Abstract
Background
Airway bleeding, either spontaneous or as a result of bronchoscopy, is associated with significant morbidity and mortality. Multiple bronchoscopic techniques are available to achieve complete hemostasis or as a bridge to definitive therapies.
Methods
We report our experience on the feasibility of endobronchial instillation of an absorbable gelatin and thrombin slurry (GTS) for the treatment of spontaneous hemoptysis and procedure-related bleeding.
Results
We identified thirteen cases in which GTS was used for endobronchial hemostasis when standard bronchoscopic measures like cold saline, epinephrine and in some cases balloon occlusion were not successful. GTS was delivered through the working channel of the bronchoscope in ten cases and through the distal port of a bronchial blocker in the remaining three. Median age was 69 years (range 52 to 79 years). Eight cases corresponded to spontaneous hemoptysis and five cases to diagnostic or therapeutic procedures. Bleeding was considered severe in nine (70%) cases. All but one case were associated with malignancy. Hemostasis was achieved in ten (77%) cases by using standard measures in addition to GTS. No patient adverse events at 30 days or damage to the equipment were identified.
Conclusion
Bronchoscopic instillation of an absorbable gelatin and thrombin slurry is feasible and may be used in cases of spontaneous or procedure-related bleeding in addition to conventional measures. It can be delivered through the working channel of the bronchoscope or through the distal port available in some bronchial blockers. Controlled studies are necessary to determine the safety and efficacy of this novel technique.
Keywords: Airway Obstruction, Bronchoscopy, Hemoptysis, Lung Cancer, Bleeding, Gelfoam, Absorbable Gelatin, Thrombin
INTRODUCTION
Hemoptysis is defined as the expectoration of blood and is classified into mild, moderate and severe based on the amount and rate of blood loss. Clinical parameters, such as need for blood product transfusion, and hemodynamic or respiratory compromise, should also be included in the overall assessment of severity, particularly since no consensus regarding cutoff values is available.1 Severe bleeding occurs in 5–15% of all cases and constitutes a medical emergency as even small volumes of blood inside the airways can lead to rapid asphyxiation. Bleeding can be idiopathic, drug-induced, related to trauma, coagulopathy and primary or secondary lung diseases; it may also present as a complication of diagnostic and interventional bronchoscopy.2
If severe hemoptysis is present, the goal should be to maintain adequate oxygenation and ventilation while attempts are made to control the bleeding. The first step is to secure the airway; this is accomplished by using the largest possible diameter endotracheal tube available or a rigid bronchoscope. Larger diameter endotracheal tubes are preferred over double lumen tubes as they provide superior access for diagnostic and therapeutic interventions. 3 If the site of bleeding is known, the bed should be tilted with the site of bleeding down (closer to the floor) to prevent spillage of blood into the non-bleeding lung.
Bronchoscopy remains a cornerstone in the management of airway bleeding, it provides diagnostic information, allows for minimally-invasive treatments to occur and guides interventional radiologists and thoracic surgeons when planning for urgent and emergent procedures if these are indicated. Mild and moderate cases of hemoptysis may be managed with flexible bronchoscopy; rigid bronchoscopy is the preferred modality in severe cases. The latter should be performed by a skilled physician and only after careful evaluation of individual cases, particularly in the setting of hemodynamic or respiratory compromise. Rigid bronchoscopy has the advantage of securing the airway and providing an access for ventilation while simultaneously allowing the use of large suction catheters and therapeutic tools. Flexible and rigid bronchoscopes are often used in combination.1,4 In certain cases the severity of bleeding may preclude the use of bronchoscopy completely; immediate consideration for angio-embolization and surgical consultation is indicated in such circumstances.
