Abstract
Objectives
The aims of this study were to define the pattern of muscle involvement in patients with immune-mediated necrotising myopathy (IMNM) relative to those with other inflammatory myopathies and to compare patients with IMNM with different autoantibodies.
Methods
All Johns Hopkins Myositis Longitudinal Cohort subjects with a thigh MRI (tMRI) who fulfilled criteria for IMNM, dermatomyositis (DM), polymyositis (PM), inclusion body myositis (IBM) or clinically amyopathic DM (CADM) were included in the study. Muscles were assessed for intramuscular and fascial oedema, atrophy and fatty replacement. Disease subgroups were compared using univariate and multivariate analyses. Patients with IMNM with anti-signal recognition particle (SRP) autoantibodies were compared with those with IMNM with anti-HMG-CoA reductase (HMGCR) autoantibodies.
Results
The study included 666 subjects (101 IMNM, 176 PM, 219 DM, 17 CADM and 153 IBM). Compared with DM or PM, IMNM was characterised by a higher proportion of thigh muscles with oedema, atrophy and fatty replacement (p<0.01). Patients with IMNM with anti-SRP had more atrophy (19%, p=0.003) and fatty replacement (18%, p=0.04) than those with anti-HMGCR. In IMNM, muscle abnormalities were especially common in the lateral rotator and gluteal groups. Fascial involvement was most widespread in DM. Fatty replacement of muscle tissue began early during the course of disease in IMNM and the other groups. An optimal combination of tMRI features had only a 55% positive predictive value for diagnosing IMNM.
Conclusions
Compared with patients with DM or PM, IMNM is characterised by more widespread muscle involvement. Anti-SRP-positive patients have more severe muscle involvement than anti-HMGCR-positive patients.
INTRODUCTION
The idiopathic inflammatory myopathies, including polymyositis (PM), dermatomyositis (DM) and inclusion body myositis (IBM), are a heterogeneous family of diseases characterised by muscle weakness, high muscle enzyme levels, autoantibodies and muscle biopsies with prominent lymphocytic infiltrates.1 As the best imaging technique to investigate soft tissue abnormalities, MRI has been used to detect unique patterns of muscle oedema, muscle atrophy, fatty replacement and fascial oedema in different types of patients with myositis.2 For example, previous studies have noted that fascial oedema seems to be more common in DM than PM or IBM.3,4 Other studies have revealed that patients with IBM have a unique pattern characterised by severe involvement of the anterior thigh compartment with selective sparing of the rectus femoris muscle;5 these patients also tend to have asymmetric muscle involvement on MRI.6 A more recent study has described a pattern of MRI findings that may be useful for diagnosing IBM and excluding other myopathies, such as PM and DM.7
In recent years, it has become widely accepted that some patients with autoimmune myopathy have muscle biopsies with prominent muscle cell necrosis and only minimal lymphocytic infiltration. This form of myositis has been termed immune-mediated necrotising myopathy (IMNM) or necrotising autoimmune myopathy and is now recognised to be distinct from PM, DM or IBM.1 Patients with IMNM typically have very high serum creatine kinase (CK) levels, a relative lack of skin or other organ system involvement and, often, autoantibodies recognising either the signal recognition particle (SRP) or HMG-CoA reductase (HMGCR).1 Some of these patients, especially those with anti-SRP, may have especially severe disease that responds poorly to immunosuppressive therapy.1
To date, no studies have used thigh MRI (tMRI) to analyse the pattern of muscle involvement in patients with IMNM. In this study, we analysed the tMRI features in a large cohort of patients with myositis, comparing IMNM with other disease categories. We also compared the tMRI features of anti-SRP-positive IMNM subject with those who had autoantibodies recognising HMGCR.
MATERIAL AND METHODS
Study population
All patients enrolled in the Johns Hopkins Myositis Center longitudinal cohort from May 2008 to April 2015 with an available tMRI, routinely performed at the first visit, were sequentially included in the study.
Standard protocol approvals and patient consents
This study was approved by the Johns Hopkins Institutional Review Board, and written informed consent was obtained from each participant.
Demographic and clinical features
The date of the tMRI and demographic features, including the sex, race and date of symptom onset, were collected through retrospective chart review. Also, patients were classified in one of five mutually exclusive clinical subgroups by retrospective chart review. Thus, patients were classified as having IMNM if they met the 2003 European Neuromuscular Centre (ENMC) criteria,8 IBM if they fulfilled Griggs’ criteria9 or clinically amyopathic DM (CADM) if they met Sontheimer’s criteria.10 If none of these three criteria were met, patients were evaluated for Bohan and Peter criteria and classified accordingly as possible, probable or definite DM or PM.11
Autoantibody analysis of patients with necrotising myositis
Testing of sera for anti-HMGCR autoantibodies was performed by ELISA and confirmed by immunoprecipitation in vitro-transcribed and translated (IVTT) HMGCR protein as previously described.12 Anti-SRP testing was performed by immunoprecipitation of IVTT-generated SRP subunits at the Johns Hopkins Rheumatic Disease Research Core Center as previously described,13 by testing at the Oklahoma Medical Research Foundation using immunoprecipitation, and/or by using Quest Diagnostics myositis panels. Other myositis-specific autoantibodies, including the antisynthetase autoantibodies, anti-MDA5, anti-Mi2 and anti-NXP2 were also tested using these methods.
