Abstract
Background
International normalized ratio (INR) and partial thromboplastin time (PTT) are used interchangeably to diagnose acute traumatic coagulopathy (ATC) but reflect disparate activation pathways. In this study we identified injury/patient characteristics and coagulation factors that drive contact pathway, tissue factor pathway (TF), and common pathway dysfunction by examining injured patients with discordant coagulopathies. We hypothesized that patients with INR/PTT discordance reflect differing phenotypes representing contact vs. tissue factor pathway perturbations, and that characterization will provide targets to guide individualized resuscitation.
Methods
Plasma samples were prospectively collected from 1262 critically-injured patients at a single Level-1 trauma center. Standard coagulation measures and an extensive panel of pro- and anti-coagulant factors were assayed and analyzed with demographic and outcome data.
Results
Fourteen percent of patients were coagulopathic on admission. Among these, 48% had abnormal INR and PTT (BOTH), 43% had isolated prolonged PTT (PTT-CONTACT), and 9% had isolated elevated INR (INR-TF). PTT-CONTACT and BOTH had lower GCS than INR-TF (p<0.001). INR-TF had decreased factor VII activity compared to PTT-CONTACT, while PTT-CONTACT had decreased factor VIII activity compared to INR-TF. All coagulopathic patients had factor V defecits but activity was lowest in BOTH suggesting an additive downstream effect of disordered activation pathways. Patients with PTT-CONTACT received half as much PRBC and FFP as other groups (p<0.001). Despite resuscitation, mortality was higher for coagulopathic patients; mortality was highest in BOTH and higher in PTT-CONTACT than INR-TF (71%, 60%, 41% p=0.04)
Conclusions
Discordant phenotypes demonstrate differential factor deficiencies consistent with dysfunction of contact vs. tissue factor pathways with additive effects from common pathway dysfunction. Recognition and treatment of pathway-specific factor deficiencies driving different coagulopathic phenotypes in injured patients may individualize resuscitation and improve outcomes.
Level of Evidence
Prognostic/therapeutic study, level III.
Keywords: Traumatic coagulopathy, phenotype, precision medicine, resuscitation
BACKGROUND
Acute traumatic coagulopathy (also known as trauma-induced coagulopathy) is common, affecting one in three severely injured patients (1, 2). Since its discovery, the trauma community has sought to characterize the underlying physiologic mechanisms and optimal treatment of this disease. Treatment strategy for the injured coagulopathic patient typically consists of plasma-based balanced resuscitation (3), but there are no validated patient-specific algorithms specifying how to tailor administration to different patterns of standard coagulation values. Despite significant advances, coagulopathy portends worse outcomes and increased mortality for affected patients (2, 4, 5). One potential cause underlying this ongoing morbidity may be that Acute traumatic coagulopathy (ATC) is not a single entity, but is actually comprised of multiple coagulopathic phenotypes that warrant distinct, targeted treatment strategies.
The hematology community recognizes multiple distinct defects in coagulation leading to heritable and acquired coagulopathy. Often, discordance between standard measurements on routine coagulation testing suggests pathology driven by pathway-specific factor deficiencies (6, 7). Diagnosis ensues and treatment is then tailored to the specific factor deficit. Although these disorders in most cases are reflective of single derangements in coagulation as opposed to the multifactorial disordered mechanisms underlying ATC, this approach highlights the contrast to the diagnostic and treatment practice employed in hemostatic resuscitation. While patients with other hematologic disorders are diagnosed and treated to target specific abnormalities, even in the era of ‘precision medicine’ traumatic coagulopathy is commonly considered as a single disease and treated in a non-targeted manner despite voluminous revolutionary work in coagulation and hemostatic resuscitation.
Although some centers now utilize thromboelastography parameters to diagnose traumatic coagulopathy, ATC was originally defined as admission prolongation of either prothrombin time/international normalized ratio (INR) or activated partial thromboplastin time (PTT)(1, 2), and these tests remain the most widely available and continue to be commonly utilized today. However, PTT and INR evaluate separate physiologic pathways that (if functioning appropriately) ultimately result in fibrin generation and while some coagulopathic trauma patients present with prolongation of both, many demonstrate isolated elevation of either PTT or INR (2, 8).
INR assesses patient response to tissue factor exposure, traditionally referred to as the “intrinsic” coagulation pathway. This pathway relies on sufficient levels of functioning factor VII to induce “common” pathway activation and eventual thrombin production (9). Partial thromboplastin time evaluates the contact activation pathway (formerly “extrinsic” pathway), measuring the response of blood to an activator which triggers sequential activation of a series of mediators including factors XII, XI, IX, and VIII culminating in “common pathway” activation (10). Deficiency in mediators of the “common” pathway including factor V, X, or II can also lead to prolongation of both PTT and INR.
Differences in the coagulation profiles of patients presenting with ATC compared to non-coagulopathic patients have been previously described and include relative deficiencies of factor II, V, VII, VIII, IX, X, activation of protein C, and elevated D-dimer (8, 11, 12). However, the extent to which injuries, factor profiles, and outcomes differ in patients presenting with discordant abnormalities in standard tests remains unknown, and may suggest critical, targetable differences in pathology. The purpose of this study was to define phenotypes of ATC. Specifically, we sought to identify patient and injury patterns that drive isolated prolongations of PTT, INR or the combination of both and to characterize the detailed coagulation profile specific to each pattern. We hypothesized that isolated PTT prolongation, isolated INR prolongation, and dual PTT and INR prolongation after trauma represent distinct “coagulation phenotypes” driven by abnormality of one or more pathway-specific composite factors which may provide potential targets for tailored resuscitation of the injured coagulopathic patient.
METHODS
Admission plasma samples were prospectively collected from 1262 adult patients who met criteria for highest-level trauma-activation at a single, urban Level-1 trauma center between March 2005 and April 2015. Exclusion criteria included pediatric patients, pregnant patients, prisoners, and patients transferred from other institutions. Patients were additionally excluded for active use of anticoagulation, anti-fibrinolytic, or antiplatelet therapy.
Admission samples were collected during initial clinical sampling upon arrival to the ED. A waiver of consent was applied for initial blood draw samples, in accordance with the approval of the Committee of Human Research at University of California San Francisco (12)
Citrated samples were assayed for international normalized ratio (INR) and partial thromboplastin time (PTT). Acute traumatic coagulopathy was defined according to previously described cutoffs of INR≥1.4 or PTT≥35 (2, 11). Patients were defined as non-coagulopathic if INR<1.4 and PTT<35. Coagulopathic patients were further categorized into one of three coagulopathic phenotype groups as follows. Patients with isolated INR elevations and normal PTT (INR≥1.4, PTT<35) were designated as “INR-TF” phenotype. Patients were categorized as “PTT-CONTACT” phenotype if they demonstrated isolated PTT elevations and normal INR (INR<1.4, PTT≥35). Patients with elevations in both standard coagulopathy measures (INR≥1.4, PTT≥35) were categorized as “BOTH.”
In addition to standard coagulation measures, samples were assayed for an extensive panel of pro- and anti-coagulant factors including Protein C (PC), D-dimer, antithrombin III (ATIII), and factors II, V, VII, VIII, IX, X using a Stago-Compact Functional Coagulation Analyzer (Diagnostica Stago; Parsippany, NJ). Although the study was initiated prior to availability of functional testing at our institution, samples collected after March 2010 were also assayed using ROTEM Delta System Analyzer, Model ET1729L to assess clot function in the presence of tissue factor alone (EXTEM) and with platelet inhibitor cytochalasin D (FIBTEM). EXTEM MCF was used as a measurement of overall functional coagulability whereas FIBTEM MCF was used to measure the functional contribution of fibrin polymerization to clot formation.
