Abstract
Background
Deeper short-interval intracortical inhibition (SICI), a marker of GABAA activity, correlates with better motor performance in patients with moderate to severe hand impairments in the chronic phase after stroke.
Objectives
We evaluated the correlation between SICI in the affected hemisphere and pinch force of the paretic hand in well-recovered patients. We also investigated the correlation between SICI and pinch force in controls.
Methods
Twenty-two subjects were included in the study. SICI was measured with a paired-pulse paradigm. The correlation between lateral pinch strength and SICI was assessed with Spearman's rho.
Results
There was a significant correlation (rho = 0.69, p = 0.014) between SICI and pinch strength in patients, but not in controls. SICI was significantly deeper in patients with greater hand weakness.
Conclusions
These preliminary findings suggest that decreased GABAA activity in M1AH correlates with better hand motor performance in well-recovered subjects with stroke in the chronic phase.
Short-interval intracortical inhibition (SICI) to transcranial magnetic stimulation (TMS) reflects activation of inhibitory, GABAAergic cortical neurons in the primary motor cortex [1]. A meta-analysis concluded that SICI is decreased in the primary motor cortex of the affected hemisphere (M1AH) early, but not in the chronic phase after stroke [2]. In this phase, in patients with moderate to severe upper limb impairments, deeper SICI correlates with better motor performance [3,4].
The aim of this study was to evaluate the relation between SICI in M1AH and pinch force of the paretic hand in subjects with stroke and excellent motor recovery. We also compared results of SICI in subjects with stroke and in controls. We expected to find distinct relations between SICI and force in the two groups, in the absence of significant differences in SICI between them.
Methods
Twelve patients (6 men) in the chronic phase after a single hemispheric ischemic stroke, and ten age-matched control subjects were included in the study. Inclusion criteria (stroke group): age, 18–99 years; hemiparesis after ischemic stroke > 6 months in a cerebral hemisphere as documented by neuroimaging; good motor recovery, defined as the ability to perform tasks included in the Jebsen-Taylor test; this test measures the time to complete tasks that demand dexterity [5]. Exclusion criteria: previous symptomatic strokes; uncontrolled medical problems; psychiatric illnesses; neglect; inability to provide informed consent due to severe aphasia or cognitive impairment; relative or absolute contraindications to TMS [6]; use of medications that could influence cortical excitability.
For control subjects, inclusion criteria were: age, sex and handedness comparable to those of patients. Exclusion criteria: history of neurologic or psychiatric disorders; uncontrolled medical problems; relative or absolute contraindications to TMS, simultaneous participation in other research protocols.
The protocol was approved by the local ethics committee, and all patients and controls provided informed consent to participate.
Thumb lateral pinch force was measured according to a standardized protocol [7] in the paretic hand of subjects with stroke, and in the homologous hand in controls.
TMS was delivered to M1AH in patients through a figure-of-8-shaped magnetic coil connected to two magnetic stimulators via a Bi-Stim 2002 module (Magstim, UK) [8]. Motor evoked potentials (MEPs) were recorded by surface electrodes placed on the thenar eminence on the abductor pollicis brevis (APB) of the paretic hand in patients. After identification of the APB hot spot, resting motor threshold (rMT) and short-interval intracortical inhibition (SICI) were measured as previously described [8]. For SICI measurements, two magnetic pulses were applied: first, a conditioning stimulus (CS; intensity set to 80% of the APB rMT). Second, a test stimulus (TS; intensity required to evoke MEPs of approximately 0.5 to 1 mV (MEPTS). The order of presentation of inhibitory (interval between CS and TS, 2 ms; 16 trials) and control trials (TS; 16 trials) was randomized. The right hemisphere in healthy subjects was used as a control for the right hemisphere of patients and the same procedure was performed for the left hemisphere.