Image acquisition
MRI was performed on a 1.5T (Avanto, Siemens Medical Solutions, Erlangen, Germany) or 3T (Verio, Siemens Medical Solutions, Erlangen, Germany) depending on scanner availability with standardised protocol of coronal and axial T1-weighted and short-tau inversion recovery (STIR) images.14 The type of image ‘weighting’ employed during image acquisition determines the MRI contrast, with T1 being fat sensitive and STIR being fluid sensitive. The imaging sequences were performed with parameters to optimise image quality and yield with similar contrast resolution irrespective of field strength.2 The field of view was from the hips to knees. Axial images were contiguously acquired throughout the thigh to allow for evaluation of the full extent of each muscle (see online supplementary appendix 1).
Image evaluation
Image analysis was performed by two experienced musculoskeletal radiologists at the Johns Hopkins Radiology Department as part of routine clinical care. One of the musculoskeletal radiologists (JAC) had more than 10 years of experience reading MRI (and interpreted the majority of cases ∼75%), and the other musculoskeletal radiologist (SA) had 3 years of experience reading MRI (in addition to a musculoskeletal fellowship).
The radiologists interpreting the scans were masked to disease activity and subgroup of inflammatory myopathy. The presence of oedema, fatty replacement, atrophy and fascial oedema was evaluated in 15 muscles of both thighs (figure 1). Muscles were grouped according to online supplementary table S1.
Figure 1.
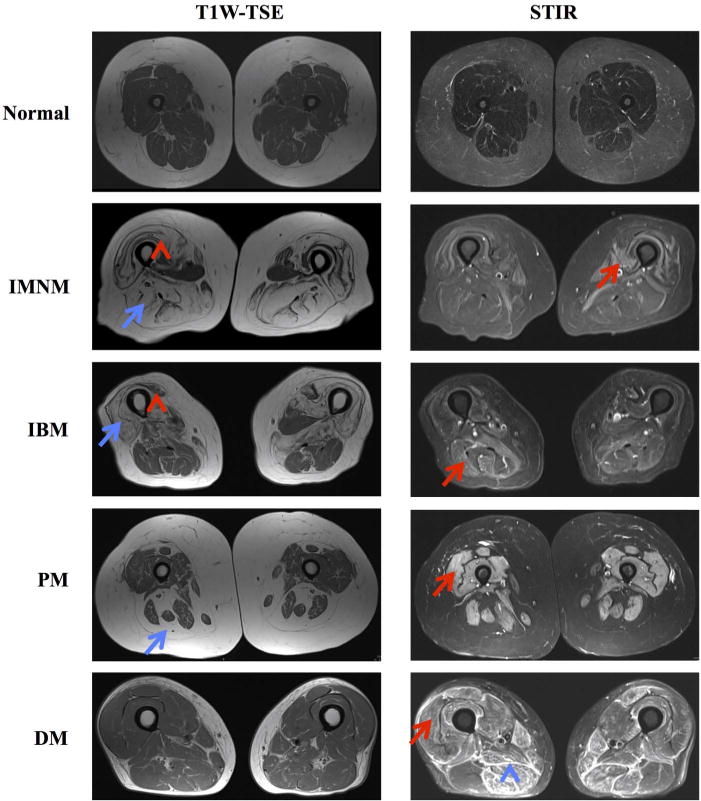
Examples of T1-weighted (T1W) turbo spin echo (TSE) and short-tau inversion recovery (STIR) sequences showing oedema (red arrows), atrophy (red arrow heads), fatty replacement (blue arrows) and fascial oedema (blue arrow heads) in patients with immune-mediated necrotising myopathy (IMNM), inclusion body myositis (IBM), polymyositis (PM) and dermatomyositis (DM).
The readings were standardised using predefined MRI definitions and pulse sequences. Consensus training was arranged by using the MRI definitions document (see online supplementary appendix 1).
Statistical analysis
Dichotomous variables were expressed as percentages and absolute frequencies, and continuous features were reported as means and SDs.
Pairwise comparisons for categorical variables between groups were made using χ2 test or Fisher’s exact test, as appropriate. Student’s t-test was used to compare continuous variables among groups and correlation was studied using Pearson’s coefficient.
The asymmetry of each tMRI feature in each patient was quantified by calculating the percentage of muscles that showed that feature in one side but not in the other.
