Abstract
BACKGROUND
Convergence Insufficiency (CI) is a common binocular vision disorder which often causes symptoms when doing near work. However, the best screening test for CI is unknown. The purpose of this study was to evaluate the ability of common tests of binocular and accommodative function to identify children with CI in a school screening setting.
METHODS
Children ages 9 to 14 were invited to participate. Positive fusional vergences (PFV), near point of convergence (NPC), accommodative amplitude, accommodative facility, Modified Thorington, and the Convergence Insufficiency Symptom Survey were evaluated.
RESULTS
Of the 282 children tested, approximately 20% had 2–3 sign CI. One half of 2–3 sign CI and 66% of 3 sign CI subjects were symptomatic. Approximately 61% of subjects with symptomatic 2–3 sign CI had an accompanying low accommodative amplitude. The largest area under the ROC curve was obtained using NPC break measurements. NPC break ≥ 6 cm for CI and NPC break ≥ 7 cm for symptomatic CI were the cut points that maximized the sum of sensitivity and specificity.
CONCLUSION
NPC break performed best in identifying children with CI.
Keywords: children, convergence insufficiency, near point of convergence, screening
Convergence Insufficiency is a common binocular vision condition with a prevalence of approximately 2 to 8% in which a person is unable to adequately converge for near work.1–4 Symptoms associated with convergence insufficiency often include blurred vision, eyestrain, headache, diplopia, frequent loss of place, difficulty concentrating on near work, and/or avoidance of near work.1,5,6 In addition, Rouse et al.7,8 found that parents of children with symptomatic convergence insufficiency report a significantly higher number of academic performance symptoms (such as difficulty completing assignments, careless mistakes, avoidance of near work, inattentiveness, and worry about school performance), as compared to parents of children with normal binocular vision.
The Convergence Insufficiency Treatment Trial (CITT) showed that approximately 80% of children with symptomatic convergence insufficiency were improved or successfully treated with vergence/accommodative therapy, demonstrating that therapy is effective in improving the signs and symptoms of convergence insufficiency.9 Furthermore, fewer performance symptoms, less parental concern and improved attention have been reported after successful or improved treatment outcome.10,11 Despite its common occurrence, many children with convergence insufficiency are unaware that they have convergence insufficiency. Screening to detect convergence insufficiency may allow identification and referral for management. However, school screenings often focus on detection of reduced visual acuity, rather than binocular vision dysfunction such as convergence insufficiency, and the best test to screen for the presence of convergence insufficiency is not known. The purpose of this study was to evaluate the ability of common tests of ocular alignment, convergence ability, and accommodative ability to identify children with convergence insufficiency in a school screening setting in which it may only be feasible to do one test. While the focus of treatment is often on symptomatic convergence insufficiency9, we evaluate screening for both symptomatic and any convergence insufficiency given that there may be interest in the detection of all children with convergence insufficiency (symptomatic and asymptomatic) to allow referral for follow up.
METHODS
The Ohio State University Institutional Review Board reviewed and approved the research study and informed consent documents. Elementary and middle schools in central Ohio were invited to participate. High schools were not invited due to the difficulty of screening children with varied schedules, essentially limiting the upper age of enrollment to age 14 years. Children younger than 9 years of age were not invited to take part in the study because the CISS is validated for children ages 9 to 18 years of age.6 All children ages 9 years and older at 8 participating schools in central Ohio were invited to participate. Signed parental permission and assent were obtained from the participating parents and children, respectively. Screenings were performed in a large, quiet room at each school. Auxiliary lighting was brought to each screening to ensure appropriate illumination. Children who wore glasses were tested while wearing their correction.
Modified Clinical Technique Screening
Modified Clinical Technique Screening was performed in order to identify and exclude children with amblyopia or significant uncorrected refractive error. Testing was performed by optometrists experienced in working with children. Distance visual acuity was tested monocularly using a light box and a logMAR ETDRS chart. Retinoscopy was performed while the child wore retinoscopy glasses and watched a video. Unilateral and alternate cover testing was performed at distance and 40 cm using a 6/9 (20/30) letter as a fixation target. Children who met the Modified Clinical Technique referral criteria for reduced distance visual acuity (6/12 [20/40] or worse in either eye), hyperopia (≥ +1.50D in either eye), astigmatism (cylinder of ≥ +1.0D in either eye), and/or anisometropia (≥ 1.0D) were excluded and referred for care by an eye care professional.12 As distance visual acuity is generally effective in identifying children with myopia and children may over-accommodate slightly on dry retinoscopy, children were not excluded for apparent low myopia (e.g. −0.50 or −0.75) on dry screening retinoscopy if visual acuity was 6/9.5 (20/32) or better in each eye. Children were excluded and referred for myopia of −0.50 or more on dry screening retinoscopy with visual acuity of 6/12 (20/40) or worse in either eye.