Bleeding as a complication of flexible bronchoscopy is uncommon, minor and severe bleeding occur in 0.19% and 0.26% of bronchoscopies respectively. 5 The risk however, increases significantly when performing endobronchial and transbronchial lung biopsies and depending on the baseline characteristics of the patient, bleeding may be seen in 1.9 – 9% of cases.6,7 With the growth of the field of interventional pulmonology, procedure-related bleeding is a complication that the advanced bronchoscopist should be ready to address.5 Bleeding encountered during therapeutic bronchoscopy for malignant airway obstruction which requires interventions is uncommon, presenting in only 0.5% of cases. 8 Procedure-related bleeding can be classified as mild, moderate or severe based on the interventions required to control it. This approach may be more suitable than the traditional quantification of bleeding since aspiration of saline solution, epinephrine and secretions makes volume assessment inaccurate, and in the case of severe or rapid bleeding, volume assessment is trumped by the bronchoscopist’s assessment of the bleeding.
DESIGN
Objective
To report the feasibility of endobronchial instillation of an absorbable gelatin and thrombin (GTS) slurry in cases of spontaneous hemoptysis and procedure-related bleeding.
Methods
After obtaining IRB/PB (Institutional Review Board / Privacy Board) approval (approval number WA0109-15), we screened the institutional operating and endoscopy room database at Memorial Sloan Kettering Cancer Center from January 2010 to April 2017 for bronchoscopic procedures performed by the Pulmonary or Thoracic Surgery services and using the keyword “Gelfoam plus”. Patient consent was waived by our internal IRB based on the retrospective nature of the study.
Bronchoscopy reports, OR and anesthesia records, and progress notes were reviewed for pertinent information. We classified airway bleeding as mild (<20ml/24h), moderate (20–100ml/24h) and severe (>100ml/24h; bleeding associated with hemodynamic compromise or respiratory failure; bleeding identified during bronchoscopy which persists despite standard bronchoscopic measures including bronchoscopic tamponade, instillation of epinephrine and cold saline and/or requires placement of balloon occlusion devices; and at the discretion of the bronchoscopist based on the rate of bleeding and assessment of individual cases). We collected hemostasis rates. Failure to achieve hemostasis was determined when persistent bleeding was identified bronchoscopically despite all measures; post-bronchoscopic balloon occlusion with an inflated balloon was required; or when immediate angio-embolization or surgery was required. We followed patients for up to 30 days for adverse events attributed to GTS instillation, recurrence of bleeding (defined as spontaneous hemoptysis or fresh blood visible in the endotracheal tube) and cause of death.
Technique description
We consider using GTS in cases of spontaneous hemoptysis or procedure-related bleeding which do not subside after standard bronchoscopic measures (SBM). We also consider it in cases in which the bleeding is severe enough that SBM may not be feasible or effective due to profuse bleeding. GTS may be used concomitantly with balloon occlusion based on the severity or persistence of the bleeding at the discretion of the bronchoscopist
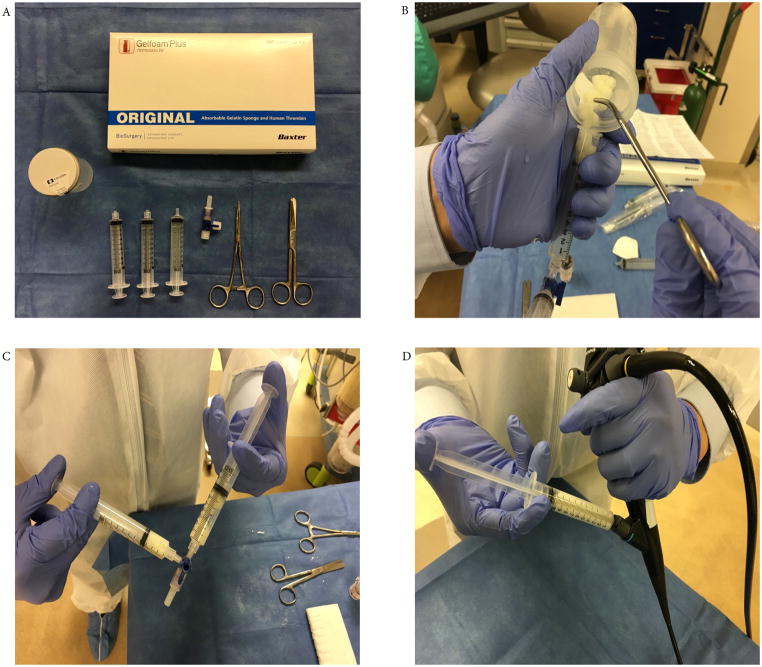
We prepare the slurry using the components available in the GELFOAM® PLUS kit (Baxter Healthcare Corporation product 1501341, listing price for one kit $189.00). This includes absorbable gelatin, lyophilized thrombin powder, two prefilled 10 ml saline solution syringes and a vial access device. (Figure 1A) Package is stored in the operating room supply area and in the bronchoscopy suite under manufacturer recommendations (temperature 15–25°C, 59–77°F); no refrigeration is required. Slurry is formed during bronchoscopy immediately before its administration. Time needed to reconstitute components is approximately one to two minutes. No particular difficulties are encountered during process and authors believe that proficiency can be attained after 2–3 procedures.
