Summary
Human cytomegalovirus (HCMV) is the most common cause of viral infection acquired in utero. Even though the infection has been studied for several decades, immune determinants important for virus control and mechanisms of long-term sequelae caused by infection are still insufficiently characterized. Animal models of congenital HCMV infection provide unique opportunity to study various aspects of human disease. In this review, we summarize current knowledge on the role of immune system in congenital CMV infection, with emphasis on lessons learned from mouse model of congenital CMV infection.
Keywords: cytomegalovirus, immune response, brain pathology, congenital CMV infection, vaccines
1. Pathogenesis of congenital CMV infection
Human cytomegalovirus (HCMV) is a widespread β-herpesvirus which infects 40–100% of the adult population worldwide [1]. Like all herpesviruses, HCMV establishes lifelong latency that can lead to periodic reactivations. Infection with HCMV is usually asymptomatic in healthy individuals [2]. However, in immunocompromised individuals and in congenitally infected children, HCMV can cause severe disease and even mortality. Thus, development of HCMV vaccine is a public health priority [3].
Even though the primary HCMV infection in healthy adults is usually asymptomatic, viremia can last for several weeks or even longer [4]. Due to broad tropism, HCMV is able to infect the majority of cell types and organs. Infection of epithelial cells in kidney and salivary glands enables virus shedding in the saliva and urine for months, supporting transmission to new hosts [5]. Epithelial cells in mucosal tissues are the first targets of virus in new host, while myeloid and endothelial cells enable dissemination of virus throughout the organism. The mechanism of HCMV transmission to fetus is still incompletely defined, however it requires infection of placenta [6].
In general, infections acquired before birth, or early after birth are characterized by higher levels of viral replication, greater risk of persistent infection and more severe disease outcome, as compared to infections acquired later in life [7]. HCMV is the leading cause of viral congenital infection, the most common non-genetic cause of hearing loss and an important cause of neurodevelopmental delay [8–11]. Congenital HCMV infection affects 0.2–2.0% of all newborns worldwide, and the rate depends on many variables, including geographic location, socioeconomic status and ethnicity [12]. Approximately 10–15% of children with congenital HCMV are symptomatic at birth [13]. The infection induces a wide array of symptoms including intrauterine growth retardation, jaundice, hepatosplenomegaly and neurodevelopmental deficits [14]. Up to 10% of infected infants can exhibit long-term neurological sequelae, including sensorineural hearing loss [15]. Despite the lack of symptoms infected infants excrete virus for years after birth indicating lack of efficient control in some organs [16]. In comparison, in immunocompetent adults viral excretion can continue for a maximum of several months [4].
The determinants of HCMV transmission to fetus and why the infection is symptomatic in some cases is still not completely understood. Infection of the fetus during the early stages of pregnancy confers higher risk for long-term adverse central nervous system (CNS) outcome, probably due to infection and damage of a less developed CNS [17, 18]. Early emergence of both arms of adaptive immune responses has been shown to be important in preventing congenital transmission. However, pre-conceptional maternal immunity to HCMV does not prevent transmission of virus to fetus nor the development of disease in the infected fetus [19]. In fact, the rates of congenital HCMV are highest in populations in which women of childbearing age have the highest HCMV seroprevalence. The infection of offspring born to women with existing immunity, thought to result from either reactivation of latent virus or infection with a new strain of HCMV, can also cause congenital HCMV disease [19].
In addition to intrauterine transmission, postnatal transmission of virus, usually via maternal breast milk, can cause significant morbidity [20]. Disease following breast milk transmission of HCMV is typically observed in low birth weight premature infants [21]. Currently there is no evidence that such infection can cause long-term neurodevelopmental sequelae [22]. When congenital and perinatal infection were compared, the level of viruria among cases of congenital HCMV was on average one log higher in symptomatic compared to asymptomatic cases, while at the same time the viruria was one log higher in infants with congenital asymptomatic infection compared to perinatally infected children [23]. However, after one year all three groups had same low-level persistent infection.
1.1. Animal models to study congenital HCMV infection
Due to strict species specificity of HCMV, animal cytomegaloviruses (CMV) have been exceptionally useful in defining immune responses to congenital HCMV infection. Mouse cytomegalovirus (MCMV) infection of mice has been the most commonly used model of HCMV infection [24]. In contrast to HCMV, MCMV does not cross the placenta [25]. Therefore, we employ a model of congenital infection in which newborn mice are infected with MCMV intraperitoneally immediately after birth [26]. It is worth mentioning that the central nervous system (CNS) in newborn mice is developmentally equivalent to the human fetus at 15 weeks of gestation, a period when HCMV infection in humans is most frequently acquired during pregnancy [25]. Upon intraperitoneal infection of newborn mice, the virus disseminates to various tissues, including the brain where infection results in widespread, focal, non-necrotizing encephalitis [26]. CNS pathology in infected newborn mice closely recapitulates the pathology occurring during the congenital HCMV infection, most evident in smaller cerebellum size and thicker external granular layer. Moreover, the infection causes hearing loss associated with inner ear inflammation and loss of spiral ganglia neurons [27].
2. Innate immune responses to congenital CMV infection
2.1. Myeloid cells
Upon infection, innate immune response is the first line of defense. Monocytes, macrophages and dendritic cells (DCs) are specialized in sensing pathogens and production of cytokines. They are important not only for direct antiviral properties but also for proper formation of adaptive immune response [28]. Myeloid cells recognize highly conserved structures called pathogen-associated molecular patterns (PAMPs) or endogenous molecules released from damaged cells termed damage-associated molecular patterns (DAMPs). PAMPs and DAMPs are recognized by germline-encoded pattern recognition receptors (PRRs). Among PRRs, Toll-like receptors (TLRs) are the most extensively studied.
TLR9 is the main sensor of MCMV, whereas TLR3 and TLR7 play a supportive role in induction of inflammatory cytokine responses [28]. In addition, HCMV glycoproteins gH and gB are agonists for TLR2, even though the importance of these interactions is still not completely clear. While myeloid cells play an important role in combating CMV infection, the virus has many ways of evading their response [28]. In addition, macrophages and conventional DCs (cDCs) are fully permissive for lytic infection with HCMV [28]. Upon infection of myeloid cells, HCMV impairs their antigen presenting ability by downregulation of MHC class I and II, and costimulatory molecules. However, rapid T cell responses develop in spite of these evasion mechanisms. It is assumed that this is due to the fact that in vivo only a very small proportion of DCs (<5% at peak of infection) is infected [29]. Upon resolution of acute infection, HCMV resides latent in monocytes and myeloid progenitors in bone marrow and is able to reactivate upon differentiation of progenitors to macrophages and DCs, consequently spreading the virus to other organs [30].
By second trimester, CD14+ monocytes and macrophages, plasmacytoid DCs and cDCs are present in the human fetus [31]. Majority of studies investigating functional potential of myeloid cells upon encounter of different stimuli found that myeloid cells in neonates produce lower amounts of proinflammatory cytokines as compared to adult counterparts [7]. In addition, fetal DCs promote regulatory T-cell induction and inhibit T-cell TNF-α production through arginase-2 [31]. Fetal immune response is skewed towards Th2 response to avoid potentially damaging Th1 response in utero [32]. Therefore, neonatal innate immune defenses are focused towards protection against extracellular rather than intracellular pathogens, which is probably essential for survival advantage in early life against fatal bacterial infections [7].
The role of myeloid cells in response to congenital CMV infection has been poorly characterized. Pregnancy by itself is associated with altered cytokine milieu [33]. Increased IL-10 production during pregnancy has been associated with increased susceptibility to fetal CMV infection [34]. Infants with congenital CMV infection have upregulated macrophage-derived chemokines in serum, as compared to healthy infants [35]. Cord blood cDCs are equally susceptible to HCMV in vitro as adult cDCs, but they produce less IL-12, IFN-β and IFN-γ, while levels of IFN-α are similar [36]. In another study it was shown that neonatal DC function was not overly compromised, but had delay in IFN response as compared to adult DCs [37]. In addition, some published data claim that single nucleotide polymorphisms in TLR4 and TLR9 may contribute to the development of congenital HCMV infection [38].
2.2. NK cells
NK cells are component of innate immune system involved in control of intracellular pathogens and tumors, able to directly kill transformed and infected cells [39]. In addition, NK cells produce cytokines which stimulate other components of immune system. NK cell activation is dictated by repertoire of activating and inhibitory receptors and balance of their engagement. Fetal NK cells develop in utero, but it is assumed they go through final stage of differentiation and maturation postnatally [40]. Unlike in adults where majority of peripheral blood NK cells are terminally mature, fetal NK cells are less mature and less cytotoxic. Human fetal NK cells display antibody dependent cytotoxicity and respond to cytokine stimulation, however, they are hyporesponsive to absence of MHC I or to allogeneic cells [40]. This unresponsiveness correlates with number of killer-cell immunoglobulin-like (KIR) receptors expressed, and with sensitivity of fetal NK cells to TGF-β mediated suppression. Similarly, in newborn mice NK cells are kept immature due to TGF-β rich environment and express low levels of Ly49 receptors (functional homologs of KIR receptors) [41]. Inability to be activated via engagement of activating receptors and to respond to changes of MHC class I levels might make newborns susceptible to viral infections since those are the primary mechanisms by which virus infected cells trigger NK cytotoxicity.