Selection of Convergence Insufficiency Screening Study Procedures
Testing that is commonly included in the diagnosis of symptomatic convergence insufficiency9 (ocular alignment, near point of convergence, positive fusional vergence) was performed in order to allow investigation of the ability of each test to identify children with 2 or 3 signs of convergence insufficiency (exophoria at near with receded near point of convergence and/or insufficient positive fusional vergence). The ability of the positive fusional vergence/phoria ratio was also investigated because this ratio is also commonly used in the diagnosis of convergence insufficiency.9 Ocular alignment was assessed using both cover testing and Modified Thorington, but only the latter was evaluated for its ability to identify children with convergence insufficiency in a screening setting because cover testing requires a high level of training and skill. The Convergence Insufficiency Symptom Survey (CISS) score was also included because convergence insufficiency is frequently associated with near work symptoms.6,13,14 Tests of accommodative ability were also included because convergence insufficiency is commonly accompanied by accommodative dysfunction, and the vergence and accommodative systems are linked.2,15
Convergence Insufficiency Screening Study Procedures
All screeners were trained prior to the screenings and adhered to protocols and scripts for each procedure. Testing to determine ocular alignment (cover testing and Modified Thorington) was performed by optometrists experienced in working with children. Unilateral and alternate cover testing was performed at distance and 40 cm using a 6/9 (20/30) letter as a fixation target. Modified Thorington (phoria near test card, Bernell, Mishawaka, IN) was performed at 40 cm. A non-elastic string was attached to the card to help maintain the correct distance during testing. Each subject viewed the Modified Thorington card with a Maddox rod placed over the right eye. The instructions that accompany the Modified Thorington were read to the subject to help ensure understanding of the test and consistency of instructions.
The CISS was used to assess symptoms and testing was performed by a trained lay screener. Screening tests of vergence and accommodative function were performed by trained student optometric clinicians. CISS, near point of convergence, positive fusional vergence, accommodative amplitude of the right eye, and accommodative facility of the right eye were performed according to CITT protocol.16 The CISS was administered before tests of vergence or accommodative function by a screener masked to the other screening tests. The child was provided a card with printed response options (i.e., Never, Infrequently [not very often], Sometimes, Fairly Often, Always) and read each question verbatim. The tester recorded the response to each of the 15 questions. Scores for responses ranged from 0 for “never” to 4 for “always”; the total score could range from 0 to 60. Monocular near visual acuities were measured using a standard equivalent Snellen near card. Near point of convergence testing was performed using a single column of letters of 6/9 (20/30) equivalent at 40 cm on a near point rule (Gulden Ophthalmics, Elkins Park, PA). The child was instructed to: “look at the letters and report when they become double or break into two, but try to keep the target one/single as long as possible.” Near point of convergence break and recovery were measured three times (recorded to the nearest half-centimeter) and the mean was used for analysis. Positive fusional vergence range was measured using a horizontal prism bar and a hand-held fixation target (Gulden Fixation Stick #15302) containing a single column of letters of 6/9 (20/30) equivalent held at 40 cm. Each subject was asked to report when the letters blurred or became double (split into 2) and to keep the target single as long as possible. Blur, break and recovery values were measured three times. The break value was used in the analysis if no blur value was reported. Because the primary goal of the study was to identify children with convergence insufficiency, only base-out fusion ranges were assessed. If diplopia was not reported but the examiner observed a loss of fusion during the measurement of near convergence ranges, the point at which fusion was lost was recorded as the “break” finding. Monocular amplitude of accommodation was assessed using a single column of letters of 6/9 (20/30) equivalent at 40 cm and a near point rule. The first sustained blur was considered the endpoint. Accommodative amplitude was measured three times (right eye only, recorded to the nearest half-centimeter) and the mean was used for analysis. Monocular accommodative facility was measured using ± 2.00 lens flippers and a single column of letters of 6/9 (20/30) equivalent on a hand-held fixation target containing a single column of letters of 6/9 (20/30) equivalent held at 40 cm. The subject was instructed to try to get the letters clear as quickly as possible, and to report (by saying “clear”) as soon as the letters were clear. Monocular accommodative facility (cycles/minute) was assessed only on the right eye. Binocular accommodative facility was also assessed using a Suppression Vectogram number 9 and Polaroid filter glasses (Stereo Optical, Chicago, IL).