Figure 1.
We first cut absorbable gelatin into small pieces (5mm × 5mm) and place them in a specimen cup. We then reconstitute thrombin powder using one of the prefilled 10 ml saline solution syringes and combine it with the gelatin in the specimen cup. Mixture is stirred until gelatin in soaked with thrombin and is then transferred into a 10cc syringe. Finally, we connect this syringe to an empty 10cc syringe via a three-way stopcock. The mixture is then agitated repeatedly between syringes until a slurry is formed. (Figure 1B and 1C)
GT is instilled through the working channel of the bronchoscope (Figure 1D), either directly onto the bleeding surface or into the distal airways, and followed by epinephrine (1:10,000 – 1:50,000) solution to assure complete delivery of the mixture. Saline can also be used for this however, we advocate using epinephrine due to the potential additional hemostatic effect. Occlusion with a bronchial blocker is done simultaneously, if deemed necessary. If a bronchial blocker with a distal port is placed, the slurry may also be instilled through this port in similar fashion at the discretion of the bronchoscopist. We maintain regular cleaning and processing protocols for bronchoscopes after using GTS. No additional processing was implemented.
RESULTS
We identified thirteen cases in which Gelfoam Plus kit was used from June 2010 to April 2017 for bronchoscopic hemostasis. One patient had GTS instillation on two separate occasions. The median age was 69 years (range 52 to 79 years); six female and seven male. Spontaneous bleeding was found in eight cases. Of these, one was secondary to bleeding from a surgical stump; others were due to central or distal malignant airway disease. Procedure-related bleeding was encountered in five cases and was related to debulking of obstructing endobronchial tumor, transbronchial lung biopsy or incidentally upon contact with an endobronchial mass. Bleeding was considered severe in nine cases (70%), moderate in two cases (15%) and mild in two cases (15%) based on our predefined classification. No patient was receiving therapeutic anticoagulation at the time of bronchoscopy and no significant coagulopathy was recorded. (Table 1)
Table 1.
Baseline Characteristics
| Case | Gender | Age | Underlying Malignancy | Indication for Bronchoscopy |
Type of Bronchoscopy |
Airway Bleeding |
Anticoagulation | INR / PT (9.4– 13.5 SEC) |
PTT (25– 38 SEC) |
Platelet Count (k/mcL) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 71 | Metastatic Sarcoma | Hemoptysis / Urgent | Flexible | Severe | No | 1.10 / 12.3 | 27.2 | 302 |
| 2 | Female | 77 | Metastatic Pancreatic Cancer | Hemoptysis / Urgent | Flexible | Moderate | No | 1.15 / 12.8 | 27.6 | 143 |
| 3* | Male | 79 | Adenocarcinoma with Unknown Primary | Hemoptysis / Emergent | Rigid/Flexible | Severe | No | 1.23 / 13.7 | 28.5 | 162 |
