Abstract
Background
Theories of executive control propose that communication between medial frontal cortex (MFC) and lateral prefrontal cortex (lPFC) is critical for learning. 6-Hz phase synchronization may be the mechanism by which neural activity between MFC and lPFC is coordinated into a functional network. Recent evidence suggests that switching from eyes closed to open may induce a change in brain-state reflected by enhanced executive control and related functional connectivity.
Objective/Hypothesis
To examine whether causal manipulation of MFC and lPFC can improve learning according to the brain-state induced by switching from eyes closed to open.
Methods
Within-subjects, sham-controlled, double-blind study of 30 healthy subjects, each receiving 6-Hz in-phase high definition transcranial alternating-current stimulation (HD-tACS) applied to MFC and right lPFC prior to performing a time estimation task.
Results
HD-tACS with eyes open improved learning ability relative to sham, whereas HD-tACS with eyes closed had no significant effect on behavior.
Conclusion
Results suggest a phase-sensitive mechanism in frontal cortex mediates components of learning performance in a state-dependent manner.
Keywords: transcranial alternating-current stimulation, learning, medial frontal cortex, lateral prefrontal cortex
1. Introduction
Executive mechanisms determine how well an individual can react to feedback from the environment and learn. Theories in neuroscience propose that communication between medial frontal cortex (MFC) and lateral prefrontal cortex (lPFC) is vital for learning [1–6]. Theta (~6 Hz) phase synchronization may provide an effective means by which information is coordinated across spatially disparate brain regions, such as MFC and lPFC, supporting neural communication and plasticity [7–11]. Recent evidence suggests that functional connectivity underlying executive processing may be altered by the brain-state of an individual [12]. Specifically, changing from eyes closed to open may induce an alteration in brain-state reflected by enhanced executive control and related functional connectivity to prepare an individual for event-related processing once visual input is present. Here, we target theta mechanisms of frontal cortex with 6-Hz alternating current and determine whether changes in brain-state can modulate the effectiveness of causally manipulating human learning with noninvasive electrical stimulation.
2. Methods
2.1. Subjects and Procedures
Thirty-two neurologically normal subjects (16 female, mean age 24) consented to procedures approved by the Boston University Institutional Review Board and were paid. Two subjects voluntarily withdrew before completing the experiment, leaving 30 subjects whose data were analyzed.
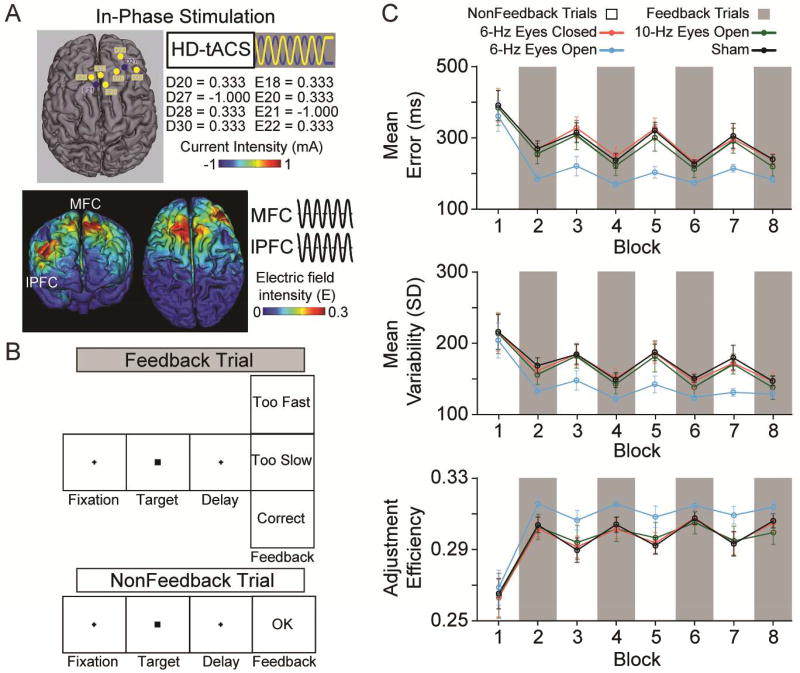
All subjects received active stimulation sessions with eyes open and eyes closed, and a sham session across three different days, separated by at least 48 hours to avoid ordering confounds related to repeated brain stimulation [13]. High definition transcranial alternating-current stimulation (HD-tACS; MxN 9-channel HD-tES, Soterix, New York, NY) was applied simultaneously over MFC and right lPFC with a 0° relative phase difference for 20 minutes using a 6-Hz bipolar sinusoidal alternating current with 1-mA intensity, peak to peak (Fig 1A). We have recently shown the effectiveness of this novel HD-tACS montage in manipulating adaptive behavior [14], however, it is unknown what role, if any, brain-state may play in modulating the stimulation-induced effects on learning.