Classification
Subjects were classified as having convergence insufficiency if they had an exophoria at near and exhibited 2 or 3 clinical signs of convergence insufficiency. The signs of convergence insufficiency were: 1) presence of exophoria 4Δ greater at near than at distance on cover testing, 2) insufficient positive fusional vergence (i.e., failing Sheard’s criterion or positive fusional vergence < 15Δ base-out) at near, and 3) a receded near point of convergence of ≥ 6.0 cm.2,14 Esophoria at near was defined as ≥ 3Δ at near. Orthophoria with convergence dysfunction was classified as orthophoria with positive fusional vergence ≤ 15Δ to base-out blur (break if no blur) and a receded near point of convergence of ≥ 6.0 cm. Subjects were classified as having low accommodative amplitude if monocular accommodative amplitude was at least 2D less than minimum expected amplitude (15 – [age * 0.25]).1,15 Performance more than one standard deviation below the normative value of 11 cycles per minute (cpm) for school-age children was classified as monocular accommodative infacility (< 6.0 cpm).1 Subjects were classified as symptomatic (CISS ≥ 16) or asymptomatic (CISS < 16) based upon the CISS score.5,6
Statistical Analysis
Data analyses were performed using Statistical Analysis System (version 9.2, SAS Institute, Cary, NC) and Statistical Package for the Social Sciences (version 15). Unless specifically stated otherwise, an alpha level of 0.05 was used to assess statistical significance. Receiver operating characteristic (ROC) curve analysis was used to evaluate the ability of each test (positive fusional vergence range blur, break, recovery; near point of convergence break, recovery; accommodative amplitude; accommodative facility; CISS; Modified Thorington; near visual acuity) and Sheard’s criterion (positive fusional vergence range blur [or break if no blur] divided by near phoria) to discriminate between subjects with and without convergence insufficiency. A receiver operating characteristic curve shows the trade-offs between the false positive rate (1-specificity) versus the true positive rate (sensitivity). The area under the receiver operating characteristic curve describes the accuracy of the clinical test to distinguish those with convergence insufficiency from those without convergence insufficiency. An area of 1.0 would signify a perfect test while an area of 0.5 is associated with a weak test (i.e. no better than flipping a fair coin). The cut-point which maximized the sum of sensitivity and specificity was also identified for tests with the largest areas under the curve which were significantly greater than 0.50. Choosing the cut-point which maximizes the sum of sensitivity and specificity equates to choosing the value of the clinical test which minimizes the sum of the false positive and false negative rates.17 Using this cut-point ensures that the correct classification is maximized or, conversely, that misclassification is minimized. Near point of convergence cut-points of 0.25 cm or 0.75 cm were rounded up to the nearest half centimeter. Regression was not performed to assess the ability of each clinical measure to identify children with accommodative dysfunction because only accommodative amplitude and facility were performed due to time limitations.
RESULTS
Three hundred forty-nine children between the ages of 9 and 14 years from 8 central Ohio private and public schools were screened. Sixty-seven subjects (19%) were excluded from the study due to inability to complete the testing due to developmental delay (n=8), reduced distance VA (n=39), and/or meeting the Modified Clinical Technique referral criteria for refractive error (n=20).
Of the 282 subjects included in the analysis, approximately 60% were female, 81.5% identified themselves as white, 11% as African American, 2.5% as other, and 5% did not report race. Two percent classified themselves as Hispanic or Latino, 76% reported not being Hispanic or Latino, and 22% did not report their ethnicity. Sixteen of the 282 subjects had a near visual acuity of 6/12 (20/40) or worse in one or both eyes, but had a refractive error of less than +1.50 on dry screening retinoscopy. Of these 16 children, 3 (18.8%) were classified as having 2–3 signs of convergence insufficiency while 11 (68.8%) had low accommodative amplitude. One hundred twenty children were identified as symptomatic (162 non-symptomatic).