The importance of NK cells in control of herpesvirus infections has been first observed in individuals lacking NK cells [42]. Similarly, NK cells are crucial in early control of MCMV infection [43]. Mouse activating NK cell receptor Ly49H is able to directly recognize MCMV-infected cells (viral protein m157) and mediate destruction of infected cells [43]. In addition, Ly49H+ NK cells expand upon infection with MCMV and form a pool of “memory NK cells” able to provide enhanced protection upon challenge [44]. Similarly"adaptive” NKG2C+ NK cells are often expanded in HCMV seropositive individuals and possess enhanced antibody dependent cellular cytotoxicity [45]. However, the role of these “adaptive” NK cells as well as the ligand(s) that mediate their expansion are still unknown. In addition, the requirement for NKG2C in induction of “adaptive” NK cells is redundant as ”adaptive” NK cells are found also in individuals with NKG2C deletion [46].
The role of NK cells during congenital HCMV infection is still poorly defined. In HCMV seropositive children increased numbers of NKG2C+ NK cells were detected [47, 48]. In addition, NK cells of these children express lower levels of activating receptors NKp30 and NKp46 and higher levels of inhibitory LILRB1 receptor [47]. NKG2C+ NK cell expansion was particularly marked in children who suffered symptomatic congenital infection [48]. Expansion of these cells seems to be amplified if T cell response is not effective or is delayed, indicating that NKG2C+ NK cell expansion could reflect inadequate T cell response. Evidence of this linkage between T cell responses and NK cell expansion was provided by findings in an immunocompromised 3-month old child with severe T cell deficiency in which NKG2C+ NK cell expansion correlated with reduction in virus load, indicating protective role of these cells [49]. However, incidence of congenital HCMV infection appears to be the same in children with NKG2C deletion as compared to children that express NKG2C [48]. In addition, it is highly unlikely that NK Ly49H/m157 interaction plays an important role in early control of MCMV in newborn mice, as this receptor is only beginning to be expressed after one week after birth [50]. As mentioned earlier, NK cells are actively being suppressed in newborn mice by TGF-β. In the absence of TGF-β, NK cells mature faster in newborn mice, express Ly49H receptor earlier and can provide protection against MCMV [41].
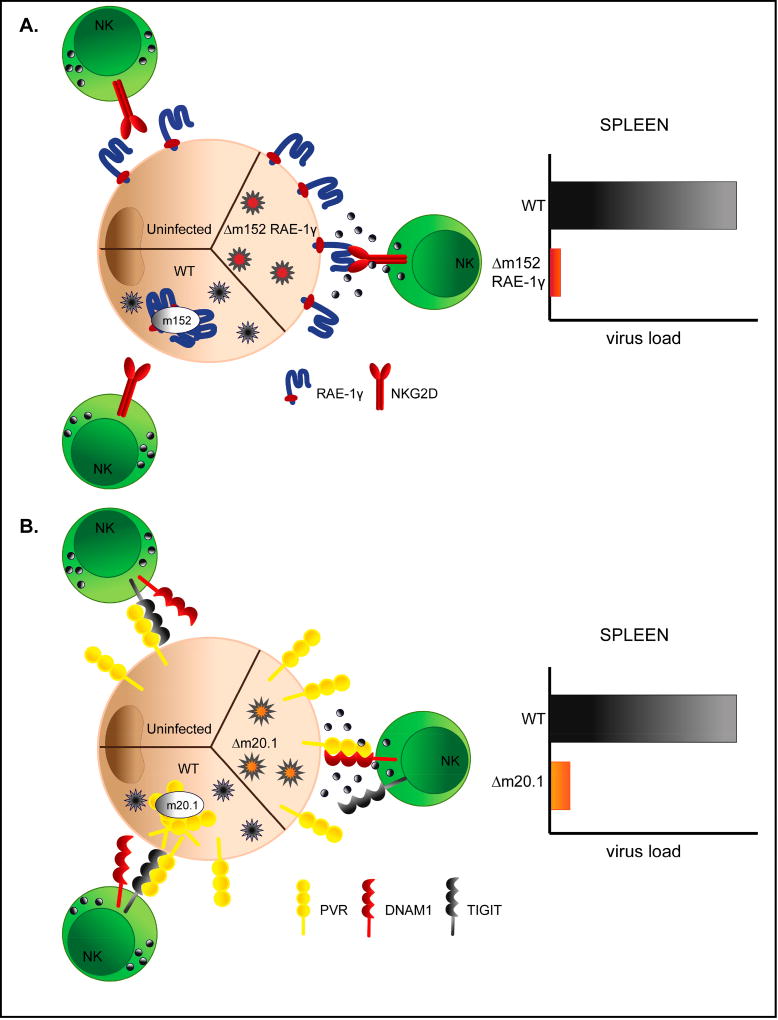
Cytomegaloviruses encode variety of genes with function directed towards evasion of NK cell recognition [43]. However, how this affects pathogenesis of congenital infection remains to be determined. We have shown that NK cell evasion is critical for efficient virus replication in newborn mice [51, 52]. Recombinant viruses expressing NKG2D ligand RAE-1γ or unable to regulate expression of PVR (CD155) are efficiently controlled in newborn mice (Fig. 1). Since NK cells in newborns are considered to be immature and actively suppressed [41], these findings suggest that suppressed NK cells possess potential to control infection which can be manifested if the activating signal strength increases. We have also shown that by exploiting such mechanisms, attenuated vaccine vectors can be generated that provide strong and durable immunity to vectored antigen [53].
Figure 1. Viral immunoevasion compromises virus control in newborn mice.
A) Efficient control of MCMV expressing NKG2D ligand RAE-1γ. WT MCMV downregulates RAE-1γ via m152 protein making the virus resistant to NKG2D dependent control in vivo. Insertion of gene encoding RAE-1γ in place of its viral inhibitor results in overexpression of RAE-1γ on the surface of infected cells and makes virus immunologically attenuated in vivo. B) MCMV inhibitor of PVR (CD155) encoded by MCMV gene m20.1 causes retention of PVR inside of the infected cell resulting in lower cell surface expression and preferential engagement of NK inhibitory receptor TIGIT. Deletion of m20.1 gene restores high cell surface expression of PVR and attenuates the virus in vivo via engagement of activating receptor DNAM-1.
It is worth mentioning that during pregnancy the uterus contains cells known as uterine or decidual NK cells. In fact, ~70 % of decidual leukocytes are NK cells. These NK cells have lower cytotoxic ability as compared to peripheral NK cells, but produce high amounts of cytokines and play important role in placentation [54]. However, these cells acquire cytotoxic potential when exposed to HCMV infected decidual fibroblasts, thus they could be important in prevention of CMV transmission in early pregnancy [55].
3. Adaptive immune responses to congenital CMV infection
3.1. T cells
T cells are major players of adaptive immune response which recognize foreign antigen in the context of MHC molecules presented on surface of infected cells or by professional antigen presenting cells. CD8+ T cells display their function through direct cytotoxicity, whereas CD4+ T cells differentiate into one of the subsets mediating distinct helper and regulatory functions. Upon encounter of foreign antigen, naïve T cells bearing specific T-cell receptor (TCR) activate and start rapid clonal expansion. Following the resolution of infection, antigen specific T cells contract, leaving behind only small portion of memory T cells ready to respond to challenge [56]. Compared to infections in adulthood, infections acquired in neonatal period lead to more restricted TCR clonotype diversity of CD8+ T cells, which impairs their recall capacity later in life [57]. In addition, neonatal CD8+ T cells do respond upon infection but preferentially turn into short lived effectors instead of long lived memory effector cells, resulting in narrowed CD8+ T cell memory repertoire [58]. CD4+ T cell response is even more affected by immature microenvironment in fetuses and newborns. In newborns, CD4+ T cell response is biased towards Th2 rather than Th1, additionally impairing CD8+ T cell mediated cytotoxicity [59].
