Abstract
Background:
Currently, there is no vaccine available for any form of leishmaniasis for human use, including visceral leishmaniasis (VL). The treatment relies on drugs associated with severe toxic side effects and increased parasite drug resistance. At present, there is a strong need to develop and implement a successful vaccine against this disease. Therefore, we evaluated immunoprophylactic potential of a cocktail of low molecular weight antigens along with various adjuvants.
Methods:
The three antigens (2015, Department of Zoology, Panjab University, Chandigarh), 31kDa, 36 kDa and 51 kDa of L. donovani were used in this study. Inbred BALB/c mice were immunized with 10 μg of cocktail antigens i.e. 31+36+51kDa alone and along with different adjuvants (ALD, saponin, and liposome). Mice were boosted twice at an interval of 2 wk and after last dose; mice were given challenge infection with 107 promastigotes. Mice have sacrificed15 d post immunization and on 30, 60, 90 post-challenge days for evaluation of different parameters.
Results:
Immunized animals showed reduced parasite load, increased DTH responses and elevated levels of IgG2a antibody. The levels of Th1 cytokines were higher as compared to Th2 cytokines in immunized animals.
Conclusion:
Best results were obtained with cocktail of 31+36+51+liposome and this combination conferred maximum protection.
Keywords: Cocktail antigens, Experimental visceral leishmaniasis, Adjuvants
Introduction
Visceral leishmaniasis is caused by dimorphic protozoan parasites of genus Leishmania. Every year 200000 to 400000 new cases of VL occur (1). In the endemic regions of visceral leishmaniasis of the world, the situation has complicated due to high toxicity and increasing drug resistance. Immunization of animals with defined subunit vaccines or live-attenuated strains and killed vaccines of Leishmania can induce significant protection (2). Although substantial efforts have been made by many laboratories, no such vaccine is available until date. The clinical trials of first-generation vaccines in humans have assessed the effect of three types of vaccines (3). Most of the vaccine studies in past have been performed using second generation vaccine candidates like GP36, 31 kDa and 32 kDa (4).
Numerous studies have been conducted with various antigens in vaccine research against leishmaniasis. The use of FML as the antigenic molecule has been described previously (5). It is a 36-kDa glycoprotein present in both stages of Leishmania parasites. The use of this antigen in serological diagnosis has resulted in 100% sensitivity and 96% specificity. The 31kDa protein of Leishmania promastigote has been used in diagnosis. In a study, the 31-kDa polypeptide was identified by 100% of the serum samples from VL patients and 73%–100% of the serum samples from patients cured of VL (6). The 51 kDa Leishmania protein has also been identified in 30% of blood samples from VL patients (7). Besides, this 51 kDa antigen has been tested for PCR based diagnosis of VL (8, 26)
An effective antileishmanial vaccine cannot consist of a single antigen (2).Therefore, cocktail vaccines comprised of multiple antigens along with suitable adjuvants have more chances to become successful. Adjuvants when added to antigens they boost the immune response. ALD, saponin and liposomes have previously been used as an adjuvant in different laboratories and have shown promising results (9,10). So, the present study was planned with a cocktail of 31+36+51kDa antigens of L. donovani along with ALD (Autoclaved L. donovani), saponin and cationic liposomes against murine visceral leishmaniasis.
Materials and Methods
The study was conducted in Department of Zoology, Panjab University, Chandigarh, India in 2015.
Parasite: The promastigotes of L. donovani of strain MHOM/IN/80/Dd8 were used. The promastigotes were subcultured after every 48-72hin NNN media supplemented with MEM.
Animals: Inbred BALB/c mice of either sex were procured from Central Animal House, Panjab University, Chandigarh. They were fed with water and mouse feed ad libitum.
Identification and electro-elution of antigens
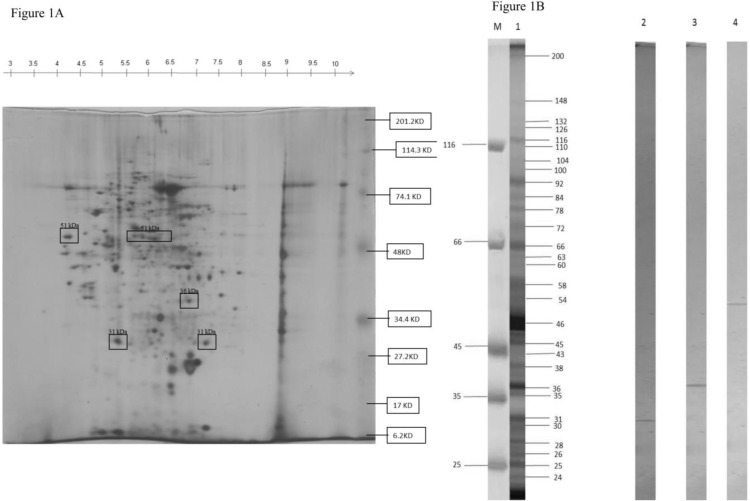
The antigens were identified using 2-D gel electrophoresis using molecular weight markers (Fig. 1A). The parasite proteins were also resolved using 1-D electrophoresis comprising only one dimension i.e. SDS-PAGE. The required bands were taken out and electro-eluted (Fig. 1B) by a procedure described previously (11).
Fig. 1:
(A) 2-D gel electrophoresis of promastigote antigens of L. donovani pH 3–10: Immobiline strip of pH 3–10 was used in 1-D isoelectric focusing
(B) SDS-PAGE of promastigote antigens of L. donovani and eluted 31, 36 and 51kDa antigens
Lane M: molecular weight markers, lane 1: promastigote antigens, lane 2: eluted 31kDa antigen, lane 3: eluted 36kDa antigen, lane 3: eluted 51kDa antigen
Preparation of vaccines
The cocktail of 31+36+51 kDa was formulated with ALD (Autoclaved L. donovani), saponin and cationic liposomes as adjuvants. The ALD antigen was prepared by method (11).
In order to formulate the antigens with ALD as an adjuvant, 250μg of eluted proteins (Nanodrop was used to estimate protein concentration) were mixed with 2.5 mg of ALD antigen. The cocktail was also formulated with saponin as an adjuvant. Saponin was used at a dosage of 100 μg/mice (12).The positively charged liposomes were formulated using the commercially available kit (Sigma, USA). Sixty-three micromoles of phosphatidylcholine, 9 μmol ofcholesterol and 18μmol of stearylamine in the ratio of (7:1:2) were used. The antigen was entrapped by the method (13). In addition, the cocktail of 31+36+51kDa without any adjuvant was also used to immunize the animals.