We defined the extent of a tMRI feature as the percentage of muscles showing each feature. We determined whether certain muscle groups were more likely to be affected by MRI abnormalities depending on the disease category.
The influence of non-modifiable risk factors (sex, race, length of illness and age at the onset of the first symptoms) on the percentage of muscles showing each of the tMRI features were assessed using fractional probit models, reporting the marginal effects (dy/dx) of each of the predictor variables (equivalent to the predicted per cent change in the dependent variable per unit of the predictor variable).15 The administration of corticosteroid, intravenous immunoglobulins, rituximab, methotrexate, azathioprine and mycophenolate were used as adjusting covariates in the models comparing anti-HMGCR with anti-SRP-associated myositis.
Also, forward multiple logistic regression was used to select which muscles and tMRI features were most informative for each clinical group. A likelihood ratio test significance of 0.01 was selected to include variables in the model to maintain a manageable number of items in each formula. The area under the receiver operating characteristic (ROC) curve of the resulting models was internally validated using 100 bootstrap samples, obtaining the optimism-corrected area under the curve (AUC).16
All statistical analyses were performed using Stata/MP V.14.0. To account for the number of statistical tests performed, a two-sided p value of 0.001 or less was considered statistically significant for the univariate analysis while 0.05 was considered significant for the multivariate analysis.
RESULTS
Patients
Eight hundred and ninety-one patients underwent tMRI and 666 of these fulfilled the criteria for one of the defined myositis groups. Among these, 101 had IMNM, 219 had DM, 176 had PM, 17 had CADM and 153 had IBM. From the 101 patients with IMNM, 50 were positive for anti-HMGCR (50%) and 22 for anti-SRP autoantibodies (22%) (figure 2). Other myositis-specific autoantibodies were detected in <3% of the patients with IMNM. As expected, there were marked differences in the age at onset, duration of disease and demographic factors among the different groups of patients. Patients with anti-SRP were younger and were more commonly under immunosuppressant treatment at the time of the tMRI (68% vs 40%, p=0.03) than those with anti-HMGCR (38.4 vs 53.3 years old, p<0.001); there were no differences between these two groups of patients with IMNM with regard to the duration of disease at the time of tMRI, race or gender (table 1).
Figure 2.
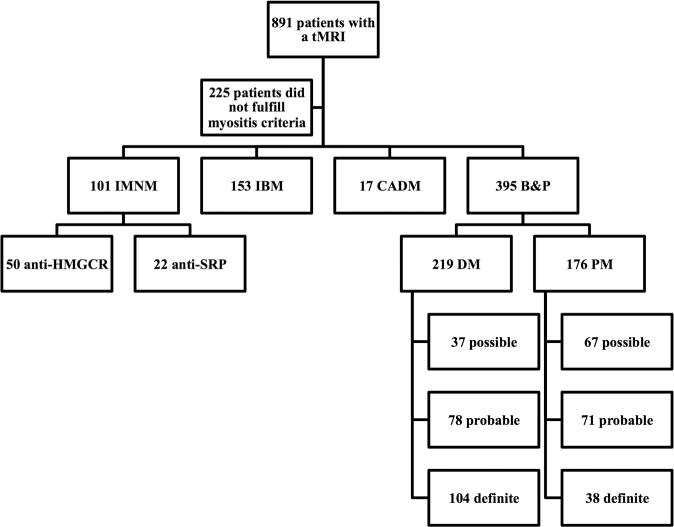
Patient flow chart. B&P, Bohan and Peter criteria; CADM, clinically amyopathic dermatomyositis; DM, dermatomyositis; IBM, inclusion body myositis; IMNM, immune-mediated necrotising myopathy; PM, polymyositis; SRP, signal recognition particle; tMRI: thigh MRI.
Table 1.
General features of the patients included in the study
| IMNM | IBM (n=153) | PM (n=176) | DM (n=219) | CADM (n=17) | Total (n=666) | |||
|---|---|---|---|---|---|---|---|---|
| Total (n=101) | HMGCR (n=50) | SRP (n=22) | ||||||
| Age at onset | 48.9 (16.0) | 53.3 (13.1)*** | 38.4 (13.1)*** | 58.4 (10.8)*** | 50.8 (15.9) | 45.4 (14.2)*** | 48.7 (14.9) | 50.4 (15.0)* |
| Length of illness (years) | 4.3 (5.8) | 4.3 (5.8) | 3.5 (4.0) | 7.8 (6.7)*** | 5.6 (7.2) | 3.8 (4.7)*** | 2.2 (3.0)* | 5.2 (6.3)* |
| Female sex | 65% (66) | 64% (32) | 86% (19) | 38% (58)*** | 66% (116) | 76% (167)*** | 82% (14) | 63% (421) |
| White | 69% (70)* | 78% (39) | 59% (13) | 88% (134)*** | 69% (122)** | 81% (177) | 71% (12) | 77% (515) |
| Black | 19% (19)* | 14% (7) | 36% (8) | 7% (10)* | 20% (35)*** | 8% (17)* | 6% (1) | 12% (82) |
| Other races | 12% (12) | 8% (4) | 5% (1) | 6% (9)* | 11% (19) | 11% (25) | 24% (4) | 10% (69) |
Continuous variables are expressed as mean (SD) and bivariate variables as percentage (absolute number). The value of each major clinical group (IMNM, IBM, PM, DM and CADM) was compared with the rest of the sample. In separate analyses, patients with anti-HMGCR and anti-SRP were compared with each other only. The χ2 test and Fisher’s exact test were used to compare bivariate variables. Continuous variables were compared using Student’s t-test. The length of illness was measured from the onset of first symptoms to the date of the thigh MRI.
