Abstract
Background
Agastache rugosa (Fisch. & C.A.Mey.) Kuntze (Korean mint) is used to treat diverse types of human disorders in traditional medicine. In recent years, its non-fermented leaf extract (ARE) has been shown to possess protective properties against ultraviolet-B (UV-B) radiation-induced photooxidative stress. The present work aimed to examine whether probiotic bacterial fermentation would potentiate the skin anti-photoaging activity of ARE or not, by comparing the protective properties of ARE and corresponding fermented extract (ARE-F) against UV-B radiation-induced photooxidative stress in HaCaT keratinocytes.
Methods
ARE-F was produced from ARE by the fermentation with Lactobacillus rhamnosus HK-9, a type of Gram-positive probiotic bacterial strain. Anti-photoaging activities were evaluated by analyzing reactive oxygen species (ROS), promatrix metalloproteinases (proMMPs), total glutathione (GSH) and total superoxide dismutase (SOD) in UV-B-irradiated HaCaT keratinocytes. Antiradical activity was determined using 2,2-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical scavenging assay.
Results
ARE-F contained higher attenuating activity on the UV-B-induced ROS generation than ARE. Similarly, ARE-F was able to diminish the UV-B-induced proMMP-9 and -2 more effectively than ARE. ARE-F displayed higher tendencies to augment the UV-B-reduced total GSH content and SOD activity than ARE. However, there were no significant difference between ARE and ARE-F in ABTS radical scavenging activities.
Conclusions
The findings suggest that the UV-B radiation-protective activity of ARE is enhanced by probiotic bacterial fermentation, which might improve the therapeutic and cosmetic values of A. rugosa leaves.
Keywords: Agastache rugosa, Anti-photoaging, Glutathione, HaCaT, Matrix metalloproteinase (MMP), Probiotic fermentation, Reactive oxygen species (ROS), Superoxide dismutase
Background
Skin aging, concisely defined as an impairment of skin integrity, is classified into the two main types: intrinsic or chronologic aging and extrinsic aging or photoaging. Intrinsic aging comes from the common process of senescence affecting all body organs, whereas photoaging occurs as a consequence of chronic exposure to environmental factors, especially solar ultraviolet (UV) radiation, and is characterized by skin fragility, wrinkle, roughness, sagging, dryness, laxity and hyper-pigmentation [1, 2].
The three types of UV radiation – UV-A, UV-B and UV-C – have different wavelength ranges. Although UV-C radiation (100–280 nm) is extremely damaging to the skin, it is almost completely absorbed by the ozone layer and thus has no adverse effects. Despite being more penetrating deeper in the skin, less energetic UV-A radiation (315–400 nm) has been considered to be less harmful. Recent studies implicate that UV-A radiation can cause skin cancer indirectly via generating highly reactive chemical intermediates, such as free radicals and ROS, which in turn can damage DNA [3]. UV-B radiation (280–315 nm) constitutes only 5% of the total solar UV energy and is considered the most damaging and genotoxic, which is chiefly responsible for various sun-induced skin disorders, including sunburn, photoaging, oxidative damage, erythema, acute inflammation, immunosuppression, and nonmelanoma and melanoma skin cancers [3, 4]. UV-B radiation can cause DNA damage as a consequence of its direct action on DNA molecule resulting in the formation of cyclobutane-pyrimidine dimers, 6–4 photoproducts, Dewar isomers and 8-hydroxy-2′-deoxyguanosine, and indirectly increase ROS levels, which, in case of overwhelming the cellular antioxidant defense capacity, leads to oxidative stress and subsequently oxidative photolesions of macromolecules, such as DNA, proteins and lipids, in the skin [5, 6]. ROS participate in diverse UV-B-induced pathophysiological processes by activating mitogen-activated protein kinase (MAPK) and nuclear factor-κB (NF-κB) [7].
Although MMPs play a role in normal tissue development, remodeling and repair, they are closely linked to tissue destruction in a number of pathological conditions, including inflammation, UV-induced skin damage and tumor invasion [8, 9]. MMP-2 and -9, belonging to the gelatinase group of MMPs, degrade the extracellar matrix (ECM) via degrading type IV collagen, the most abundant components of the basement membrane which is important for maintaining tissue organization, providing structural support for cells and influencing cell signaling and polarity, and influence skin wrinkle formation and skin thickness [10]. The exposure of human skin to UV radiation up-regulates the synthesis of various MMPs, including MMP-1, − 2, − 3, − 7, − 8, − 9 and − 12, which are implicated in photoaging [10]. MMP-2 and -9 are expressed at elevated levels in a variety of cell types under oxidative stress [11, 12].
A. rugosa is a perennial herb belonging to the mint family (Lamiaceae) cultivated in East Asian Countries, including Korea, and has been used to treat colds, anorexia, cholera, vomiting, miasma and other kinds of disorders [13]. A variety of essential oils, such as methyleugenol, estragole and eugenol, and diverse types of flavonoids, such as tilianin, acacetin, linarin, agastachoside and rosmarinic acid, have been identified from A. rugosa [14]. Two diterpenoid compounds, agastanol and agastaquinone, and two lignin compounds, agastinol and agastenol, were also identified from A. rugosa [15, 16]. A range of biological and pharmacological activities of A. rugosa, including antimicrobial, antifungal, insecticidal, antiviral, antihypertensive, anti-inflammatory, anticancer, antioxidative, antiatherogenic and vasorelaxant activities, have been documented [17–20].
Probiotic bacterial fermentation, emerging as one of crucial processing tools in cosmetic technologies, is used to diminish toxicities of cosmetic resources, enhance absorption into the skin by altering their molecular structures, and improve their desirable pharmacological activities [21].
Throughout the previous work, ARE was reported to have an attenuating potential against the UV-B-induced photoaging of human skin [22]. In the present work, it is demonstrated that the skin anti-photoaging activity of ARE can be potentiated by probiotic bacterial fermentation. This finding might possibly broaden the usefulness of A. rugosa in therapeutic as well as cosmetic applications.
Methods
Chemicals
Bradford reagent, bovine serum albumin, ascorbic acid (AA), 2,2-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), ammonium persulfate, 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA), 5,5′-dithiobis (2-nitrobenzoic acid) (DTNB), glutathione reductase (GR), catalase, xanthine, xanthine oxidase and NADPH were from Sigma-Aldrich Chemical Co. (St Louis, MO, USA). Cell lysis buffer [25 mM Tris-phosphate (pH 7.8), 2 mM 1,2-diaminocyclohexane-N,N,Nv,Nv-tetraacetic acid, 2 mM dithiothreitol, 10% glycerol, 1% Triton X-100] was from Promega Korea (Seoul, Korea).
Plant material
Dried A. rugosa leaves, obtained from a local market in Chuncheon, Korea, were authenticated by Prof. Ki-Oug Yoo (Department of Biological Sciences, College of Natural Sciences, Kangwon National University, Chuncheon, Korea). A voucher specimen (No. KWNU90446) was deposited at the herbarium in the same department.
Preparation of ARE and ARE-F
As previously described [22], ARE was prepared as follows. Dried A. rugosa leaves, ground under liquid nitrogen, were extracted under reflux by placing in a water bath at 90 °C for 4 h. After being filtered through a filter paper, the hot water extract was evaporated to dryness in a freeze dryer, and the extract powder was designated as ARE. The yield was approximately 10.4%.