Immunization and challenge infection
Mice were categorized into different groups for experimental purpose. Group 1 comprised of normal control mice, group 2 were infected with 1×107 promastigotes of L. donovani intravenously. Ten micrograms of cocktail antigens along with different adjuvants i.e. 31+36+51kDa; 31+36+51+ALD; 31+36+51+saponin and 31+36+51+liposome were used to immunize animals. The animals were immunized subcutaneously. After two weeks of final booster (2 boosters were given), animals were challenged with 1×107 promastigotes. Different parameters were evaluated at 15 d post immunization and 30, 60 and 90 post-challenge days.
Assessment of infection
Mice were sacrificed after 15 d post immunization and on 30, 60, 90 post challenge/ post infection days. The parasite load was counted by examining Giemsa stained impression smears of liverand expressed in terms of Leishman Donovan Units (LDU) (14).
Delayed-type hypersensitivity response
Two days before the day of sacrifice, 40 μL of leishmanin and PBS was injected (i.e.) in the right and left footpad of mice, respectively. After 48 h, the thickness of right and left footpad was measured through verniercalliper. The DTH response was calculated by previously prescribed method (15).
Assessment of antibody response
Serum specific IgG1 and IgG2a levels were assessed by conventional enzyme-linked immunosorbent assay (ELISA) by the method (16).
Cytokine assays
Th1 and Th2 cytokine levels were assessed in frozen serum samples (−70°C) using commercially available kits from Diaclone, France.
Statistical analysis
The statistical analysis was done by one-way ANOVA using SPSS software (ver.16, (Chicago, IL, USA).
Ethics statement
The ethical clearance for conducting these experiments was taken from the Institutional Animal Ethics Committee of Panjab University, Chandigarh (Approval number-IAEC/284-295/3.9.12).
Results
DTH levels
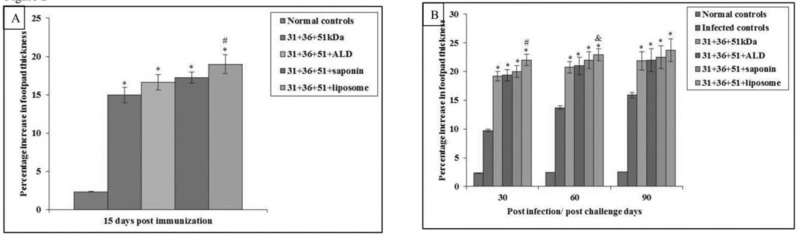
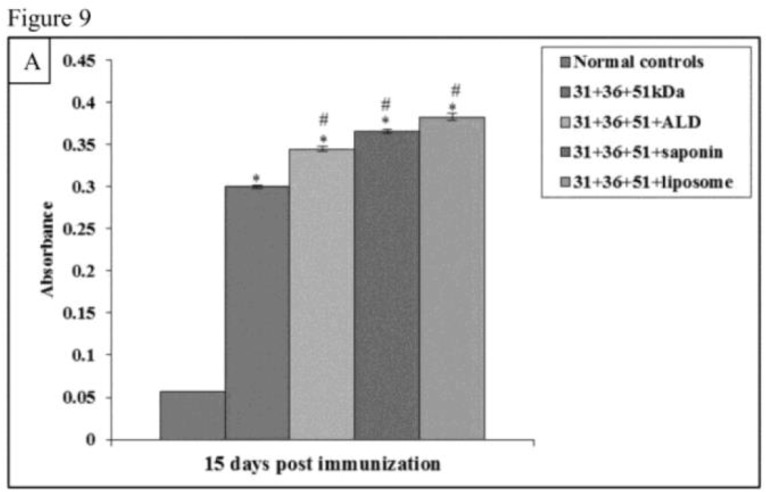
Immunization of mice with cocktail of three antigens i.e. 31 kDa, 36 kDa and 51 kDaalone and along with adjuvants ALD, saponin and liposome, elevated the percentage increase in footpad thickness (prechallenge) significantly in comparison to normal controls (P<0.001). When a comparison was made between animals immunized with only antigens i.e. 31+36+51kDa with other groups of animals, significantly increased DTH responses were seen in animals immunized with 31+36+51+liposome(P<0.001) (Fig.2A).
Fig. 2:
A) Percentage increase in footpad thickness (DTH response) in normal and immunized mice on 15 d post immunization. The data is presented as mean±S.D. of six mice per group.
P value: Normal controls vs. 31+36+51kDa; 31+36+51+ALD; 31+36+51+saponin; 31+36+51+liposome. *(P<0.001)
P value: 31+36+51 kDa vs. 31+36+51+ALD; 31+36+51+saponin; 31+36+51+liposome. #(P<0.001)
B) Percentage increase in footpad thickness (DTH response) in infected and immunized mice on different post challenge days. The data is presented as mean±S.D. of six mice per group
P-value: Infected controls vs. 31+36+51kDa; 31+36+51+ALD; 31+36+51+saponin; 31+36+51+liposome *(P<0.001)
P-value: 31+36+51kDa vs. 31+36+51+ALD; 31+36+51+saponin; 31+36+51+liposome. #(P<0.001), &(P<0.05)
The DTH responses increased significantly in immunized animals as compared to the infected controls on all post challenge days (P<0.001). Addition of adjuvants i.e. ALD, saponin, and liposome further increased the DTH responses. Peak DTH responses were seen in mice immunized with cocktail of 31+36+51+liposome and the difference was significantly higher (P<0.001, 0.05) as compared to those immunized with cocktail of 31+36+51kDa except on day 90 (Fig.2B).