<0.05;
<0.01;
<0.001.
CADM, clinically amyopathic dermatomyositis; DM, dermatomyositis; HMGCR, HMG-CoA reductase; IBM, inclusion body myositis; IMNM, immune-mediated necrotising myopathy; PM, polymyositis; SRP, signal recognition particle.
Extent and laterality of tMRI features by univariate analysis
MRI revealed that patients with IMNM had more extensive oedema (56%) than those with either PM (29%) or DM (30%) (all p<0.0001). Moreover, patients with IMNM showed a trend towards more atrophy (23%) and fatty replacement (38%) than those with either PM or DM (all p<0.02). As expected, CADM showed the least extensive muscle involvement by tMRI (table 2).
Table 2.
Extent of thigh MRI findings among clinical subsets
| IMNM | IBM (n=153) Mean (SD) |
PM (n=176) Mean (SD) |
DM (n=219) Mean (SD) |
CADM (n=17) Mean (SD) |
Total (n=666) Mean (SD) |
|||
|---|---|---|---|---|---|---|---|---|
| Total (n=101) Mean (SD) |
HMGCR (n=50) Mean (SD) |
SRP (n=22) Mean (SD) |
||||||
| Oedema | 55.5 (32.2)*** | 58.9 (31.8) | 65.8 (28.9) | 48.1 (24.6)*** | 29.4 (30.5)*** | 30.1 (36.7)*** | 6.1 (18.5)*** | 37.3 (33.5) |
| Atrophy | 23.2 (28.7)** | 21.7 (28.9)* | 38.2 (30.2)* | 32.2 (26.7)*** | 12.7 (24.6)* | 5.7 (16.7)*** | 2.5 (7.4)* | 16.2 (25.5) |
| Fatty replacement | 38.0 (33.1)* | 34.4 (30.9) | 49.1 (31.2) | 50.1 (27.3)*** | 28.3 (31.1) | 17.5 (27.0)*** | 7.1 (12.8)** | 30.7 (31.6) |
| Fascial oedema | 6.2 (15.1)* | 5.1 (15.2) | 6.0 (12.2) | 6.0 (12.0)** | 5.8 (11.8)** | 16.5 (24.3)*** | 8.6 (17.0) | 9.5 (18.1) |
Mean percentage of each major clinical group (IMNM, IBM, PM, DM and CADM) compared with the rest of the sample using Student’s t-test. In separate analyses, patients with anti-HMGCR and anti-SRP were compared to each other only.
<0.05;
<0.01;
<0.001.
CADM, clinically amyopathic dermatomyositis; DM, dermatomyositis; HMGCR, HMG-CoA reductase; IBM, inclusion body myositis; IMNM, immune-mediated necrotising myopathy; PM, polymyositis; SRP, signal recognition particle.
Interestingly, patients with IMNM showed a trend towards having more asymmetry in the percentage of muscles showing fatty replacement (2.6%, SD 6%, p<0.01) than those with DM or PM. Patients with anti-SRP showed more asymmetry than those with anti-HMGCR for all the tMRI features, but the difference did not reach statistical significance. Similar to IMNM, IBM also showed greater asymmetry in the percentage of muscles with atrophy (2.5%, SD 5.2%, p<0.001) compared with PM or DM. Of note, DM showed a trend towards more asymmetry in fascial oedema compared with all the other groups (2.2%, SD 4.7%, p<0.05). These differences in the laterality of muscle involvement did not seem to have a preference for a particular side (see online supplementary table S2).
