DOI] [PubMed] [Google Scholar]
- 64.Wigner N.N.A., Luderer H.H.F., Cox M.M.K., Sooy K., Gerstenfeld L.L.C., Demay M.M.B. Acute phosphate restriction leads to impaired fracture healing and resistance to BMP-2. J Bone Miner Res. 2010;25:724–733. doi: 10.1359/jbmr.091021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu E.E.S., Zalutskaya A., Chae B.B.T., Zhu E.E.D., Gori F., Demay M.M.B. Phosphate interacts with PTHrP to regulate endochondral bone formation. Endocrinology. 2014;155:3750–3756. doi: 10.1210/en.2014-1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lyon T., Scheele W., Bhandari M., Koval K.K.J., Sanchez E.E.G., Christensen J. Efficacy and safety of recombinant human bone morphogenetic protein-2/calcium phosphate matrix for closed tibial diaphyseal fracture: a double-blind, randomized, controlled phase-II/III trial. J Bone Joint Surg Am. 2013;95:2088–2096. doi: 10.2106/JBJS.L.01545. [DOI] [PubMed] [Google Scholar]
- 67.Kato H., Faria T.T.N., Stannard B., Roberts C.C.T., Jr., LeRoith D. Role of tyrosine kinase activity in signal transduction by the insulin-like growth factor-I (IGF-I) receptor. Characterization of kinase-deficient IGF-I receptors and the action of an IGF-I-mimetic antibody (alpha IR-3) J Biol Chem. 1993;268:2655–2661. [PubMed] [Google Scholar]
- 68.Kawai M., Rosen C.C.J. The insulin-like growth factor system in bone: basic and clinical implications. Endocrinol Metab Clin North Am. 2012;41:323–333. doi: 10.1016/j.ecl.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu J.J.P., Baker J., Perkins A.A.S., Robertson E.E.J., Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r) Cell. 1993;75:59–72. [PubMed] [Google Scholar]
- 70.Bikle D., Majumdar S., Laib A., Powell-Braxton L., Rosen C., Beamer W. The skeletal structure of insulin-like growth factor I-deficient mice. J Bone Miner Res. 2001;16:2320–2329. doi: 10.1359/jbmr.2001.16.12.2320. [DOI] [PubMed] [Google Scholar]
- 71.He J., Rosen C.C.J., Adams D.D.J., Kream B.B.E. Postnatal growth and bone mass in mice with IGF-I haploinsufficiency. Bone. 2006;38:826–835. doi: 10.1016/j.bone.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 72.Wang T., Wang Y., Menendez A., Fong C., Babey M., Tahimic C.C.G. Osteoblast-specific loss of IGF1R signaling results in impaired endochondral bone formation during fracture healing. J Bone Miner Res. 2015;30:1572–1584. doi: 10.1002/jbmr.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lau K.K.W., Rundle C.C.H., Zhou X.X.D., Baylink D.D.J., Sheng M.M.H. Conditional deletion of IGF-I in osteocytes unexpectedly accelerates bony union of the fracture gap in mice. Bone. 2016;92:18–28. doi: 10.1016/j.bone.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 74.Koh A., Niikura T., Lee S.S.Y., Oe K., Koga T., Dogaki Y. Differential gene expression and immunolocalization of insulin-like growth factors and insulin-like growth factor binding proteins between experimental nonunions and standard healing fractures. J Orthop Res. 2011;29:1820–1826. doi: 10.1002/jor.21457. [DOI] [PubMed] [Google Scholar]
- 75.Weiss S., Henle P., Bidlingmaier M., Moghaddam A., Kasten P., Zimmermann G. Systemic response of the GH/IGF-I axis in timely versus delayed fracture healing. Growth Horm IGF Res. 2008;18:205–212. doi: 10.1016/j.ghir.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 76.Chen Q.Q.Q., Wang W.W.M. Expression of FGF-2 and IGF-1 in diabetic rats with fracture. Asian Pac J Trop Med. 2014;7:71–75. doi: 10.1016/S1995-7645(13)60195-9. [DOI] [PubMed] [Google Scholar]