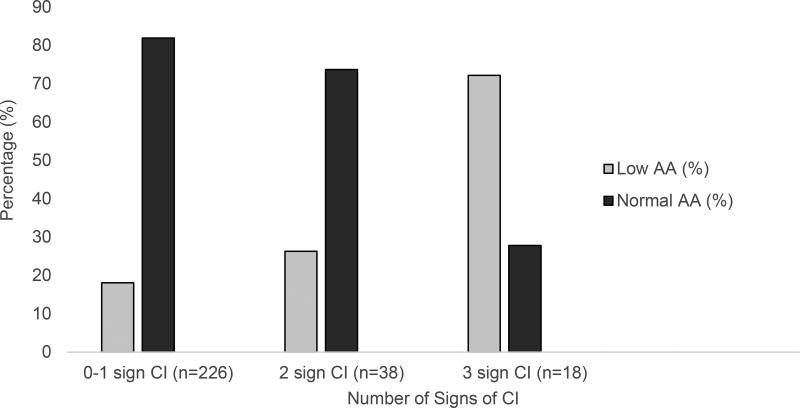
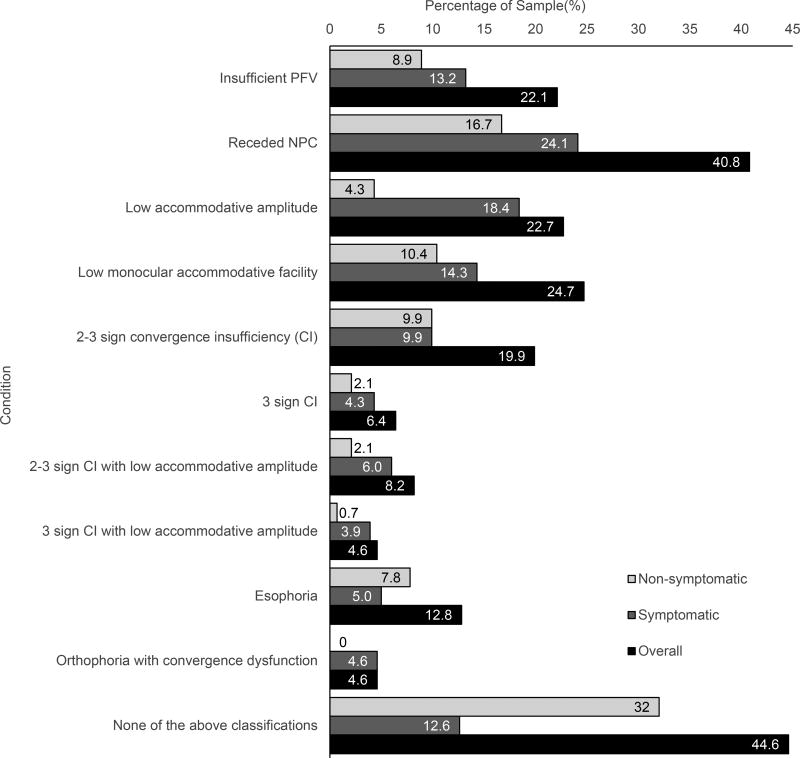
Table 1 shows mean, median and range values for positive fusional vergence, near point of convergence, accommodative amplitude, accommodative facility, Modified Thorington, and cover test that characterize the study population. A wide range of findings were observed for each test. The association between convergence insufficiency and low amplitude of accommodation and the percentages of subjects classified as having binocular or accommodative dysfunction by symptom level are shown in Figures 1 and 2, respectively. Low amplitude was observed in 26% (10/38) of those with 2 signs and in 72% (13/18) of those with 3 signs of convergence insufficiency (Figure 1). Approximately 81% (52/64) of the subjects with low accommodative amplitude, 50% (28/56) of those with 2 to 3 signs of convergence insufficiency and 66% (12/18) of those with 3 signs of convergence insufficiency were symptomatic (Figure 2).
Table 1.