Demographics, resuscitation data, laboratory results, and outcomes were collected and matched to coagulation profiles for each patient. Data collected included: demographic information (age, gender, Body Mass Index (BMI)); injury characteristics (mechanism, injury severity as measured by Injury Severity Score (ISS)(13), Glasgow Coma Score (GCS)(14)); physiologic and laboratory data (temperature, base deficit, platelet count, fibrinogen, transfusion of packed red blood cells (PRBC), plasma (FFP), platelets within the first 72 hours of admission; hospital-related outcomes (“ventilator-free” days, multiple organ failure (MOF), and death). Multi-organ failure was defined using the Denver Post-injury Multiple Organ Failure Score (15).
Descriptive statistics are reported as means and standard deviations for normally distributed data and medians with interquartile ranges for skewed and ordinal data. Binary variables are described with percentages. Differences between groups were evaluated using Chi2 and Fischer’s exact test for proportions and categorical variables, Student’s t test or one-way analysis of variance for normally distributed data, and Wilcoxon rank sum or Kruskal Wallis one-way analysis of variance for ordinal data and variables with non-parametric distributions. Univariate and multiple linear regressions were used to further assess relationships between coagulopathic phenotype and differentially expressed coagulopathic markers and transfused blood products. Kaplan-Meier survival analysis was used to generate time-to-death curves stratified by coagulopathic phenotype. An [α] alpha level <0.05 was considered significant for all analyses. Statistical analysis was performed using Stata 14 (StataCorp, College Station, TX).
RESULTS
COAGULOPATHIC VS. NON-COAGULOPATHIC PATIENTS
Of 1262 eligible patients, 14% (181 patients) presented with coagulopathy. Consistent with the reports of other investigators, the coagulopathic patients in our cohort demonstrated significant differences in patient and injury characteristics, coagulation profiles, and worse outcomes than non-coagulopathic patients. (Table 1)
Table 1.
Demographics, Coagulation Profiles, and Outcomes for Coagulopathic versus Non-Coagulopathic Patients after Injury
| Non-Coagulopathic | Coagulopathic | ||
|---|---|---|---|
| N= 1081 | N=181 | p-value | |
| Age (years) | 34 (25-50) | 36 (24-53) | 0.460 |
| Male (%) | 83.3 ± 37.3 | 73.5 ± 44.3 | 0.002 |
| Blunt Mechanism (%) | 52.9 ± 49.9 | 67.2 ± 47.1 | <0.001 |
| Injury Severity Score | 10 (2-26) | 29.5 (20-43) | <0.001 |
| GCS* | 14 (8-15) | 5 (3-12) | <0.001 |
| Base Deficit* (mEq/L) | −3.33 ± 5.77 | −8.06 ± 7.00 | <0.001 |
| FIBTEM MCF* (mm) | 14 (11-17) | 10 (7-15) | <0.001 |
| EXTEM MCF* (mm) | 64 (60-67) | 59 (52-63) | <0.001 |
| Factor Activity* (%) | |||
| Factor II | 76.0 ± 19.5 | 58.4 ± 19.7 | <0.001 |
| Factor V | 52 (37-70) | 22 (15-34) | <0.001 |
| Factor VII | 81.5 (62-103) | 63 (44-94) | <0.001 |
| Factor VIII | 170 (99-277) | 128 (69-204) | <0.001 |
| Factor IX | 125 ± 40.5 | 92.9 ± 40.8 | <0.001 |
| Factor X | 79.7 ± 21.7 | 58.8 ± 22.1 | <0.001 |
| Fibrinogen* (mg/dL) | 236 ± 94.9 | 154 ± 90.1 | <0.001 |
| D-dimer* (ug/dL) | 1.08 (0.33-4.3) | 7.05 (4-9.55) | <0.001 |
| Antithrombin III* (% activity) | 88.6 ± 27.4 | 66.9 ± 29.5 | <0.001 |
| Protein C* (% activity) | 91.2 ± 26.8 | 70.5 ± 30.8 | <0.001 |
| 72-Hour Transfusion (units) | |||
| PRBC | 2.34 ± 5.96 | 10.9 ± 15.0 | <0.001 |
| FFP | 1.30 ± 4.21 | 8.00 ± 11.9 | <0.001 |
| Platelets | 0.21 ± 0.74 | 1.24 ± 3.02 | <0.001 |
| Multi-Organ Failure (%) | 7.4 ± 26.2 | 16.0 ± 36.8 | <0.001 |
| Discharge Mortality (%) | 9.2 ± 28.9 | 63.5 ± 48.3 | <0.001 |
Legend: Data are presented as mean ±SD for normally distributed variables or median (IQR) for non-parametric/ordinal variables. Variables with
designation were measured at admission. INR, International Normalized Ratio; PTT, partial thromboplastin time: Non-Coagulopathic, INR<1.4 and PTT<35; Coagulopathic, INR≥1.4 and/or PTT≥35; GCS, Glasgow Coma Scale; MCF, Maximal Clot Firmness; mm, millimeters; PRBC, packed red blood cells; FFP, fresh frozen plasma; PRBC, FFP, and platelet totals are units transfused within 72 hours of admission.
COAGULOPATHIC PHENOTYPES
DEMOGRAPHICS AND INJURY CHARACTERISTICS
Among patients with admission coagulopathy, 9% (17 patients) presented with isolated elevated INR (INR-TF), 43% (77 patients) presented with isolated prolonged PTT (PTT-CONTACT), and 48% had abnormal INR and PTT (BOTH). Elevated PTT groups (PTT-CONTACT and BOTH) had lower GCS than INR-TF patients. These groups also trended toward increased injury severity but this was not statistically or clinically significant. There were no other differences in patient demographics or injury characteristics between coagulopathic phenotypes (Table 2).
Table 2.