Overall, fatty replacement extent was moderately correlated with the extent of atrophy (R=0.5, p<0.001) and oedema (R=0.4, p<0.001) while the extent of oedema was associated with the extent of atrophy (R=0.3, p<0.001) and fascial oedema (R=0.3, p<0.001). IBM did not show a significant association between oedema and atrophy or fascial oedema (both p>0.05), while in DM oedema was more correlated with fascial oedema than in the rest of the clinical groups (R=0.5, p<0.001) (see online supplementary table S3).
tMRI features by muscle group
Next, we determined whether certain muscle groups were more likely to be affected by MRI abnormalities depending on the disease category. Indeed, IMNM subjects had atrophy and fatty replacement preferentially in the lateral rotators, glutei, medial compartment and posterior compartment. In contrast, patients with IBM had oedema, fatty replacement and atrophy predominantly in the anterior, medial and posterior compartments. Patients with DM had more prevalent fascial oedema in the anterior, medial and posterior compartments compared with the rest of the patients. Finally, patients with PM showed no defined pattern of involvement for any of the tMRI features. Within the IMNM subgroups, patients with anti-SRP showed a trend towards more extensive oedema, atrophy and fatty replacement in the lateral rotator group, more atrophy and fatty replacement in the anterior compartment, and more atrophy in the medial compartment compared with those with anti-HMGCR (all p values between 0.001 and 0.05) (see online supplementary table S4).
Multivariate analysis of tMRI features
Multivariate analysis demonstrated that IMNM had significantly more extensive oedema, atrophy and fatty replacement than DM, PM or CADM (all p<0.01) independent of the age at onset, duration of illness, sex or race of the subject (table 3); tMRI features were not statistically different between patients with IMNM and IBM (all p>0.05). Within the IMNM subgroup, anti-SRP-positive patients had more extensive atrophy (19%, p=0.003) and fatty replacement (18%, p=0.04) than anti-HMGCR-positive patients independent of the age at onset, duration of the disease, sex, race and treatment at the time of the tMRI (table 4).
Table 3.
Multivariate analysis of the extent of the different thigh MRI features (percentage of muscles involved) among different clinical subsets using fractional probit regression
| Oedema dy/dx (95% CI) | Atrophy dy/dx (95% CI) | Fatty replacement dy/dx (95% CI) | Fascial oedema dy/dx (95% CI) | |
|---|---|---|---|---|
| Clinical subset (referenced to IMNM) | ||||
| IBM | −5.82 (−13.31 to 1.66) | 0.92 (−4.23 to 6.06) | 1.52 (−5.56 to 8.61) | 1.68 (−3.87 to 7.23) |
| PM | −23.72 (−30.63 to −16.80)*** | −10.92 (−16.38 to −5.46)*** | −10.77 (−17.69 to −3.86)** | 0.05 (−4.53 to 4.64) |
| DM | −22.14 (−29.36 to −14.93)*** | −18.24 (−24.00 to −12.47)*** | −17.39 (−24.21 to −10.57)*** | 8.82 (4.53 to 13.11)*** |
| CADM | −61.46 (−85.87 to −37.05)*** | −25.76 (−39.13 to −12.39)*** | −31.18 (−46.55 to −15.80)*** | 1.85 (−6.86 to 10.57) |
| Age at onset (10 years) | 0.73 (−1.10 to 2.56) | 2.41 (0.84 to 3.97)** | 2.05 (0.34 to 3.77)* | −0.88 (−1.76 to 0.00) |
| Time from onset to MRI (logarithm of months) | −8.83 (−14.34 to −3.33)** | 10.44 (6.13 to 14.74)*** | 13.90 (8.90 to 18.89)*** | −4.82 (−7.39 to −2.26)*** |
| Sex (female) | −9.60 (−14.58 to −4.61)*** | 3.24 (−0.45 to 6.92) | −8.61 (−13.11 to −4.11)*** | −2.92 (−5.72 to −0.11)* |
| Race (referenced to white patients) | ||||
| Black | 6.11 (−1.70 to 13.92) | 0.84 (−5.26 to 6.95) | 8.18 (1.59 to 14.77)* | −0.49 (−4.77 to 3.80) |
| Other races | 2.26 (−5.86 to 10.37) | 4.08 (−1.70 to 9.86) | −0.02 (−7.69 to 7.66) | −0.51 (−4.74 to 3.73) |
dy/dx, marginal effect (% change of the dependent variable for each one point of the predictor variables).
<0.05;
<0.01;
<0.001.
CADM, clinically amyopathic dermatomyositis; DM, dermatomyositis; IBM, inclusion body myositis; IMNM, immune-mediated necrotising myopathy; PM, polymyositis.
Table 4.