Studies in mouse model have shown that CD8+ and CD4+ T cells play a central role in the resolution of acute MCMV infection in adult mice [60, 61]. Correspondingly, adoptive transfer of HCMV-specific T cell clones provides efficient control of infection in immunocompromised individuals [62–65]. Following CMV infection, T cells specific for certain epitopes are sustained at high frequencies and increase with age, encompassing as much as 10% of CD8+ T and CD4+ T cell memory reservoir. This observation was termed “memory inflation” and is more pronounced in CD8+ T cell compartment [62]. Latent CMV infection is also characterized by the appearance of virus-specific cytotoxic CD4+ T cells lacking expression of CD28 (CD4+CD28−) [66]. These virus-specific CD4+CD28− T cells show specific tissue localization and provide protection against reactivation [67, 68].
Congenital HCMV infection induces strong CD8+ T cell response which develops as early as 22nd week of gestation [69–73]. In the mouse model of congenital infection, CD8+ T cells are essential for resolution of MCMV infection, and depletion of CD8+ T cells results in fatal outcome [74]. Despite being able to respond, CD8+ T cells in young infants with active HCMV infection have more focused peptide specificity and lower peptide avidity compared to adults, however the peptide specificity seems to broaden over time [75]. Similarly, infection of newborn mice results in diversified T cell repertoire [76]. Fetal HCMV specific CD8+ T cells are phenotypically similar when compared to adult CD8+ T cells and are able to produce perforin, granzyme and IFN-γ upon stimulation, but to a lower extent than their adult counterparts [69, 72, 73]. The reduced polyfunctionality of both CD4+ and CD8+ T cells could be due to a certain level of exhaustion characterized by expression of PD1 in congenitally infected infants [77]. Interestingly, blockade of PD1 receptor can enhance neonatal T cell responses in vitro, opening the possibility for therapeutic intervention. The magnitude of the CD4+ T cell response in fetus correlates with the severity of CMV disease [78]. Unlike CD8+ T cells, HCMV specific CD4+ T cells are very low or even undetectable in infants with congenital HCMV infection [79, 80]. In addition, they show reduced ability to produce IFN-γ and IL-2 [81]. Impaired CD4+ T cell responses in early life could interfere with virus clearance and enhance shedding for long periods after primary infection, as CD4+ T cells are important for control of MCMV in salivary glands [61].
Beside CD4+ and CD8+ T cells, γδ T cells differentiate and expand in utero upon HCMV infection, express IFN-γ and show antiviral activity in vitro [82]. However, the contribution of these cells to virus control during congenital infection remains to be determined.
3.2. Antiviral antibodies
The B cell compartment also shows distinct features in early life. Naive cells are dominant population of B cells upon birth, with memory B cells increasing in size during the first six months of life [83]. In general, newborns use a biased antibody gene repertoire with a low frequency of somatic mutations, which probably contributes to poor affinity maturation and impaired functional antibody response [84].
Maternal HCMV-specific antibodies have been proposed to decrease the risk of placental HCMV transmission, although the nature of the magnitude and specificity of protective antiviral antibodies remains poorly understood [85]. Similarly, pregnant women developing early high avidity antibodies appear to be at lower risk of vertical HCMV transmission [85, 86]. The major targets of antibodies are viral glycoproteins expressed on the surface of virions and important for viral entry, primarily gB and gH/gL/pUL(128-131A) (pentameric complex) [87]. gB antibody titers are similar in transmitting and non-transmitting mothers, but antibodies to pentameric complex are increased in the groups of non-transmitting mothers, suggesting that antibodies targeting pentameric complex could be important for prevention of HCMV transmission [88]. Over the last decade, it has been argued that the pentameric complex of HCMV is targeted by very potent neutralizing antibodies, while gB is targeted mainly by non-neutralizing antibodies [89].
Up to now two groups of vaccines have been developed against HCMV [87]. One group includes live attenuated vaccines (Towne, AD169 and Towne/Toledo chimeric viruses) and dense bodies, while the second includes vaccines exploiting viral subunits. Laboratory strains Towne and AD169 have large deletion in ULb’ region, and lack pentameric complex as a result of in vitro passaging [87]. New vaccine approaches are incorporating pentameric complex as well. One such example is AD169 virion vaccine with restored pentameric complex [90]. Immunization with replication-defective variant of this vaccine shows antiviral activity against a panel of distinct clinical isolates of HCMV and elicits durable neutralizing antibody response in Rhesus macaques [90, 91]. In mouse model it was shown that gH/gL/gO complex is essential for in vivo infection, while the alternative gH/gL complex can compensate for the spread once the infection is established [92]. Therefore, targeting of gH/gL/gO complex might also be important to prevent transmission of virus.
Passive immunization was also tested for prevention of vertical transmission of CMV. Administration of CMV-specific hyperimmune globulin (HIG) to pregnant women initially appeared to lower the risk of congenital HCMV infection [93]. In addition, several case reports have provided evidence that timely HIG administration is able to reduce severity of CMV-associated fetal anomalies [94]. However, in a double-blind placebo-controlled study no significant impact of administration of anti-CMV immunoglobulin was found [95].
Maternal antibodies efficiently cross the maternal-fetal interface, conferring passive immunity to infections. We have shown that transplacentally transferred maternal antibodies provide complete protection to newborn mice upon challenge with the same strain of MCMV [51]. Transfer of maternal IgGs is facilitated by transcytosis via the neonatal Fc receptor in syncytiotrophoblasts of the placenta which progressively increases in the last trimester of gestation [96]. However, antibodies could even facilitate transmission of CMV across the placental barrier, as it was shown that complex of IgG and virion can be transcytosed across the surface of trophoblast cells via the neonatal Fc receptor, opening the possibility for viral subversion of antibodies [97].
HCMV can readily reinfect pregnant women with existing HCMV immunity [19]. One of the explanations for this is that the reinfection of immunocompetent women occurs with new strain(s) of HCMV, encoding different antigenic determinants [98, 99]. In these studies it was observed that the newly acquired virus was also transmitted to the fetus. Therefore, current literature suggests that the pre-existing antibodies to HCMV can reduce the frequency of transmission, but they cannot prevent infection with new HCMV strain.
Low total IgM levels, but not HCMV specific IgM levels, in newborns are characteristic of symptomatic congenital HCMV infection [100]. CMV-specific, high-avidity IgG with neutralizing activity was similar in the fetal bloodstream as compared with the maternal circulation and viral DNA in the placenta was reduced in the presence of high-avidity IgG [101]. Infants with an increased numbers of B cells present at birth had a reduced incidence of long-term impairment, suggesting that antibodies may be critical for effective anti-HCMV immunity upon congenital infection [102]. In a mouse model, passive transfer of immune sera or monoclonal antibodies to infected newborn mice significantly reduced viral load in infected newborn mice [103].
4. Immune responses to CMV infection in the CNS: A double-edged sword
The developing brain is inherently more susceptible to MCMV infection as compared to adult brain, independently of immune system [104]. Virtually all cell types in the brain have some degree of susceptibility to CMV infection [105]. However, only astrocytes and endothelial cells support productive infection in vitro. In addition, neuronal stem cells and neuronal precursor cells are permissive for CMV infection, and infection in the brain results in reduced proliferation, altered differentiation and apoptosis of neuronal stem cells [106, 107]. Following resolution of acute infection, latent MCMV persists in the brain of infected neonatal mice and can be reactivated using in-vitro culture of brain slices from persistently infected mice [108].
In a mouse model of congenital CMV infection, MCMV infection is characterized by neurodevelopmental abnormalities that recapitulate key features of the congenital HCMV infection [26]. The abnormalities in CNS development that are most readily observable are in the cerebellum, probably because that region of mouse brain undergoes extensive postnatal development [26]. MCMV infection in the brain results in focal non-necrotizing encephalitis, with no specific cellular tropism, similar to the histopathology reported in studies of brain tissues from infants with congenital HCMV infection [26]. In addition, infection induces a strong inflammatory response in the brain characterized by the activation of microglia (brain resident macrophages), recruitment of activated peripheral immune cells and the expression of pro-inflammatory cytokines [25]. Deficits in cerebral development are symmetric and global and it was therefore assumed that inflammation induced by MCMV infection is responsible for deficits in CNS development [26]. Indeed, treatment of mice with anti-inflammatory drugs (glucocorticoids, corticosteroids) or with TNF-α neutralizing antibodies limits morphogenic abnormalities in the CNS without affecting level of virus replication in brain [109, 110].