Fig. 1. Methods, Design, and Results.
A, The right lateralized 8-channel in-phase high definition transcranial alternating-current stimulation (HD-tACS) montage and current-flow model shown on 3D reconstructions of the cortical surface. The yellow (positive) and blue (negative) circles in the montage reflect the polarity of electrical current applied over medial frontal cortex (MFC) and right lateral prefrontal cortex (lPFC) target regions. B, The sequence of events on feedback and nonfeedback trials in the time estimation task. C, Performance measures of absolute error magnitude, response variability, and adjustment efficiency across blocks of feedback (gray) and nonfeedback (white) trials after the 6-Hz eyes closed (red), 6-Hz eyes open (blue), and 10-Hz eyes open (green) active conditions, and the sham (black) condition in the same subjects. Error bars show ± 1 standard error of the mean.
Various controls were instituted consistent with the authors’ previous work [15–21]. All subjects received sham stimulation lasting 30 seconds, ramping up and down at the beginning and end of the 20-minute period to simulate the tingling sensation often experienced by subjects during active stimulation. Second, a double-blind method was used where a second experimenter set the mode (e.g., active or sham) on the stimulator, and otherwise did not interact with the subject or experimenter who performed data collection. Third, the order of the sham and active tACS was counterbalanced across subjects. The absence of order effects was confirmed using ANOVAs on the behavioral measures (described below) using the between-subjects factor of stimulation order. No significant effects (Fs(5, 20) < 0.770, ps > 0.468) or interactions (2-way, Fs(10, 40) < 0.794, ps > 0.486; 3-way, Fs(30, 120) < 0.960, ps > 0.439) of stimulation order reached significance.
Immediately after stimulation, subjects performed a modified time estimation task (Fig. 1B) [22, 23], in which they had to learn to estimate a 1.7 second lapse. Each trial began with central fixation (0.4° × 0.4°, <0.01 cd/m2, 300–900 ms), followed by a central cue (square subtending 1° × 1°, 10 cd/m2) indicating a button press was required with the right thumb after 1.7 seconds. Visual feedback (1000 ms), presented 600 ms after response informed subjects whether they were “too fast,” “too slow,” or “correct.” Initially, a correct response was one ± 200 ms around target time (1500 to 1900 ms). However, after a correct response this time window shrunk by ± 20 ms, or increased by ± 20 ms if the response was incorrect, which ensured a similar number of trials across feedback conditions.
To examine learning, we included blocks of trials without valid feedback, in which the word “OK” was presented for 1000 ms. The task contained blocks of 80 trials with valid feedback and blocks of 20 trials without valid feedback. Performance on nonfeedback trials allowed us to examine the maintenance of the internal representation of the time interval learned during the preceding valid feedback trials.
2.2. Data Analysis and Statistics
Error magnitude was measured as the mean of the absolute difference between the subjects’ estimations and the target time interval. Response variability was measured as the standard deviation of the error (i.e., the difference between subjects’ estimation and the target) in each block. Both metrics are critical indices of learning performance [24]. Further, we used adjustment efficiency to examine how efficiently the subjects adjusted their estimations based on feedback. Adjustment efficiency was calculated using the following equation:
where e is the absolute error in the current (i) or preceding (i − 1) trials. Adjustment efficiency provides information on how well the adjustments were made, on average, across stimulation conditions during the feedback and nonfeedback blocks, and was computed for each trial and averaged for each block.
We used separate repeated measures ANOVAs with within-subjects factors of feedback (valid vs. invalid), time (block 1 vs. block 2 vs. block 3 vs. block 4), and stimulation (eyes open vs. eyes closed vs. sham) for each dependent measure (i.e., error magnitude, response variability, and adjustment efficiency). We adjusted p-values using the Greenhouse-Geisser epsilon correction for nonsphericity when the sphericity assumption was violated [25].