ARE, resuspended in distilled water, was incubated at 30 °C for 5 days with L. rhamnosus HK-9 (KACC 11254P, Korea), centrifuged at 5000 g for 20 min to discard bacterial cells, and concentrated using a freeze dryer to generate fermented extract powder, designated as ARE-F. Prior to the experiments, both ARE and ARE-F were dissolved in dimethyl sulfoxide, and control cells were treated with vehicle only (0.1% dimethyl sulfoxide). The vehicle itself had no damaging effect on the viabilities of HaCaT cells.
Cell culture
A human immortalized HaCaT keratinocyte cell line (ATCC No. CRL-2309) was obtained from American Type Culture Collection (Manassas, VA, USA) and grown in Dulbecco’s Modified Eagle’s Medium (DMEM) with 10% heat-inactivated fetal bovine serum (FBS), 100 units/mL penicillin and 100 μg/mL streptomycin in a humidified atmosphere with 5% CO2 at 37 °C.
UV-B irradiation
As a UV-B source, an ultraviolet lamp (peak, 312 nm; model VL-6 M, Vilber Lourmat, Marine, France) was used with a radiometer (model VLX-3 W) equipped with a sensor (bandwidth, 280 to 320 nm; model CX-312). In the present work, HaCaT keratinocyte culture at 25 °C were irradiated with solar simulated UV-B radiation at 70 mJ/cm2, an intensity chosen to induce a photooxidative stress through a preliminary experiment.
Preparation of cellular lysate
At 18 h after irradiation, adherent cells, washed twice with PBS and stored on ice for 5 min, were collected using a cell scraper, centrifuged at 5000 g for 10 min, resuspended in cell lysis buffer and stored for 30 min on ice. Cellular lysate was taken out after centrifugation at 5000 g for 15 min.
Protein content in cellular lysates was quantitated with the Bradford protein assay [23] using bovine serum albumin as a reference standard to construct a calibration curve.
Quantitation of intracellular ROS
As previously described [24], a redox-sensitive fluorescent probe DCFH-DA, which generates the fluorescent 2′,7′-dichlorofluorescein (DCF; λexcitation = 485 nm, λemission = 530 nm) upon enzymatic reduction and subsequent oxidation by ROS, was utilized to detect intracellular ROS. After HaCaT keratinocytes were treated with ARE and ARE-F and/or 20 μM DCFH-DA for 30 min at 37 °C, they were washed twice with 1 mL FBS-free DMEM. The cells, resuspended in 1 mL FBS-free DMEM, were irradiated with UV-B radiation. Immediately after the irradiation, the intracellular ROS levels were determined by monitoring the DCF fluorescence using a multi-mode microplate reader (Synergy™ Mx, BioTek Instruments, Winooki, VT, USA).
Western blotting analysis
Western blotting analyses were conducted to detect proMMP-2 and -9 in cellular lysate using anti-MMP-2 (ALX-210-753, Enzo Life Sciences, Farmingdale, NY, USA) and anti-MMP-9 (3852S, Cell Signaling Technology, Danvers, MA, USA) antibodies. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH), used as an internal loading standard, was detected using anti-GAPDH antibody (LF-PA0212, AbFrontier, Seoul, Korea). Cellular lysates were separated on 10% (w/v) SDS-PAGE and electrotransferred to PVDF membrane. After the blotted membrane was probed with primary antibodies overnight at 4 °C, it was incubated with secondary antibody (goat anti-rabbit IgG-pAb-HRP-conjugate; ADI-SAB-300, Enzo Life Sciences, Farmingdale, NY, USA) for 1 h at room temperature, and developed using an enhanced West-save up™ (AbFrontier, Seoul, Korea).
Determination of total GSH
Total GSH content in cellular lysates was determined using an enzymatic recycling assay based on GR [25]. The reaction mixture (200 μL), which contained 175 mM KH2PO4, 6.3 mM EDTA, 0.21 mM NADPH, 0.6 mM DTNB, 0.5 units/mL GR and cellular lysate, was incubated at 25 °C. A change in absorbance at 412 nm was monitored using a microplate reader. Total GSH contents were calculated from a calibration curve constructed with various GSH concentrations, and normalized to the total protein content of cellular lysates, which gave rise to total GSH content in μg/mg protein.
Determination of SOD activity
Total SOD activity in cellular lysates was determined as the reduction of cytochrome c with xanthine/xanthine oxidase system [26]. The reaction mixture (200 μL), contained 50 mM phosphate buffer (pH 7.4), 0.01 units/mL xanthine oxidase, 0.1 mM EDTA, 1 μM catalase, 0.05 mM xanthine, 20 μM cytochrome c and cellular lysate. A change in absorbance at 550 nm was monitored using a microplate reader. The SOD activity was normalized by determining the protein content of cellular lysates, and represented as ΔA550/min/mg protein.
Quantitation of antiradical activity
Antiradical activities of ARE and ARE-F were detected using ABTS radical scavenging assay [27] with a slight modification. ABTS radical cations (ABTS+), produced by reacting ABTS stock solution (0.07 mM) with 0.12 mM ammonium persulfate, were kept to stand in the dark at room temperature for 16 h before use. The varying concentrations of ARE and ARE-F (each 10 μL) were mixed with 290 μL of ABTS+ solution and the final volume was made up to 1 mL with ethanol. The reaction mixture was incubated for 15 min in the dark at room temperature. AA was used as a positive control. The absorbance was measured at 745 nm and the percent inhibition by ARE and ARE-F was calculated using the formula, Inhibition (%) = [(Control - Test)/Control] × 100. The AA concentration eliciting 50% scavenging of ABTS+ radicals (SC50) was calculated.
Statistical analysis
The data were expressed as mean ± SD. Differences between experimental groups were analyzed using one-way ANOVA followed by post hoc Tukey HSD test for multiple comparisons. A P value < 0.05 was considered statistically significant.
Results
Reactive oxygen species (ROS)
ROS are generated by exogenous (environmental) sources, such as UV irradiation and heat exposure, in addition to their production as natural byproducts of the normal cellular metabolism of oxygen. Oxidative stress, which refers to the excessively elevated intracellular levels of ROS and participate in a myriad of pathologies, arises from an imbalance between the production of ROS and the cellular capability to scavenge the reactive intermediates and to repair the resulting damage. ROS levels, one of principal markers of oxidative stress, are considered to reflect the degree of oxidative stress.
When HaCaT keratinocytes were irradiated with 70 mJ/cm2 UV-B radiation, the intracellular ROS levels went up to approximately 15.3-fold (Fig. 1). This result could prove that UV-B irradiation, at the intensity chosen in this work, can impose a state of oxidative stress on HaCaT keratinocytes. However, the radiation intensity used couldn’t interfere with the viabilities of HaCaT keratinocytes, which was detected using an MTT assay (data not shown). When HaCaT keratinocytes were subjected to varying concentrations (5, 20 and 80 μg/mL) of ARE prior to the irradiation, ARE attenuated the UV-B-induced ROS levels to. 57.4, 55.7 and 32.8%, respectively (Fig. 1a). In the same pretreatment, ARE-F, at the concentrations of 5, 20 or 80 μg/mL, could attenuate the UV-B-induced ROS levels to 60.7, 36.1 and 13.1%, respectively (Fig. 1b). ARE exhibited an IC50 value of 27.0 μg/mL, whereas that of ARE-F was determined to be 17.4 μg/mL. Collectively, ARE-F has an enhanced attenuating activity on the UV-B-induced ROS levels than ARE, implying that the intracellular antioxidative activity of ARE can be augmented by probiotic bacterial fermentation.