MCMV infection in brain induces strong IFN-stimulated genes response, and treatment of primary brain cultures with type I IFNs or IFN-γ reduces number of MCMV infected cells [111]. Following MCMV infection, microglia cells polarize towards proinflammatory state and produce antiviral molecules [74]. Beside activation of microglia, MCMV infection in the brain is associated with infiltration of NK cells, monocytes/macrophages and T cells (Fig. 2A). Infiltrating monocytes, together with microglia, seem to be the main source of pro-inflammatory cytokine TNF-α, central for induction of inflammation in brain [110]. While it seems that monocytes do not contribute significantly to virus clearance, it seems that they play important role in induction of neurodevelopmental abnormalities. Depletion of NK cells in newborn mice infected with MCMV intracerebrally results in increased virus load in brain [112]. Furthermore, blockade of NO synthase similarly resulted in increased virus load in brain [112]. In addition, NK cells and NOS2 positive macrophages co-localized in proximity of infected cells in brain. In contrast, MCMV infected neurons were not surrounded with these immune cells, indicating active immunoevasion [112]. Production of iNOS and proinflammatory cytokines by activated meningeal macrophages and parenchymal microglia is also associated with reduced stemness of neural progenitor stem cells in MCMV infected newborn brains [113]. In addition, there is some evidence that miRNAs could play a role in control of MCMV in newborn mice as in Dicer-deficient mice, unable to generate miRNAs, higher levels of MCMV can be detected in the brain of newborn mice [114].
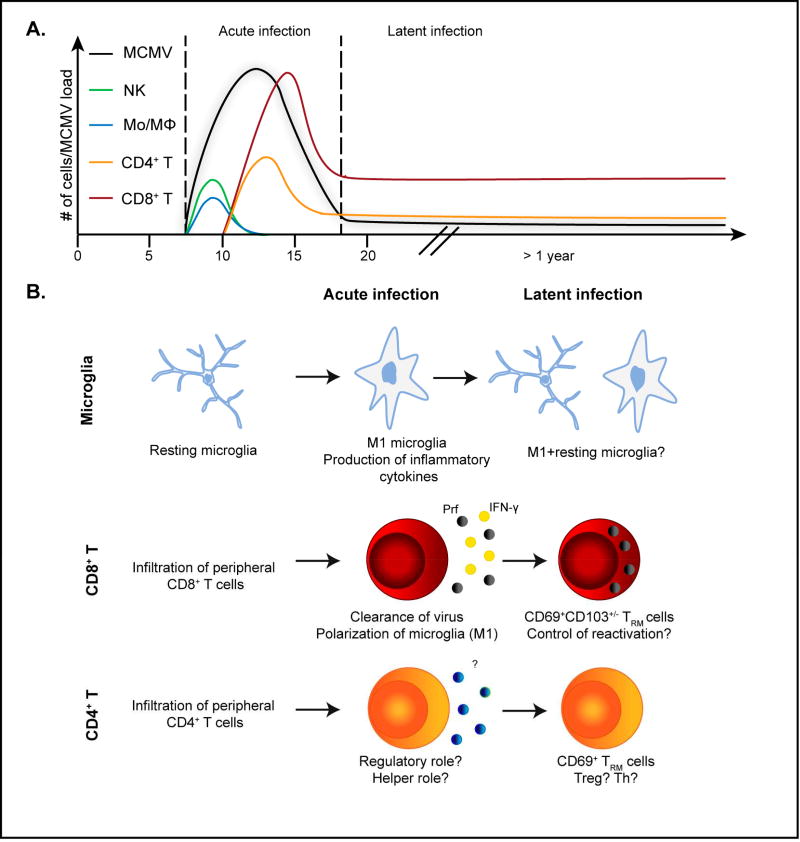
Figure 2. Immune response in the brain of MCMV-infected newborn mice.
A) Kinetics of infiltrating immune cells and virus in the brain of neonatal mice infected intraperitoneally. Upon infiltration in brain, MCMV replicates up to day 20 after infection. Infection of brain results in rapid infiltration of NK cells and monocytes/macrophages (Mo/MΦ), followed by infiltration of T cells. Upon resolution of acute infection, latent virus persist in brain, as well as CD8+ and CD4+ T cells. B) MCMV infection of newborn mice permanently changes immune cell homeostasis in brain. Microglia: Upon infection, resting microglia polarizes towards proinflammatory, M1, phenotype which is maintained for prolonged period of time. CD8+ T and CD4+ T cells: Upon infection of brain, activated CD8+ T and CD4+ T cells 26 infiltrate the brain. While CD8+ T cells control the infection (Perforin, IFN-γ), the role of CD4+ T cells is not determined. Upon resolution of acute infection, CD8+ T and CD4+ T cells are maintained in the brain as tissue resident memory cells (TRM cells).
Adaptive immunity plays a central role in control of CMV in the brain. CD8+ T cells are essential for resolution of MCMV infection in the brain of infected newborn mice [74]. In addition, adoptive transfer of virus specific CD8+ T cells decreases neurodevelopmental changes (Jonjić et al, unpublished). Passive immunization of MCMV-infected newborn mice also protects against infection of brain and neurodevelopmental abnormalities [103]. In brains of MCMV-infected newborn mice treated with immune serum or antiviral monoclonal antibodies, the titer of infectious virus was reduced as well as mononuclear cell infiltrates and CNS pathology. Following the resolution of acute MCMV infection in brain, the virus-specific T cells persist in the brain of infected newborn mice long-term, essentially for the lifetime of mice (Jonjic et al unpublished and [76]). These cells phenotypically represent tissue resident memory T cells (TRM cells) as they express CD69 and CD103 (Fig. 2B). Whether these cells contribute to control of latent virus and brain pathology during virus reactivation remains to be determined. However, persistence of TRM cells along with long-term polarization of microglia suggests that MCMV infection in the brain permanently changes immune homeostasis in the brain (Fig. 2B). Whether this persistent virus infection could contribute to development of neurodegenerative CNS disease or other chronic CNS disease remains to be determined. Our published data suggest that MCMV can significantly impact neurodegenerative diseases in CNS as we could observe that mice resistant to experimental autoimmune encephalitis (EAE), become sensitive to EAE upon MCMV infection [115]. Similarly, MCMV infection in EAE susceptible C57BL/6 mice exacerbates EAE symptoms, which correlates with expansion of CD4+CD28− T cells [116].
CMV encephalitis has been studied in intracranially infected adult mice as well. In this paragraph we list major findings in this model, however it remains to be determined to what extent do these findings reflect congenital disease. Upon intracerebral infection of adult mice, CD8+ T cells are essential for control of virus in brain via perforin [105]. CD8+ T cells infiltrating infected brain produce IFN-γ that polarizes microglia cells towards pro-inflammatory phenotype but also induces PDL-1 on microglia, which in turn inhibits production of IL-2 and IFN-γ by T cells [117, 118]. The PD-1:PDL-1 pathway also promotes generation of CD8+ TRM cells [117, 119]. Treg cell depletion results in increased proliferation of T cells, but impaired generation of functional CD8+ TRM cells, and prolongs chronic reactive phenotypes of the resident glial cells [120, 121]. B cells also accumulate and persist in the brain of intracerebrally infected mice, and reduce virus reactivation [122]. Furthermore, upon intracerebral infection of adult mice IL-10 is essential for survival [105]. Absence of IL-10 is associated with increased IFN-γ response and reduced lymphocyte infiltration while the virus control in the brain was unchanged, however the mechanistic role of IL-10 remains elusive.
As in the case of congenital HCMV infection, perinatal MCMV infection induces hearing loss associated with inner ear inflammation and loss of spiral ganglia neurons [27]. Similarly, intracerebral inoculation of MCMV in neonatal mice results in hearing loss associated with loss of hair cells, even though these cells are not infected with MCMV, and this correlated with proinflammatory response [123].
5. Concluding remarks
HCMV infection is the most common congenital viral infection, and can result in neurological sequelae and mental retardation. Development of vaccine could prevent intrauterine transmission of HCMV and/or disease. For optimal designing of CMV vaccine, we also need to improve our understanding of key factors responsible for developmental of neurological sequelae, as only 10% are symptomatic at birth and significant proportion of asymptomatic has long term sequelae as well. Thus, there is an unmet need to define basic mechanisms of CNS infection and disease in congenital CMV infection. Such studies could determine critical parameters in virus entry into the CNS and mechanisms of control of acute and chronic CNS infection that could be more precisely targeted by prophylactic vaccines and antiviral agents. Lessons learned from mouse model indicate that beside neurodevelopmental impairment induced by infection, immune cell homeostasis in CNS is subject of continuous changes resulting in induction of tissue resident lymphocyte populations. It will be important to determine how these cells impact the pathogenesis. These studies combined with better understating of immune response in fetal and neonatal period will likely further define the nature of optimal CMV vaccine.
Acknowledgments
We apologize to our colleagues whose work was not cited due to space limitations. W.J.B. and S.J. are supported by NIH (1 R01 DC015980-01A1). AK is supported by the Croatian Science Foundation under the project 7132.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interests:
The authors declare no conflict of interests regarding the publication of this paper.