Between-group comparisons were made with unpaired t tests or Mann-Whitney tests according to distribution of the data. Correlations between behavioral and TMS measures were evaluated with Spearman’s rho. P-values ≤ 0.05 were considered statistically significant.
Results
There were no significant differences between age, sex or handedness between subjects with stroke and controls (p>0.05). JTT performance was significantly worse in the stroke group (64.9 ± 24.0 seconds) than in controls (34.5 ± 5.3 seconds; p<0.001). Average (±standard deviation) time from stroke was 3.1±1.2 years. Eight patients had corticosubcortical strokes and four, exclusively subcortical lesions. The stroke lesion involved M1 in two patients.
Pinch force was non-significantly worse in patients (55.4 ± 16.0 Newtons) than in controls (67.6 ± 14.0 Newtons; p=0.08). There were no significant differences between rMT (controls, 58.6 ±10.4%; patients, 54.3±13.1% p=0.29) or SICI (controls, 62.3 ±71.7%; patients, 84.2±104.2%; p=0.18) between the two groups.
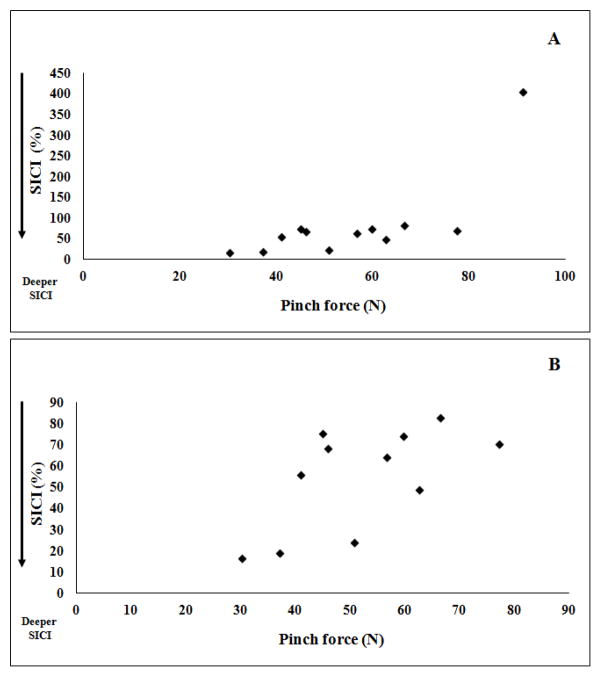
Figure 1A shows that SICI in M1AH was significantly deeper in patients with greater hand weakness (rho=0.69; p=0.014). In the strongest patient, there was facilitation instead of inhibition (Figure 1A). After removal of this outlier the correlation became weaker (rho=0.591; p=0.056), but the direction of the effect persisted (Figure 1B). In controls, no significant correlation was found (rho=0.47; p=0.16).
Figure 1.
Correlation between short-interval intracortical inhibition (SICI) and pinch force (in Newtons) in patients, with (A) or without (B) inclusion of results from the strongest patient (outlier). In this patient, the stroke lesion involved M1 and there was facilitation instead of inhibition.
Discussion
For the first time, we report a significant correlation between SICI in M1AH and pinch force in subjects with excellent motor recovery in the chronic phase after stroke. In line with results of a meta-analysis [2], there were no significant differences in SICI between patients with stroke and controls. Comparable rMTs between the two groups were expected, considering that patients had very mild motor impairments [9].
In controls, the absence of a significant correlation between pinch strength and SICI may be explained by a ceiling effect. In patients, SICI in M1AH was deeper in subjects with stroke and lower levels of pinch strength. This result contrasts with those reported in subjects with moderate to severe hand motor impairment in the chronic phase. In these subjects, increased SICI in M1AH correlates with better Motricity Index and Wolf Motor Function Test-Functional Ability scores [3] as well as finger motor function [4]. Increased SICI in M1AH also correlates with decreased spasticity in patients with poor recovery [3] and may be beneficial to focus neural activity when reorganization leads to patterns that differ substantially from normal. On the other hand, decreased inhibition may favor recruitment of cortical neurons and enhance motor performance in well-recovered patients.