Fig. 1.
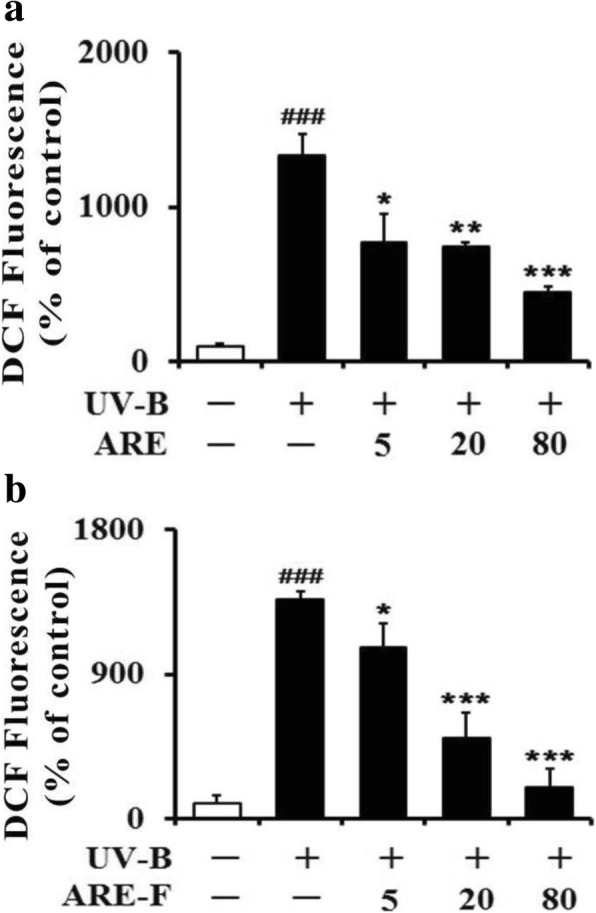
Suppressive effects of ARE, (a) and ARE-F (b) on the ROS levels in HaCaT keratinocytes under UV-B irradiation. HaCaT cells were subjected to the varying concentrations (0, 5, 20 and 80 μg/mL) of ARE (a) and ARE-F (b) for 1 h before the irradiation. The ROS levels were determined using DCFH-DA in a microplate fluorometer, and represented as DCF fluorescence, expressed as a percentage (%) of the corresponding non-irradiated control. ###P < 0.001 versus the non-irradiated control. *P < 0.05; **P < 0.01; ***P < 0.001 versus the non-treated control (UV-B irradiation alone)
Promatrix metalloprtoenase-9 (proMMP-9) and − 2 (proMMP-2)
The skin photoaging activity of UV-B radiation is mediated by the enhancement of diverse MMPs by UV-B-induced ROS. MMP-1, − 9 and − 2, known to be directly involved in the impairment of ECM components, were shown to be up-regulated by UV-B radiation [10]. Even the precursor forms (proMMP-9 and -2) of MMP-9 and -2 were previously identified to be enhanced in HaCaT keratinocytes by UV-B irradiation [28]. As shown in Fig. 2, the UV-B irradiation alone could give rise to approximately 2.0-fold elevation in the proMMP-9 protein level over that in the non-irradiated control cells. HaCaT keratinocytes were subjected to varying concentrations (0, 5, 20 and 80 μg/mL) of ARE prior to UV-B irradiation. ARE at the concentrations of 5, 20 and 80 μg/mL made the UV-B-induced proMMP-9 elevation reduce to 96.1, 72.5 and 54.9% of that from the UV-B irradiation alone, respectively (Fig. 2a). As shown in Fig. 2b, the attenuating activity of ARE-F was also examined in a similar way. ARE-F at the concentrations of 5, 20 and 80 μg/mL was able to attenuate the UV-B-induced proMMP-9 elevation to 39.6, 32.1 and 20.8%, respectively (Fig. 2b). The IC50 values of ARE and ARE-F in the attenuating activities on proMMP-9 were 91.8 and 11.2 μg/mL, respectively. In brief, ARE-F has higher attenuating activity on the UV-B-induced proMMP-9 elevation than ARE.
Fig. 2.
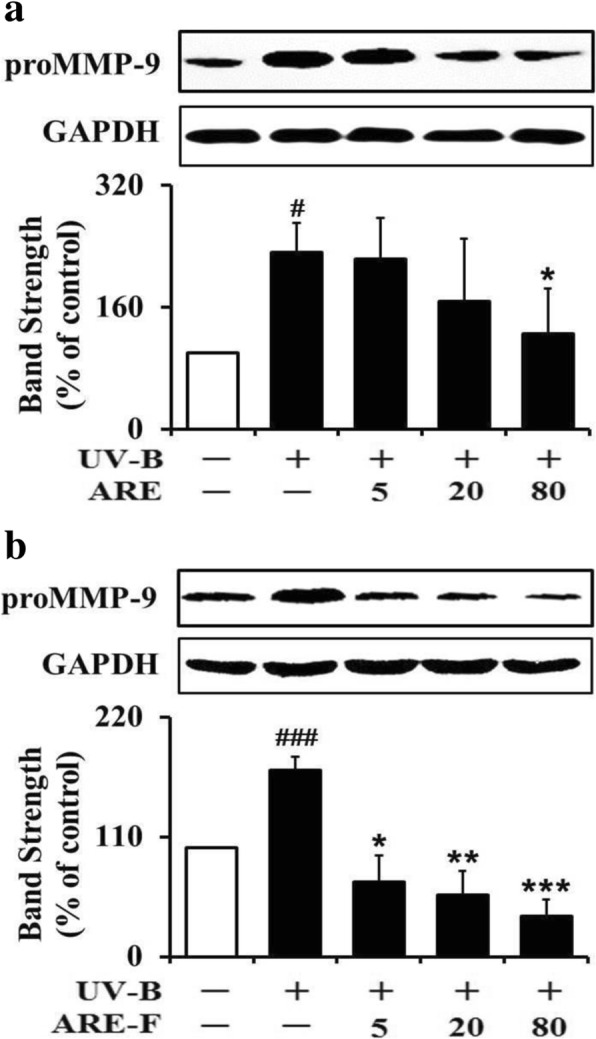
Suppressive effects of ARE (a) and ARE-F (b) on the proMMP-9 elevation in HaCaT keratinocytes under UV-B irradiation. HaCaT cells were subjected to the varying concentrations (0, 2, 20 and 80 μg/mL) of ARE (a) and ARE-F (b) for 1 h before the irradiation. The proMMP-9 proteins in cellular lysates, detected using western blotting analysis, were expressed as a percentage (%) of the corresponding non-irradiated control. In the lower panels of both a and b, the band strength was determined with densitometry using the ImageJ software which can be downloaded from the NIH website. #P < 0.05; ###P < 0.001 versus the non-irradiated control. *P < 0.05; **P < 0.01; ***P < 0.001 versus the non-treated control (UV-B irradiation alone)
As shown in Fig. 3, the attenuating activities of both ARE and ARE-F on the UV-B-induced proMMP-2 levels were also measured and compared. The UV-B irradiation alone induced the proMMP-2 protein levels to about 1.7-fold over the non-irradiated control value (Fig. 3). ARE at the concentrations of 5, 20 and 80 μg/mL attenuated the UV-B-induced proMMP-2 protein to 69.5, 57.6 and 39.0% of that of the UV-B irradiation only (Fig. 3a). In an analogous experiment, ARE-F at the concentrations of 5, 20 and 80 μg/mL could attenuate the UV-B-induced proMMP-2 protein levels to 74.1, 37.0 and 26.0%, respectively, of that of the UV-B irradiation only (Fig. 3b). The IC50 values of ARE and ARE-F were determined to be 48.3 and 27.9 μg/mL. Taken together, the proMMP-2-downregulating activity of ARE is also enhanced by probiotic fermentation.