References
- 1.Hyde TB, Schmid DS, Cannon MJ. Cytomegalovirus seroconversion rates and risk factors: implications for congenital CMV. Rev Med Virol. 2010;20:311–26. doi: 10.1002/rmv.659. [DOI] [PubMed] [Google Scholar]
- 2.Bate SL, Dollard SC, Cannon MJ. Cytomegalovirus seroprevalence in the United States: the national health and nutrition examination surveys, 1988-2004. Clin Infect Dis. 2010;50:1439–47. doi: 10.1086/652438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arvin AM, Fast P, Myers M, Plotkin S, Rabinovich R, Committee NVA Vaccine development to prevent cytomegalovirus disease: report from the National Vaccine Advisory Committee. Clin Infect Dis. 2004;39:233–9. doi: 10.1086/421999. [DOI] [PubMed] [Google Scholar]
- 4.Zanghellini F, Boppana SB, Emery VC, Griffiths PD, Pass RF. Asymptomatic primary cytomegalovirus infection: virologic and immunologic features. J Infect Dis. 1999;180:702–7. doi: 10.1086/314939. [DOI] [PubMed] [Google Scholar]
- 5.Sinzger C, Digel M, Jahn G. Cytomegalovirus cell tropism. Curr Top Microbiol Immunol. 2008;325:63–83. doi: 10.1007/978-3-540-77349-8_4. [DOI] [PubMed] [Google Scholar]
- 6.Pereira L, Maidji E. Cytomegalovirus infection in the human placenta: maternal immunity and developmentally regulated receptors on trophoblasts converge. Curr Top Microbiol Immunol. 2008;325:383–95. doi: 10.1007/978-3-540-77349-8_21. [DOI] [PubMed] [Google Scholar]
- 7.Prendergast AJ, Klenerman P, Goulder PJ. The impact of differential antiviral immunity in children and adults. Nat Rev Immunol. 2012;12:636–48. doi: 10.1038/nri3277. [DOI] [PubMed] [Google Scholar]
- 8.Grosse SD, Ross DS, Dollard SC. Congenital cytomegalovirus (CMV) infection as a cause of permanent bilateral hearing loss: a quantitative assessment. J Clin Virol. 2008;41:57–62. doi: 10.1016/j.jcv.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Declau F, Boudewyns A, Van den Ende J, Peeters A, van den Heyning P. Etiologic and audiologic evaluations after universal neonatal hearing screening: analysis of 170 referred neonates. Pediatrics. 2008;121:1119–26. doi: 10.1542/peds.2007-1479. [DOI] [PubMed] [Google Scholar]
- 10.Morton CC, Nance WE. Newborn hearing screening--a silent revolution. N Engl J Med. 2006;354:2151–64. doi: 10.1056/NEJMra050700. [DOI] [PubMed] [Google Scholar]
- 11.Nance WE, Lim BG, Dodson KM. Importance of congenital cytomegalovirus infections as a cause for pre-lingual hearing loss. J Clin Virol. 2006;35:221–5. doi: 10.1016/j.jcv.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 12.Kenneson A, Cannon MJ. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol. 2007;17:253–76. doi: 10.1002/rmv.535. [DOI] [PubMed] [Google Scholar]
- 13.Boppana SB, Pass RF, Britt WJ, Stagno S, Alford CA. Symptomatic congenital cytomegalovirus infection: neonatal morbidity and mortality. Pediatr Infect Dis J. 1992;11:93–9. doi: 10.1097/00006454-199202000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Ross SA, Boppana SB. Congenital cytomegalovirus infection: outcome and diagnosis. Semin Pediatr Infect Dis. 2005;16:44–9. doi: 10.1053/j.spid.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 15.Fowler KB. Congenital cytomegalovirus infection: audiologic outcome. Clin Infect Dis. 2013;57(Suppl 4):S182–4. doi: 10.1093/cid/cit609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pass RF, Stagno S, Britt WJ, Alford CA. Specific cell-mediated immunity and the natural history of congenital infection with cytomegalovirus. J Infect Dis. 1983;148:953–61. doi: 10.1093/infdis/148.6.953. [DOI] [PubMed] [Google Scholar]
- 17.Enders G, Daiminger A, Bader U, Exler S, Enders M. Intrauterine transmission and clinical outcome of 248 pregnancies with primary cytomegalovirus infection in relation to gestational age. J Clin Virol. 2011;52:244–6. doi: 10.1016/j.jcv.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Pass RF, Fowler KB, Boppana SB, Britt WJ, Stagno S. Congenital cytomegalovirus infection following first trimester maternal infection: symptoms at birth and outcome. J Clin Virol. 2006;35:216–20. doi: 10.1016/j.jcv.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 19.Britt WJ. Congenital human cytomegalovirus infection and the enigma of maternal immunity. J Virol. 2017;91:e02392–16. doi: 10.1128/JVI.02392-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dworsky M, Yow M, Stagno S, Pass RF, Alford C. Cytomegalovirus infection of breast milk and transmission in infancy. Pediatrics. 1983;72:295–9. [PubMed] [Google Scholar]
- 21.Maschmann J, Hamprecht K, Dietz K, Jahn G, Speer CP. Cytomegalovirus infection of extremely low-birth weight infants via breast milk. Clin Infect Dis. 2001;33:1998–2003. doi: 10.1086/324345. [DOI] [PubMed] [Google Scholar]
- 22.Vollmer B, Seibold-Weiger K, Schmitz-Salue C, Hamprecht K, Goelz R, Krageloh-Mann I, et al. Postnatally acquired cytomegalovirus infection via breast milk: effects on hearing and development in preterm infants. Pediatr Infect Dis J. 2004;23:322–7. doi: 10.1097/00006454-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Stagno S, Reynolds DW, Tsiantos A, Fuccillo DA, Long W, Alford CA. Comparative serial virologic and serologic studies of symptomatic and subclinical congenitally and natally acquired cytomegalovirus infections. J Infect Dis. 1975;132:568–77. doi: 10.1093/infdis/132.5.568. [DOI] [PubMed] [Google Scholar]
- 24.Reddehase MJ, Simon CO, Seckert CK, Lemmermann N, Grzimek NK. Murine model of cytomegalovirus latency and reactivation. Curr Top Microbiol Immunol. 2008;325:315–31. doi: 10.1007/978-3-540-77349-8_18. [DOI] [PubMed] [Google Scholar]
- 25.Slavuljica I, Kveštak D, Huszthy PC, Kosmac K, Britt WJ, Jonjić S. Immunobiology of congenital cytomegalovirus infection of the central nervous system—the murine cytomegalovirus model. Cell Mol Immunol. 2015;12:180–91. doi: 10.1038/cmi.2014.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koontz T, Bralic M, Tomac J, Pernjak-Pugel E, Bantug G, Jonjic S, et al. Altered development of the brain after focal herpesvirus infection of the central nervous system. J Exp Med. 2008;205:423–35. doi: 10.1084/jem.20071489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bradford RD, Yoo YG, Golemac M, Pugel EP, Jonjic S, Britt WJ. Murine CMV-induced hearing loss is associated with inner ear inflammation and loss of spiral ganglia neurons. PLoS Pathog. 2015;11:e1004774. doi: 10.1371/journal.ppat.1004774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brinkmann MM, Dağ F, Hengel H, Messerle M, Kalinke U, Čičin-Šain L. Cytomegalovirus immune evasion of myeloid lineage cells. Med Microbiol Immunol. 2015;204:367–82. doi: 10.1007/s00430-015-0403-4. [DOI] [PubMed] [Google Scholar]
- 29.Dalod M, Hamilton T, Salomon R, Salazar-Mather TP, Henry SC, Hamilton JD, et al. Dendritic cell responses to early murine cytomegalovirus infection: subset functional specialization and differential regulation by interferon alpha/beta. J Exp Med. 2003;197:885–98. doi: 10.1084/jem.20021522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poole E, Sinclair J. Sleepless latency of human cytomegalovirus. Med Microbiol Immunol. 2015;204:421–9. doi: 10.1007/s00430-015-0401-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGovern N, Shin A, Low G, Low D, Duan K, Yao LJ, et al. Human fetal dendritic cells promote prenatal T-cell immune suppression through arginase-2. Nature. 2017;546:662–6. doi: 10.1038/nature22795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mold JE, Michaelsson J, Burt TD, Muench MO, Beckerman KP, Busch MP, et al. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science. 2008;322:1562–5. doi: 10.1126/science.1164511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mor G, Aldo P, Alvero AB. The unique immunological and microbial aspects of pregnancy. Nat Rev Immunol. 2017;17:469–82. doi: 10.1038/nri.2017.64. [DOI] [PubMed] [Google Scholar]