Baseline Patient and Injury Characteristics, Admission Laboratory Values, and Outcomes by Coagulopathic Phenotype
| INR-TF | PTT-CONTACT | BOTH | ||
|---|---|---|---|---|
| N=17 | N=77 | N=88 | p-value | |
| Age (years) | 36 (24-46) | 40 (28-54) | 32.5 (22-52) | 0.091 |
| Male (%) | 82.4 ± 39.3 | 66.2 ± 47.6 | 78.2 ± 41.6 | 0.178 |
| BMI (kg/m2) | 27.8 ± 4.62 | 25.0 ± 4.81 | 26.4 ± 5.20 | 0.764 |
| Blunt Mechanism | 64.7 ± 49.3 | 73.7 ± 44.3 | 62.1 ± 48.8 | 0.283 |
| Injury Severity Score | 19.5 (13.5-31.1) | 30 (18-38) | 29 (25-45) | 0.056 |
| GCS* | 12.3 ± 3.50 | 7.09 ± 4.68 | 6.42 ± 4.50 | <0.001 |
| Crystalloid (mL) | 100 (0-500) | 50 (0-250) | 175 (50-300) | 0.333 |
| Temperature* (°C) | 36.2 (35.6-36.6) | 35.6 (35-36.6) | 36 (35.1-36.5) | 0.692 |
| Base Deficit* (mEq/L) | −7.92 ± 7.62 | −6.61 ± 6.94 | −9.38 ± 6.77 | 0.859 |
| Platelets* (1000/μL) | 236 ± 73.6 | 283 ± 98.5 | 201 ± 83.6 | 0.202 |
| FIBTEM MCF* (mm) | 13 (12-14) | 11 (8-15) | 8 (5-10) | 0.031 |
| EXTEM MCF* (mm) | 64 (53-64) | 61.5 (56.5-65.5) | 54 (49-59.5) | 0.002 |
| Vent-Free Days (days) | 0 (0-27) | 0 (0-22) | 0 (0-10) | 0.093 |
| Multi-Organ Failure (%) | 23.5 ± 43.7 | 11.7 ± 32.3 | 18.4 ± 39.0 | 0.322 |
| Mortality (%) | ||||
| 24 Hour | 11.8 ± 33.2 | 31.2 ± 46.6 | 44.8 ± 50.0 | 0.017 |
| Discharge | 41.2 ± 50.7 | 59.7 ± 49.4 | 71.3 ± 45.5 | 0.041 |
Legend: Data are presented as mean ±SD for normally distributed variables or median (IQR) for non-parametric/ordinal variables. Variables with
designation were measured at admission. INR, International Normalized Ratio; PTT, partial thromboplastin time; INR-TF, INR≥1.4 and PTT<35; PTT-CONTACT, INR<1.4 and PTT≥35; BOTH, INR≥1.4 and PTT≥35; BMI, Body Mass Index; GCS, Glascow Coma Scale; Crystalloid, pre-hospital crystalloid; MCF, Maximal Clot Firmness; mm, millimeters; Vent-Free Days are number of days not spent on a ventilator within the first 28 days of hospitalization. For complete data regarding coagulopathic profile, please see SDC Table 2.
COAGULATION PROFILE
Coagulopathic phenotype groups demonstrated differences in factor activity levels consistent with their specific coagulation activation pathways (Figure 2). INR-TF and BOTH patients had decreased factor VII activity compared to PTT-CONTACT. Patients with perturbations in the contact pathway (BOTH and PTT-CONTACT) had decreased admission factor VIII activity compared to INR-TF. There were no significant differences in factor IX between groups (p=0.571) (Figure 2). All coagulopathic patients had critically depressed common pathway factor V activity but levels were lowest in BOTH. There were no significant differences between coagulopathic groups for common pathway factors II or X activity. Unadjusted and multiple linear regression of coagulopathic group on factor levels confirmed these patterns. (SDC Table 1). In multiple linear regression, an approximately equal decrease in factor V was predicted by INR-TF and BOTH.
Figure 2. Differential Factor Activity by Coagulopathic Phenotypes.
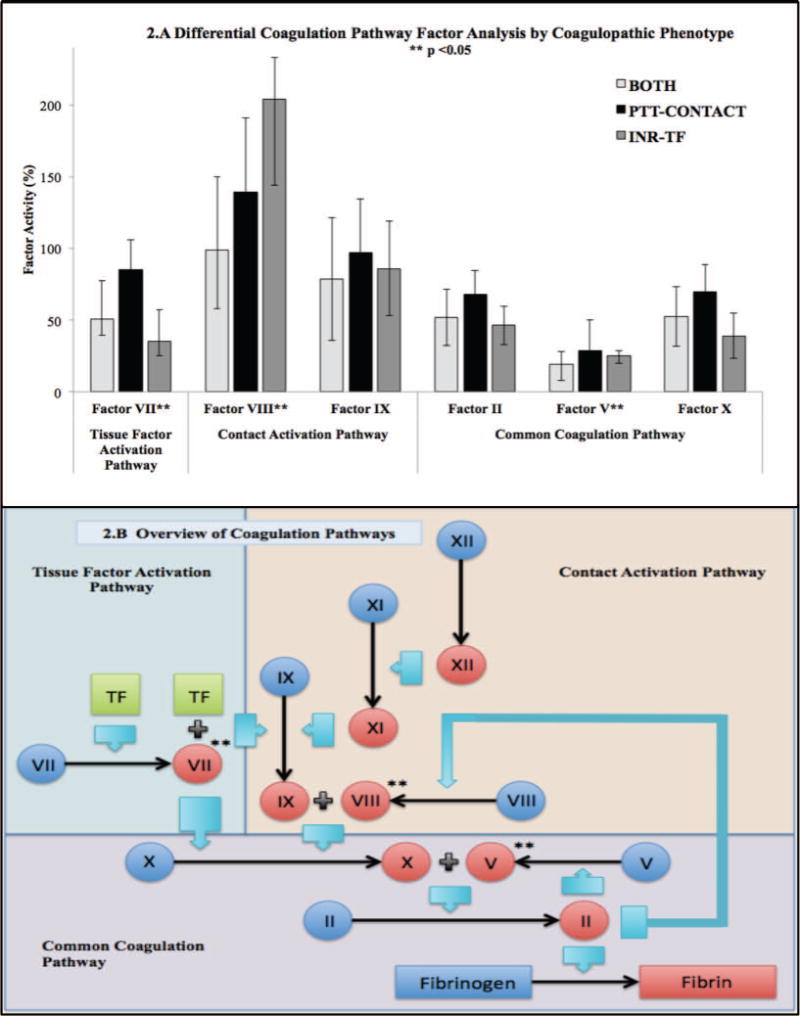
2.A Coagulation Pathway Factor Analysis by Coagulopathic Phenotype. Bars demonstrate means for normally distributed variables (Factor II, IX, and X) and medians for non-parametric variables (Factors V, VII, and VIII). Error bars indicate ±SD or IQR respectively. All activity levels were drawn at admission. INR, International Normalized Ratio; PTT, partial thromboplastin time; INR-TF, INR≥1.4 and PTT<35; PTT-CONTACT, INR<1.4 and PTT≥35; BOTH, INR≥1.4 and PTT≥35. 2.B Overview of Coagulation Pathways. Simplified schematic of the coagulation cascade highlighting points of differential factor activity between coagulopathic phenotypes. Variables designated with ** demonstrated significant differences between groups at an alpha level p<0.05. TF, Tissue Factor; II, Factor II; V, Factor V; VII, Factor VII; VIII, Factor VIII; IX, Factor IX; X, Factor X.
Coagulopathic phenotypes also demonstrated differential expression of fibrinogen, D-dimer, antithrombin III, and Protein C (Figure 1). Fibrinogen levels were highest for PTT-CONTACT, lower for INR-TF, and lowest for BOTH. D-dimer was elevated in PTT-CONTACT and BOTH compared to INR-TF. Antithrombin III activity was lowest in the BOTH phenotype. Finally, Protein C was lower in elevated INR phenotypes compared to PTT-CONTACT. Univariate and multiple linear regression of coagulopthic phenotypes on these markers revealed similar prediction patterns with the following exceptions: 1) only BOTH predicted significant decrease in fibrinogen levels on univariate and multiple regression, and 2) BOTH and PTT-CONTACT predicted decrease in ATIII on univariate regression, however only BOTH predicted ATIII depression on multiple regression analysis (SDC Tables 2–5).
Figure 1. Coagulation Markers by Coagulopathic Phenotype.