Fig. 3.
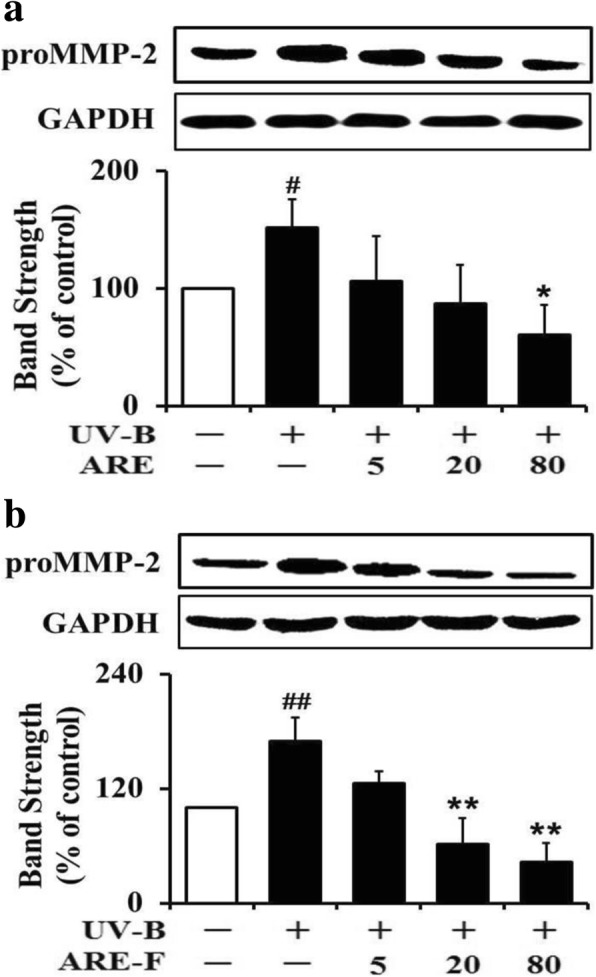
Suppressive effects of ARE (a) and ARE-F (b) on the proMMP-2 elevation in HaCaT keratinocytes under UV-B irradiation. HaCaT cells were subjected to the varying concentrations (0, 5, 20 and 80 μg/mL) of ARE (a) and ARE-F (b) for 1 h before the irradiation. The proMMP-2 proteins in cellular lysates, detected using western blotting analysis, were expressed as a percentage (%) of the corresponding non-irradiated control. In the lower panels of both a and b, the band strength was determined with densitometry using the ImageJ software which can be downloaded from the NIH website. #P < 0.05; ##P < 0.01 versus the non-irradiated control. *P < 0.05; **P < 0.01 versus the non-treated control (UV-B irradiation alone)
Total glutathione (GSH)
GSH plays a protective property against oxidative stress in various cell types, including skin cells and has its protective property against oxidative stress via directly or indirectly scavenging ROS. In the previous work, total GSH, including oxidized and reduced GSH, was shown to decline in UV-B-irradiated keratinocytes [29]. Likewise, total GSH levels were significantly decreased in HaCaT keratinocytes under UV-B irradiation, compared to the non-irradiated control value (Fig. 4). ARE at the concentrations of 5, 20 and 80 μg/mL enhanced the UV-B-reduced total GSH levels to 1.1-, 1.6- and 1.7-fold of the non-treated value, respectively (Fig. 4a). Similarly, ARE-F at the concentrations of 5, 20 and 80 μg/mL was able to enhance the UV-B-reduced total GSH levels to 1.1-, 1.4- and 1.9-fold of the non-treated value, respectively (Fig. 4b). ARE and ARE-F at 80 μg/mL could restore the UV-B-reduced total GSH levels to 80.0 and 111.2%, respectively, of the non-irradiated value (Fig. 4). This finding implies that the GSH-restoring activity of ARE on the UV-B-reduced total GSH levels is enhanced by probiotic fermentation.
Fig. 4.
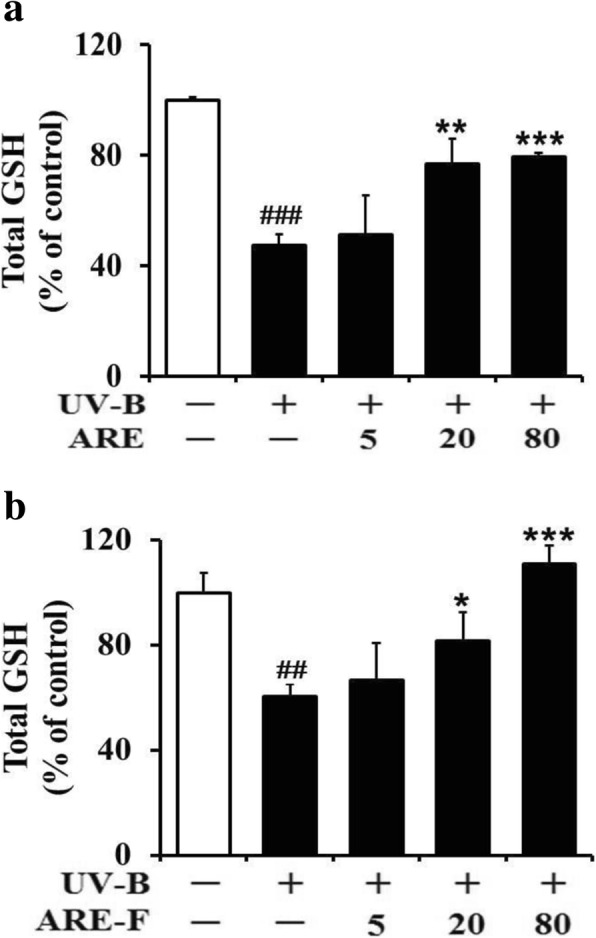
Enhancing effects of ARE (a) and ARE-F (b) on the total GSH attenuation in HaCaT keratinocytes under UV-B irradiation. HaCaT cells were subjected to the varying concentrations (0, 5, 20 and 80 μg/mL) of ARE (a) and ARE-F (b) for 1 h before the irradiation. The total GSH content in the cellular lysates, quantitated with an enzymatic recycling assay using GR, was expressed as a percentage (%) of the corresponding non-irradiated control. ##P < 0.01; ###P < 0.001 versus the non-irradiated control. *P < 0.05; **P < 0.01; ***P < 0.001 versus the non-treated control (UV-B irradiation alone)
Total superoxide dismutase (SOD)
SOD, deeply involved in the defensive mechanisms against oxidative stress, was previously identified to decline in UV-B-irradiated keratinocytes [30]. In this work, UV-B irradiation markedly diminished the total SOD activity, compared to the non-irradiated value (Fig. 5). As shown in Fig. 5a, ARE at the concentrations of 5, 20 and 80 μg/mL enhanced the UV-B-reduced SOD activity to 1.3-, 1.4 and 1.4-fold over that of the UV-B irradiation only. ARE-F at the concentrations of 5, 20 and 80 μg/mL was able to increase the UV-B-reduced total SOD activity to 1.1-, 1.6- and 1.8-fold, respectively, over that of the UV-B irradiation only (Fig. 5b). ARE and ARE-F at the concentration of 80 μg/mL could restore the UV-B-reduced total SOD activity levels to 70.4 and 110.2%, respectively, over that of the non-irradiation only (Fig. 5). Taken together, the SOD-restoring activity of ARE is up-regulated by probiotic fermentation.