Multivariate analysis of the extent of the different thigh MRI features (percentage of muscles involved) in patients with anti-HMGCR-associated myositis compared with those with anti-SRP-associated myositis using fractional probit regression
| Oedema dy/dx (95% CI) | Atrophy dy/dx (95% CI) | Fatty replacement dy/dx (95% CI) | Fascial oedema dy/dx (95% CI) | |
|---|---|---|---|---|
| IMNM autoantibody group (anti-SRP vs anti-HMGCR) | 6.92 (−9.74 to 23.58) | 19.18 (6.52 to 31.84)** | 17.64 (0.59 to 34.70)* | 6.59 (−1.38 to 14.56) |
| Age at onset (10 years) | −2.04 (−7.33 to 3.25) | 0.28 (−4.69 to 5.25) | 0.06 (−5.53 to 5.65) | 1.35 (−1.85 to 4.56) |
| Time from onset to MRI (logarithm of months) | −21.98 (−35.02 to −8.93)*** | 11.21 (−2.96 to 25.38) | 20.50 (6.34 to 34.66)** | −2.32 (−9.08 to 4.45) |
| Sex (female) | −10.63 (−26.29 to 5.04) | 5.06 (−8.39 to 18.50) | −2.68 (−17.65 to 12.30) | −6.19 (−13.14 to 0.76) |
| Race (referenced to white patients) | ||||
| Black | 8.41 (−8.93 to 25.75) | 4.42 (−8.56 to 17.40) | 11.98 (−4.41 to 28.37) | −4.47 (−10.05 to 1.10) |
| Other races | 6.18 (−16.62 to 28.97) | 16.62 (−11.24 to 44.48) | −24.72 (−48.05 to −1.39)* | −3.18 (−9.81 to 3.45) |
dy/dx, marginal effect (% change of the dependent variable for each one point of the predictor variables).
Multivariate analysis adjusted for treatment (administration of corticosteroid, intravenous immunoglobulins, rituximab, mycophenolate, methotrexate or azathioprine).
<0.05;
<0.01;
<0.001.
HMGCR, HMG-CoA reductase; IMNM, immune-mediated necrotising myopathy; SRP, signal recognition particle.
Not surprisingly, in all myositis subgroups, as the length of time between disease onset and imaging increased, the extent of oedema decreased and both atrophy and fatty replacement increased (all p<0.01). Moreover, graphical analysis revealed that these changes were faster immediately after the onset of the disease than later on (and required a logarithmic transformation of the length of illness to linearise its relationship with the tMRI features) (table 3).
Excluding the patients with only ‘possible’ DM and PM from the analysis did not change the results (see online supplementary table S5).
tMRI features most associated with each myositis subgroup
We used forward logistic regression to select individual muscles and tMRI features that were most uniquely associated with IMNM compared with the other clinical subgroups. This analysis revealed that adductor brevis oedema and obturator externus atrophy were especially common in IMNM. In contrast, fascial oedema in the semitendinosus was particularly rare in IMNM compared with the other subgroups.
We performed similar analyses to identify tMRI features that are preferentially associated with the other myositis subgroups. In IBM, fatty replacement of the vastus lateralis and atrophy of the vastus medialis were more prevalent than in other subgroups; in contrast, oedema in the obturator internus was particularly rare in IBM. Patients with PM had no defining pattern of muscle involvement by tMRI; however, oedema and fascial oedema in the rectus femoris were particularly uncommon with this diagnosis. Interestingly, these analyses revealed that fascial oedema is the hallmark tMRI feature of DM. Indeed, fascial oedema surrounding the rectus femoris and the semimembranosus were the most supportive features for DM, while the presence of atrophy in the vastus medialis and oedema in the biceps femoris were the two features most unlikely to be found in this subgroup of patients (see online supplementary table S6).
We next determined how well the patterns of muscle involvement using tMRI could be used to diagnose the different myositis subgroups. However, after selecting the most balanced cut-off for the logistic regression formulas using Youden’s index, we estimated that the positive predictive value of these formulas was suboptimal, with a value >60% only in patients with IBM. Nonetheless, the negative predictive values of these formulas were excellent in IBM (94.7%) and IMNM (93.1%) and very good in DM (88.3%). (see online supplementary table S7 and figure S1).
DISCUSSION
Patients with IMNM comprise a significant fraction of patients with idiopathic inflammatory myopathies. Indeed, 15% of patients from Johns Hopkins Myositis Center Longitudinal Cohort meeting criteria for this study had IMNM. Although a number of prior reports have used muscle MRI to characterise muscle abnormalities in patients with PM, DM, IBM4,5,7,17–21 and even anti-SRP,22 no studies have systematically investigated the MRI findings in patients with IMNM compared with the other clinical groups. Here, we demonstrate that patients with IMNM have significantly more widespread muscle oedema, atrophy and fatty replacement compared with those with PM and DM. Our analysis also reveals that patients with IMNM have a characteristic pattern of muscle involvement. Taken together, these findings reinforce the idea that IMNM represents a unique form of myositis that can be distinguished from PM based on autoantibodies and muscle biopsy findings, and on the extent and pattern of muscle involvement. Moreover, comparing the two most common autoantibody groups in IMNM we have found that patients with anti-SRP show evidence of more severe muscle involvement than those with anti-HMGCR, reinforcing that autoantibodies define distinct groups and serve as important prognostic factors in patients with myositis.