Descriptive statistics for measures of binocular and accommodative function
| Test | n† | Mean | Std | Median | Range |
|---|---|---|---|---|---|
| Positive Fusional Vergence (Δ) Blur | 282 | 24.68 | 11.94 | 22.67 | 2.33, 45.00 |
| Positive Fusional Vergence (Δ) Break | 282 | 30.27 | 11.95 | 31.67 | 5.33, 50.00 |
| Positive Fusional Vergence (Δ) Recovery | 282 | 24.32 | 11.67 | 21.83 | 0.33, 45.00 |
| Near Point of Convergence (cm) Break | 282 | 6.90 | 5.35 | 5.00 | 0.5, 32.5 |
| Near Point of Convergence (cm) Recovery | 282 | 9.73 | 6.38 | 7.50 | 3.0, 37.5 |
| Accommodative Amplitude (D) | |||||
| Overall | 282 | 15.18 | 6.45 | 16.50 | 3.0, 25.0 |
| 9 year olds | 69 | 14.64 | 7.59 | 16.50 | 3.0, 25.0 |
| 10 year olds | 76 | 15.42 | 6.64 | 16.50 | 3.0, 25.0 |
| 11 year olds | 44 | 14.67 | 5.87 | 14.50 | 3.0, 25.0 |
| 12 year olds | 41 | 15.43 | 5.85 | 15.50 | 3.5, 25.0 |
| 13 year olds | 27 | 16.57 | 5.22 | 16.50 | 6.5, 25.0 |
| 14 year olds | 25 | 14.86 | 5.89 | 14.50 | 3.0, 25.0 |
| Accommodative Facility OD | 279 | 9.31 | 5.03 | 10.00 | 0, 25.0 |
| Accommodative Facility OU | 277 | 6.87 | 4.76 | 7.00 | 0, 28.0 |
| Modified Thorington ‡ | 281 | −1.15 | 4.17 | −1.00 | −18.0, 24.0 |
| Cover Test at distance ‡ | 282 | −0.23 | 2.38 | 0.00 | −15.0, 16.0 |
| Cover Test at near ‡ | 281 | −0.94 | 6.04 | 0.00 | −20.0, 25.0 |
| CISS Score | 282 | 16.36 | 12.28 | 14.00 | 0.0, 55.0 |
Variations from n=282 are a result of some children needing to leave the screening early to attend class;
Exodeviations indicated by minus sign; (Δ) = prism diopter; D = diopter; cm = centimeter
Figure 1.
Percentages of children with convergence insufficiency and low amplitude of accommodation
CI = Convergence insufficiency; AA = Amplitude of accommodation
Figure 2.
Percentages of subjects classified with binocular or accommodative dysfunction, by symptom level
Identification of Convergence Insufficiency
Receiver operating characteristic curve analysis was performed to evaluate the ability of each test to identify subjects who were classified as having convergence insufficiency. Tables 2 and 3 provide a summary of the tests which achieved the largest area under the curve and the cut-point which maximized the sum of sensitivity and specificity. (Tests not listed never achieved the largest area under the curve.) The test finding which most frequently achieved one of the largest areas under the curve was near point of convergence break. Near point of convergence break was also the best test for identification of convergence insufficiency when defined as receded near point of convergence and reduced positive fusional vergence at near in the presence of any phoria (area under curve 0.835; cut-point ≥ 6 cm; sensitivity = 0.847; specificity = 0.743).18 Near point of convergence recovery was also often among the best tests, but had smaller areas under the curve than near point of convergence break. The ratio of positive fusional vergence to phoria was among the top 3 tests for identifying both 3 sign convergence insufficiency and symptomatic 3 sign convergence insufficiency. Amplitude of accommodation was among the top 3 tests for identifying those with symptomatic 3 sign convergence insufficiency.
Table 2.
Test results found to have the greatest area under the curve in ROC analysis
| Measure | Clinical test | ||
|---|---|---|---|
|
| |||
| Test Rank | 1 | 2 | 3 |
| Screening for 2–3 sign CI | |||
| Test | MT phoria (Δ) | NPC break (cm) | NPC recovery (cm) |
| AUC (p-value) | 0.795 (< 0.001) | 0.766 (< 0.001) | 0.744 (< 0.001) |
| Cutpoint | ≥ 3 exo | ≥ 6.0 | ≥ 8.5 |
| Sensitivity | 0.625 | 0.804 | 0.750 |
| Specificity | 0.858 | 0.690 | 0.664 |
|
| |||
| Screening for 3 sign CI | |||