3. Results
The brain-state induced by opening versus closing eyes determined whether learning was modulated by the multi-focal HD-tACS to frontal cortex. This was supported by the critical stimulation × feedback × time interactions on error magnitude (F(6, 174) = 3.208, P = 0.017), response variability (F(6, 174) = 2.780, P = 0.030), and adjustment efficiency (F(6, 174) = 3.040, P = 0.020). Parsing these interactions revealed that the eyes-open condition was driving the effects (error magnitude, F(3, 87) = 4.157, P = 0.011; response variability, F(3, 87) = 3.229, P = 0.032; adjustment efficiency, F(3, 87) = 3.059, P = 0.038), whereas when the same subjects received stimulation with eyes closed no changes in learning were observed relative to sham (error magnitude, F(3, 87) = 0.072, P = 0.922; response variability, F(3, 87) = 0.088, P = 0.926; adjustment efficiency, F(3, 87) = 0.393, P = 0.707).
To assess the frequency specificity of the effects, we invited back all subjects to participate in an additional eyes-open condition in which MFC-right-lPFC was targeted with 10-Hz alternating current. Of the twenty-two subjects who returned, we found no significant impact of 10-Hz stimulation on error magnitude (F(3, 63) = 0.150, P = 0.843), response variability (F(3, 63) = 0.164, P = 0.857), or adjustment efficiency (F(3, 63) = 0.545, P = 0.606), suggesting that the functional connectivity in frontal cortex underlying learning and adaptive behavior may be established along particular frequency channels of neural communication, such as those in the theta range (4 – 8 Hz), but not others in the alpha range (9 – 13 Hz).
4. Discussion
We propose that stimulation with eyes open induced behavioral improvements by preferentially synchronizing active neuronal networks in frontal cortex in an activity-selective fashion. That is, the stimulation may have capitalized on the active theta synchronization underlying the heightened executive processes induced by the eyes-open condition, leading to a facilitation of neuroplastic changes in theta functional connectivity between MFC and lPFC important for flexible behavior. The results are consistent with theories of executive function [1–6] and oscillatory neural communication [7–11], highlight the importance of subject-defined parameters such as brain-state in determining the behavioral impact of neuromodulation protocols targeting frontal cortex, and contribute to the advancement of tACS as a potential clinical tool for improving cognition in patient populations.
Highlights.
Eyes open 6-Hz tACS targeting MFC and right lPFC improves learning
Eyes closed 6-Hz tACS to the same regions does not modulate learning
Brain-state determines the behavioral effectiveness of tACS to frontal cortex
Acknowledgments
This work was supported by a grant from the National Institutes of Health (R01-MH-114877).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
None.
References
- 1.Brown JW, Braver TS. Learned predictions of error likelihood in the anterior cingulate cortex. Science. 2005;307:1118–21. doi: 10.1126/science.1105783. [DOI] [PubMed] [Google Scholar]
- 2.Shenhav A, Cohen JD, Botvinick MM. Dorsal anterior cingulate cortex and the value of control. Nat Neurosci. 2016;19:1286–91. doi: 10.1038/nn.4384. [DOI] [PubMed] [Google Scholar]
- 3.Shenhav A, Botvinick MM, Cohen JD. The expected value of control: An integrative theory of anterior cingulate cortex function. Neuron. 2013;79:217–40. doi: 10.1016/j.neuron.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller EK, Buschman TJ. Cortical circuits for the control of attention. Cur Opin Neurobiol. 2013;23:216–22. doi: 10.1016/j.conb.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–7. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- 6.Sheth SA, Mian MK, Patel SR, Asaad WF, Williams ZM, Dougherty DD, et al. Human dorsal anterior cingulate cortex neurons mediate ongoing behavioural adaptation. Nature. 2012;488:218–21. doi: 10.1038/nature11239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siegel M, Donner TH, Engel AK. Spectral fingerprints of large-scale neuronal interactions. Nat Rev Neurosci. 2012;13:121–34. doi: 10.1038/nrn3137. [DOI] [PubMed] [Google Scholar]