Parasite load
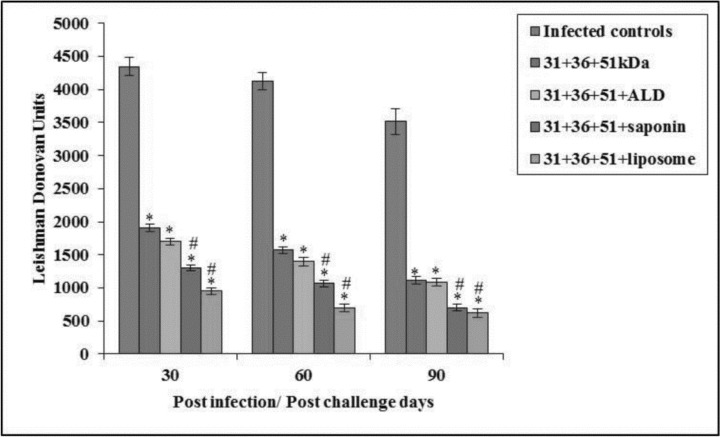
Parasite clearance was monitored in liver.The parasite load in immunized animals was significantly lesser as compared to the infected controls (P<0.001). The cocktail of 31+36+51+liposome imparted maximum protection with 78.16% to 82.37% reduction in LDU. In animals immunized with 31+36+51+saponin parasite load declined by 70.11% (30 post-challenge day) to 80.10% (90 post-challenge day). Further lesser amount of protection was conferred by immunization with 31+36+51+ALD with decline of parasite load by 69.01% on 90 post-challengedays. Least amount of protection was conferred by immunization with 31+36+51kDa showing 68.34% reduction on 90 post-challenge days as compared to the infected controls. When LDU of mice immunized with cocktails of 31+36+51+saponin and 31+36+51+liposome were compared to those immunized with 31+36+51kDa alone, significantly lower values were observed (P<0.001)(Fig.3).
Fig. 3:
Parasite load in liver in terms of LDU in infected and immunized mice on different post challenge days. The data is presented as mean±S.D. of six mice per group
P-value: Infected controls vs. 31+36+51kDa; 31+36+51+ALD; 31+36+51+saponin; 31+36+51+liposome *(P<0.001)
P value: 31+36+51kDa vs. 31+36+51+ALD; 31+36+51+saponin; 31+36+51+liposome. #(P<0.001)
Cytokine responses: Th1 cytokines
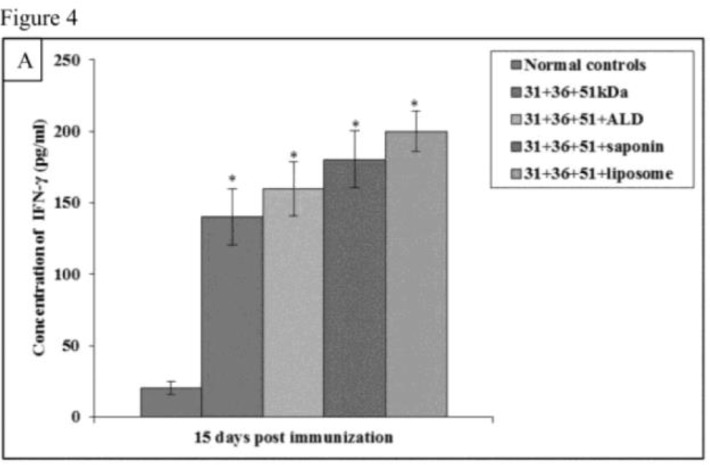
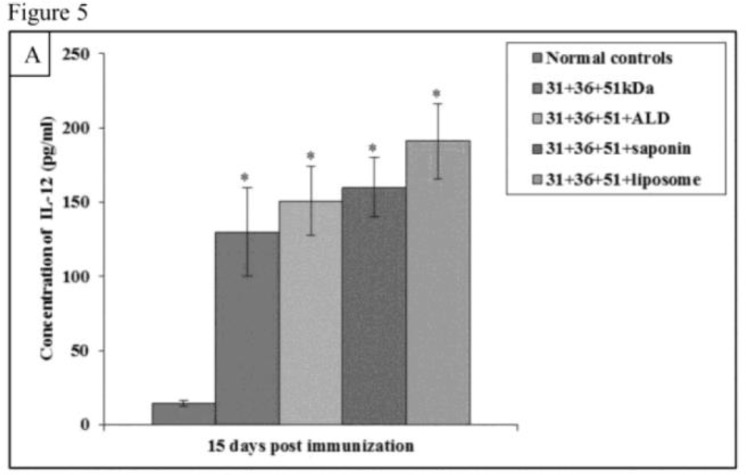
Immunization of mice with cocktail of 31+36+51kDa alone and along with adjuvants ALD, saponin, and liposome lead to significant increase in the pre-challenge IFN-γ and IL-12 levels as compared to normal controls (P<0.001)(Fig. 4A and 5A).
Fig.4A:
IFN-γ concentration in normal and immunized mice on 15 d post immunization. The data is presented as mean±S.D. of six mice per group
P-value: Normal controls vs. 31+36+51kDa; 31+36+51+ALD; 31+36+51+saponin; 31+36+51+liposome. *(P<0.001)
P-value: 31+36+51kDa vs. 31+36+51+ALD; 31+36+51+saponin; 31+36+51+liposome
Fig.5A:
IL-12 concentration in normal and immunized mice on 15 d post immunization. The data is presented as mean±S.D. of six mice per group
P-value: Normal controls vs. 31+36+51kDa; 31+36+51+ALD; 31+36+51+saponin; 31+36+51+liposome*P(<0.001)
P-value: 31+36+51kDa vs. 31+36+51+ALD; 31+36+51+saponin; 31+36+51+liposome
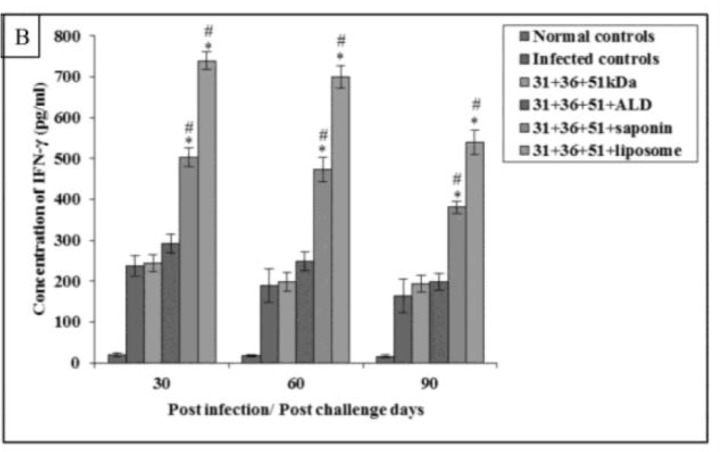
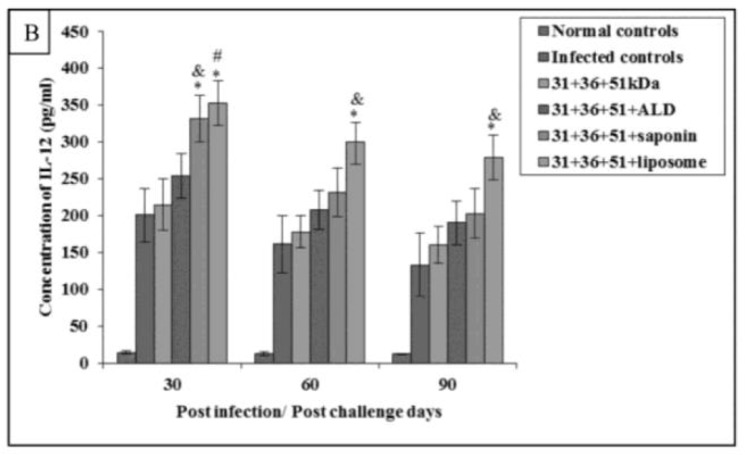
The concentration of these cytokines was significantly higher in immunized animals (31+36+51+saponin and 31+36+51+liposome) as compared to the infected animals on all post challenge days (P<0.001) except IL-12 concentration in animals immunized with 31+36+51+saponin on 60 and 90post-challenge days. Maximum levels of Th1 cytokines was observed in animals immunized with 31+36+51+liposome. A significant increase was found in mice immunized with cocktail of 31+36+51+saponin and 31+36+51+liposome as compared to those immunized with 31+36+51kDa alone on all post challenge days (P<0.001, 0.05) except for IL-12 levels in animals immunized with cocktail of 31+36+51+saponin on day 60 and 90 as compared to those immunized with 31+36+51kDa alone (Fig.4B and 5B).