- 77.Granero-Molto F., Myers T.T.J., Weis J.J.A., Longobardi L., Li T., Yan Y. Mesenchymal stem cells expressing insulin-like growth factor-I (MSCIGF) promote fracture healing and restore new bone formation in Irs1 knockout mice: analyses of MSCIGF autocrine and paracrine regenerative effects. Stem Cell. 2011;29:1537–1548. doi: 10.1002/stem.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Myers T.T.J., Yan Y., Granero-Molto F., Weis J.J.A., Longobardi L., Li T. Systemically delivered insulin-like growth factor-I enhances mesenchymal stem cell-dependent fracture healing. Growth Factors. 2012;30:230–241. doi: 10.3109/08977194.2012.683188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Raschke M., Wildemann B., Inden P., Bail H., Flyvbjerg A., Hoffmann J. Insulin-like growth factor-1 and transforming growth factor-beta1 accelerates osteotomy healing using polylactide-coated implants as a delivery system: a biomechanical and histological study in minipigs. Bone. 2002;30:144–151. doi: 10.1016/s8756-3282(01)00640-8. [DOI] [PubMed] [Google Scholar]
- 80.Schmidmaier G., Wildemann B., Heeger J., Gabelein T., Flyvbjerg A., Bail H.H.J. Improvement of fracture healing by systemic administration of growth hormone and local application of insulin-like growth factor-1 and transforming growth factor-beta1. Bone. 2002;31:165–172. doi: 10.1016/s8756-3282(02)00798-6. [DOI] [PubMed] [Google Scholar]
- 81.Fowlkes J.J.L., Thrailkill K.K.M., Liu L., Wahl E.E.C., Bunn R.R.C., Cockrell G.G.E. Effects of systemic and local administration of recombinant human IGF-I (rhIGF-I) on de novo bone formation in an aged mouse model. J Bone Miner Res. 2006;21:1359–1366. doi: 10.1359/JBMR.060618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Boonen S., Rosen C., Bouillon R., Sommer A., McKay M., Rosen D. Musculoskeletal effects of the recombinant human IGF-I/IGF binding protein-3 complex in osteoporotic patients with proximal femoral fracture: a double-blind, placebo-controlled pilot study. J Clin Endocrinol Metab. 2002;87:1593–1599. doi: 10.1210/jcem.87.4.8426. [DOI] [PubMed] [Google Scholar]
- 83.Kosaki N., Takaishi H., Kamekura S., Kimura T., Okada Y., Minqi L. Impaired bone fracture healing in matrix metalloproteinase-13 deficient mice. Biochem Biophys Res Commun. 2007;354:846–851. doi: 10.1016/j.bbrc.2006.12.234. [DOI] [PubMed] [Google Scholar]
- 84.Colnot C., Thompson Z., Miclau T., Werb Z., Helms J.J.A. Altered fracture repair in the absence of MMP9. Development. 2003;130:4123–4133. doi: 10.1242/dev.00559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lieu S., Hansen E., Dedini R., Behonick D., Werb Z., Miclau T. Impaired remodeling phase of fracture repair in the absence of matrix metalloproteinase-2. Dis Model Mech. 2011;4:203–211. doi: 10.1242/dmm.006304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Henle P., Zimmermann G., Weiss S. Matrix metalloproteinases and failed fracture healing. Bone. 2005;37:791–798. doi: 10.1016/j.bone.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 87.Wigner N.N.A., Kulkarni N., Yakavonis M., Young M., Tinsley B., Meeks B. Urine matrix metalloproteinases (MMPs) as biomarkers for the progression of fracture healing. Injury. 2012;43:274–278. doi: 10.1016/j.injury.2011.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fajardo M., Liu C.C.J., Ilalov K., Egol K.K.A. Matrix metalloproteinases that associate with and cleave bone morphogenetic protein-2 in vitro are elevated in hypertrophic fracture nonunion tissue. J Orthop Trauma. 2010;24:557–563. doi: 10.1097/BOT.0b013e3181ed294c. [DOI] [PubMed] [Google Scholar]