Fig. 5.
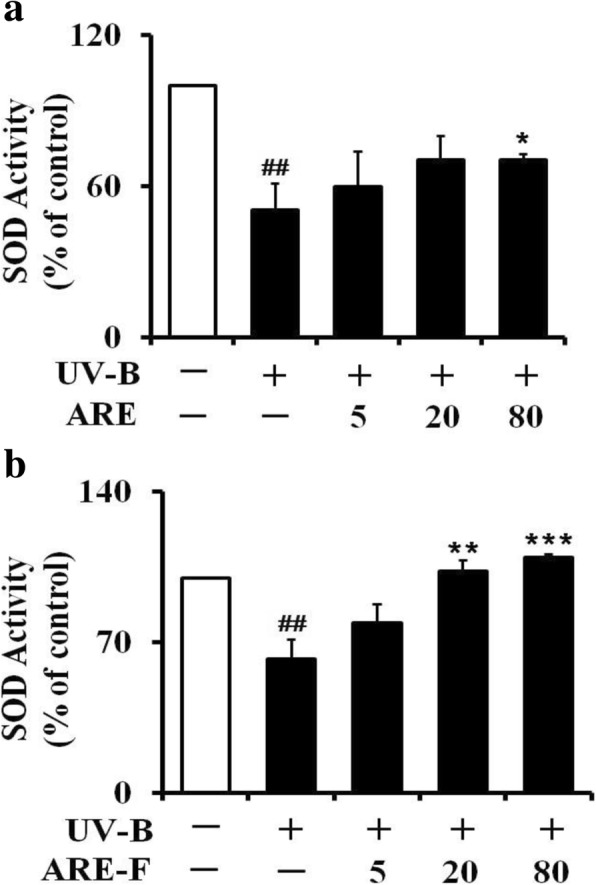
Enhancing effects of ARE (a) and ARE-F (b) on the total SOD activity attenuation in HaCaT keratinocytes under UV-B irradiation. HaCaT cells were subjected to the varying concentrations (0, 5, 20 and 80 μg/mL) of ARE (a) and ARE-F (b) for 1 h before the irradiation. The total SOD activity in the cellular lysates, expressed as a percentage (%) of the corresponding non-irradiated control, was determined using a spectrophotometric assay. ##P < 0.01 versus the non-irradiated control. *P < 0.05; **P < 0.01; ***P < 0.001 versus the non-treated control (UV-B irradiation alone)
In vitro antiradical activity
In order to compare the antiradical activities of ARE and ARE-F, the ABTS radical scavenging assay was conducted. AA, used as a positive control, was found to display an SC50 of 49.0 μg/mL (Fig. 6). Both ARE and ARE-F exhibited relatively weak ABTS radical scavenging activities, compared to AA and they didn’t display a significant difference in ABTS radical scavenging activities (Fig. 6). This result suggests that the potentiation of the skin anti-photoaging properties of ARE by probiotic fermentation might not be irrespective of a change in in vitro antiradical activity.
Fig. 6.
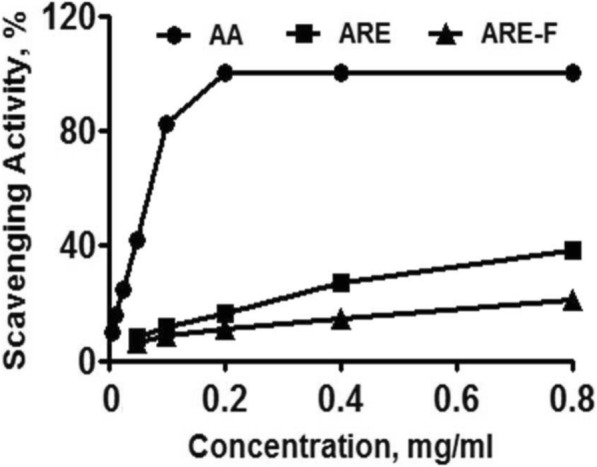
The ABTS radical scavenging activities of ARE and ARE-F. AA was used as a positive control. Each value shows the mean ± SD of the three independent experiments repeated in triplicate
Discussion
Through the recent years, the biological and pharmacological efficacies of A. rugosa have been continuously and extensively studied. An extract of A. rugosa leaves protects RAW264.7 cells from hydrogen peroxide-induced injury via the induction of protein kinase G-dependent heme oxygenase-1 [31]. Essential oils of A. rugosa leaves, whose major compounds are p-menthan-3-one and estragole, have antimicrobial, antifilm and antitumor activities [32]. Demethyleugenol β-glucopyranoside, isolated from A. rugosa, possesses has an ameliorating activity on skin pigmentation by decreasing melanin synthesis via down-regulating microphthalmia-associated transcription factor and sex-determinating region Y-box 9 and subsequently resulting in a decrease in melanogenic enzymes, such as tyrosinase, tyrosinase-related protein 1 and dopachrome tautomerase [33]. An ethanol extract of A. rugosa leaves has an antimelanogenic activity through the suppression of tyrosinase, which is enhanced by the fermentation with L. rhamnosus and L. paracasei [34]. Addition of yeast extract, as an elicitor, to the A. rugosa culture enhances the accumulation of rosmarinic acid, a main phenylpropanoid of A. rugosa, through the up-regulation of phenylpropanoid biosynthetic pathway genes, such as hydroxyl phenylpyruvate reductase and rosmarinic acid synthase genes [35]. A hot water extract of A. rugosa leaves have skin anti-photoaging property against UV-B-induced photooxidative stress in human keratinocytes via up-regulating GSH and SOD [22]. These findings further support the valuable application potential of A. rugosa in the manufacture of functional cosmetics. Enhancement of the desirable efficacies of A. rugosa would make its application more convenient and more economical.
Since useful phytochemicals, as nature’s chemicals, are believed to be more safe than synthetic chemicals, they increasingly attract great interest in the healthcare, food, flavor and cosmetics industries. Fermentation of phytochemicals is a crucial processing method and is attracting a lot of attention since it may have the advantage of having novel and augmented biological functions. Desirable cosmetic ingredients need to contain attractive properties, including antioxidant, anti-inflammatory, skin whitening, anti-aging, moisturizing, bio-anti-wrinkle activities and so on [36]. During fermentation processing, microbes, including lactic acid bacteria, can improve the cellular functions of plant materials through their enzymatic activities, which promotes the production of a variety of metabolites and/or the release of functional components that are cryptic in non-fermented materials [37]. Bioconversion using whole cells is usually more stable, inexpensive and convenient than that using a purified enzyme [38]. Some biosurfactant extracts from Chinese medical herb fermentation exhibit favorable antioxidative, emulsifying and moisturizing properties in cosmetic formulations [39].