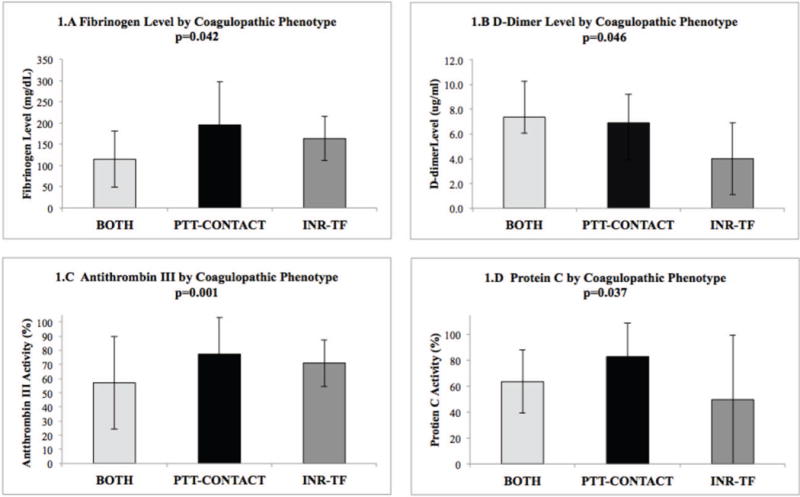
1.A Fibrinogen Level by Coagulopathic Phenotype 1.B D-Dimer Level by Coagulopathic Phenotype 1.C Antithrombin III by Coagulopathic Phenotype 1.D Protein C by Coagulopathic Phenotype. Bars demonstrate means for normally distributed variables (Fibrinogen, Antithrombin III, and Protein C) and median for non-parametric variables (D-dimer). Error bars indicate ±SD or IQR respectively. INR, International Normalized Ratio; PTT, partial thromboplastin time; INR-TF, INR≥1.4 and PTT<35; PTT-CONTACT, INR<1.4 and PTT≥35; BOTH, INR≥1.4 and PTT≥35.
Functional measures of overall coagulability (EXTEM MCF) and fibrin polymerization (FIBTEM MCF) were depressed in patients with BOTH relative to INR-TF and PTT-CONTACT (Table 2).
TRANSFUSION REQUIREMENTS
There were disparities in product transfusion between coagulopathic phenotypes (Figure 3). PTT-CONTACT received less PRBC and FFP than other groups at 24 and 72 hours. Similar relationships between phenotype and product transfusion were predicted by univariate and multiple linear regression (SDC Tables 6–7). In fact, PTT-CONTACT did not statistically predict PRBC transfusion at either timepoint in multiple linear regression. PTT-CONTACT also received the fewest platelet units of any phenotype at 24 hours (p=0.040), but no statistical difference between phenotypes was seen for platelet transfusion at 72 h (p=0.087) .
Figure 3. Transfusion by Coagulopathic Group at 24 and 72 Hours After Injury.
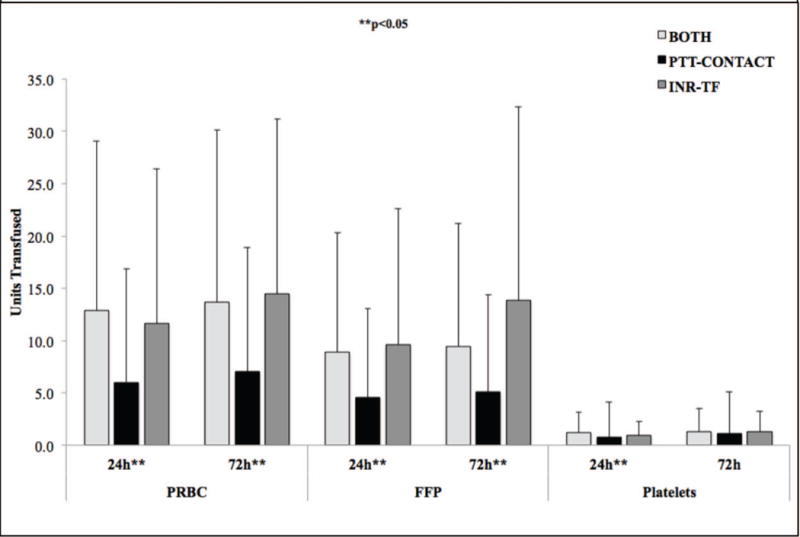
Legend: Bars are presented as means, with error bars indicating ±SD. INR, International Normalized Ratio; PTT, partial thromboplastin time; INR-TF, INR≥1.4 and PTT<35; PTT-CONTACT, INR<1.4 and PTT≥35; BOTH, INR≥1.4 and PTT≥35; PRBC, packed red blood cells; FFP, fresh frozen plasma; 24h, 24 hours after admission; 72h, 72 hours after admission. PRBC, FFP, and platelet totals represent units transfused within 24 or 72 hours of admission.
OUTCOMES
There were no differences in ventilator-free days or multi-organ system failure between phenotypic groups (Table 2). Twenty-four hour and discharge mortality was lowest for INR-TF, higher for PTT-CONTACT and highest for BOTH (Table 2). In Kaplan Meier Survival analysis adjusted for injury severity, estimated survival rates were again highest for INR-TF, lower for PTT-CONTACT, and lowest for BOTH with statistical difference between curves (Figure 4).
Figure 4. Survival After Injury by Coagulopathic Phenotype Adjusted for Injury Severity.
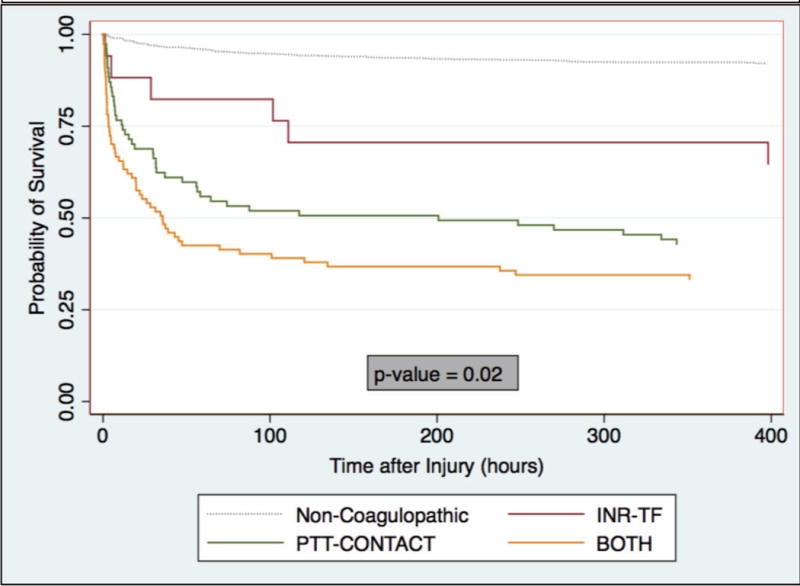
Kaplan Meier Estimator of survival after injury by coagulopathic phenotype adjusted for injury severity score. Log-rank test for equality of survivor functions performed for coagulopathic phenotype curves only (p-value). Non-coagulopathic curve displayed for reference. INR, International Normalized Ratio; PTT, partial thromboplastin time; INR-TF, INR≥1.4 and PTT<35; PTT-CONTACT, INR<1.4 and PTT≥35; BOTH, INR≥1.4 and PTT≥35.