| Test | PFV / Phoria | PFV blur (Δ) | NPC break (cm) |
| AUC (p-value) | 0.938 (< 0.001) | 0.863 (< 0.001) | 0.853 (< 0.001) |
| Cutpoint | ≤ 1.90 | < 14 | ≥ 6.0 |
| Sensitivity | 0.889 | 0.778 | 1.000 |
| Specificity | 0.908 | 0.837 | 0.633 |
|
| |||
| Screening for symptomatic 2–3 sign CI | |||
| Test | CISS | NPC break (cm) | NPC recovery (cm) |
| AUC (p-value) | 0.823 (< 0.001) | 0.781 (< 0.001] | 0.777 (< 0.001) |
| Cutpoint | ≥ 16 | ≥ 7.5 | ≥ 9.0 |
| Sensitivity | 1.000 | 0.679 | 0.750 |
| Specificity | 0.638 | 0.783 | 0.669 |
|
| |||
| Screening for symptomatic 3 sign CI | |||
| Test | AA (D) | PFV / Phoria | NPC break (cm) |
| AUC (p-value) | 0.937 (< 0.001) | 0.925 (< 0.001) | 0.916 (< 0.001) |
| Cutpoint | ≤ 10.0 | ≤ 1.9 | ≥ 9.5 |
| Sensitivity | 1.000 | 0.833 | 0.833 |
| Specificity | 0.800 | 0.886 | 0.859 |
|
| |||
| Screening for symptomatic 2–3 sign CI with accommodative dysfunction | |||
| Test | NPC break (cm) | NPC recovery (cm) | CISS |
| AUC (p-value) | 0.915 (< 0.001) | 0.895 (< 0.001) | 0.850 (< 0.001) |
| Cutpoint | ≥ 7.5 | ≥ 10.0 | ≥ 16 |
| Sensitivity | 0.941 | 0.941 | 1.000 |
| Specificity | 0.780 | 0.731 | 0.614 |
AUC = Area under ROC curve; MT = Modified Thorington; NPC = Near Point of Convergence; PFV = Positive Fusional Vergence; AA = Accommodative amplitude; CISS = Convergence Insufficiency Symptom Survey score
Table 3.
Summary of best screening tests according to classification of CI
| Clinical Test |
Test used to define CI (w/ or w/o symptoms) |
2–3 sign CI | 3 sign CI |
symptomatic 2–3 sign CI |
symptomatic 3 sign CI |
symptomatic 2–3 sign CI with accommodative dysfunction |
|---|---|---|---|---|---|---|
| NPC break (cm) | X | X | X | X | X | X |
| NPC recovery (cm) | X | X | X | |||
| PFV / Phoria | X | X | X | |||
| Phoria (Δ) (MT) | X† | X | ||||
| PFV blur (Δ) | X | X | ||||
| CISS | X | X | X | |||
| Accommodative Amplitude (D) | X | |||||
| Accommodative Facility |
CI = Convergence Insufficiency; MT = Modified Thorington; NPC = Near Point of Convergence; CISS = Convergence Insufficiency Symptom Survey score
Phoria as measured by cover testing was used to define convergence insufficiency and positive fusional vergence/phoria.
DISCUSSION
In this study, approximately 20% of subjects had 2 to 3 signs of convergence insufficiency and 6% showed 3 signs of convergence insufficiency. Similar to Rouse et al.2 this study found a high frequency of accommodative dysfunction accompanying convergence insufficiency. This study found that approximately 61% of children with symptomatic 2 or 3 sign convergence insufficiency had an accompanying low accommodative amplitude which is comparable to the 55–78.9% and to the 26–77.8% association reported by Rouse et al.2 and Borsting et al.3 for 2 or 3 sign convergence insufficiency, respectively.
These results support the association between convergence insufficiency and accommodative disorders with near work symptoms such as eyestrain, blurred vision, double vision and frequent loss of place, which may have an impact on the amount, quality, and efficiency of near work someone is able to perform.3,6,19–21 In this study, approximately 81% of the subjects with low accommodative amplitude, 50% of the 2 to 3 sign convergence insufficiency subjects and 66% of the 3 sign convergence insufficiency subjects were symptomatic. These numbers are similar to those reported by Rouse,14 who found that 47% of those with suspect (1–2 sign) convergence insufficiency, and 72.7% of those with definite (3 sign) convergence insufficiency were symptomatic. It is possible that some subjects who were not symptomatic had suppression, but this was not assessed directly in this study.