Fermentation with lactic acid bacteria is one of the most valuable tools to exploit the desirable functions of plant resources and to enrich them with bioactive compounds [40]. Fermentation with several lactic acid bacteria has been used to improve the antimicrobial, antioxidant and immunomodulatory activities of natural compounds of plant and microbial origins [41]. Phenolic compounds are well-known secondary metabolites of various plants that have been subjected to the lactic acid bacteria fermentation for studying the accompanying changes [42]. Bioconversion of baicalin and wogonoside to baicalein and wogonin, two main flavonoids exhibiting increased beneficial pharmacological properties in Scutellaria baicalensis, is significantly enhanced during fermentation using β-glucuronidase from L. brevis RO1 [43]. Myrtle berries homogenate, fermented with L. plantarum C2, was found to contain the increased antioxidant activity, probably based up on the increased concentrations of total phenols (mainly, gallic acid and ellagic acid), anthocyanins and flavonoids (mainly, myricetin and quercetin) [44]. Dried funori, a non-toxic, all-natural starch derived from seaweed (Gloiopeltis furcata and Gloiopeltis tenax), displays a significant enhancement in the superoxide anion radical-scavenging capacity after the fermentation with L. plantarum S-SU1 [45]. Fermentation of Psidium guajava fruit extract using L. plantarum NCIM 2912 enhances its antioxidant potential as well as total phenolics and short and medium chain fatty acid contents [46]. Fermenting a red ginseng extract with L. brevis enhances contents of ginsenoside metabolites, such as Rg3, Rg5, Rk1, compound K, Rh1, F2 Rg2, uronic acid, polyphenols and flavonoids, and subsequently offers increased anti-wrinkle and whitening efficacies and diminished toxicological potency [47]. L. plantarum– and Bifidobacterium bifidum-fermented aqueous extracts of Acanthopanax koreanum roots exhibit enhanced antioxidant and antisenescent activities against the exposure to UV-B irradiation and hydrogen peroxide, implying the improved anti-wrinkle effect on human skin [21]. The aloe fermentation supernatant fermented by L. plantarum possess enhanced antioxidant, antibacterial and anti-inflammatory activities [48]. In this work, we demonstrate that the skin anti-photoaging properties of a hot water extract of A. rugosa leaves are significantly augmented by the fermentation with L. rhamnosus HK-9, although the underlying mechanism currently remains uncertain. However, this result may elevate its application potential in cosmetics industries.
In this work, ARE-F tended to exhibit a diminished ABTS radical scavenging activity, compared to ARE, which might be contrary to its intracellular defensive properties in HaCaT keratinocytes. Although the cause of this discrepancy remains to be clarified, some findings on the reduction of antioxidant components and activities during fermentation were previously reported. The fermentation of strawberry must with Saccharomyces cerevisiae was found to diminish the ABTS and 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activities [49]. The liquid state fermentations of in vitro sprout and shoot cultures of java tea with L. acidophilus and L. plantarum cause a significant reduction in the levels of rosmarinic acid and total phenolic compounds and a loss of antioxidant activities, such as DPPH and ABTS scavenging activities and SOD-like activity [50, 51]. In these experiments, the degree of reduction in some antioxidant components and activities was found to depend on the fermentation parameters, such as probiotic strains, fermentation temperature and fermentation period. Further approaches in future would help understand the discrepancy obtained in this work.
Conclusions
In conclusion, the present work demonstrates that the fermentation of A. rugosa leaf extract with a probiotic Lactobacillus strain improves its skin anti-photoaging properties through further augmenting UV-B-reduced total GSH and SOD activity levels and increasingly attenuating UV-B-induced ROS and MMP-2 and -9 levels in some sequential order. These findings imply the potential augmentation of the skin anti-photoaging properties of A. rugosa by probiotic fermentation, which can expand its usefulness in various applications, including cosmetic manufacture.
Acknowledgements
The authors are very grateful to Ms. Su Hee Lee for her technical assistance.
Funding
This study was supported by a grant of the Korean Health Technology R&D Project (Grant No.HN14C0081), the Ministry of Health & Welfare, Republic of Korea.
Availability of data and materials
The data and materials of this work are available to other researchers upon request.
Abbreviations
- AA
ascorbic acid
- ABTS
2,2-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)
- ARE
a non-fermented hot water extract of A. rugosa leaves
- ARE-F
a fermented hot water extract of A. rugosa leaves
- DCF, 2′,7′-dichlorofluorescein
DCFH-DA, 2′,7′-dichlorodihydrofluorescein diacetate
- DMEM
Dulbecco’s modified Eagle’s medium
- DNTB
5,5′-dithiobis (2-nitrobenzoic acid)
- FBS
fetal bovine serum
- GSH
glutathione
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GR
Glutathione reductase
- IC50
50% inhibitory concentration
- MMP
Matrix metalloproteinase
- PBS
Phosphate-buffered saline
- proMMP
promatrix metalloproteinase
- ROS
Reactive oxygen species
- SC50
50% scavenging concentration
- SOD
Superoxide dismutase
- UV-B
Ultraviolet-B
Authors’ contributions
DS, YHH, HWL, DDK and CJL participated in the design of this work. DS, YHH, KJ and YL performed the experiments. KJ and YL analyzed the data. HWL, DDK and CJL supervised this work and wrote the manuscript. All authors read the final manuscript and approved it for submission.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Daehyun Shin and Yoonjin Lee contributed equally to this work.
Contributor Information
Daehyun Shin, Email: dhshin@cosmocos.com.
Yoonjin Lee, Email: yjlee@cosmocos.com.
Yu-Hua Huang, Email: yuhua_8206@naver.com.
Hye-Won Lim, Email: wendy99ca@naver.com.
Kyounghee Jang, Email: khjang@cosmocos.com.
Dae-Duk Kim, Email: ddkim@snu.ac.kr.
Chang-Jin Lim, Email: cjlim@kangwon.ac.kr.