Fig.4B:
IFN-γconcentration in infected and immunized mice on different post challenge days. The data is presented as mean±S.D. of six mice per group
P-value: Infected controls vs. 31+36+51kDa; 31+36+51+ALD; 31+36+51+saponin; 31+36+51+liposome. *(P<0.001)
P-value: 31+36+51kDa vs. 31+36+51+ALD; 31+36+51+saponin; 31+36+51+liposome. #(P<0.001)
Fig.5B:
IL-12 concentration in infected and immunized mice on different post challenge days. The data is presented as mean±S.D. of six mice per group
P-value: Infected controls vs. 31+36+51kDa; 31+36+51+ALD; 31+36+51+saponin; 31+36+51+liposome. *P (<0.001)
P value: 31+36+51kDa vs. 31+36+51+ALD; 31+36+51+saponin; 31+36+51+liposome. #(P<0.001), & (P<0.05)
Th2 cytokines
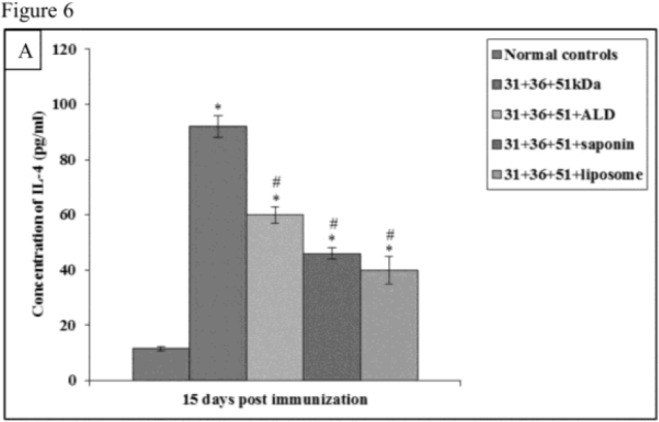
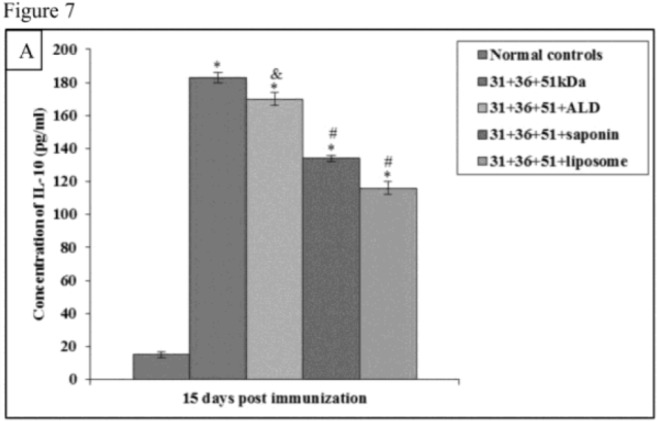
In animals immunized with cocktail of 31+36+51kDa alone and along with adjuvants ALD, saponin and liposome, the prechallenge IL-4 and IL-10 levels were significantly high as compared to normal controls (P<0.001) in all the groups (Fig.6A and 7A).
Fig.6A:
IL-4 concentration in normal and immunized mice on 15 d post immunization. The data is presented as mean±S.D. of six mice per group.
P-value: Normal controls vs. 31+36+51kDa; 31+36+51+ALD; 31+36+51+saponin; 31+36+51+liposome. *(P<0.001).
P-value: 31+36+51kDa vs. 31+36+51+ALD; 31+36+51+saponin; 31+36+51+liposome. #(P<0.001).
Fig.7A:
IL-10 concentration in normal and immunized mice on 15 d post immunization. The data is presented as mean±S.D. of six mice per group.
P-value: Normal controls vs. 31+36+51kDa; 31+36+51+ALD; 31+36+51+saponin; 31+36+51+liposome. *(P<0.001)
P-value: 31+36+51kDa vs. 31+36+51+ALD; 31+36+51+saponin; 31+36+51+liposome. #(P<0.001), &(P<0.05).
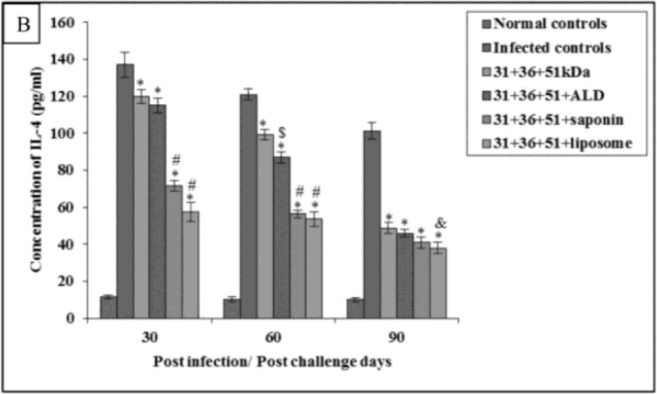
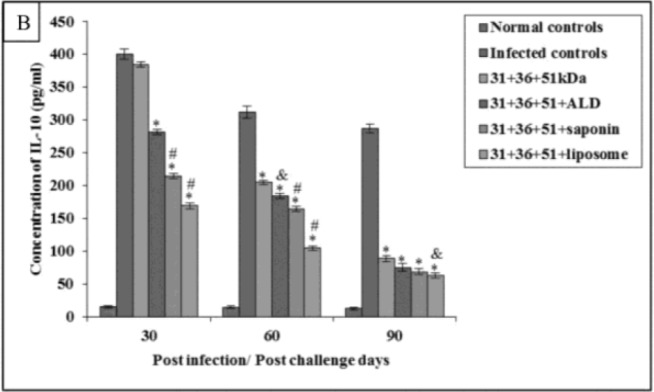
Immunized animals showed lesser concentration of IL-4 and IL-10 in comparison to the infected controls.