We evaluated the ability of common tests of alignment, vergence and accommodative function to identify children with convergence insufficiency. Although measures of vergence and alignment were included in the classification of convergence insufficiency, these results show which of these commonly used tests perform best in identifying children with convergence insufficiency in a screening setting when it may only be possible to perform one test. Near point of convergence break consistently achieved one of the highest areas under the curve for detection of convergence insufficiency and was, therefore, one of the best tests for identification of convergence insufficiency in a screening setting. It is interesting to note that a study by Rouse et al.22 revealed that near point of convergence is often used as the sole means of diagnosing convergence insufficiency in clinical practice. Near point of convergence (along with binocular accommodative facility) has also been reported to have the best diagnostic validity for identification of symptomatic, high near exophoria in subjects ages 19 to 35 years.23 The test with the highest sum of sensitivity and specificity was also most frequently near point of convergence break, however the best cut-point (to maximize the sum of sensitivity and specificity) varied. The best referral cut-points for near point of convergence were similar to those used in the CITT studies, however, this may be in part due to the use of CITT cut-points in classification.9 A near point of convergence break ≥ 6 cm had the best sum of sensitivity and specificity for identifying children with convergence insufficiency (2–3 sign convergence insufficiency, 3 sign convergence insufficiency) while a near point of convergence break ≥ 7.5 cm generally had the highest sum of sensitivity and specificity for symptomatic convergence insufficiency. The near point of convergence cut-point of ≥ 6 cm is in agreement with the 6 cm cut-off used for convergence insufficiency classification in the CITT16 and both cut-points are in accord with the 6–10 cm cut-off for school screenings recommended by Hayes et al.24
For detection of convergence insufficiency with 3 signs, the ratio of positive fusional vergence over the phoria also performed among the best tests, but this would require testing both positive fusional vergence and phoria and determination of the ratio. Amplitude of accommodation was among the best tests for detection of symptomatic 3 sign convergence insufficiency. CISS score was among the best tests for the detection of symptomatic convergence insufficiency, although this may be due to its inclusion in the classifications for symptomatic convergence insufficiency. Furthermore, specificity for CISS was low (approximately 61 to 63%) and 12.6% of children had a symptomatic score in the absence of meeting the criteria for any binocular or accommodative dysfunction. It is noteworthy that the CISS was not designed as a screening test for use in the absence of clinical testing, rather it was designed and validated for use in differentiating between those with symptomatic convergence insufficiency and normal binocular vision when used in conjunction with clinical signs, and for use in monitoring changes in symptoms with treatment.5,6
These results suggest that near point of convergence testing with a referral criterion of 6 cm or greater for break (reported double or observation of loss of fusion) performs best for identifying children with convergence insufficiency in general and a near point of convergence with a referral criterion of 7.5 cm or greater for break performs best for identifying children with symptomatic convergence insufficiency (two or three signs). Amplitude of accommodation with a referral criterion of 10D or less was also among the best tests for identifying children with symptomatic 3 sign convergence insufficiency. Near point of convergence and amplitude of accommodation testing are tests that nurse and/or lay screeners may be able to be trained to perform, therefore, further research should evaluate the effectiveness of screening for convergence insufficiency with one or both of these tests when performed by nurse and/or lay screeners.
Acknowledgments
The authors would like to thank the following people for assisting in conducting the school screenings: Molly Biddle, Drs. Shane Foster, Michael Kennedy, Cayti McDaniel, Kristin Oblad, Joseph Osmond, Amy Anderson, Marielle Serenda, and Ivy Madson.
Partial funding provided by T35-EY07151 and the Ohio Lions Eye Research Foundation.
Footnotes
DISCLOSURES
The authors declare that they have no conflict of interest.
