Abstract
Background:
The protozoan Trichomonas vaginalis is a sexually transmitted disease (STD). Metronidazole is a chosen drug for the treatment. This study evaluated the anti trichomonal activity of alcoholic extracts of combination Verbascum thapsus and Ginger officinale.
Methods:
This experimental study was conducted in the Parasitology Laboratory, Kashan University of Medical Sciences, Kashan, Iran in 2015, on 23 women with suspected trichomoniasis referring to Kashan clinical centers. Medium TYI-S-33 was used for culture of three T. vaginalis isolates. Different concentrations (25, 50, 100, 200, 400, 800 μg/ml) of V. thapsus and G. officinale ethanol extract added to Trichomonas trophozoites in 48-well plates and metronidazole considered as positive control and the negative control was TYI-S33 containing Trichomonas trophozoites without any drug. In all of mentioned groups, trophozoites number counted 12, 24, 48 h after culture. Results were analyzed using ANOVA statistical test, to evaluate the toxicity of extract, measured by MTT assay. Induced apoptosis of T. vaginalis after treatment with different concentrations of extract was determined by Flow Cytometry.
Results:
IC50 of alcoholic extract of combination V. thapsus and G. officinale and metronidazole after 24h was 73.80 μg/ml and 0.0326 μg/ml, respectively. The toxicity percentage of 25–800 μg/ml concentrations of this combination were between 0.2–1.98. In different concentrations of extract (25,50,100,200 and 400 μg/ml) apoptosis percent after 48h was 18.97 to 77.19 and necrosis percent was calculated 1.35, 3.18, 3.10, 1.16 and 4.09, respectively.
Conclusion:
Alcoholic extract of combination V. thapsus and G. officinale induces programmed death in T. vaginalis. Due to no toxicity on macrophages, it can be examined in vivo studies.
Keywords: Trichomonas vaginalis, Alcoholic extract, Verbascum thapsus, Ginger officinale, In vitro
Introduction
Trichomonas vaginalis is a protozoan parasite that causes human trichomoniasis, a very sexually transmitted disease (STD) with a significant incidence worldwide. This flagellated protozoan parasite can infect the urinary tract-genital in women and men (1). Symptomatic women usually have common sites of infection include the vagina, urethra and endocervix and clinical features include vaginal discharge, dysuria, itching, vulvar irritation and abdominal pain, T. vaginalis in patients with AIDS associated with inflammation and cervical cancer (2, 3).
The prevalence rate of trichomoniasis in Iran is 2 to 8% and may be up to 30% in high-risk populations (4). In recent years, metronidazole is used in the treatment of this infection as the most effective drug. Some reports of potential carcinogenic and teratogenic effects on the fetus and the incidence of drug resistance have been confirmed (5, 6). Many attempts have been made for evaluation of the effects of plants on the T. vaginalis (7, 8). Verbascum thapsus belongs to the family of Scorphulariaceae seen throughout the world. Compounds of this plant reduce cyclooxygenase activity and other bioactive substances including phenyl ethanol, glycosoaminoglycans, glycosides, saponins, and it is antiseptic and anti-inflammatory properties and is known for its skin wound healing. In addition, the plant is used to treat diarrhea and urogenital system infection (9, 10).
Ginger belongs to the family of the Zingiberaceae, and contains flavonoids, saponins, tannins and alkaloids with powerful antioxidant properties. Ginger is used to treat nausea, vomiting, arthritis, rheumatoid arthritis and osteomyelitis applications. This plant has anti-bacterial and anti-viral effects (11). With the prevalence of this parasite and the effects of herbal medicines on this disease, the present study aimed to determine the effectiveness of alcoholic extract of combination V. thapsus and Ginger officinale compared with the metronidazole in vitro.