The concentration of IL-4 and IL-10 was significantly lower (P<0.001) in immunized animals as compared to the infected controls except for IL-10 levels in animals immunized with a cocktail of 31+36+51kDa on 30 post-challengedays. Maximum concentration of these cytokines was observed in animals immunized with 31+36+51kDa. Among immunized animals, minimum levels of this cytokine were observed in animals immunized with 31+36+51+liposome. A significant decrease (P<0.001, 0.05) in IL-4 andIL-10 levels was seen in animals immunized with cocktail of 31+36+51+ALD on 60 post-challengedaysand 31+36+51+saponin (except day 90) as compared to those immunized with 31+36+51kDa alone. Moreover, the levels of both the cytokines (IL-4 and IL-10) were also found to be significantly lower in mice immunized with cocktail of 31+36+51+liposome as compared to 31+36+51 kDa alone on all post challenge days (P<0.001, 0.05) (Fig.6B and 7B).
Fig.6B:
IL-4 concentration in infected and immunized mice on different post challenge days. The data is presented as mean±S.D. of six mice per group.
P-value: Infected controls vs. 31+36+51kDa; 31+36+51+ALD; 31+36+51+saponin; 31+36+51+liposome. *(P<0.001), $(P<0.05)
P-value: 31+36+51kDa vs. 31+36+51+ALD; 31+36+51+saponin; 31+36+51+liposome. #(P<0.001), &(P<0.05).
Fig.7B:
IL-10 concentration in infected and immunized mice on different post challenge days. The data is presented as mean±S.D. of six mice per group.
P-value: Infected controls vs. 31+36+51kDa; 31+36+51+ALD; 31+36+51+saponin; 31+36+51+liposome. *(P<0.001).
P-value: 31+36+51kDa vs. 31+36+51+ALD; 31+36+51+saponin; 31+36+51+liposome. #(P<0.001), &(P<0.05).
Antibody Responses
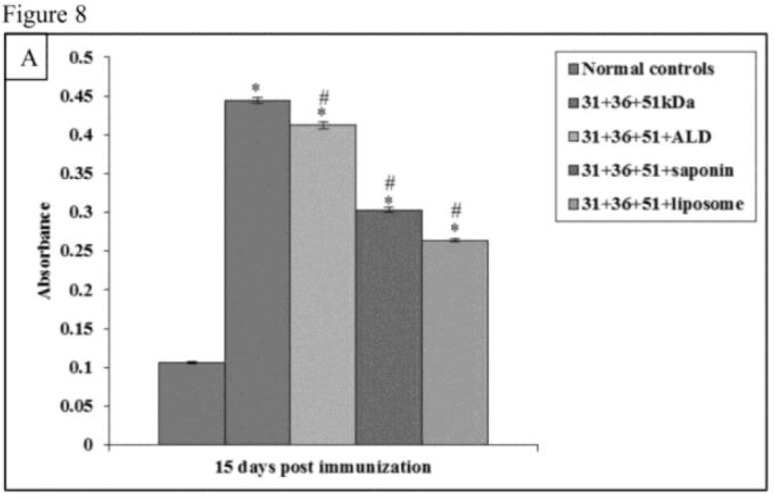
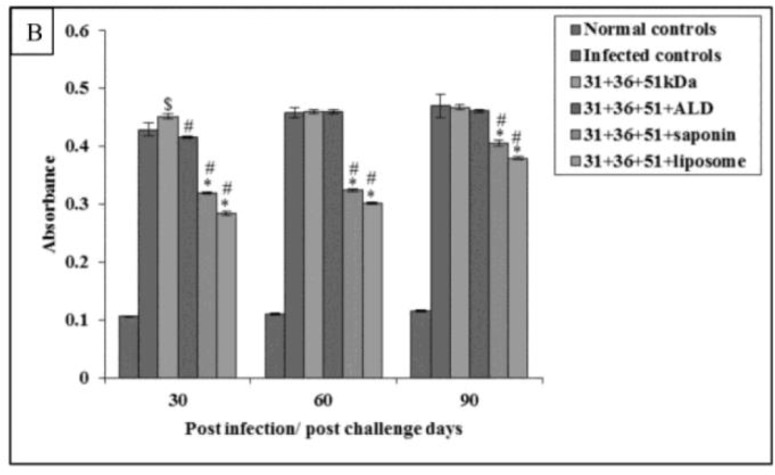
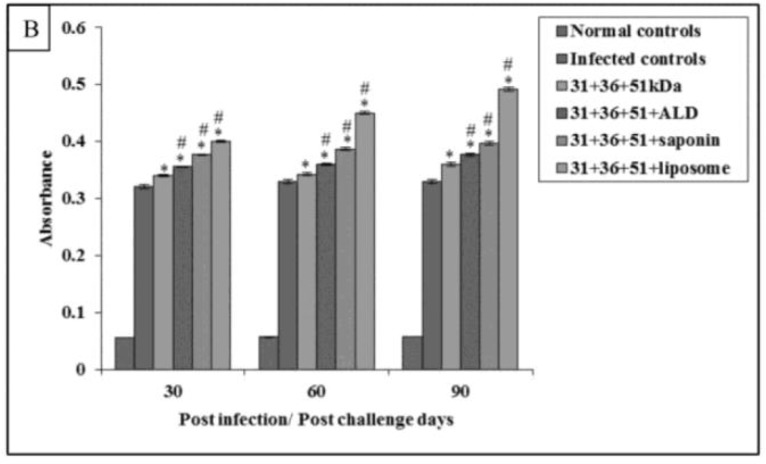
The pre-challenge antibody levels were significantly high in all the immunized groups as compared to normal controls (P<0.001). When the animals immunized with only antigens i.e. 31+36+51kDa were compared with other groups of animals significantly decreased levels of IgG1 and increased levels of IgG2a were observed in animals immunized with 31+36+51+ALD, 31+36+51+saponin, and 31+36+51+liposome (P<0.001) (Fig.8A and 9A).