- 89.Ai-Aql Z.Z.S., Alagl A.A.S., Graves D.D.T., Gerstenfeld L.L.C., Einhorn T.T.A. Molecular mechanisms controlling bone formation during fracture healing and distraction osteogenesis. J Dent Res. 2008;87:107–118. doi: 10.1177/154405910808700215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chu T., Liu Y., Wang Z., Zhu P., Jiao W., Wen J. Sustained vascular endothelial growth factor blockade by antivascular endothelial growth factor antibodies results in nonunion in the process of fracture healing in rabbits. J Trauma. 2009;66:1180–1183. doi: 10.1097/TA.0b013e31818b4e61. [DOI] [PubMed] [Google Scholar]
- 91.Garcia P., Pieruschka A., Klein M., Tami A., Histing T., Holstein J.J.H. Temporal and spatial vascularization patterns of unions and nonunions: role of vascular endothelial growth factor and bone morphogenetic proteins. J Bone Joint Surg Am. 2012;94:49–58. doi: 10.2106/JBJS.J.00795. [DOI] [PubMed] [Google Scholar]
- 92.Sarahrudi K., Thomas A., Braunsteiner T., Wolf H., Vecsei V., Aharinejad S. VEGF serum concentrations in patients with long bone fractures: a comparison between impaired and normal fracture healing. J Orthop Res. 2009;27:1293–1297. doi: 10.1002/jor.20906. [DOI] [PubMed] [Google Scholar]
- 93.Ozturk B.B.Y., Inci I., Egri S., Ozturk A.A.M., Yetkin H., Goktas G. The treatment of segmental bone defects in rabbit tibiae with vascular endothelial growth factor (VEGF)-loaded gelatin/hydroxyapatite "cryogel" scaffold. Eur J Orthop Surg Traumatol. 2013;23:767–774. doi: 10.1007/s00590-012-1070-4. [DOI] [PubMed] [Google Scholar]
- 94.Ogilvie C.C.M., Lu C., Marcucio R., Lee M., Thompson Z., Hu D. Vascular endothelial growth factor improves bone repair in a murine nonunion model. Iowa Orthop J. 2012;32:90–94. [PMC free article] [PubMed] [Google Scholar]
- 95.Corbett S.S.A., Hukkanen M., Batten J., McCarthy I.I.D., Polak J.J.M., Hughes S.S.P. Nitric oxide in fracture repair. Differential localisation, expression and activity of nitric oxide synthases. J Bone Joint Surg Br. 1999;81:531–537. doi: 10.1302/0301-620x.81b3.8852. [DOI] [PubMed] [Google Scholar]
- 96.Zhu W., Diwan A.A.D., Lin J.J.H., Murrell G.G.A. Nitric oxide synthase isoforms during fracture healing. J Bone Miner Res. 2001;16:535–540. doi: 10.1359/jbmr.2001.16.3.535. [DOI] [PubMed] [Google Scholar]
- 97.Tomlinson R.R.E., Shoghi K.K.I., Silva M.M.J. Nitric oxide-mediated vasodilation increases blood flow during the early stages of stress fracture healing. J Appl Physiol. 2014;116:416–424. doi: 10.1152/japplphysiol.00957.2013. 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chae H.H.J., Park R.R.K., Chung H.H.T., Kang J.J.S., Kim M.M.S., Choi D.D.Y. Nitric oxide is a regulator of bone remodelling. J Pharm Pharmacol. 1997;49:897–902. doi: 10.1111/j.2042-7158.1997.tb06132.x. [DOI] [PubMed] [Google Scholar]
- 99.Corbett S.S.A., McCarthy I.I.D., Batten J., Hukkanen M., Polak J.J.M., Hughes S.S.P. Nitric oxide mediated vasoreactivity during fracture repair. Clin Orthop Relat Res. 1999:247–253. doi: 10.1097/00003086-199908000-00030. [DOI] [PubMed] [Google Scholar]
- 100.Meesters D.D.M., Neubert S., Wijnands K.K.A., Heyer F.F.L., Zeiter S., Ito K. Deficiency of inducible and endothelial nitric oxide synthase results in diminished bone formation and delayed union and nonunion development. Bone. 2016;83:111–118. doi: 10.1016/j.bone.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 101.Wijnands K.K.A., Brink P.P.R., Weijers P.P.H., Dejong C.C.H., Poeze M. Impaired fracture healing associated with amino acid disturbances. Am J Clin Nutr. 2012;95:1270–1277. doi: 10.3945/ajcn.110.009209. [DOI] [PubMed] [Google Scholar]