In addition to MRI features that were specific to IMNM, we identified some characteristics that apply to all forms of myositis. For example, we found that fatty replacement occurs relatively early in all forms of myositis and spreads to additional muscle groups most quickly during the early phases of disease. This novel observation is consistent with the importance of early therapeutic intervention with immunosuppressive agents so as to maximise the chances of limiting the spread of disease in patients with PM, DM and IMNM. Unfortunately, to date, no therapies have been shown to affect disease progression in IBM.
Several articles have noted that fascial oedema appears to be a characteristic of DM muscle MRI.3 4 However, the current study is the first to conclusively demonstrate that fascial oedema is more common and widespread in DM compared with other forms of myositis. This finding is consistent with muscle biopsies from patients with DM, which often show inflammation within the perimysium.
We used forward logistic regression models in an attempt to identify diagnostic patterns of muscle involvement in patients with each myositis subtype. Ultimately, these models yielded patterns that had only modest positive predictive values for identifying the different forms of myositis. Of note, we found that models for PM performed especially poorly, which is consistent with PM including an especially heterogeneous population of patients.
This study has a number of limitations. First, because most patients had only one muscle MRI, we were unable to perform longitudinal studies on individual patients. Nonetheless, the large sample size, with patients undergoing MRI at various times during their disease course, allowed us to use statistical methods to model how the duration of disease affects muscle MRI features in different myositis subsets. Second, our study only included information about whether the features analysed here were present within a given muscle; even though the tMRI protocol included semiquantitative assessment at an individual muscle level for each radiologic feature (three levels of extent for all tMRI features), we considered that analysing the presence/absence of each tMRI feature would increase the reproducibility of our findings and simplify the methodology of our analysis. Therefore, we analysed patterns of muscle involvement and the spread of involvement to additional muscles over time, but we did not analyse the extent of the muscle features within a given muscle. Third, only about 50% of the patients (666 out of 1312 patients) had an available tMRI performed at Hopkins. Various reasons lead to the lack of a tMRI, including scheduling issues, availability of a recent tMRI outside Hopkins and patient consent. However, the proportion of patients in each clinical group with a tMRI was similar (IMNM: 55.5%; IBM: 57.1%; PM: 51.2%; DM: 46.3%; CADM: 37.8%). Given the type of features that we analysed and the final large sample size, it is unlikely that selection bias could have significantly influenced our results. Finally, interobserver reproducibility was not formally assessed but the clinical practice for myositis interpretations was to use a standardised set of definitions developed and agreed upon by the two participating radiologists in consensus so as to minimise this bias.
These limitations notwithstanding, this study demonstrates that patients with IMNM have especially extensive muscle involvement compared with other forms of myositis, that patients with IMNM have a characteristic pattern of muscle abnormalities involving hip rotators and glutei, and that patients with DM have the most widespread fascial involvement. Our analysis also revealed that in IMNM the spread of abnormalities (including fatty replacement) to additional muscles within the thigh occurs most quickly near the onset of disease and that patients with anti-SRP have more severe muscle involvement compared with patients with anti-HMGCR.
Supplementary Material
Acknowledgments
Funding This research was supported in part by the Intramural Research Program of the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health. The Myositis Research Database is supported by the Huayi and Siuling Zhang Discovery Fund.
Footnotes
Correction notice This article has been corrected since it was published Online First. Details of the co-corresponding author have been included.
Contributors All authors listed have contributed sufficiently to the project to be included as authors and to take public responsibility for its content, and all those who are qualified to be authors are listed in the author byline.
Competing interests None declared.
Patient consent Obtained.
Provenance and peer review Not commissioned; externally peer reviewed.
Ethics approval This study was approved by the Johns Hopkins Institutional Review Board.