DISCUSSION
Acute traumatic coagulopathy is common after severe injury and contributes to increased bleeding, mortality and poor outcomes in survivors(2, 8, 11, 12) Considerable work toward mechanistic characterization has ensued since ATC was first described yet despite better understanding, the ability to diagnose separate phenotypes and apply targeted treatment remains limited in practice (8, 11, 12). Hemostatic resuscitation has focused on one-size-fits-all plasma-based resuscitation protocols. These protocols have resulted in considerable mortality reductions, however in the age of precision medicine we have the opportunity to further improve outcomes by individualizing diagnosis and applying targeted treatment.
Recent prospective, randomized data from the Denver Health group have demonstrated that utilizing functional testing to guide resuscitation for patients with severe injuries and physiologic collapse led to decreased mortality compared with guidance based on conventional testing(16). This data provides support for the idea that ATC is not simply a single entity and is a perfect example of the efficacy of shifting towards a paradigm of individualizing care to patient physiology. However, abnormal PTT was not utilized as one of the conventional criteria for resuscitation, which, in light of the data presented here, may have significantly contributed to higher mortality in the conventional assay group. We propose that the optimal, precision approach would include an understanding of the biologic underpinnings of all commonly collected assays, with integration of this information in order to best target individualized treatment.
In this prospective observational cohort we provide the first evidence that conventional assay phenotypes provide specific mechanistic information with the potential to inform and tailor resuscitation presenting with coagulopathy. Discordant standard coagulation assays INR-TF vs. PTT-CONTACT vs. BOTH, have different associated demographic and injury patterns and different outcomes. INR-TF phenotype occurs in patients with preserved admission GCS and demonstrates a coagulopathic profile marked by low Protein C and depressed factor VII activity. PTT-CONTACT is characterized by low initial GCS and a coagulopathic profile notable for relative factor VIII deficiency with preserved protein C, and elevated D-dimer. Patients with PTT- CONTACT coagulopathy received less PRBC and FFP resuscitation than other phenotypes but demonstrated high mortality rates. Finally, the BOTH phenotype appears to demonstrate the most severe coagulopathy and highest mortality after injury. BOTH is characterized by depressed GCS at admission, with a coagulopathic profile significant for a combination of depressed factor VII and factor VIII, critically deficient factor V, low fibrinogen and antithrombin III, and elevated D-dimer.
These differences provide insight into potential targets for treatment beyond the standard balanced plasma based resuscitation.
DEMOGRAPHICS AND INJURY CHARACTERISTICS
The association between depressed GCS and elevated PTT phenotypes provides indirect evidence that traumatic brain injury may play a role in perturbation of the contact activation pathway. Early post-TBI coagulopathy has canonically attributed to consumptive coagulopathy in the tissue factor pathway following tissue-factor release from damaged brain tissue (17). However, we demonstrate that low GCS is associated with prolonged PTT even without concomitant INR derangement, suggesting that TF pathway consumption does not fully explain post-TBI coagulopathy. Other research has also questioned the primacy of the tissue-factor release hypothesis (18, 19). Of course, low GCS is not a perfect surrogate for brain injury, highlighting the need to examine coagulopathic phenotypes specifically in a TBI population. Improved understanding of the mechanisms underlying post-TBI coagulopathy could ultimately provide the basis for a targeted approach to preventing progression of intracranial hemorrhage.
IMPLICATIONS OF COAGULATION PROFILES DIFFERENCES
Differentially expressed coagulation markers provide insight into the mechanistic differences between coagulopathic phenotypes and more importantly, may suggest potential targets for tailored resuscitation practice. Our data suggests that depressed factor VII activity drives perturbations of the tissue-factor activation pathway after injury and consequently, could provide a potential target for attenuating ATC in this subgroup. Use of activated recombinant factor VII (rfVIIa) initially received considerable attention but was never broadly accepted by the trauma community due to multiple studies suggesting questionable efficacy in prevention of mortality and increased thrombosis (20, 21). In the majority of these studies, rfVIIa was used in patients undergoing massive transfusion at late stages in their coagulopathic course, rather than targeted toward patients with elevated INR on admission (22). While the present study does not provide sufficient evidence to recommend empiric factor VII administration, we do suggest that prospective phenotypic-specific repletion warrants further investigation.
Although factor VIII levels are known to be elevated following severe injury (23), patients with PTT elevation coagulopathy uniquely demonstrate relative depressions in median factor VIII activity compared to non-coagulopathic patients(8). This has several interesting implications for resuscitation. First, while in healthy controls, factor activity levels of 30% are commonly accepted as sufficient for adequate coagulation, the natural history and sufficient factor levels for functional clotting after injury has never been delineated. This data suggests that higher than expected levels of functional factor VIII may be necessary for adequate coagulation in the trauma setting. Furthermore, abnormalities in the contact activation pathway may be driven by relative deficits in factor VIII activity. Interestingly, this association is found in PTT-CONTACT despite relatively preserved Protein C levels (low aPC). Although aPC is known to down-regulate factors V and VIII(11), our data suggests that factor VIII depletion in PTT-CONTACT may occur via another mechanism or, alternatively, that even low amounts of aPC activity are sufficient to downregulate factor VIII to levels insufficient for post trauma hemostasis. Of note, factor VIII levels in BOTH are even lower than in PTT-CONTACT, which may be attributable to a combination of mechanisms including exacerbation of the inhibitory influence of aPC, which is elevated in BOTH. Early targeted repletion of factor VIII in patients with elevated PTT phenotypes may correct perturbations in this pathway and prevent coagulopathy from worsening. Fresh frozen plasma, the primary product utilized in the current treatment of coagulopathy, tends to have relatively low levels of factor VIII (24) and does not provide an ideal source for repletion.
Critical factor V deficiency was present in all coagulopathic groups. We hypothesize that dual perturbation of contact and tissue factor activation pathways with deficits of multiple pathway mediators resulted in additive downstream depression resulting in the most severe deficiencies among patients in the BOTH cohort. However, factor V repletion represents an important resuscitation target for all coagulopathic groups, which may partially explain the efficacy of FFP in the treatment of traumatic coagulopathy. This data provides mechanistic evidence that all coagulopathic groups likely will continue to derive benefit from FFP resuscitation, although, as suggested above, additional adjuncts may be necessary to adequately treat other, phenotype-specific, factor deficiencies. Additionally, this implicates the potential utility of aPC pathway inhibition.
Fibrinogen deficit also appeared to be most severe in the setting of dual contact and tissue-factor activation abnormality. Of note, admission fibrinogen levels in BOTH approached traditional thresholds for transfusion, therefore we would advocate maintaining a high index of suspicion for hyperfibrinolysis among patients presenting with BOTH and trending fibrinogen closely throughout early resuscitation in this population with repletion as necessary, particularly in situations where functional assays are unavailable.
Antithrombin III is a key regulator of endogenous anticoagulation. ATIII activity deficit is known to correlate with increasing injury severity (25) which largely accounts for the low ATIII seen in patients with ATC. However, when adjusted for GCS and injury severity, only dual perturbation of contact and tissue-factor pathways remains associated with depressed ATIII. Patients presenting with Acute Traumatic Coagulopathy are known to have increased rates of thrombotic complications throughout hospitalization (26). Further investigation is needed to determine whether presentation with BOTH phenotype specifically portends increased, potentially modifiable, thrombotic risk. This deficit may underscore the necessity of aggressive and early FFP resuscitation in this group.