Conclusion
These preliminary findings indicate that decreased GABAA activity in M1AH correlates with better recovery of distal hand muscle strength in well-recovered subjects with stroke. It is possible that effects of up- or down-regulation of GABAA activity may lead to different outcomes, according to severity of motor impairments.
Further studies should compare the functional relevance of SICI across subjects with different levels of motor recovery. A barrier to perform such studies is that MEPs may not be obtained, or their amplitudes may be very small in patients with moderate to severe hand motor impairments. Small test MEPs are more readily suppressed than large MEPs [10]. Test MEPs must then be matched in order to allow appropriate comparisons in SICI as well as in the relation between SICI and motor performance, between groups of patients with different levels of motor impairments.
Highlights.
Deeper SICI correlated with lower pinch force in well-recovered subjects with stroke.
SICI was comparable in well-recovered subjects with stroke and in controls.
Decreased GABAA activity in M1 may be beneficial to generation of force in stroke.
Acknowledgments
Funding
The work was supported by the National Institutes of Health (1R21 TW006706) and the Brazilian National Council for Scientific and Technological Development (CNPq 477916/2006-6); KNF received a scholarship from CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior). The funding agencies had no roles in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Footnotes
Declaration of interest
Conflicts of interest: none.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Talelli P, Greenwood RJ, Rothwell JC. Arm function after stroke: neurophysiological correlates and recovery mechanisms assessed by transcranial magnetic stimulation. Clin Neurophysiol. 2006;117:1641–59. doi: 10.1016/j.clinph.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 2.McDonnell MN, Stinear CM. TMS measures of motor cortex function after stroke: A meta-analysis. Brain Stimul. 2017;10:721–734. doi: 10.1016/j.brs.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 3.Marconi B, Filippi GM, Koch G, Giacobbe V, Pecchioli C, Versace V, et al. Long-term effects on cortical excitability and motor recovery induced by repeated muscle vibration in chronic stroke patients. Neurorehabil Neural Repair. 2011;25:48–60. doi: 10.1177/1545968310376757. [DOI] [PubMed] [Google Scholar]
- 4.Honaga K, Fujiwara T, Tsuji T, Hase K, Ushiba J, Liu M. State of intracortical inhibitory interneuron activity in patients with chronic stroke. Clin Neurophysiol. 2013;124:364–70. doi: 10.1016/j.clinph.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Ferreiro KN, Santos RL, Conforto AB. Psychometric properties of the portuguese version of the Jebsen-Taylor test for adults with mild hemiparesis. Rev Bras Fisioter. 2010;14:377–82. doi: 10.1590/s1413-35552010005000018. [DOI] [PubMed] [Google Scholar]
- 6.Rossi S, Hallett M, Rossini PM, Pascual-Leone A Group SoTC. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120:2008–39. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mathiowetz V, Kashman N, Volland G, Weber K, Dowe M, Rogers S. Grip and pinch strength: normative data for adults. Arch Phys Med Rehabil. 1985;66:69–74. [PubMed] [Google Scholar]
- 8.Mello EA, Cohen LG, Monteiro Dos Anjos S, Conti J, Andrade KN, Tovar Moll F, et al. Increase in short-interval intracortical facilitation of the motor cortex after low-frequency repetitive magnetic stimulation of the unaffected hemisphere in the subacute phase after stroke. Neural Plast. 2015;2015:407320. doi: 10.1155/2015/407320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pennisi G, Alagona G, Rapisarda G, Nicoletti F, Costanzo E, Ferri R, et al. Transcranial magnetic stimulation after pure motor stroke. Clin Neurophysiol. 2002;113:1536–1543. doi: 10.1016/s1388-2457(02)00255-9. [DOI] [PubMed] [Google Scholar]
- 10.Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, et al. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]