- 34.Muller WJ, Jones CA, Koelle DM. Immunobiology of herpes simplex virus and cytomegalovirus infections of the fetus and newborn. Curr Immunol Rev. 2010;6:38–55. doi: 10.2174/157339510790231833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Z, Tian Y, Wang B, Yan Z, Qian D, Ding S, et al. Serum proteomics with SELDI-TOF-MS in congenital human cytomegalovirus hepatitis. J Med Virol. 2007;79:1500–5. doi: 10.1002/jmv.20927. [DOI] [PubMed] [Google Scholar]
- 36.Renneson J, Dutta B, Goriely S, Danis B, Lecomte S, Laes JF, et al. IL-12 and type I IFN response of neonatal myeloid DC to human CMV infection. Eur J Immunol. 2009;39:2789–99. doi: 10.1002/eji.200939414. [DOI] [PubMed] [Google Scholar]
- 37.Dantoft W, Martinez-Vicente P, Jafali J, Perez-Martinez L, Martin K, Kotzamanis K, et al. Genomic programming of human neonatal dendritic cells in congenital systemic and in vitro cytomegalovirus infection reveal plastic and robust immune pathway biology responses. Front Immunol. 2017;8:1146. doi: 10.3389/fimmu.2017.01146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wujcicka W, Paradowska E, Studzińska M, Gaj Z, Wilczyński J, Leśnikowski Z, et al. TLR9 2848 GA heterozygotic status possibly predisposes fetuses and newborns to congenital infection with human cytomegalovirus. PLoS One. 2015;10:e0122831. doi: 10.1371/journal.pone.0122831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008;9:495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ivarsson MA, Loh L, Marquardt N, Kekalainen E, Berglin L, Bjorkstrom NK, et al. Differentiation and functional regulation of human fetal NK cells. J Clin Invest. 2013;123:3889–901. doi: 10.1172/JCI68989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marcoe JP, Lim JR, Schaubert KL, Fodil-Cornu N, Matka M, McCubbrey AL, et al. TGF-β is responsible for NK cell immaturity during ontogeny and increased susceptibility to infection during mouse infancy. Nat Immunol. 2012;13:843–50. doi: 10.1038/ni.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Biron CA, Byron KS, Sullivan JL. Severe herpesvirus infections in an adolescent without natural killer cells. N Engl J Med. 1989;320:1731–5. doi: 10.1056/NEJM198906293202605. [DOI] [PubMed] [Google Scholar]
- 43.Brizic I, Lenac Rovis T, Krmpotic A, Jonjic S. MCMV avoidance of recognition and control by NK cells. Semin Immunopathol. 2014;36:641–50. doi: 10.1007/s00281-014-0441-9. [DOI] [PubMed] [Google Scholar]
- 44.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457:557–61. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hammer Q, Romagnani C. About training and memory: NK-cell adaptation to viral infections. Adv Immunol. 2017;133:171–207. doi: 10.1016/bs.ai.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 46.Liu LL, Landskron J, Ask EH, Enqvist M, Sohlberg E, Traherne JA, et al. Critical role of CD2 costimulation in adaptive natural killer cell responses revealed in NKG2C-deficient humans. Cell Rep. 2016;15:1088–99. doi: 10.1016/j.celrep.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Monsivais-Urenda A, Noyola-Cherpitel D, Hernandez-Salinas A, Garcia-Sepulveda C, Romo N, Baranda L, et al. Influence of human cytomegalovirus infection on the NK cell receptor repertoire in children. Eur J Immunol. 2010;40:1418–27. doi: 10.1002/eji.200939898. [DOI] [PubMed] [Google Scholar]
- 48.Noyola DE, Fortuny C, Muntasell A, Noguera-Julian A, Munoz-Almagro C, Alarcon A, et al. Influence of congenital human cytomegalovirus infection and the NKG2C genotype on NK-cell subset distribution in children. Eur J Immunol. 2012;42:3256–66. doi: 10.1002/eji.201242752. [DOI] [PubMed] [Google Scholar]
- 49.Kuijpers TW, Baars PA, Dantin C, van den Burg M, van Lier RA, Roosnek E. Human NK cells can control CMV infection in the absence of T cells. Blood. 2008;112:914–5. doi: 10.1182/blood-2008-05-157354. [DOI] [PubMed] [Google Scholar]
- 50.Wu X, Chen Y, Wei H, Sun R, Tian Z. Development of murine hepatic NK cells during ontogeny: comparison with spleen NK cells. Clin Dev Immunol. 2012;2012:759765. doi: 10.1155/2012/759765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Slavuljica I, Busche A, Babić M, Mitrović M, Gašparović I, Cekinović D, et al. Recombinant mouse cytomegalovirus expressing a ligand for the NKG2D receptor is attenuated and has improved vaccine properties. J Clin Invest. 2010;120:4532–45. doi: 10.1172/JCI43961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lenac Rovis T, Kucan Brlic P, Kaynan N, Juranic Lisnic V, Brizic I, Jordan S, et al. Inflammatory monocytes and NK cells play a crucial role in DNAM-1-dependent control of cytomegalovirus infection. J Exp Med. 2016;213:1835–50. doi: 10.1084/jem.20151899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tršan T, Vuković K, Filipović P, Brizić AL, Lemmermann NAW, Schober K, et al. Cytomegalovirus vector expressing RAE-1γ induces enhanced anti-tumor capacity of murine CD8(+) T cells. Eur J Immunol. 2017;47:1354–67. doi: 10.1002/eji.201746964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gaynor LM, Colucci F. Uterine natural killer cells: Functional distinctions and influence on pregnancy in humans and mice. Front Immunol. 2017;8:467. doi: 10.3389/fimmu.2017.00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Siewiera J, El Costa H, Tabiasco J, Berrebi A, Cartron G, Le Bouteiller P, et al. Human cytomegalovirus infection elicits new decidual natural killer cell effector functions. PLoS Pathog. 2013;9:e1003257. doi: 10.1371/journal.ppat.1003257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harty JT, Badovinac VP. Shaping and reshaping CD8+ T-cell memory. Nat Rev Immunol. 2008;8:107–19. doi: 10.1038/nri2251. [DOI] [PubMed] [Google Scholar]
- 57.Rudd BD, Venturi V, Smith NL, Nzingha K, Goldberg EL, Li G, et al. Acute neonatal infections 'lock-in' a suboptimal CD8+ T cell repertoire with impaired recall responses. PLoS Pathog. 2013;9:e1003572. doi: 10.1371/journal.ppat.1003572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smith NL, Wissink E, Wang J, Pinello JF, Davenport MP, Grimson A, et al. Rapid proliferation and differentiation impairs the development of memory CD8+ T cells in early life. J Immunol. 2014;193:177–84. doi: 10.4049/jimmunol.1400553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li L, Lee HH, Bell JJ, Gregg RK, Ellis JS, Gessner A, et al. IL-4 utilizes an alternative receptor to drive apoptosis of Th1 cells and skews neonatal immunity toward Th2. Immunity. 2004;20:429–40. doi: 10.1016/s1074-7613(04)00072-x. [DOI] [PubMed] [Google Scholar]
- 60.Reddehase MJ, Weiland F, Munch K, Jonjic S, Luske A, Koszinowski UH. Interstitial murine cytomegalovirus pneumonia after irradiation: characterization of cells that limit viral replication during established infection of the lungs. J Virol. 1985;55:264–73. doi: 10.1128/jvi.55.2.264-273.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jonjić S, Mutter W, Weiland F, Reddehase MJ, Koszinowski UH. Site-restricted persistent cytomegalovirus infection after selective long-term depletion of CD4+ T lymphocytes. J Exp Med. 1989;169:1199–212. doi: 10.1084/jem.169.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Klenerman P, Oxenius A. T cell responses to cytomegalovirus. Nat Rev Immunol. 2016;16:367–77. doi: 10.1038/nri.2016.38. [DOI] [PubMed] [Google Scholar]
- 63.Schmitt A, Tonn T, Busch DH, Grigoleit GU, Einsele H, Odendahl M, et al. Adoptive transfer and selective reconstitution of streptamer-selected cytomegalovirus-specific CD8+ T cells leads to virus clearance in patients after allogeneic peripheral blood stem cell transplantation. Transfusion. 2011;51:591–9. doi: 10.1111/j.1537-2995.2010.02940.x. [DOI] [PubMed] [Google Scholar]
- 64.Stemberger C, Graef P, Odendahl M, Albrecht J, Dossinger G, Anderl F, et al. Lowest numbers of primary CD8(+) T cells can reconstitute protective immunity upon adoptive immunotherapy. Blood. 2014;124:628–37. doi: 10.1182/blood-2013-12-547349. [DOI] [PubMed] [Google Scholar]
- 65.Neuenhahn M, Albrecht J, Odendahl M, Schlott F, Dossinger G, Schiemann M, et al. Transfer of minimally manipulated CMV-specific T cells from stem cell or third-party donors to treat CMV infection after allo-HSCT. Leukemia. 2017;31:2161–71. doi: 10.1038/leu.2017.16. [DOI] [PubMed] [Google Scholar]