| 4* | Male | 79 | Adenocarcinoma with Unknown Primary | Hemoptysis / Urgent | Rigid/Flexible | Moderate | No | 1.19 / 13.2 | 30.5 | 130 |
| 5 | Male | 70 | Metastatic Sarcoma | Hemoptysis / Urgent | Rigid/Flexible | Severe | No | 1.17 / 13.1 | 29.3 | 110 |
| 6 | Male | 52 | Lung Adenocarcinoma | Hemoptysis / Urgent | Flexible | Mild | No | 1.07 / 11.9 | 27.9 | 212 |
| 7 | Male | 74 | Squamous cell lung cancer | Hemoptysis/ Urgent | Rigid/Flexible | Severe | No | 1.17 / 13.1 | 37.7 | 149 |
| 8 | Female | 53 | Metastatic Breast Cancer with Lymphangitic Spread | Diffuse infiltrates / Elective | Flexible | Severe | No | 0.97 / 10.8 | 30.4 | 96 |
| 9 | Female | 58 | Acute Myeloid Leukemia | Diffuse infiltrates / Elective | Flexible | Severe | No | 0.97 / 10.8 | 16.6 | 146 |
| 10 | Female | 72 | Metastatic Renal Cell Cancer | MAO / Emergent | Rigid/flexible | Mild | No | 1.4 / 15.6 | 25.3 | 417 |
| 11 | Female | 69 | Metastatic Renal Cell Cancer | MAO / Elective | Rigid/Flexible | Severe | No | 1.04 / 11.6 | 27.6 | 230 |
| 12 | Male | 62 | NSCLC (poorly differentiated) | MAO / Urgent | Rigid/Flexible | Severe | No | 1.16 / 12.8 | 25.2 | 187 |
| 13 | Female | 54 | Combined Small and Large Cell Neuroendocrine Lung Cancer | Hemoptysis/Emergent | Flexible | Severe | No | 1.4 / 15.4 | 32.4 | 224 |
Case 3 and 4 correspond to the same patient in two separate occasions
MAO (Malignant Airway Obstruction)
Three cases had GTS instillation through the distal port of a bronchial blocker. Remaining cases had instillation through the working channel of a flexible bronchoscope. Of these, four had concomitant Fogarty balloon occlusion. Hemostasis was achieved bronchoscopically in ten cases (77%). A bronchial blocker was left in place preemptively in two of these cases but remained deflated. Most cases used additional SBM to achieve hemostasis. Epinephrine was used in eleven cases (84%) and tamponade with an embolectomy balloon (Fogarty® Edwards Lifesciences, 8/14 French model number 62080814F) and/or a bronchial blocker (Arndt Endobronchial Blocker® Cook Medical, 9 French model number A-AEBS-9.0-78-SPH-AS) was used in nine cases (70%). Two cases also used endobronchial oxidized cellulose polymer (Surgicel® Ethicon) placed bronchoscopically, and two cases used more than one Gelfoam Plus kit. Recurrence of bleeding was seen in 50% of cases in which hemostasis was initially achieved, a procedure (therapeutic flexible bronchoscopy) was required in only one of these.
Failure to achieve hemostasis was encountered in three cases (23%), one with spontaneous hemoptysis and two with procedure-related bleeding. All of these cases required post-bronchoscopy bronchial balloon occlusion. Two required referral to interventional radiology for embolization and one had spontaneous resolution without further interventions. No patients required surgery in our study. (Table 2)
Table 2.