References
- 1.Jenkins G. Molecular mechanisms of skin ageing. Mech Ageing Dev. 2002;123(7):801–810. doi: 10.1016/S0047-6374(01)00425-0. [DOI] [PubMed] [Google Scholar]
- 2.Mohamed MA, Jung M, Lee SM, Lee TH, Kim J. Protective effect of Disporum sessile D.Don extract against UVB-induced photoaging via suppressing MMP-1 expression and collagen degradation in human skin cells. J Photochem Photobiol B. 2014;133:73–79. doi: 10.1016/j.jphotobiol.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Hseu YC, Chou CW, Senthil Kumar KJ, Fu KT, Wang HM, Hsu LS, et al. Ellagic acid protects human keratinocyte (HaCaT) cells against UVA-induced oxidative stress and apoptosis through the upregulation of the HO-1 and Nrf-2 antioxidant genes. Food Chem Toxicol. 2012;50(5):1245–1255. doi: 10.1016/j.fct.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 4.Piao MJ, Kim KC, Zheng J, Yao CW, Cha JW, Boo SJ, et al. The ethyl acetate fraction of Sargassum muticum attenuates ultraviolet B radiation-induced apoptotic cell death via regulation of MAPK- and caspase-dependent signaling pathways in human HaCaT keratinocytes. Pharm Biol. 2014;52(9):1110–1118. doi: 10.3109/13880209.2013.879186. [DOI] [PubMed] [Google Scholar]
- 5.F'guyer S, Afaq F, Mukhtar H. Photochemoprevention of skin cancer by botanical agents. Photodermatol Photoimmunol Photomed. 2003;19(2):56–72. doi: 10.1034/j.1600-0781.2003.00019.x. [DOI] [PubMed] [Google Scholar]
- 6.Cadet J, Sage E, Douki T. Ultraviolet radiation-mediated damage to cellular DNA. Mutat Res. 2005;571(1–2):3–17. doi: 10.1016/j.mrfmmm.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 7.Aoki-Yoshida A, Aoki R, Takayama Y. Protective effect of pyruvate against UVB-induced damage in HaCaT human keratinocytes. J Biosci Bioeng. 2013;115(4):442–448. doi: 10.1016/j.jbiosc.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Fisher GJ, Wang ZQ, Datta SC, Varani J, Kang S, Voorhees JJ. Pathophysiology of premature skin aging induced by ultraviolet light. N Engl J Med. 1997;337(20):1419–1428. doi: 10.1056/NEJM199711133372003. [DOI] [PubMed] [Google Scholar]
- 9.Varani J, Hattori Y, Chi Y, Schmidt T, Perone P, Zeigler ME, et al. Collagenolytic and gelatinolytic matrix metalloproteinases and their inhibitors in basal cell carcinoma of skin: comparison with normal skin. Br J Cancer. 2000;82(3):657–665. doi: 10.1054/bjoc.1999.0978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inomata S, Matsunaga Y, Amano S, Takada K, Kobayashi K, Tsunenaga M, et al. Possible involvement of gelatinases in basement membrane damage and wrinkle formation in chronically ultraviolet B-exposed hairless mouse. J Invest Dermatol. 2003;120(1):128–134. doi: 10.1046/j.1523-1747.2003.12021.x. [DOI] [PubMed] [Google Scholar]
- 11.Reel B, Oktay G, Ozkal S, Islekel H, Ozer E, Ozsarlak-Sozer G, et al. MMP-2 and MMP-9 alteration in response to collaring in rabbits: the effects of endothelin receptor antagonism. J Cardiovasc Pharmacol Ther. 2009;14(4):292–301. doi: 10.1177/1074248409343690. [DOI] [PubMed] [Google Scholar]
- 12.Piao MJ, Susara Ruwan Kumara MH, Kim KC, Kang KA, Kang HK, Lee NH, et al. Diphlorethohydroxycarmalol suppresses ultraviolet B-induced matrix metalloproteinases via inhibition of JNK and ERK signaling in human keratinocytes. Biomol Ther (Seoul) 2015;23(6):557–563. doi: 10.4062/biomolther.2015.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim YB, Kim JK, Uddin MR, Xu H, Park WT, Tuan PA, et al. Metabolomics analysis and biosynthesis of rosmarinic acid in Agastache rugosa Kuntze treated with methyl jasmonate. PLoS One. 2013;8(5):e64199. doi: 10.1371/journal.pone.0064199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li HQ, Liu QZ, Liu ZL, Du SS, Deng ZW. Chemical composition and nematicidal activity of essential oil of Agastache rugosa against Meloidogyne incognita. Molecules. 2013;18(4):4170–4180. doi: 10.3390/molecules18044170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Min BS, Hattori M, Lee HK, Kim YH. Inhibitory constituents against HIV-1 protease from Agastache rugosa. Arch Pharm Res. 1999;22(1):75–77. doi: 10.1007/BF02976440. [DOI] [PubMed] [Google Scholar]
- 16.Lee C, Kim H, Kho Y. Agastinol and agastenol, novel lignans from Agastache rugosa and their evaluation in an apoptosis inhibition assay. J Nat Prod. 2002;65(3):414–416. doi: 10.1021/np010425e. [DOI] [PubMed] [Google Scholar]
- 17.Hong JJ, Choi JH, Oh SR, Lee HK, Park JH, Lee KY, et al. Inhibition of cytokine-induced vascular cell adhesion molecule-1 expression; possible mechanism for anti-atherogenic effect of Agastache rugosa. FEBS Lett. 2001;495(3):142–147. doi: 10.1016/S0014-5793(01)02379-1. [DOI] [PubMed] [Google Scholar]
- 18.Gao LP, Wei HL, Zhao HS, Xiao SY, Zheng RL. Antiapoptotic and antioxidant effects of rosmarinic acid in astrocytes. Pharmazie. 2005;60(1):62–65. [PubMed] [Google Scholar]
- 19.Swarup V, Ghosh J, Ghosh S, Saxena A, Basu A. Antiviral and anti-inflammatory effects of rosmarinic acid in an experimental murine model of Japanese encephalitis. Antimicrob Agents Chemother. 2007;51(9):3367–3370. doi: 10.1128/AAC.00041-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tuan PA, Park WT, Xu H, Park NI, Park SU. Accumulation of tilianin and rosmarinic acid and expression of phenylpropanoid biosynthetic genes in Agastache rugosa. J Agric Food Chem. 2012;60(23):5945–5951. doi: 10.1021/jf300833m. [DOI] [PubMed] [Google Scholar]
- 21.Park MJ, Bae YS. Fermented Acanthopanax koreanum root extract reduces UVB- and H2O-induced senescence in human skin fibroblast cells. J Microbiol Biotechnol. 2016;26(7):1224–1233. doi: 10.4014/jmb.1602.02049. [DOI] [PubMed] [Google Scholar]
- 22.Oh Y, Lim HW, Huang YH, Kwon HS, Jin CD, Kim K, et al. Attenuating properties of Agastache rugosa leaf extract against ultraviolet-B-induced photoaging via up-regulating glutathione and superoxide dismutase in a human keratinocyte cell line. J Photochem Photobiol B. 2016;163:170–176. doi: 10.1016/j.jphotobiol.2016.08.026. [DOI] [PubMed] [Google Scholar]
- 23.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1–2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 24.Royall JA, Ischiropoulos H. Evaluation of 2′,7′-dichlorofluorescin and dihydrorhodamine 123 as fluorescent probes for intracellular H2O2 in cultured endothelial cells. Arch Biochem Biophys. 1993;302(2):348–355. doi: 10.1006/abbi.1993.1222. [DOI] [PubMed] [Google Scholar]
- 25.Nakagawa K, Saijo N, Tsuchida S, Sakai M, Tsunokawa Y, Yokota J, et al. Glutathione-S-transferase π as a determinant of drug resistance in transfectant cell lines. J Biol Chem. 1990;265(8):4296–4301. [PubMed] [Google Scholar]
- 26.Lee YY, Kim HG, Jung HI, Shin YH, Hong SM, Park EH, et al. Activities of antioxidant and redox enzymes in human normal hepatic and hepatoma cell lines. Mol Cells. 2002;14(2):305–311. [PubMed] [Google Scholar]