- 66.van Leeuwen EM, Remmerswaal EB, Heemskerk MH, ten Berge IJ, van Lier RA. Strong selection of virus-specific cytotoxic CD4+ T-cell clones during primary human cytomegalovirus infection. Blood. 2006;108:3121–7. doi: 10.1182/blood-2006-03-006809. [DOI] [PubMed] [Google Scholar]
- 67.Pachnio A, Ciaurriz M, Begum J, Lal N, Zuo J, Beggs A, et al. Cytomegalovirus infection leads to development of high frequencies of cytotoxic virus-specific CD4+ T cells targeted to vascular endothelium. PLoS Pathog. 2016;12:e1005832. doi: 10.1371/journal.ppat.1005832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Verma S, Weiskopf D, Gupta A, McDonald B, Peters B, Sette A, et al. Cytomegalovirus-specific CD4 T cells are cytolytic and mediate vaccine protection. J Virol. 2015;90:650–8. doi: 10.1128/JVI.02123-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Marchant A, Appay V, Van Der Sande M, Dulphy N, Liesnard C, Kidd M, et al. Mature CD8(+) T lymphocyte response to viral infection during fetal life. J Clin Invest. 2003;111:1747–55. doi: 10.1172/JCI17470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gibson L, Piccinini G, Lilleri D, Revello MG, Wang Z, Markel S, et al. Human cytomegalovirus proteins pp65 and immediate early protein 1 are common targets for CD8+ T cell responses in children with congenital or postnatal human cytomegalovirus infection. J Immunol. 2004;172:2256–64. doi: 10.4049/jimmunol.172.4.2256. [DOI] [PubMed] [Google Scholar]
- 71.Miles DJ, van der Sande M, Jeffries D, Kaye S, Ismaili J, Ojuola O, et al. Cytomegalovirus infection in Gambian infants leads to profound CD8 T-cell differentiation. J Virol. 2007;81:5766–76. doi: 10.1128/JVI.00052-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pedron B, Guerin V, Jacquemard F, Munier A, Daffos F, Thulliez P, et al. Comparison of CD8+ T Cell responses to cytomegalovirus between human fetuses and their transmitter mothers. J Infect Dis. 2007;196:1033–43. doi: 10.1086/521196. [DOI] [PubMed] [Google Scholar]
- 73.Elbou Ould MA, Luton D, Yadini M, Pedron B, Aujard Y, Jacqz-Aigrain E, et al. Cellular immune response of fetuses to cytomegalovirus. Pediatr Res. 2004;55:280–6. doi: 10.1203/01.PDR.0000104150.85437.FE. [DOI] [PubMed] [Google Scholar]
- 74.Bantug GR, Cekinovic D, Bradford R, Koontz T, Jonjic S, Britt WJ. CD8+ T lymphocytes control murine cytomegalovirus replication in the central nervous system of newborn animals. J Immunol. 2008;181:2111–23. doi: 10.4049/jimmunol.181.3.2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gibson L, Dooley S, Trzmielina S, Somasundaran M, Fisher D, Revello MG, et al. Cytomegalovirus (CMV) IE1- and pp65-specific CD8+ T cell responses broaden over time after primary CMV infection in infants. J Infect Dis. 2007;195:1789–98. doi: 10.1086/518042. [DOI] [PubMed] [Google Scholar]
- 76.Venturi V, Nzingha K, Amos TG, Charles WC, Dekhtiarenko I, Cicin-Sain L, et al. The Neonatal CD8+ T Cell Repertoire Rapidly Diversifies during Persistent Viral Infection. J Immunol. 2016;196:1604–16. doi: 10.4049/jimmunol.1501867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huygens A, Lecomte S, Tackoen M, Olislagers V, Delmarcelle Y, Burny W, et al. Functional exhaustion limits CD4+ and CD8+ T-cell responses to congenital cytomegalovirus infection. J Infect Dis. 2015;212:484–94. doi: 10.1093/infdis/jiv071. [DOI] [PubMed] [Google Scholar]
- 78.Fujikawa T, Numazaki K, Asanuma H, Tsutsumi H. Human cytomegalovirus infection during pregnancy and detection of specific T cells by intracellular cytokine staining. Int J Infect Dis. 2003;7:215–21. doi: 10.1016/s1201-9712(03)90055-5. [DOI] [PubMed] [Google Scholar]
- 79.Lidehall AK, Engman ML, Sund F, Malm G, Lewensohn-Fuchs I, Ewald U, et al. Cytomegalovirus-specific CD4 and CD8 T cell responses in infants and children. Scand J Immunol. 2013;77:135–43. doi: 10.1111/sji.12013. [DOI] [PubMed] [Google Scholar]
- 80.Hayashi N, Kimura H, Morishima T, Tanaka N, Tsurumi T, Kuzushima K. Flow cytometric analysis of cytomegalovirus-specific cell-mediated immunity in the congenital infection. J Med Virol. 2003;71:251–8. doi: 10.1002/jmv.10477. [DOI] [PubMed] [Google Scholar]
- 81.Tu W, Chen S, Sharp M, Dekker C, Manganello AM, Tongson EC, et al. Persistent and selective deficiency of CD4+ T cell immunity to cytomegalovirus in immunocompetent young children. J Immunol. 2004;172:3260–7. doi: 10.4049/jimmunol.172.5.3260. [DOI] [PubMed] [Google Scholar]
- 82.Vermijlen D, Brouwer M, Donner C, Liesnard C, Tackoen M, Van Rysselberge M, et al. Human cytomegalovirus elicits fetal gammadelta T cell responses in utero. J Exp Med. 2010;207:807–21. doi: 10.1084/jem.20090348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Avanzini MA, Maccario R, Belloni C, Carrera G, Bertaina A, Cagliuso M, et al. B lymphocyte subsets and their functional activity in the early months of life. Int J Immunopathol Pharmacol. 2010;23:247–54. doi: 10.1177/039463201002300122. [DOI] [PubMed] [Google Scholar]
- 84.Williams JV, Weitkamp JH, Blum DL, LaFleur BJ, Crowe JE. The human neonatal B cell response to respiratory syncytial virus uses a biased antibody variable gene repertoire that lacks somatic mutations. Mol Immunol. 2009;47:407–14. doi: 10.1016/j.molimm.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Boppana SB, Britt WJ. Antiviral antibody responses and intrauterine transmission after primary maternal cytomegalovirus infection. J Infect Dis. 1995;171:1115–21. doi: 10.1093/infdis/171.5.1115. [DOI] [PubMed] [Google Scholar]
- 86.Furione M, Rognoni V, Sarasini A, Zavattoni M, Lilleri D, Gerna G, et al. Slow increase in IgG avidity correlates with prevention of human cytomegalovirus transmission to the fetus. J Med Virol. 2013;85:1960–7. doi: 10.1002/jmv.23691. [DOI] [PubMed] [Google Scholar]
- 87.Wang D, Fu TM. Progress on human cytomegalovirus vaccines for prevention of congenital infection and disease. Curr Opin Virol. 2014;6:13–23. doi: 10.1016/j.coviro.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 88.Lilleri D, Kabanova A, Revello MG, Percivalle E, Sarasini A, Genini E, et al. Fetal human cytomegalovirus transmission correlates with delayed maternal antibodies to gH/gL/pUL128-130-131 complex during primary infection. PLoS One. 2013;8:e59863. doi: 10.1371/journal.pone.0059863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kabanova A, Perez L, Lilleri D, Marcandalli J, Agatic G, Becattini S, et al. Antibody-driven design of a human cytomegalovirus gHgLpUL128L subunit vaccine that selectively elicits potent neutralizing antibodies. Proc Natl Acad Sci U S A. 2014;111:17965–70. doi: 10.1073/pnas.1415310111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang D, Freed DC, He X, Li F, Tang A, Cox KS, et al. A replication-defective human cytomegalovirus vaccine for prevention of congenital infection. Sci Transl Med. 2016;8:362ra145. doi: 10.1126/scitranslmed.aaf9387. [DOI] [PubMed] [Google Scholar]
- 91.Ha S, Li F, Troutman MC, Freed DC, Tang A, Loughney JW, et al. Neutralization of diverse human cytomegalovirus strains conferred by antibodies targeting viral gH/gL/pUL128-131 pentameric complex. J Virol. 2017:91. doi: 10.1128/JVI.02033-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lemmermann NA, Krmpotic A, Podlech J, Brizic I, Prager A, Adler H, et al. Non-redundant and redundant roles of cytomegalovirus gH/gL complexes in host organ entry and intra-tissue spread. PLoS Pathog. 2015;11:e1004640. doi: 10.1371/journal.ppat.1004640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nigro G, Adler SP, La Torre R, Best AM, Group CCC Passive immunization during pregnancy for congenital cytomegalovirus infection. N Engl J Med. 2005;353:1350–62. doi: 10.1056/NEJMoa043337. [DOI] [PubMed] [Google Scholar]
- 94.Juckstock J, Rothenburger M, Friese K, Traunmuller F. Passive immunization against congenital cytomegalovirus infection: Current state of knowledge. Pharmacology. 2015;95:209–17. doi: 10.1159/000381626. [DOI] [PubMed] [Google Scholar]