References
- 1.Scheiman M, Wick B. Clinical Management of Binocular Vision: Heterophoric, Accommodative, and Eye Movement Disorders. 3. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 2.Rouse MW, Borsting E, Hyman L, et al. Frequency of convergence insufficiency among fifth and sixth graders. The Convergence Insufficiency and Reading Study (CIRS) group. Optom Vis Sci. 1999;76:643–9. doi: 10.1097/00006324-199909000-00022. [DOI] [PubMed] [Google Scholar]
- 3.Borsting E, Rouse MW, Deland PN, et al. Association of symptoms and convergence and accommodative insufficiency in school-age children. Optometry. 2003;74:25–34. [PubMed] [Google Scholar]
- 4.Letourneau JE, Ducic S. Prevalence of convergence insufficiency among elementary school children. Can J Optom. 1988;50:194–7. [Google Scholar]
- 5.Borsting EJ, Rouse MW, Mitchell GL, et al. Validity and reliability of the revised convergence insufficiency symptom survey in children aged 9 to 18 years. Optom Vis Sci. 2003;80:832–8. doi: 10.1097/00006324-200312000-00014. [DOI] [PubMed] [Google Scholar]
- 6.Rouse M, Borsting E, Mitchell GL, et al. Validity of the convergence insufficiency symptom survey: a confirmatory study. Optom Vis Sci. 2009;86:357–63. doi: 10.1097/OPX.0b013e3181989252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rouse M, Borsting E, Mitchell GL, et al. Academic behaviors in children with convergence insufficiency with and without parent-reported ADHD. Optom Vis Sci. 2009;86:1169–77. doi: 10.1097/OPX.0b013e3181baad13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borsting E, Rouse M, Chu R. Measuring ADHD behaviors in children with symptomatic accommodative dysfunction or convergence insufficiency: a preliminary study. Optometry. 2005;76:588–92. doi: 10.1016/j.optm.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Convergence Insufficiency Treatment Trial Study Group. Randomized clinical trial of treatments for symptomatic convergence insufficiency in children. Arch Ophthalmol. 2008;126:1336–49. doi: 10.1001/archopht.126.10.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borsting E, Mitchell GL, Kulp MT, et al. Improvement in academic behaviors after successful treatment of convergence insufficiency. Optom Vis Sci. 2012;89:12–8. doi: 10.1097/OPX.0b013e318238ffc3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borsting E, Mitchell GL, Arnold LE, et al. Behavioral and emotional problems associated with convergence insufficiency in children: an open trial. J Atten Disord. 2016;20:836–44. doi: 10.1177/1087054713511528. [DOI] [PubMed] [Google Scholar]
- 12.Blum HL, Peters HB, Bettman JW, et al. Design and evaluation of a vision screening program for elementary school children. Am J Public Health Nations Health. 1959;49:1670–81. doi: 10.2105/ajph.49.12.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barnhardt C, Cotter SA, Mitchell GL, et al. Symptoms in children with convergence insufficiency: before and after treatment. Optom Vis Sci. 2012;89:1512–20. doi: 10.1097/OPX.0b013e318269c8f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rouse MW, Hyman L, Hussein M, et al. Frequency of convergence insufficiency in optometry clinic settings. Convergence Insufficiency and Reading Study (CIRS) Group. Optom Vis Sci. 1998;75:88–96. doi: 10.1097/00006324-199802000-00012. [DOI] [PubMed] [Google Scholar]
- 15.Scheiman M, Cotter S, Kulp MT, et al. Treatment of accommodative dysfunction in children: results from a randomized clinical trial. Optom Vis Sci. 2011;88:1343–52. doi: 10.1097/OPX.0b013e31822f4d7c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Convergence Insufficiency Treatment Trial Study Group. The convergence insufficiency treatment trial: design, methods, and baseline data. Ophthalmic epidemiol. 2008;15:24–36. doi: 10.1080/09286580701772037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaivanto K. Maximization of the sum of sensitivity and specificity as a diagnostic cutpoint criterion. J Clin Epidemiol. 2008;61:517–8. doi: 10.1016/j.jclinepi.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 18.von Noorden GK, Campos E. Binocular Vision and Ocular Motility. 6. Philadelphia: Mosby; 2002. [Google Scholar]
- 19.von Noorden GK, Brown DJ, Parks M. Associated convergence and accommodative insufficiency. Doc Ophthalmol. 1973;34:393–403. doi: 10.1007/BF00151826. [DOI] [PubMed] [Google Scholar]
- 20.Granet DB, Gomi CF, Ventura R, et al. The relationship between convergence insufficiency and ADHD. Strabismus. 2005;13:163–8. doi: 10.1080/09273970500455436. [DOI] [PubMed] [Google Scholar]
- 21.Abdi S, Rydberg A. Asthenopia in schoolchildren, orthoptic and ophthalmological findings and treatment. Doc Ophthalmol. 2005;111:65–72. doi: 10.1007/s10633-005-4722-4. [DOI] [PubMed] [Google Scholar]
- 22.Rouse M, Hyman L, Hussein M, et al. How do you make the diagnosis of convergence insufficiency? Survey results. J Optom Vis Devel. 1997;28:91–7. [Google Scholar]
- 23.Cacho-Martinez P, Garcia-Munoz A, Ruiz-Cantero MT. Diagnostic validity of clinical signs associated with a large exophoria at near. J Ophthalmol. 2013;2013:549–435. doi: 10.1155/2013/549435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayes GJ, Cohen BE, Rouse MW, et al. Normative values for the nearpoint of convergence of elementary schoolchildren. Optom Vis Sci. 1998;75:506–12. doi: 10.1097/00006324-199807000-00019. [DOI] [PubMed] [Google Scholar]