Finally, Protein C correlates inversely with activated Protein C (aPC), a key driver of Acute Traumatic Coagulopathy which functions, in part, via inactivation of factor V and factor VIII (11). Our data suggests that aPC, which is highest among elevated INR phenotypes, may play a substantial role in disregulation of the tissue-factor activation pathway. This observation provides clinical corroboration for recent research demonstrating attenuation of aPC following tissue-factor pathway inhibition in a murine model (27).
FUNCTIONAL TESTING
Among all admission functional testing, only FIBTEM MCF for the BOTH phenotype reached the manufacturers threshold for hypcoagulability, which may partially be attributable to the lack of validated ROTEM cutoffs in the trauma population. However, as described above the coagulopathic groups identified by standard coagulation tests were deficient in multiple coagulation markers, and demonstrated clinical coagulopathy including higher transfusion requirements, and worse outcomes than non-coagulopathic patients. Although, as previous studies have suggested, many coagulopathic patients will share both abnormal ROTEM and traditional coagulation tests, our data suggests that these assays may not infrequently identify different subsets of patients with coagulopathy suggesting that they should be used concomitantly to best direct diagnosis and targeted treatment.
OUTCOMES
Product resuscitation differed between coagulopathic phenotype groups, with preferential PRBC and FFP resuscitation for phenotypes presenting with some component of elevated INR coagulopathy. It is possible that PTT-CONTACT patients did not receive as many PRBC because these patients achieved earlier hemostasis; unfortunately the present study did not collect information regarding blood loss or surgical hemostasis. However, particularly in consideration of the high early mortality rates for this group, we suspect that PTT-CONTACT patients were substantially under-resuscitated compared to those with INR abnormalities.
Mortality rates differed considerably between coagulopathic phenotypes and were lowest for INR-TF, higher for PTT-CONTACT, and highest for BOTH throughout admission. Kaplan Meier survival analysis of death demonstrated a similar pattern for survival rates among coagulopathic phenotypes after adjustment for injury severity. While it is important to recognize that death is an highly complex outcome with multiple interacting predictors and it is highly unlikely that the cause of death in these patients is solely or primarily attributable to ATC. However this data does provide evidence that these phenotypes playing a significant, if incomplete role, in survival. All phenotypes were predicted to have decreased survival compared to non-coagulopathic patients, emphasizing the need for improved, individualized treatment strategies after injury.
LIMITATIONS
Limitations of this study include those inherent to observational study design and a single center cohort and highlight the utility for further prospective investigations regarding the implications of coagulopathic phenotypes among the broader trauma community. The power of our statistical analysis was limited by a relatively small number of coagulopathic patients. Furthermore, the cutoffs for coagulopathy were selected according to thresholds previously described in the literature, but we recognize that they are not absolute and different cutoffs could be selected. We would hypothesize that with more extreme thresholds coagulation factor deficits, transfusion requirements and even higher mortality would be expected. Finally, we recognize that while this paper focuses on phenotypes delineated by standard coagulation testing there are other variables which play key roles in coagulopathic physiology including platelet function and fibrinolysis, which were not investigated here. It is also possible that additional key explanatory variables exist which are important to the understanding of coagulopathic phenotype but which were not measured in this study.
CONCLUSIONS
We demonstrate that patients diagnosed with Acute Traumatic Coagulopathy comprise distinct coagulopathic phenotypes which can be characterized by discordance in standard coagulation assays. These phenotypes demonstrate distinct biologic profiles which recommend pathway-specific potential treatment targets which could be potentially employed in a precision medicine approach to treatment of coagulopathy. We propose the need for additional prospective investigation to address the efficacy of phenotype-specific therapy for the treatment of traumatic coagulopathy. Through the recognition of the physiology and implications of distinct, readily diagnosable coagulopathic groups within the overarching disease of Acute Traumatic Coagulopathy, clinicians have the opportunity to expand the arsenal of individualizing risk assessment and make informed, mechanistically-grounded decisions regarding treatment.
Supplementary Material
A. Unadjusted univariate linear regression of factors V, VII, VIII. Coef, coefficient; CI, confidence interval; p, p-value; R2 coefficient of determination; GCS, Glasgow Coma Scale; BD base deficit. INR, International Normalized Ratio; PTT, partial thromboplastin time; Coagulopathic Phenotypes: INR-TF, INR≥1.4 and PTT<35; PTT-CONTACT, INR<1.4 and PTT≥35; BOTH, INR≥1.4 and PTT≥35. Variables with * designation were measured at admission. B. Multiple linear regression of factor V, VII, and VIII on coagulopathic phenotype adjusted for multiple phenotypes, male, blunt, injury severity, and base deficit.
A. Unadjusted univariate linear regression on fibrinogen. Coef, coefficient; CI, confidence interval; p, p-value; R2 coefficient of determination; GCS, Glasgow Coma Scale; BD base deficit. INR, International Normalized Ratio; PTT, partial thromboplastin time; Coagulopathic Phenotypes: INR-TF, INR≥1.4 and PTT<35; PTT-CONTACT, INR<1.4 and PTT≥35; BOTH, INR≥1.4 and PTT≥35. Variables with * designation were measured at admission. B. Multiple linear regression of fibrinogen on coagulopathic phenotype adjusted for multiple phenotypes, injury severity, and base deficit.
A. Unadjusted univariate linear regression of D-dimer. Coef, coefficient; CI, confidence interval; p, p-value; R2 coefficient of determination; GCS, Glasgow Coma Scale; BD base deficit. INR, International Normalized Ratio; PTT, partial thromboplastin time; Coagulopathic Phenotypes: INR-TF, INR≥1.4 and PTT<35; PTT-CONTACT, INR<1.4 and PTT≥35; BOTH, INR≥1.4 and PTT≥35. Variables with * designation were measured at admission. B. Multiple linear regression of D-dimer on coagulopathic phenotype adjusted for multiple phenotypes, male, blunt mechanism, injury severity, admission GSC, and base deficit.
A. Unadjusted univariate linear regression of antithrombin III. Coef, coefficient; CI, confidence interval; p, p-value; R2 coefficient of determination; GCS, Glasgow Coma Scale; BD base deficit. INR, International Normalized Ratio; PTT, partial thromboplastin time; Coagulopathic Phenotypes: INR-TF, INR≥1.4 and PTT<35; PTT-CONTACT, INR<1.4 and PTT≥35; BOTH, INR≥1.4 and PTT≥35. Variables with * designation were measured at admission. B. Multiple linear regression of antithrombin III on coagulopathic phenotype adjusted for multiple phenotypes, blunt mechanism, injury severity, admission GSC, and base deficit.
A. Unadjusted univariate linear regression of Protein C. Coef, coefficient; CI, confidence interval; p, p-value; R2 coefficient of determination; GCS, Glasgow Coma Scale; BD base deficit. INR, International Normalized Ratio; PTT, partial thromboplastin time; Coagulopathic Phenotypes: INR-TF, INR≥1.4 and PTT<35; PTT-CONTACT, INR<1.4 and PTT≥35; BOTH, INR≥1.4 and PTT≥35. Variables with * designation were measured at admission. B. Multiple linear regression of Protein C on coagulopathic phenotype adjusted for multiple phenotypes, injury severity, admission GCS, and base deficit.