- 102.Luiking Y.Y.C., Poeze M., Ramsay G., Deutz N.N.E. Reduced citrulline production in sepsis is related to diminished de novo arginine and nitric oxide production. Am J Clin Nutr. 2009;89:142–152. doi: 10.3945/ajcn.2007.25765. [DOI] [PubMed] [Google Scholar]
- 103.Lee R.R.H., Efron D., Tantry U., Barbul A. Nitric oxide in the healing wound: a time-course study. J Surg Res. 2001;101:104–108. doi: 10.1006/jsre.2001.6261. [DOI] [PubMed] [Google Scholar]
- 104.Schaffer M.M.R., Tantry U., van Wesep R.R.A., Barbul A. Nitric oxide metabolism in wounds. J Surg Res. 1997;71:25–31. doi: 10.1006/jsre.1997.5137. [DOI] [PubMed] [Google Scholar]
- 105.Kdolsky R.R.K., Mohr W., Savidis-Dacho H., Beer R., Puig S., Reihsner R. The influence of oral L-arginine on fracture healing: an animal study. Wien Klin Wochenschr. 2005;117:693–701. doi: 10.1007/s00508-005-0431-y. [DOI] [PubMed] [Google Scholar]
- 106.Rajfer R.R.A., Kilic A., Neviaser A.A.S., Schulte L.L.M., Hlaing S.S.M., Landeros J. Enhancement of fracture healing in the rat, modulated by compounds that stimulate inducible nitric oxide synthase: acceleration of fracture healing via inducible nitric oxide synthase. Bone Joint Res. 2017;6:90–97. doi: 10.1302/2046-3758.62.BJR-2016-0164.R2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wise J.J.K., Sena K., Vranizan K., Pollock J.J.F., Healy K.K.E., Hughes W.W.F. Temporal gene expression profiling during rat femoral marrow ablation-induced intramembranous bone regeneration. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lauing K.K.L., Roper P.P.M., Nauer R.R.K., Callaci J.J.J. Acute alcohol exposure impairs fracture healing and deregulates beta-catenin signaling in the fracture callus. Alcohol Clin Exp Res. 2012;36:2095–2103. doi: 10.1111/j.1530-0277.2012.01830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lauing K.K.L., Sundaramurthy S., Nauer R.R.K., Callaci J.J.J. Exogenous activation of Wnt/beta-catenin signaling attenuates binge alcohol-induced deficient bone fracture healing. Alcohol Alcohol. 2014;49:399–408. doi: 10.1093/alcalc/agu006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Roper P.P.M., Abbasnia P., Vuchkovska A., Natoli R.R.M., Callaci J.J.J. Alcohol-related deficient fracture healing is associated with activation of FoxO transcription factors in mice. J Orthop Res. 2016;34:2106–2115. doi: 10.1002/jor.23235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Alzahrani M.M.M., Rauch F., Hamdy R.R.C. Does sclerostin depletion stimulate fracture healing in a mouse model? Clin Orthop Relat Res. 2016;474:1294–1302. doi: 10.1007/s11999-015-4640-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jawad M.M.U., Fritton K.K.E., Ma T., Ren P.P.G., Goodman S.S.B., Ke H.H.Z. Effects of sclerostin antibody on healing of a non-critical size femoral bone defect. J Orthop Res. 2013;31:155–163. doi: 10.1002/jor.22186. [DOI] [PubMed] [Google Scholar]
- 113.Tinsley B.B.A., Dukas A., Pensak M.M.J., Adams D.D.J., Tang A.A.H., Ominsky M.M.S. Systemic administration of sclerostin antibody enhances bone morphogenetic protein-induced femoral defect repair in a rat model. J Bone Joint Surg Am. 2015;97:1852–1859. doi: 10.2106/JBJS.O.00171. [DOI] [PubMed] [Google Scholar]
- 114.Jin H., Wang B., Li J., Xie W., Mao Q., Li S. Anti-DKK1 antibody promotes bone fracture healing through activation of beta-catenin signaling. Bone. 2015;71:63–75. doi: 10.1016/j.bone.2014.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]