Fig.8A:
Levels of Leishmania-specific antibody (IgG1) in serum samples in normal and immunized mice on 15 d post immunization. The data is presented as mean±S.D. of six mice per group
P-value: Normal controls vs. 31+36+51kDa; 31+36+51+ALD; 31+36+51+saponin; 31+36+51+liposome *(P<0.001).
P-value: 31+36+51kDa vs. 31+36+51+ALD; 31+36+51+saponin; 31+36+51+liposome. #(P<0.001).
Fig.9A:
Levels of Leishmania-specific antibody (IgG2a) in serum samples in normal and immunized mice on 15 d post immunization. The data is presented as mean±S.D. of six mice per group
P-value: Normal controls vs. 31+36+51kDa; 31+36+51+ALD; 31+36+51+saponin; 31+36+51+liposome.*(P<0.001)
P-value: 31+36+51kDa vs. 31+36+51+ALD; 31+36+51+saponin; 31+36+51+liposome. #(P<0.001)
Maximum levels of IgG1 were obtained in infected controls group. Among immunized group maximum, IgG1 levels were obtained in animals immunized with 31+36+51kDa followed by groups of animals immunized with 31+36+51+ALD, 31+36+51+saponin, and 31+36+51+liposome. The IgG1 response in immunized animals was significantly lesser as compared to infected controls except in 31+36+51kDa and 31+36+51+ALD group on 60 and 90 post-challenge days. The IgG1 levels were also significantly lesser in mice immunized with 31+36+51+ALD (30 post-challenge day), 31+36+51+saponin and 31+36+51+liposome as compared to those immunized with 31+36+51kDa alone (P<0.001, 0.05) (Fig.8B).
Fig.8B:
Levels of Leishmania-specific antibody (IgG1) in serum samples in infected and immunized mice on different post challenge days. The data is presented as mean±S.D. of six mice per group.
P-value: Infected controls vs. 31+36+51kDa; 31+36+51+ALD; 31+36+51+saponin; 31+36+51+liposome. *(P<0.001), $(P<0.05)
P-value: 31+36+51kDa vs. 31+36+51+ALD; 31+36+51+saponin; 31+36+51+liposome. #(P<0.001)
The maximum absorbance values of IgG2a were observed group of animals vaccinated with a cocktail of 31+36+51+liposome followed by those vaccinated with 31+36+51+saponin, 31+36+51+ALD, and 31+36+51kDa. The IgG2a levels were also significantly greater in mice immunized with 31+36+51+ALD, 31+36+51+saponin and 31+36+51+liposome as compared to those immunized with 31+36+51kDa alone (P<0.001) (Fig.9B).
Fig.9B:
Levels of Leishmania-specific antibody (IgG2a) in serum samples in infected and immunized mice on different post challenge days. The data is presented as mean±S.D. of six mice per group
P-value: Infected controls vs. 31+36+51kDa; 31+36+51+ALD; 31+36+51+saponin; 31+36+51+liposome. *(P<0.001)
P-value: 31+36+51kDa vs. 31+36+51+ALD; 31+36+51+saponin; 31+36+51+liposome. #(P<0.001)
Discussion
Among the tropical diseases, leishmaniasis is second in mortality and fourth in morbidity (17). The present scenario for vaccination is based on use of recombinant proteins (18). Different studies have been carried out with autoclaved Leishmania major and L. donovani antigens and encouraging results were observed in primates and murine models by many workers (19). Inoculation of killed vaccines leads to loss of infection-induced immunity in mice (20).
As concerns the first generation vaccines, single dose of alum-precipitated ALM along with BCG was found to be safe (21). Significant studies have also been reported by some workers in field of canine visceral leishmaniasis (22, 23). Some of the antigens included in the immunodominant 30- to 36-kDa fraction of L. infantum were detected by using proteomic approach (24).Hence, keeping in mind the significance of low molecular weight antigens, the present study has been designed.
To test novel therapeutic and immunoprophylactic agents, murine models of leishmaniasis have been used extensively (25). In our study mice were injected intravenously with 107 promastigotes and sacrificed on various post challenge days. Maximum hepatic parasite load was seen in infected control group on 30 post-challenge days.
Thereafter, it was found to decline. It is in accordance with the earlier studies of L. donovani infection in BALB/c mice (26). In immunized animals, parasite load declined significantly as compared to infected controls. Maximum decline in parasite load i.e. 78.16% to 82.37% was found in group of animals immunized with a cocktail of 31+36+51+liposome. These results are in accordance with an earlier study in which formulation of single antigens (i.e. LD31, LD51, LD72, LD91) with liposome reduced parasite load by 74%, 72%, 65% and 46% respectively (27). Minimum reduction in parasite load was obtained when animals were immunized with a cocktail of 31+36+51kDa. In our study, when ALD was used as an adjuvant with a cocktail of 31+36+51kDa the percentage reduction of 60.91%–69.01% was achieved. These results are in line with a previous past study from our laboratory in which BALB/c mice immunized with a cocktail of Hsp70+Hsp 83+ALD induced 67%–91.90% decline in LDU as compared to the infected controls against murine VL (28). In the present study, maximum reduction in parasite load was obtained when liposome was used as an adjuvant as compared to saponin and ALD. Results are similar to a study (29) where mice inoculated with 2.5 μg of gp63 in liposomes reduced parasite load significantly.
Maximum DTH responses were observed in mice immunized with 31+36+51+liposome. Our results are in continuation with work (27) where mice immunized with liposomal LD31 and LD51 alone exhibited significant DTH responses (P<0.0001) as compared to control groups. In another study immunization of hamsters with proteins, P4-7 induced significantly higher levels of DTH responses on 45, 90 and 120 post-challenge days (30). Maximum DTH responses were observed with the use of liposome and it was followed by saponin and ALD. Results are similar with study (27) where mice immunized with liposomal LD31 and LD51 alone exhibited significant DTH responses. Our results are also similar to a study (31) in which mice immunized with rNH36 and FML along with saponin as adjuvant (100 μg) showed significant DTH responses.
In leishmaniasis, cellular immune responses play main role in recovery from infection. Th1 type of immune responses is crucial in preventing the growth of intracellular parasite like Leishmania (32). The antigen presenting cells pick up the leishmanial antigens (33). These cells activate specific T cells in the production of different cytokines and induce the macrophages to kill the parasites (33). IL-12 is a major immunoregulatory cytokine which initiates and maintain Th1 type of immune response and plays a significant role in the induction of IFN-γ production by T and NK cells (34).