A. Unadjusted univariate linear regression of packed red blood cells (PRBC) transfused (I.)24 hours and (II.) 72 hours after injury. Coef, coefficient; CI, confidence interval; p, p-value; R2 coefficient of determination; GCS, Glasgow Coma Scale; BD base deficit. INR, International Normalized Ratio; PTT, partial thromboplastin time; Coagulopathic Phenotypes: INR-TF, INR≥1.4 and PTT<35; PTT-CONTACT, INR<1.4 and PTT≥35; BOTH, INR≥1.4 and PTT≥35. Variables with * designation were measured at admission. B. Multiple linear regression of PRBC transfused at (I.)24 hours and (II.) 72 hours after injury on coagulopathic phenotype adjusted for multiple phenotypes, injury severity, GCS, and base deficit.
A. Unadjusted univariate linear regression of fresh frozen plasma (FFP) transfused (I.)24 hours and (II.) 72 hours after injury. Coef, coefficient; CI, confidence interval; p, p-value; R2 coefficient of determination; GCS, Glasgow Coma Scale; BD base deficit. INR, International Normalized Ratio; PTT, partial thromboplastin time; Coagulopathic Phenotypes: INR-TF, INR≥1.4 and PTT<35; PTT-CONTACT, INR<1.4 and PTT≥35; BOTH, INR≥1.4 and PTT≥35. Variables with * designation were measured at admission. B. Multiple linear regression of FFP transfused at (I.)24 hours and (II.) 72 hours after injury on coagulopathic phenotype adjusted for multiple phenotypes, injury severity, GCS, and base deficit.
Acknowledgments
Funding: Supported by PCORI R-IMC-1306-02735 (MJC), NIH #K01ES026834 (RAC)
Footnotes
Conflicts of interest: None
This study was presented at the 46th annual meeting of the Western Trauma Association, February 28–March 4, 2016, in Lake Tahoe, California. Second place winner of the Earl G. Young Resident Research Competition.
AUTHOR CONTRIBUTIONS: SAC, LZK, RAC, and MJC contributed to study design, data collection, data analysis, data interpretation, and writing.
BMH, RCK, ASC, MFN, CMH, and CSC contributed to study design and data collection.
References
- 1.Brohi K, Singh J, Heron M, Coats T. Acute traumatic coagulopathy. J Trauma. 2003;54(6):1127–30. doi: 10.1097/01.TA.0000069184.82147.06. [DOI] [PubMed] [Google Scholar]
- 2.MacLeod JB, Lynn M, McKenney MG, Cohn SM, Murtha M. Early coagulopathy predicts mortality in trauma. J Trauma. 2003;55(1):39–44. doi: 10.1097/01.TA.0000075338.21177.EF. [DOI] [PubMed] [Google Scholar]
- 3.Spinella PC, Holcomb JB. Resuscitation and transfusion principles for traumatic hemorrhagic shock. Blood Rev. 2009;23(6):231–40. doi: 10.1016/j.blre.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niles SE, McLaughlin DF, Perkins JG, Wade CE, Li Y, Spinella PC, Holcomb JB. Increased mortality associated with the early coagulopathy of trauma in combat casualties. J Trauma. 2008;64(6):1459–63. doi: 10.1097/TA.0b013e318174e8bc. discussion 63-5. [DOI] [PubMed] [Google Scholar]
- 5.Maegele M, Lefering R, Yucel N, Tjardes T, Rixen D, Paffrath T, Simanski C, Neugebauer E, Bouillon B, AG Polytrauma of the German Trauma Society (DGU) Early coagulopathy in multiple injury: an analysis from the German Trauma Registry on 8724 patients. Injury. 2007;38(3):298–304. doi: 10.1016/j.injury.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Acharya SS. Rare bleeding disorders in children: identification and primary care management. Pediatrics. 2013;132(5):882–92. doi: 10.1542/peds.2012-3662. [DOI] [PubMed] [Google Scholar]
- 7.Suchman AL, Griner PF. Diagnostic uses of the activated partial thromboplastin time and prothrombin time. Ann Intern Med. 1986;104(6):810–6. doi: 10.7326/0003-4819-104-6-810. [DOI] [PubMed] [Google Scholar]
- 8.Cohen MJ, Kutcher M, Redick B, Nelson M, Call M, Knudson MM, Schreiber MA, Bulger EM, Muskat P, Alarcon LH, et al. Clinical and mechanistic drivers of acute traumatic coagulopathy. J Trauma Acute Care Surg. 2013;75(1 Suppl 1):S40–7. doi: 10.1097/TA.0b013e31828fa43d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davie EW, Ratnoff OD. Waterfall Sequence for Intrinsic Blood Clotting. Science. 1964;145(3638):1310–2. doi: 10.1126/science.145.3638.1310. [DOI] [PubMed] [Google Scholar]
- 10.Macfarlane RG. An Enzyme Cascade in the Blood Clotting Mechanism, and Its Function as a Biochemical Amplifier. Nature. 1964;202:498–9. doi: 10.1038/202498a0. [DOI] [PubMed] [Google Scholar]
- 11.Cohen MJ, Call M, Nelson M, Calfee CS, Esmon CT, Brohi K, Pittet JF. Critical role of activated protein C in early coagulopathy and later organ failure, infection and death in trauma patients. Ann Surg. 2012;255(2):379–85. doi: 10.1097/SLA.0b013e318235d9e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kutcher ME, Ferguson AR, Cohen MJ. A principal component analysis of coagulation after trauma. J Trauma Acute Care Surg. 2013;74(5):1223–9. doi: 10.1097/TA.0b013e31828b7fa1. discussion 9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baker SP, O’Neill B, Haddon W, Jr, Long WB. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974;14(3):187–96. [PubMed] [Google Scholar]
- 14.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2(7872):81–4. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 15.Sauaia A, Moore EE, Johnson JL, Ciesla DJ, Biffl WL, Banerjee A. Validation of postinjury multiple organ failure scores. Shock. 2009;31(5):438–47. doi: 10.1097/SHK.0b013e31818ba4c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez E, Moore EE, Moore HB, Chapman MP, Chin TL, Ghasabyan A, Wohlauer MV, Barnett CC, Bensard DD, Biffl WL, et al. Goal-directed Hemostatic Resuscitation of Trauma-induced Coagulopathy: A Pragmatic Randomized Clinical Trial Comparing a Viscoelastic Assay to Conventional Coagulation Assays. Ann Surg. 2016;263(6):1051–9. doi: 10.1097/SLA.0000000000001608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hulka F, Mullins RJ, Frank EH. Blunt brain injury activates the coagulation process. Arch Surg. 1996;131(9):923–7. doi: 10.1001/archsurg.1996.01430210021004. discussion 7-8. [DOI] [PubMed] [Google Scholar]
- 18.Laroche M, Kutcher ME, Huang MC, Cohen MJ, Manley GT. Coagulopathy after traumatic brain injury. Neurosurgery. 2012;70(6):1334–45. doi: 10.1227/NEU.0b013e31824d179b. [DOI] [PubMed] [Google Scholar]
- 19.Cohen MJ, Brohi K, Ganter MT, Manley GT, Mackersie RC, Pittet JF. Early coagulopathy after traumatic brain injury: the role of hypoperfusion and the protein C pathway. J Trauma. 2007;63(6):1254–61. doi: 10.1097/TA.0b013e318156ee4c. discussion 61-2. [DOI] [PubMed] [Google Scholar]