- 27.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26(9–10):1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 28.Kim MS, Oh GH, Kim MJ, Hwang JK. Fucosterol inhibits matrix metalloproteinase expression and promotes type-1 procollagen production in UVB-induced HaCaT cells. Photochem Photobiol. 2013;89(4):911–918. doi: 10.1111/php.12061. [DOI] [PubMed] [Google Scholar]
- 29.Zhu M, Bowden GT. Molecular mechanism(s) for UV-B irradiation-induced glutathione depletion in cultured human keratinocytes. Photochem Photobiol. 2004;80(2):191–196. doi: 10.1562/2004-02-26-RA-091.1. [DOI] [PubMed] [Google Scholar]
- 30.Iizawa O, Kato T, Tagami H, Akamatsu H, Niwa Y. Long-term follow-up study of changes in lipid peroxide levels and the activity of superoxide dismutase, catalase and glutathione peroxidase in mouse skin after acute and chronic UV irradiation. Arch Dermatol Res. 1994;286(1):47–52. doi: 10.1007/BF00375843. [DOI] [PubMed] [Google Scholar]
- 31.Oh HM, Kang YJ, Lee YS, Park MK, Kim SH, Kim HJ, et al. Protein kinase G-dependent heme oxygenase-1 induction by Agastache rugosa leaf extract protects RAW264.7 cells from hydrogen peroxide-induced injury. J Ethnopharmacol. 2006;103(2):229–235. doi: 10.1016/j.jep.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 32.Haiyan G, Lijuan H, Shaoyu L, Chen Z, Ashraf MA. Antimicrobial, antibiofilm and antitumor activities of essential oil of Agastache rugosa from Xinjiang, China. Saudi J Biol Sci. 2016;23(4):524–530. doi: 10.1016/j.sjbs.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee TH, Park S, Yoo G, Jang C, Kim MH, Kim SH, et al. Demethyleugenol β-glucopyranoside isolated from Agastache rugosa decreases melanin synthesis via down-regulation of MITF and SOX9. J Agric Food Chem. 2016;64(41):7733–7742. doi: 10.1021/acs.jafc.6b03256. [DOI] [PubMed] [Google Scholar]
- 34.Kim NY, Kwon HS, Lee HY. Effect of inhibition on tyrosinase and melanogenesis of Agastache rugosa Kuntze by lactic acid bacteria fermentation. J Cosmet Dermatol. 2017;16(3):407–415. doi: 10.1111/jocd.12264. [DOI] [PubMed] [Google Scholar]
- 35.Park MJ, Bae YS. Fermented Acanthopanax koreanum root extract reduces UVB- and H2O2-induced senescence in human skin fibroblast cells. J Microbiol Biotechnol. 2016;26(7):1224–1233. doi: 10.4014/jmb.1602.02049. [DOI] [PubMed] [Google Scholar]
- 36.Katina K, Laitila A, Juvonen R, Liukkonen KH, Kariluoto S, Piironen V, et al. Bran fermentation as a means to enhance technological properties and bioactivity of rye. Food Microbiol. 2007;24(2):175–186. doi: 10.1016/j.fm.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 37.Gobbetti M, Cagno RD, De Angelis M. Functional microorganisms for functional food quality. Crit Rev Food Sci Nutr. 2010;50(8):716–727. doi: 10.1080/10408398.2010.499770. [DOI] [PubMed] [Google Scholar]
- 38.Faber K. Biotransformation in organic chemistry: a textbook. 5. Germany: Springer; 2004. pp. 25–26. [Google Scholar]
- 39.Chen C, Lin T, Shieh Y. Emulsification and antioxidation of biosurfactant extracts from Chinese medicinal herbs fermentation in vitro. J Biosci Bioeng. 2015;120(4):387–395. doi: 10.1016/j.jbiosc.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 40.Pellati F, Benvenuti S, Magro L, Melegari M, Soragni F. Analysis of phenolic compounds and radical scavenging activity of Echinacea spp. J Pharm Biomed Anal. 2004;35(2):289–301. doi: 10.1016/S0731-7085(03)00645-9. [DOI] [PubMed] [Google Scholar]
- 41.Rizzello CG, Coda R, Macías DS, Pinto D, Marzani B, Filannino P, et al. Lactic acid fermentation as a tool to enhance the functional features of Echinacea spp. Microb Cell Factories. 2013;12(1):44. doi: 10.1186/1475-2859-12-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu S, Han Y, Zhou Z. Lactic acid bacteria in traditional fermented Chinese foods. Food Res Int. 2011;44(3):643–651. doi: 10.1016/j.foodres.2010.12.034. [DOI] [Google Scholar]
- 43.Xu C, Ji GE. Bioconversion of flavones during fermentation in milk containing Scutellaria baicalensis extract by Lactobacillus brevis. J Microbiol Biotechnol. 2013;23(10):1422–1427. doi: 10.4014/jmb.1305.05001. [DOI] [PubMed] [Google Scholar]
- 44.Curiel JA, Pinto D, Marzani B, Filannino P, Farris GA, Gobbetti M, et al. Lactic acid fermentation as a tool to enhance the antioxidant properties of Myrtus communis berries. Microb Cell Factories. 2015;14(1):67. doi: 10.1186/s12934-015-0250-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuda T, Nemoto M, Kawahara M, Oshio S, Takahashi H, Kimura B. Induction of the superoxide anion radical scavenging capacity of dried 'funori' Gloiopeltis furcata by Lactobacillus plantarum S-SU1 fermentation. Food Funct. 2015;6(8):2535–2541. doi: 10.1039/C5FO00668F. [DOI] [PubMed] [Google Scholar]
- 46.Bhat R, Suryanarayana LC, Chandrashekara KA, Krishnan PA, Kush A, Ravikumar P. Lactobacillus plantarum mediated fermentation of Psidium guajava L. fruit extract. J Biosci Bioeng. 2015;119(4):430–432. doi: 10.1016/j.jbiosc.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 47.Lee HS, Kim MR, Park Y, Park HJ, Chang UJ, Kim SY, et al. Fermenting red ginseng enhances its safety and efficacy as a novel skin care anti-aging ingredient: in vitro and animal study. J Med Food. 2012;15(11):1015–1023. doi: 10.1089/jmf.2012.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jiang M, Deng K, Jiang C, Fu M, Guo C, Wang X, et al. Evaluation of the antioxidative, antibacterial, and anti-inflammatory effects of the aloe fermentation supernatant containing Lactobacillus plantarum HM218749.1. Mediat Inflamm. 2016;2016:2945650. doi: 10.1155/2016/2945650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee PJ, Chen S. Effect of adding ball-milled achenes to must on bioactive compounds and antioxidant activities in fruit wine. J Food Sci Technol. 2016;53(3):1551–1560. doi: 10.1007/s13197-015-2073-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hunaefi D, Akumo DN, Riedel H, Smetanska I. The effect of Lactobacillus plantarum ATCC 8014 and Lactobacillus acidophilus NCFM fermentation on antioxidant properties of selected in vitro sprout culture of Orthosiphon aristatus (java tea) as a model study. Antioxidants (Basel) 2012;1(1):4–32. doi: 10.3390/antiox1010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hunaefi D, Riedel H, Akumo DN, Gruda N, Smetanska I. Effect of lactic acid fermentation on rosmarinic acid and antioxidant properties of in vitro shoot culture of Orthosiphon aristatus as a model study. Food Biotechnol. 2013;27(2):152–177. doi: 10.1080/08905436.2013.781948. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data and materials of this work are available to other researchers upon request.