Bronchoscopic Findings and Hemostasis
| Case | Cause of Bleeding | Epinephrine | Balloon Occlusion | Bronchial Blocker | Surgicel | Hemostasis Achieved | Recurrence | Further therapy |
|---|---|---|---|---|---|---|---|---|
| 1 | Spontaneous - Malignant Distal Airway | Yes | Yes | No | No | Yes | No | - |
| 2 | Spontaneous - Malignant Distal Airway and Bronchiectasis | Yes | No | No | No | Yes | 2 days | - |
| 3* | Spontaneous - Malignant Distal Airway | Yes | Yes | Yes (deflated) | No | Yes | 24h | Repeat bronchoscopy |
| 4* | Spontaneous - Malignant Distal Airway | Yes | Yes | No | No | Yes | 24h ‡ | - |
| 5 | Spontaneous - Central MAO | Yes | Yes | No | Yes | Yes | 24h | - |
| 6 | Spontaneous - Benign Surgical Stump | No | No | No | No | Yes | No | - |
| 7 | Spontaneous - Malignant Central and Distal Airway | Yes | Yes | Yes (inflated) | Yes | No | - | Embolization at 24h |
| 8 | Procedure Related - TBBx with underlying Malignant Distal Airway | Yes | Yes | Yes (deflated) | No | Yes | 3 days ‡ | - |
| 9 | Procedure Related - TBBx with underlying Benign Parenchymal Disease | Yes | Yes | Yes (inflated) | No | No | - | - |
| 10 | Procedure Related - Debulking of Central MAO | Yes | No | No | No | Yes | No | - |
| 11 | Procedure Related - Upon contact of Central MAO | Yes | Yes | No | No | Yes | No | - |
| 12 | Procedure Related - Debulking of Central MAO and Pseudoaneurysm | No | Yes | Yes (inflated) | No | No | - | Embolization immediately |
| 13 | Spontaneous - Malignant Central and Distal Airway | Yes | No | No | No | Yes | No | - |
MAO (Malignant Airway Obstruction)
TBBx (Transbronchial Lung Biopsy)
died within 30 days
Case 3 and 4 correspond to same patient in two separate occasions
Most cases presented abnormal findings on chest X-ray, such as parenchymal lesions, bleeding, atelectasis and pleural effusions, before bronchoscopy and GTS instillation. No readily discernible changes were noted in the cases where comparative images were available. In the cases where no preceding abnormal radiographic findings were visible, it is difficult to ascertain if changes were due to GTS instillation with subsequent atelectasis, or to other factors such as bleeding or balloon occlusion. Three patients died within 30 days; two in the group that achieved hemostasis and one in the group which did not. Evident recurrent contributory bleeding at the time of death was seen in the latter case; all deaths were related to their underlying malignancy. No complications attributable to the use of GTS were identified; these included post-obstructive pneumonia, respiratory failure attributed to airway obstruction, dislodgement of GTS clot, inability to remove clot if subsequent bronchoscopy was performed. Four cases had repeat bronchoscopy to ascertain hemostasis and/or to deflate a bronchial blocker 24–48h after initial instillation. Three of these had visible clot which was removed successfully and without difficulty, one had no visible clot. No dislodgment into a different segment was appreciated in any case. Finally, no damage to bronchoscope equipment attributable to GTS instillation was encountered.
DISCUSSION
Multiple bronchoscopic techniques can be used to achieve permanent hemostasis or as temporary measures while more definitive treatments are pursued. Iced saline lavage with 500–1000ml has been successfully used in cases of hemoptysis.9 Small volumes of cold saline (10–15ml) and/or topical epinephrine (1:10000 – 1:50000) are also recommended to achieve hemostasis in cases of mild to moderate bleeding complicating transbronchial lung biopsy.7 Attention should be paid as hypertension and life-threatening arrhythmias can occur when using epinephrine bronchoscopically.10 Antidiuretic hormone derivatives were evaluated as alternatives to epinephrine; terlipressin was related to insignificant changes in heart rate and blood pressure while ornipressin was not related to hemodynamic changes.11
In cases of severe bleeding however, topical epinephrine and cold saline are washed off due to profuse bleeding and thus their vasoconstrictive effect is diminished by the inability to reach the intended target. In such instances, balloon-occlusion devices may prove life-saving. These are catheters with a distal inflatable balloon which is selectively placed in the intended airway. The right or left mainstem bronchi and bronchus intermedius are preferred over distal airways due to ease of placement and lower risk of dislodgment. Once placed, they can isolate the bleeding segment, protect the non-bleeding lung and allow further interventions to be planned, all while providing a tamponading effect. Different balloon occlusion devices are available. An embolectomy balloon can be inserted through the working channel of a flexible bronchoscope (8 French catheter requires at least a 2.8mm working channel) or alongside the endotracheal tube if intubation has been performed. This is a temporary measure only as the catheter is not anchored and may dislodge inadvertently. If prolonged occlusion is needed, a bronchial blocker can be placed through an endotracheal tube and secured to it via a special adapter. The balloon may also remain deflated in order to prevent mucosal necrosis, and be inflated if bleeding recurs. A distal port which is available in bronchial blockers can be used for aspiration and instillation of products.1,4,12
Thermal therapies, such as argon plasma coagulation (APC) or lasers are valuable tools to control bleeding. However, their benefit is limited to cases in which the bleeding surface is identifiable and their energy can be focused. In cases of distal or profuse bleeding, their benefit is lessened.