- 20.Hauser CJ, Boffard K, Dutton R, Bernard GR, Croce MA, Holcomb JB, et al. Results of the CONTROL trial: efficacy and safety of recombinant activated Factor VII in the management of refractory traumatic hemorrhage. J Trauma. 2010;69(3):489–500. doi: 10.1097/TA.0b013e3181edf36e. [DOI] [PubMed] [Google Scholar]
- 21.Levi M, Peters M, Buller HR. Efficacy and safety of recombinant factor VIIa for treatment of severe bleeding: a systematic review. Crit Care Med. 2005;33(4):883–90. doi: 10.1097/01.ccm.0000159087.85970.38. [DOI] [PubMed] [Google Scholar]
- 22.Knudson MM, Cohen MJ, Reidy R, Jaeger S, Bacchetti P, Jin C, Wade CE, Holcomb JB. Trauma, transfusions, and use of recombinant factor VIIa: A multicenter case registry report of 380 patients from the Western Trauma Association. J Am Coll Surg. 2011;212(1):87–95. doi: 10.1016/j.jamcollsurg.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 23.Dunbar NM, Chandler WL. Thrombin generation in trauma patients. Transfusion. 2009;49(12):2652–60. doi: 10.1111/j.1537-2995.2009.02335.x. [DOI] [PubMed] [Google Scholar]
- 24.Downes KA, Wilson E, Yomtovian R, Sarode R. Serial measurement of clotting factors in thawed plasma stored for 5 days. Transfusion. 2001;41(4):570. doi: 10.1046/j.1537-2995.2001.41040570.x. [DOI] [PubMed] [Google Scholar]
- 25.Miller RS, Weatherford DA, Stein D, Crane MM, Stein M. Antithrombin III and trauma patients: factors that determine low levels. J Trauma. 1994;37(3):442–5. [PubMed] [Google Scholar]
- 26.Knudson MM, Collins JA, Goodman SB, McCrory DW. Thromboembolism following multiple trauma. J Trauma. 1992;32(1):2–11. doi: 10.1097/00005373-199201000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Howard BM, Miyazawa BY, Dong W, Cedron WJ, Vilardi RF, Ruf W, Cohen MJ. The tissue factor pathway mediates both activation of coagulation and coagulopathy after injury. J Trauma Acute Care Surg. 2015;79(6):1009–14. doi: 10.1097/TA.0000000000000707. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A. Unadjusted univariate linear regression of factors V, VII, VIII. Coef, coefficient; CI, confidence interval; p, p-value; R2 coefficient of determination; GCS, Glasgow Coma Scale; BD base deficit. INR, International Normalized Ratio; PTT, partial thromboplastin time; Coagulopathic Phenotypes: INR-TF, INR≥1.4 and PTT<35; PTT-CONTACT, INR<1.4 and PTT≥35; BOTH, INR≥1.4 and PTT≥35. Variables with * designation were measured at admission. B. Multiple linear regression of factor V, VII, and VIII on coagulopathic phenotype adjusted for multiple phenotypes, male, blunt, injury severity, and base deficit.
A. Unadjusted univariate linear regression on fibrinogen. Coef, coefficient; CI, confidence interval; p, p-value; R2 coefficient of determination; GCS, Glasgow Coma Scale; BD base deficit. INR, International Normalized Ratio; PTT, partial thromboplastin time; Coagulopathic Phenotypes: INR-TF, INR≥1.4 and PTT<35; PTT-CONTACT, INR<1.4 and PTT≥35; BOTH, INR≥1.4 and PTT≥35. Variables with * designation were measured at admission. B. Multiple linear regression of fibrinogen on coagulopathic phenotype adjusted for multiple phenotypes, injury severity, and base deficit.
A. Unadjusted univariate linear regression of D-dimer. Coef, coefficient; CI, confidence interval; p, p-value; R2 coefficient of determination; GCS, Glasgow Coma Scale; BD base deficit. INR, International Normalized Ratio; PTT, partial thromboplastin time; Coagulopathic Phenotypes: INR-TF, INR≥1.4 and PTT<35; PTT-CONTACT, INR<1.4 and PTT≥35; BOTH, INR≥1.4 and PTT≥35. Variables with * designation were measured at admission. B. Multiple linear regression of D-dimer on coagulopathic phenotype adjusted for multiple phenotypes, male, blunt mechanism, injury severity, admission GSC, and base deficit.
A. Unadjusted univariate linear regression of antithrombin III. Coef, coefficient; CI, confidence interval; p, p-value; R2 coefficient of determination; GCS, Glasgow Coma Scale; BD base deficit. INR, International Normalized Ratio; PTT, partial thromboplastin time; Coagulopathic Phenotypes: INR-TF, INR≥1.4 and PTT<35; PTT-CONTACT, INR<1.4 and PTT≥35; BOTH, INR≥1.4 and PTT≥35. Variables with * designation were measured at admission. B. Multiple linear regression of antithrombin III on coagulopathic phenotype adjusted for multiple phenotypes, blunt mechanism, injury severity, admission GSC, and base deficit.
A. Unadjusted univariate linear regression of Protein C. Coef, coefficient; CI, confidence interval; p, p-value; R2 coefficient of determination; GCS, Glasgow Coma Scale; BD base deficit. INR, International Normalized Ratio; PTT, partial thromboplastin time; Coagulopathic Phenotypes: INR-TF, INR≥1.4 and PTT<35; PTT-CONTACT, INR<1.4 and PTT≥35; BOTH, INR≥1.4 and PTT≥35. Variables with * designation were measured at admission. B. Multiple linear regression of Protein C on coagulopathic phenotype adjusted for multiple phenotypes, injury severity, admission GCS, and base deficit.
A. Unadjusted univariate linear regression of packed red blood cells (PRBC) transfused (I.)24 hours and (II.) 72 hours after injury. Coef, coefficient; CI, confidence interval; p, p-value; R2 coefficient of determination; GCS, Glasgow Coma Scale; BD base deficit. INR, International Normalized Ratio; PTT, partial thromboplastin time; Coagulopathic Phenotypes: INR-TF, INR≥1.4 and PTT<35; PTT-CONTACT, INR<1.4 and PTT≥35; BOTH, INR≥1.4 and PTT≥35. Variables with * designation were measured at admission. B. Multiple linear regression of PRBC transfused at (I.)24 hours and (II.) 72 hours after injury on coagulopathic phenotype adjusted for multiple phenotypes, injury severity, GCS, and base deficit.
A. Unadjusted univariate linear regression of fresh frozen plasma (FFP) transfused (I.)24 hours and (II.) 72 hours after injury. Coef, coefficient; CI, confidence interval; p, p-value; R2 coefficient of determination; GCS, Glasgow Coma Scale; BD base deficit. INR, International Normalized Ratio; PTT, partial thromboplastin time; Coagulopathic Phenotypes: INR-TF, INR≥1.4 and PTT<35; PTT-CONTACT, INR<1.4 and PTT≥35; BOTH, INR≥1.4 and PTT≥35. Variables with * designation were measured at admission. B. Multiple linear regression of FFP transfused at (I.)24 hours and (II.) 72 hours after injury on coagulopathic phenotype adjusted for multiple phenotypes, injury severity, GCS, and base deficit.