In the present study, Th1 type of immune response was observed with elevated levels of Th1 cytokines. The best results were obtained with cocktail of 31+36+51+liposome. Our findings are in accordance with a study (35) in which vaccination of hamsters with cocktail of liposomal rCPA, rCPB and rCPC (2.5 μg each) plus MPL-TDM induced strong Th1 responses. According to the study (36), immunization of mice with Lip-rgp63-CpG ODN significantly raised the levels of IFN-γ. Our results are similar with study (37) where immunization of mice with LAg (leishmanial antigens) along with liposomes produced significantly high IFN-γ levels in comparison to the controls.
In our studies when mice were immunized using saponin as an adjuvant increased level of Th1 cytokines were observed as compared to infected controls. Decreased levels of IL-10 and IL-4 were reported on immunization of BALB/c mice with immunogenic preparations composed of L. amazonensis or L. braziliensis along with saponin against intravenous challenge with L. chagasi promastigotes (38). The present study is in concordance with the study (31) where immunization of BALB/c mice with NH36 DNA vaccine resulted in two to five-fold increase in IFN-γ producing CD4(+) T cells against leishmaniasis. As an adjuvant, liposome and saponins were more effective in producing Th1 specific cytokines as compared to ALD.
Appearance of anti-leishmanial antibodies in the sera of patients is another important characteristic feature of Leishmania infection. In comparison to infected controls, elevated levels of IgG2a were observed in the serum samples of immunized animals. In our study, maximum levels of IgG2a were observed in mice immunized with a cocktail of 31+36+51+liposome. Results of our study are in consistence with a study (39) where mice immunized with SLA+CpGs-ODN along with liposome showed stronger IgG2a antibody response. Similar results were reported from our laboratory in which elevated levels of IgG2a were observed in mice vaccinated with liposome-encapsulated 78kDa antigen as compared to infected control group (11). Promising results were also obtained when saponin was used as an adjuvant. Our results are similar to an earlier study in which immunization of mice with gp36 + saponin conferred protective immune response against visceral leishmaniasis with increased production of IgG2a (4).
From the present study, immunization of mice with a cocktail of 31+36+51 kDa (alone and along with various adjuvants) was protective and highly immunogenic against experimental visceral leishmaniasis. Best results were attained when liposome was used as an adjuvant.
Conclusion
In the present situation development of either prophylactic or preventive vaccine has now become necessity to combat disease progression worldwide. Therefore, it is essential to identify new potential antigens. An effort has been made in this study to discover a poly-protein vaccine which when combined with suitable adjuvant confers long-lasting protection against the disease.
Acknowledgements
The authors acknowledge UGC-MANF fellowship programme for its financial support.
Footnotes
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.WHO (2015). Leishmaniasis fact sheet number 375. http://www.who.int/mediacentre/factsheets/fs375/en/.
- 2.Kedzierski L. Leishmaniasis Vaccine: Where are We Today? J Glob Infect Dis. 2010; 2(2:177–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Modabber F. First generation vaccine in clinical development: moving but what next? Curr Opin Anti-infect Inv Drug. 2000; 2: 35–39. [Google Scholar]
- 4.Paraguai de Souza E, Bernardo RR, Palatnik M, Palatnik de Sousa CB. Vaccination of Balb/c mice against experimental visceral leishmaniasis with the GP36 glycoprotein antigen of Leishmania donovani. Vaccine. 2001;19(23–24):3104–15. [DOI] [PubMed] [Google Scholar]
- 5.Palatnik-de-Sousa CB, Gomes EM, Paraguai-de-Souza E, Palatnik M, Luz K, Borojevic R. Leishmania donovani: titration of antibodies to the fucose-mannose ligand as an aid in diagnosis and prognosis of visceral leishmaniasis. Trans R Soc Trop Med Hyg. 1995; 89(4):390–3. [DOI] [PubMed] [Google Scholar]
- 6.Rolland-Burger L, Rolland X, Grieve CW, Monjour L. Immunoblot analysis of the humoral immune response to Leishmania donovani infantum polypeptides in human visceral leishmaniasis. J Clin Microbiol. 1991; 29(7):1429–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mary C, Ange G, Dunan S, Lamouroux D, Quilici M. Characterization of a circulating antigen involved in immune complexes in visceral leishmaniasis patients. Am J Trop Med Hyg. 1993;49(4):492–501. [DOI] [PubMed] [Google Scholar]
- 8.Berrahal F, Mary C, Roze M, et al. Canine leishmaniasis: identification of asymptomatic carriers by polymerase chain reaction and immunoblotting. Am J Trop Med Hyg. 1996; 55(3):273–7. [DOI] [PubMed] [Google Scholar]
- 9.Oda K, Matsuda H, Murakami T, et al. Adjuvant and haemolytic activities of 47 saponins derived from medicinal and food plants. Biol Chem. 2000; 381(1):67–74. [DOI] [PubMed] [Google Scholar]
- 10.Allison AG, Gregoriadis G. Liposomes as immunological adjuvants. Nature. 1974; 252(5480):252. [DOI] [PubMed] [Google Scholar]
- 11.Nagill R, Kaur S. Enhanced efficacy and immunogenicity of 78 kDa antigen formulated in various adjuvants against murine visceral leishmaniasis. Vaccine. 2010; 28(23):4002–12. [DOI] [PubMed] [Google Scholar]
- 12.Aguilar-Be I, da Silva Zardo R, Paraguai de Souza E, et al. Cross-protective efficacy of a prophylactic Leishmania donovani DNA vaccine against visceral and cutaneous murine leishmaniasis. Infect Immun. 2005; 73(2):812–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Afrin F, Ali N. Adjuvanticity and protective immunity elicited by Leishmania donovani antigens encapsulated in positively charged liposomes. Infect Immun. 1997; 65(6):2371–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bradley DJ, Kirkley J. Regulation of Leishmania population within host 1. The variable course of L. donovani infections in mice. Clin Exp Immunol. 1977;30(1):119–29. [PMC free article] [PubMed] [Google Scholar]