Multiple newer techniques have been used to control bleeding bronchoscopically. These include instillation of tranexamic acid, local delivery of silicone spigots or oxidized regenerated cellulose mesh, and other biologically active agents.13,14,15,16,17 In one study, the combination of thrombin and fibrinogen achieved immediate local control in all patients with severe spontaneous hemoptysis; long-term control was achieved in 70%.18 Instillation of n-butyl cyanoacrylate to form a sealant glue led to local control in all patients presenting with massive hemoptysis.19 None of the patients in these reports however, had bleeding related to therapeutic bronchoscopy and in only one of them the etiology of bleeding was bronchogenic carcinoma.
To our knowledge this is the first report of using GTS bronchoscopically to achieve hemostasis. The decision to proceed with instillation is based on the individual case, clinical judgement, and experience of the bronchoscopist, rather than pre-defined limits of blood loss volume. We consider using it in cases of hemoptysis or procedure-related bleeding which do not subside after SBM; it may be used alone and concomitantly with balloon occlusion. Two cases did not receive epinephrine before GTS. One case was due to severe bleeding due to a pulmonary artery pseudoaneurysm, in this case bleeding was profuse and immediate balloon occlusion was required. Attempting to control such profuse bleeding with standard measures would not have been clinically indicated. The second case corresponded to bleeding originating from a surgical stump with possible fistulization. Thoracic surgeon considered that a more permanent approach was required and therefore GTS was used instead of epinephrine.
Absorbable gelatin is a dried hydrocolloid made from porcine-derived collagen and presented in powder or sponge forms. It can absorb 40 times its weight and expands to 200% its initial dimensions. It becomes liquefied within a week and is completely absorbed in four to six weeks. It is applied alone or in combination with thrombin directly onto the bleeding surfaces to achieve hemostasis. Its use is extensively reported in surgical literature.20,21 Thrombin was approved for topical use in surgery by the US Food and Drug Administration (FDA) in the 1970’s. It was initially generated from bovine source however, due to higher risk of antibody formation it is now pooled from human plasma. Risk of viral infection transmission is still present, although very low. Thrombin is presented as a lyophilized sterile, non-pyrogenic powder which is reconstituted with saline solution.
GTS can be instilled via the working channel of the bronchoscope and directed to the source of bleeding (i.e. segmental bronchus when performing transbronchial biopsy or central airway target for malignant airway obstruction); this can be followed by balloon occlusion if deemed necessary by the bronchoscopist. It can also be delivered via the distal port of a bronchial blocker when one is in place.
We believe the ease of delivery, expansibility and clot formation properties of GTS to be some of the reasons it can help achieve hemostasis. It can be particularly useful in addition to SBM in cases of distal, unidentifiable bleeding originating from subsegmental airways (i.e. spontaneous bleeding from distal malignant airway disease, bronchiectasis, and post transbronchial biopsy) as the delivery can be directed to the culprit area. It may also be beneficial in cases of sudden bleeding due to procedures, particularly in conjunction with balloon occlusion when rapid measures are required. Solution can be prepared while balloon occlusion in underway.
Hemostasis was achieved in ten (77%) of our cases. SBM were used in most cases, in two cases Surgicel was also used bronchoscopically. Hemostasis rates could therefore be related to any specific strategy or to the combination. Further studies comparing SBM and the combination with GTS would be valuable. Despite a high recurrence rate, only one case required further interventions. Overall, hemoptysis due to malignancy is likely to recur and therefore recurrence may not be an adequate outcome to investigate. Need for angio-embolization or surgery and death from asphyxiation may be more adequate parameters to measure in comparative studies.
In three of four cases where bronchoscopy was performed to verify hemostasis and/or deflate a bronchial blocker we identified GTS clot formation in the intended area, this was successfully and easily removed. Whether dislodgment occurred in any of the cases in which a repeat bronchoscopy was not performed remains unknown. We cannot confirm complete dissolution of the mixture in 4–6 weeks as a repeat bronchoscopy was not routinely performed at this interval. Further studies investigating how rapidly GTS is absorbed in the tracheobronchial tree are needed. No immediate or remote complications attributable to this technique were identified.