- 95.Revello MG, Lazzarotto T, Guerra B, Spinillo A, Ferrazzi E, Kustermann A, et al. A randomized trial of hyperimmune globulin to prevent congenital cytomegalovirus. N Engl J Med. 2014;370:1316–26. doi: 10.1056/NEJMoa1310214. [DOI] [PubMed] [Google Scholar]
- 96.Simister NE. Placental transport of immunoglobulin G. Vaccine. 2003;21:3365–9. doi: 10.1016/s0264-410x(03)00334-7. [DOI] [PubMed] [Google Scholar]
- 97.Maidji E, McDonagh S, Genbacev O, Tabata T, Pereira L. Maternal antibodies enhance or prevent cytomegalovirus infection in the placenta by neonatal Fc receptor-mediated transcytosis. Am J Pathol. 2006;168:1210–26. doi: 10.2353/ajpath.2006.050482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Boppana SB, Rivera LB, Fowler KB, Mach M, Britt WJ. Intrauterine transmission of cytomegalovirus to infants of women with preconceptional immunity. N Engl J Med. 2001;344:1366–71. doi: 10.1056/NEJM200105033441804. [DOI] [PubMed] [Google Scholar]
- 99.Yamamoto AY, Mussi-Pinhata MM, Boppana SB, Novak Z, Wagatsuma VM, Oliveira PeF, et al. Human cytomegalovirus reinfection is associated with intrauterine transmission in a highly cytomegalovirus-immune maternal population. Am J Obstet Gynecol. 2010;202:297.e1–8. doi: 10.1016/j.ajog.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kobayashi Y, Morioka I, Koda T, Nakamachi Y, Okazaki Y, Noguchi Y, et al. Low total IgM values and high cytomegalovirus loads in the blood of newborns with symptomatic congenital cytomegalovirus infection. J Perinat Med. 2015;43:239–43. doi: 10.1515/jpm-2014-0071. [DOI] [PubMed] [Google Scholar]
- 101.Nozawa N, Fang-Hoover J, Tabata T, Maidji E, Pereira L. Cytomegalovirus-specific, high-avidity IgG with neutralizing activity in maternal circulation enriched in the fetal bloodstream. J Clin Virol. 2009;46(Suppl 4):S58–63. doi: 10.1016/j.jcv.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rovito R, Korndewal MJ, van Zelm MC, Ziagkos D, Wessels E, van der Burg M, et al. T and B cell markers in dried blood spots of neonates with congenital cytomegalovirus infection: B cell numbers at birth are associated with long-term outcomes. J Immunol. 2017;198:102–9. doi: 10.4049/jimmunol.1601182. [DOI] [PubMed] [Google Scholar]
- 103.Cekinović D, Golemac M, Pugel EP, Tomac J, Cicin-Sain L, Slavuljica I, et al. Passive immunization reduces murine cytomegalovirus-induced brain pathology in newborn mice. J Virol. 2008;82:12172–80. doi: 10.1128/JVI.01214-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.van den Pol AN, Reuter JD, Santarelli JG. Enhanced cytomegalovirus infection of developing brain independent of the adaptive immune system. J Virol. 2002;76:8842–54. doi: 10.1128/JVI.76.17.8842-8854.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cheeran MC, Lokensgard JR, Schleiss MR. Neuropathogenesis of congenital cytomegalovirus infection: disease mechanisms and prospects for intervention. Clin Microbiol Rev. 2009;22:99–126. doi: 10.1128/CMR.00023-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mutnal MB, Cheeran MC, Hu S, Lokensgard JR. Murine cytomegalovirus infection of neural stem cells alters neurogenesis in the developing brain. PLoS One. 2011;6:e16211. doi: 10.1371/journal.pone.0016211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Odeberg J, Wolmer N, Falci S, Westgren M, Seiger A, Soderberg-Naucler C. Human cytomegalovirus inhibits neuronal differentiation and induces apoptosis in human neural precursor cells. J Virol. 2006;80:8929–39. doi: 10.1128/JVI.00676-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tsutsui Y, Kawasaki H, Kosugi I. Reactivation of latent cytomegalovirus infection in mouse brain cells detected after transfer to brain slice cultures. J Virol. 2002;76:7247–54. doi: 10.1128/JVI.76.14.7247-7254.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kosmac K, Bantug GR, Pugel EP, Cekinovic D, Jonjic S, Britt WJ. Glucocorticoid treatment of MCMV infected newborn mice attenuates CNS inflammation and limits deficits in cerebellar development. PLoS Pathog. 2013;9:e1003200. doi: 10.1371/journal.ppat.1003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Seleme MC, Kosmac K, Jonjic S, Britt WJ. Tumor necrosis factor alpha-induced recruitment of inflammatory mononuclear cells leads to inflammation and altered brain development in murine cytomegalovirus-infected newborn mice. J Virol. 2017;91:e01983–16. doi: 10.1128/JVI.01983-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.van den Pol AN, Robek MD, Ghosh PK, Ozduman K, Bandi P, Whim MD, et al. Cytomegalovirus induces interferon-stimulated gene expression and is attenuated by interferon in the developing brain. J Virol. 2007;81:332–48. doi: 10.1128/JVI.01592-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kosugi I, Kawasaki H, Arai Y, Tsutsui Y. Innate immune responses to cytomegalovirus infection in the developing mouse brain and their evasion by virus-infected neurons. Am J Pathol. 2002;161:919–28. doi: 10.1016/S0002-9440(10)64252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sakao-Suzuki M, Kawasaki H, Akamatsu T, Meguro S, Miyajima H, Iwashita T, et al. Aberrant fetal macrophage/microglial reactions to cytomegalovirus infection. Ann Clin Transl Neurol. 2014;1:570–88. doi: 10.1002/acn3.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ostermann E, Macquin C, Krezel W, Bahram S, Georgel P. Increased viral dissemination in the brain and lethality in MCMV-infected, dicer-deficient neonates. Viruses. 2015;7:2308–20. doi: 10.3390/v7052308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Milovanovic J, Popovic B, Milovanovic M, Kvestak D, Arsenijevic A, Stojanovic B, et al. Murine cytomegalovirus infection induces susceptibility to EAE in resistant BALB/c mice. Front Immunol. 2017;8:192. doi: 10.3389/fimmu.2017.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vanheusden M, Broux B, Welten SP, Peeters LM, Panagioti E, Van Wijmeersch B, et al. Cytomegalovirus infection exacerbates autoimmune mediated neuroinflammation. Sci Rep. 2017;7:663. doi: 10.1038/s41598-017-00645-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schachtele SJ, Hu S, Sheng WS, Mutnal MB, Lokensgard JR. Glial cells suppress postencephalitic CD8+ T lymphocytes through PD-L1. Glia. 2014;62:1582–94. doi: 10.1002/glia.22701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mutnal MB, Hu S, Little MR, Lokensgard JR. Memory T cells persisting in the brain following MCMV infection induce long-term microglial activation via interferon-γ. J Neurovirol. 2011;17:424–37. doi: 10.1007/s13365-011-0042-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Prasad S, Hu S, Sheng WS, Chauhan P, Singh A, Lokensgard JR. The PD-1: PD-L1 pathway promotes development of brain-resident memory T cells following acute viral encephalitis. J Neuroinflammation. 2017;14:82. doi: 10.1186/s12974-017-0860-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lokensgard JR, Schachtele SJ, Mutnal MB, Sheng WS, Prasad S, Hu S. Chronic reactive gliosis following regulatory T cell depletion during acute MCMV encephalitis. Glia. 2015 doi: 10.1002/glia.22868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Prasad S, Hu S, Sheng WS, Singh A, Lokensgard JR. Tregs modulate lymphocyte proliferation, activation, and resident-memory T-cell accumulation within the brain during MCMV infection. PLoS One. 2015;10:e0145457. doi: 10.1371/journal.pone.0145457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mutnal MB, Hu S, Lokensgard JR. Persistent humoral immune responses in the CNS limit recovery of reactivated murine cytomegalovirus. PLoS One. 2012;7:e33143. doi: 10.1371/journal.pone.0033143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schachtele SJ, Mutnal MB, Schleiss MR, Lokensgard JR. Cytomegalovirus-induced sensorineural hearing loss with persistent cochlear inflammation in neonatal mice. J Neurovirol. 2011;17:201–11. doi: 10.1007/s13365-011-0024-7. [DOI] [PMC free article] [PubMed] [Google Scholar]