References
- 1.Dalakas MC. Inflammatory muscle diseases. N Engl J Med. 2015;373:393–4. doi: 10.1056/NEJMc1506827. [DOI] [PubMed] [Google Scholar]
- 2.Del Grande F, Carrino JA, Del Grande M, et al. Magnetic resonance imaging of inflammatory myopathies. Top Magn Reson Imaging. 2011;22:39–43. doi: 10.1097/RMR.0b013e31825b2c35. [DOI] [PubMed] [Google Scholar]
- 3.Garcia J. MRI in inflammatory myopathies. Skeletal Radiol. 2000;29:425–38. doi: 10.1007/s002560000238. [DOI] [PubMed] [Google Scholar]
- 4.Yoshida K, Kurosaka D, Joh K, et al. Fasciitis as a common lesion of dermatomyositis, demonstrated early after disease onset by en bloc biopsy combined with magnetic resonance imaging. Arthritis Rheum. 2010;62:3751–9. doi: 10.1002/art.27704. [DOI] [PubMed] [Google Scholar]
- 5.Cox FM, Reijnierse M, Van Rijswijk CS, et al. Magnetic resonance imaging of skeletal muscles in sporadic inclusion body myositis. Rheumatology (Oxf) 2011;50:1153–61. doi: 10.1093/rheumatology/ker001. [DOI] [PubMed] [Google Scholar]
- 6.Dion E, Cherin P, Payan C, et al. Magnetic resonance imaging criteria for distinguishing between inclusion body myositis and polymyositis. J Rheumatol. 2002;29:1897–906. [PubMed] [Google Scholar]
- 7.Tasca G, Monforte M, De Fino C, et al. Magnetic resonance imaging pattern recognition in sporadic inclusion-body myositis. Muscle Nerve. 2015;52:956–62. doi: 10.1002/mus.24661. [DOI] [PubMed] [Google Scholar]
- 8.Hoogendijk JE, Amato AA, Lecky BR, et al. 119th ENMC international workshop: trial design in adult idiopathic inflammatory myopathies, with the exception of inclusion body myositis, 10–12 October 2003, Naarden, the Netherlands. Neuromuscul Disord. 2004;14:337–45. doi: 10.1016/j.nmd.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Griggs RC, Askanas V, Dimauro S, et al. Inclusion body myositis and myopathies. Ann Neurol. 1995;38:705–13. doi: 10.1002/ana.410380504. [DOI] [PubMed] [Google Scholar]
- 10.Sontheimer RD. Would a new name hasten the acceptance of amyopathic dermatomyositis (dermatomyositis sine myositis) as a distinctive subset within the idiopathic inflammatory dermatomyopathies spectrum of clinical illness? J Am Acad Dermatol. 2002;46:626–36. doi: 10.1067/mjd.2002.120621. [DOI] [PubMed] [Google Scholar]
- 11.Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts) N Engl J Med. 1975;292:403–7. doi: 10.1056/NEJM197502202920807. [DOI] [PubMed] [Google Scholar]
- 12.Mammen AL, Pak K, Williams EK, et al. Rarity of anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase antibodies in statin users, including those with self-limited musculoskeletal side effects. Arthritis Care Res (Hoboken) 2012;64:269–72. doi: 10.1002/acr.20662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valiyil R, Casciola-Rosen L, Hong G, et al. Rituximab therapy for myopathy associated with anti-signal recognition particle antibodies: a case series. Arthritis Care Res (Hoboken) 2010;62:1328–34. doi: 10.1002/acr.20219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuo GP, Carrino JA. Skeletal muscle imaging and inflammatory myopathies. Curr Opin Rheumatol. 2007;19:530–5. doi: 10.1097/BOR.0b013e3282efdc66. [DOI] [PubMed] [Google Scholar]
- 15.Papke LE, Wooldridge JM. Econometric methods for fractional response variables With an application to 401(K) plan participation rates. J Appl Econ. 1996;11:619–32. [Google Scholar]
- 16.Efron B, Tibshirani R. An introduction to the bootstrap. New York: Chapman & Hall; 1993. [Google Scholar]
- 17.Yao L, Yip AL, Shrader JA, et al. Magnetic resonance measurement of muscle T2, fat-corrected T2 and fat fraction in the assessment of idiopathic inflammatory myopathies. Rheumatology (Oxf) 2016;55:441–9. doi: 10.1093/rheumatology/kev344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’connell MJ, Powell T, Brennan D, et al. Whole-body MR imaging in the diagnosis of polymyositis. AJR Am J Roentgenol. 2002;179:967–71. doi: 10.2214/ajr.179.4.1790967. [DOI] [PubMed] [Google Scholar]
- 19.Schulze M, Kotter I, Ernemann U, et al. MRI findings in inflammatory muscle diseases and their noninflammatory mimics. AJR Am J Roentgenol. 2009;192:1708–16. doi: 10.2214/AJR.08.1764. [DOI] [PubMed] [Google Scholar]
- 20.Van De Vlekkert J, Maas M, Hoogendijk JE, et al. Combining MRI and muscle biopsy improves diagnostic accuracy in subacute-onset idiopathic inflammatory myopathy. Muscle Nerve. 2015;51:253–8. doi: 10.1002/mus.24307. [DOI] [PubMed] [Google Scholar]
- 21.Filli L, Maurer B, Manoliu A, et al. Whole-body MRI in adult inflammatory myopathies: do we need imaging of the trunk? Eur Radiol. 2015;25:3499–507. doi: 10.1007/s00330-015-3783-3. [DOI] [PubMed] [Google Scholar]
- 22.Zheng Y, Liu L, Wang L, et al. Magnetic resonance imaging changes of thigh muscles in myopathy with antibodies to signal recognition particle. Rheumatology (Oxf) 2015;54:1017–24. doi: 10.1093/rheumatology/keu422. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