The main limitations of our study include its retrospective, non-controlled design and small sample size. SBM were used in the majority of our patients and therefore the hemostasis rate seen in our cases could be attributed to their respective effects and not due to GTS alone. Procedures were performed at a referral cancer center by fellowship-trained interventional pulmonologists or thoracic surgeons, all with extensive experience in performing flexible and rigid bronchoscopy and in managing patients with large-volume hemoptysis. Finally, general anesthesia was used in all cases and most were done in an operating room.
During our study, we found that instillation of GTS through the working channel of the bronchoscope or distal port of a bronchial blocker is feasible. Preparation of the slurry is simple and can be performed by an assistant in the bronchoscopy suite or operating room. It can be used in cases of spontaneous hemoptysis and procedure-related bleeding. Larger studies are required before general recommendations can be made.
CONCLUSION
Endobronchial instillation of an absorbable gelatin and thrombin slurry for the management of spontaneous hemoptysis and procedure-related bleeding is feasible. The slurry may be delivered through the working channel of the flexible bronchoscope or through the distal port on a bronchial blocker. Further studies are needed before routine use is recommended.
Abbreviations
- GTS
gelatin thrombin slurry
- SBM
standard bronchoscopic measures
Footnotes
Author Contributions:
ARP, MC and RPL contributed substantially to the study design, data analysis and interpretation, and the writing of the manuscript.
Financial/nonfinancial disclosures and potential conflict of interests:
A. Rolando Peralta, MD: none
Mohit Chawla, MD, FCCP: none
Robert P. Lee, MD: none
Other Contributions: none
Disclosure of Funding:
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA00874
Contributor Information
A. Rolando Peralta, Department of Medicine, Pulmonary Service, Section of Interventional Pulmonology, Memorial Sloan Kettering Cancer Center, New York, NY.
Mohit Chawla, Department of Medicine, Pulmonary Service, Section of Interventional Pulmonology, Memorial Sloan Kettering Cancer Center.
Robert P. Lee, Department of Medicine, Pulmonary Service, Section of Interventional Pulmonology, Memorial Sloan Kettering Cancer Center.
References
- 1.Yendamuri S. Massive Airway Hemorrhage. Thoracic Surgery Clinics. 2015;25(3):255–260. doi: 10.1016/j.thorsurg.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 2.Jean-Baptiste E. Clinical assessment and management of massive hemoptysis. Crit Care Med. 2000;28(5):1642–1647. doi: 10.1097/00003246-200005000-00066. [DOI] [PubMed] [Google Scholar]
- 3.Simoff MJ, Lally B, Slade MG, et al. Symptom management in patients with lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e455S–497S. doi: 10.1378/chest.12-2366. [DOI] [PubMed] [Google Scholar]
- 4.Sakr L, Dutau H. Massive hemoptysis: an update on the role of bronchoscopy in diagnosis and management. Respiration; international review of thoracic diseases. 2010;80(1):38–58. doi: 10.1159/000274492. [DOI] [PubMed] [Google Scholar]
- 5.Facciolongo N, Patelli M, Gasparini S, et al. Incidence of complications in bronchoscopy. Multicentre prospective study of 20,986 bronchoscopies. Monaldi archives for chest disease = Archivio Monaldi per le malattie del torace. 2009;71(1):8–14. doi: 10.4081/monaldi.2009.370. [DOI] [PubMed] [Google Scholar]
- 6.Du Rand IA, Blaikley J, Booton R, et al. Summary of the British Thoracic Society guideline for diagnostic flexible bronchoscopy in adults. Thorax. 2013;68(8):786–787. doi: 10.1136/thoraxjnl-2013-203629. [DOI] [PubMed] [Google Scholar]