- 15.Kaur S, Kaur T, Garg N, Mukherjee S, Raina P, Athokpam V. Effect of dose and route of inoculation on the generation of CD4+ Th1/Th2 type of immune response in murine visceral leishmaniasis. Parasitol Res. 2008; 103(6):1413–9. [DOI] [PubMed] [Google Scholar]
- 16.Ravindran R, Anam K, Bairagi BC, et al. Characterization of immunoglobulin and its subclass response to Indian Kala Azar infection before and after chemotherapy. Infect Immun. 2004;72(2):863–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bern C, Maguire JH, Alvar J. Complexities of assessing the disease burden attributable to leishmaniasis. PLoS Negl Trop Dis. 2008; 2(10):e313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ravindran R, Ali N. Progress in vaccine research and possible effector mechanisms in visceral leishmaniasis. Curr Mol Med. 2004; 4(6):697–709. [DOI] [PubMed] [Google Scholar]
- 19.Misra A, Dube A, Srivastava B, et al. Successful vaccination against Leishmania donovani infection in Indian langur using alum-precipitated autoclaved Leishmania major with BCG. Vaccine. 2001; 19(25–26):3485–92. [DOI] [PubMed] [Google Scholar]
- 20.Okwor I, Liu D, Beverley SM, Uzonna JE. Inoculation of killed Leishmania major into immune mice rapidly disrupts immunity to a secondary challenge via IL-10-mediated process. Proc Natl Acad Sci U S A. 2009; 106(33):13951–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohebali M, Khamesipour A, Mobedi I, Zarei Z, Hashemi-Fesharki R. Double-blind randomized efficacy field trial of alum precipitated autoclaved Leishmania major vaccine mixed with BCG against canine visceral leishmaniasis in Meshkin-Shahr district, I.R. Iran. Vaccine. 2004;22(29–30):4097–100. [DOI] [PubMed] [Google Scholar]
- 22.Mohebali M, Fallah E, Jamshidi Sh, Hajjaran H. Vaccine trial against canine visceral leishmaniasis in the Islamic Republic of Iran. East. Mediterr. Health J. 1998; 4: 234–238. [Google Scholar]
- 23.Marty P, Lelièvre A, Quaranta JF, et al. Detection by Western blot of four antigens characterizing acute clinical leishmaniasis due to Leishmania infantum. Trans R Soc Trop Med Hyg. 1995; 89(6):690–1. [DOI] [PubMed] [Google Scholar]
- 24.Kamoun-Essghaier S, Guizani I, Strub JM, et al. Proteomic approach for characterization of immunodominant membrane-associated 30-to 36-kilodalton fraction antigens of Leishmania infantum promastigotes, reacting with sera from mediterranean visceral Leishmaniasis patients. Clin Diagn Lab Immunol. 2005; 12(2):310–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reiner NE. Host-parasite relationship in murine leishmaniasis: pathophysiological and immunological changes. Infect Immun. 1982; 38(3):1223–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Engwerda CR, Murphy ML, Cotterell SE, Smelt SC, Kaye PM. Neutralization of IL-12 demonstrates the existence of discrete organ-specific phases in the control of Leishmania donovani. Eur J Immunol. 1998; 28(2):669–80. [DOI] [PubMed] [Google Scholar]
- 27.Bhowmick S, Ali N. Identification of novel Leishmania donovani antigens that help define correlates of vaccine-mediated protection in visceral leishmaniasis. PLoS One. 2009; 4(6):e5820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaur J, Kaur T, Kaur S. Studies on the protective efficacy and immunogenicity of Hsp70 and Hsp83 based vaccine formulations in Leishmania donovani infected BALB/c mice. Acta Trop. 2011;119(1):50–6. [DOI] [PubMed] [Google Scholar]
- 29.Bhowmick S, Ravindran R, Ali N. gp63 in stable cationic liposomes confers sustained vaccine immunity to susceptible BALB/c mice infected with Leishmania donovani. Infect Immun. 2008; 76(3):1003–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumari S, Samant M, Misra P, Khare P, Sisodia B, Shasany AK, Dube A. Th1-stimulatory polyproteins of soluble Leishmania donovani promastigotes ranging from 89.9 to 97.1 kDa offers long-lasting protection against experimental visceral leishmaniasis. Vaccine. 2008; 26(45):5700–11. [DOI] [PubMed] [Google Scholar]
- 31.Aguilar-Be I, da Silva Zardo R, Paraguai de Souza E, et al. Cross-protective efficacy of a prophylactic Leishmania donovani DNA vaccine against visceral and cutaneous murine leishmaniasis. Infect Immun. 2005; 73(2):812–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carvalho EM, Johnson WD, Barreto E, Marsden PD, Costa JL, Reed S, Rocha H. Cell mediated immunity in American cutaneous and mucosal leishmaniasis. J Immunol. 1985; 135(6):4144–8. [PubMed] [Google Scholar]
- 33.El-On J. Current status and perspectives of the immunotherapy of leishmaniasis. Isr Med Assoc J. 2009; 11(10):623–8. [PubMed] [Google Scholar]
- 34.Kobayashi M, Fitz L, Ryan M, et al. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J Exp Med. 1989; 170(3):827–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Das A, Ali N. Combining cationic liposomal delivery with MPL-TDM for cysteine protease cocktail vaccination against Leishmania donovani: evidence for antigen synergy and protection. PLoS Negl Trop Dis. 2014; 8(8):e3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jaafari MR, Badiee A, Khamesipour A, et al. The role of CpG ODN in enhancement of immune response and protection in BALB/c mice immunized with recombinant major surface glycoprotein of Leishmania (rgp63) encapsulated in cationic liposome. Vaccine. 2007; 25(32):6107–17. [DOI] [PubMed] [Google Scholar]
- 37.Mazumdar T, Anam K, Ali N. A mixed Th1/Th2 response elicited by a liposomal formulation of Leishmania vaccine instructs Th1 responses and resistance to Leishmania donovani in susceptible BALB/c mice. Vaccine. 2004; 22(9–10):1162–71. [DOI] [PubMed] [Google Scholar]
- 38.Grenfell RF, Marques-da-Silva EA, Souza-Testasicca MC, et al. Antigenic extracts of Leishmania braziliensis and Leishmania amazonensis associated with saponin partially protects BALB/c mice against Leishmania chagasi infection by suppressing IL-10 and IL-4 production. Mem Inst Oswaldo Cruz. 2010;105(6):818–22. [DOI] [PubMed] [Google Scholar]
- 39.Heravi Shargh V, Jaafari MR, Khamesipour A, et al. Cationic liposomes containing soluble Leishmania antigens (SLA) plus CpG ODNs induce protection against murine model of leishmaniasis. Parasitol Res. 2012; 111(1):105–14. [DOI] [PubMed] [Google Scholar]