- 8.Engel AK, Fries P, Singer W. Dynamic predictions: oscillations and synchrony in top-down processing. Nat Rev Neurosci. 2001;2:704–16. doi: 10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- 9.Varela F, Lachaux JP, Rodriguez E, Martinerie J. The brainweb: phase synchronization and large-scale integration. Nat Rev Neurosci. 2001;2:229–39. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- 10.Salinas E, Sejnowski T. Correlated neuronal activity and the flow of neural information. Nat Rev Neurosci. 2001;2:539–50. doi: 10.1038/35086012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fell J, Axmacher N. The role of phase synchronization in memory processes. Nat Rev Neurosci. 2011;12(2):105–18. doi: 10.1038/nrn2979. [DOI] [PubMed] [Google Scholar]
- 12.Riedl V, Utz L, Castrillón G, Grimmer T, Rauschecker JP, Ploner M, et al. Metabolic connectivity mapping reveals effective connectivity in the resting human brain. Proc Natl Acad Sci USA. 2016;113(2):428–33. doi: 10.1073/pnas.1513752113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monte-Silva K, Kuo MF, Hessenthaler S, Fresnoza S, Liebetanz D, Paulus W, et al. Induction of late LTP-like plasticity in the human motor cortex by repeated non-invasive brain stimulation. Brain Stim. 2013;6(3):424–32. doi: 10.1016/j.brs.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 14.Reinhart RMG. Disruption and rescue of interareal theta phase coupling and adaptive behavior. Proc Natl Acad Sci USA. 2017;114(43):11542–7. doi: 10.1073/pnas.1710257114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reinhart RMG, Zhu J, Park S, Woodman GF. Synchronizing theta oscillations with direct-current stimulation strengthens adaptive control in the human brain. Proc Natl Acad Sci USA. 2015;112(30):9448–53. doi: 10.1073/pnas.1504196112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reinhart RMG, Zhu J, Park S, Woodman GF. Medial-frontal stimulation enhances learning in schizophrenia by restoring prediction-error signaling. J Neurosci. 2015;35(35):12232–40. doi: 10.1523/JNEUROSCI.1717-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reinhart RMG, Xiao W, McClenahan L, Woodman GF. Electrical stimulation of visual cortex can immediately improve spatial vision. Curr Biol. 2016;25(14):1867–72. doi: 10.1016/j.cub.2016.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reinhart RMG, Woodman GF. Enhancing long-term memory with stimulation tunes visual attention in one trial. Proc Natl Acad Sci USA. 2015;112(2):625–30. doi: 10.1073/pnas.1417259112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reinhart RMG, Woodman GF. Causal control of medial-frontal cortex governs electrophysiological and behavioral indices of performance monitoring and learning. J Neurosci. 2014;34(12):4214–27. doi: 10.1523/JNEUROSCI.5421-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reinhart RMG, Woodman GF. The surprising temporal specificity following direct-current stimulation. Trends Neurosci. 2015;38(8):459–61. doi: 10.1016/j.tins.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reinhart RMG, Cosman JD, Keisuke F, Woodman GF. Using transcranial direct-current stimulation (tDCS) to understand cognitive processing. Atten Percept Psychophys. 2017;79(1):3–23. doi: 10.3758/s13414-016-1224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miltner WHR, Braun CH, Coles MGH. Event-related brain potentials following incorrect feedback in a time-estimation task: evidence for a "generic" neural system for error detection. J Cog Neurosci. 1997;9:788–98. doi: 10.1162/jocn.1997.9.6.788. [DOI] [PubMed] [Google Scholar]
- 23.Luft CDB, Nolte G, Bhattacharya J. High-learners present larger mid-frontal theta power and connectivity in response to incorrect performance feedback. J Neurosci. 2013;33(5):2029–38. doi: 10.1523/JNEUROSCI.2565-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolpert DM, Diedrichsen J, Flanagan JR. Principles of sensorimotor learning. Nat Rev Neurosci. 2011;12:739–51. doi: 10.1038/nrn3112. [DOI] [PubMed] [Google Scholar]
- 25.Jennings JR, Wood CC. The e-adjustment procedure for repeated-measures analyses of variance. Psychophysiol. 1976;13:277–8. doi: 10.1111/j.1469-8986.1976.tb00116.x. [DOI] [PubMed] [Google Scholar]