- 7.Lee P, Mehta AC, Mathur PN. Management of complications from diagnostic and interventional bronchoscopy. Respirology (Carlton, Vic) 2009;14(7):940–953. doi: 10.1111/j.1440-1843.2009.01617.x. [DOI] [PubMed] [Google Scholar]
- 8.Ost DE, Ernst A, Grosu HB, et al. Complications Following Therapeutic Bronchoscopy for Malignant Central Airway Obstruction: Results of the AQuIRE Registry. Chest. 2015;148(2):450–471. doi: 10.1378/chest.14-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conlan AA, Hurwitz SS, Krige L, Nicolaou N, Pool R. Massive hemoptysis. Review of 123 cases. J Thorac Cardiovasc Surg. 1983;85(1):120–124. [PubMed] [Google Scholar]
- 10.Steinfort DP, Herth FJ, Eberhardt R, Irving LB. Potentially fatal arrhythmia complicating endobronchial epinephrine for control of iatrogenic bleeding. American journal of respiratory and critical care medicine. 2012;185(9):1028–1030. doi: 10.1164/ajrccm.185.9.1028. [DOI] [PubMed] [Google Scholar]
- 11.Tuller C, Tuller D, Tamm M, Brutsche MH. Hemodynamic effects of endobronchial application of ornipressin versus terlipressin. Respiration; international review of thoracic diseases. 2004;71(4):397–401. doi: 10.1159/000079646. [DOI] [PubMed] [Google Scholar]
- 12.Hass AR. Principles and Practice of Interventional Pulmonology. 1. Springer-Verlag; New York: 2013. [Google Scholar]
- 13.Solomonov A, Fruchter O, Zuckerman T, Brenner B, Yigla M. Pulmonary hemorrhage: A novel mode of therapy. Respiratory medicine. 2009;103(8):1196–1200. doi: 10.1016/j.rmed.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Dutau H, Palot A, Haas A, Decamps I, Durieux O. Endobronchial embolization with a silicone spigot as a temporary treatment for massive hemoptysis: a new bronchoscopic approach of the disease. Respiration; international review of thoracic diseases. 2006;73(6):830–832. doi: 10.1159/000092954. [DOI] [PubMed] [Google Scholar]
- 15.Valipour A, Kreuzer A, Koller H, Koessler W, Burghuber OC. Bronchoscopy-guided topical hemostatic tamponade therapy for the management of life-threatening hemoptysis. Chest. 2005;127(6):2113–2118. doi: 10.1378/chest.127.6.2113. [DOI] [PubMed] [Google Scholar]
- 16.Bense L. Intrabronchial selective coagulative treatment of hemoptysis. Report of three cases. Chest. 1990;97(4):990–996. doi: 10.1378/chest.97.4.990. [DOI] [PubMed] [Google Scholar]
- 17.Tsukamoto T, Sasaki H, Nakamura H. Treatment of hemoptysis patients by thrombin and fibrinogen-thrombin infusion therapy using a fiberoptic bronchoscope. Chest. 1989;96(3):473–476. doi: 10.1378/chest.96.3.473. [DOI] [PubMed] [Google Scholar]
- 18.de Gracia J, de la Rosa D, Catalan E, Alvarez A, Bravo C, Morell F. Use of endoscopic fibrinogen-thrombin in the treatment of severe hemoptysis. Respiratory medicine. 2003;97(7):790–795. doi: 10.1016/s0954-6111(03)00032-5. [DOI] [PubMed] [Google Scholar]
- 19.Bhattacharyya P, Dutta A, Samanta AN, Chowdhury SR. New Procedure: Bronchoscopic Endobronchial Sealing: A New Mode of Managing Hemoptysis. Chest. 2002;121(6):2066–2069. doi: 10.1378/chest.121.6.2066. [DOI] [PubMed] [Google Scholar]
- 20.Vyas KS, Saha SP. Comparison of hemostatic agents used in vascular surgery. Expert opinion on biological therapy. 2013;13(12):1663–1672. doi: 10.1517/14712598.2013.848193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rossaint R, Bouillon B, Cerny V, et al. The European guideline on management of major bleeding and coagulopathy following trauma: fourth edition. Critical Care. 2016;20(1):100. doi: 10.1186/s13054-016-1265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]