Materials and Methods
Parasite Culture
This experimental study was conducted in Parasitology Laboratry, Kashan University of Medical Sciences, Kashan, Iran 2015 on women with suspected trichomoniasis referred to health centers based on clinical examination and microscopic examination of wet vaginal secretions of infected persons. From 23 samples were infected by T. vaginalis three isolates were selected for study. The parasites were axenically grown in standard TYI-S33 (trypticase-yeast extract-maltose) medium (pH:6.8) supplemented with 10% FCS, vitamin mixture and 100U/ml penicillin and 100 μg/mL streptomycin mixture at 37 °C.
Preparation of Plant Extracts
V. thapsus and G. officinale plants were obtained from the market and were approved by agricultural experts and then extracted according to the standards of British pharmacology, using the Percolation method (13).
Anti-trichomonal assay
Trophozoites were cultured in TYM-S-33 media in 48-well plates (5×104cell/well) as triplicate, different concentrations (25,50,100,200,400,800μg/ml) of combination V. thapsus and G. officinale ethanol extract and metronidazole (0.025,0.05,0.1,0.2,0.4 μg/ml) were added to each well individually. Metronidazole was used in injected form that made in manufacturing companies of Tabriz in Iran at a concentration of 0.5%. The number of parasites in each well plate was counted after 12, 24, and 48 h by trypan blue staining. In the negative control group, trophozoites were cultured in TYI-S33 without any drug. After counting of parasites, IC50 was determined by Graph Pad prism5 and growth inhibition percentage was calculated using the following formula:
A=Average number of trophozoites in control group. B=Average number of trophozoites in test group.
The present study was done according to the local ethics review committee of Kashan University of Medical Sciences that approved this work.
Flow Cytometry Analysis of Cell Death
Treated parasites were collected after 48h and centrifuged at 2000 rpm for 5 min. Then supernatant was removed, and 500μl binding buffer, 5μl Annexin-V and 5μl Propidium iodide (PI) were added to the residue. The samples incubated at laboratory temperature and dark condition for 5min. Absorbed color intensity in cells was observed by flow cytometry (2005 by Partec GmbH Munster, Germany) and the results were analyzed by FlowJo software, and the rate of apoptosis was determined.
Toxicity evaluation of combination V. Thapsus and G. officinale ethanol extract
Peritoneal macrophages obtained from mice and 105 cells/well macrophages were cultivated on each well of the 96-well plates and treated with different concentrations of the combination V. thapsus and G. officinale ethanol extract for 12, 24 and 48h at 37 °C. After this time, 20 μl MTT reagents (5 mg/ml, pH 7.4) in fresh TYI-S-33 culture medium were added to each sample. Then the plates were incubated for 3–5h at 37 °C under 5% CO2. After this time, the supernatant was removed from wells and 100 μl of DMSO was added to each well. After 15 min, absorption (OD) of each well was read at 570 nm by an ELISA reader. The amount of killed macrophages was determined based on the optical absorbance in test and control groups using the following formula (14).
AB is the OD of the blank well, AC is the OD of the untreated samples and AT is the OD of treated samples.
Results
The effect of this combination was measured at different concentrations (from 25 to 800 μg/ml) and at 12, 24 and 48h. There were no any motile and alive trophozoites at 800μg/ml concentration of extract after 48 h. IC50 for extract and metronidazole was calculated 73.8 μg/ml and 0.0326 μg/ml respectively (Table 1).
Table 1:
Comparison of the effects of different concentrations of the V. thapsus and G. officinale ethanol extract on T. vaginalis, 12 h, 24 h and 48 h after exposure
| Concentrations (μg/ml) | T. vaginalis trophozoite (× 104), Mean±SD | ||
|---|---|---|---|
| 12 h | 24 h | 48 h | |
| 25 | 40.7 ± 0.57 | 35.6±6.02 | 31.7±3.78 |
| 50 | 35.33± 4.5 | 29.7± 1.52 | 26.3±5.50 |
| 100 | 29.7± 0.57 | 26.3±2.51 | 20.7± 7.02 |
| 200 | 25.3 ± 4.5 | 20.3±0.57 | 16.7±1.53 |
| 400 | 17±3 | 10.7±1.15 | 4.7 ± 0.57 |
| 800 | 0.67±0.57 | 0.57±0.33 | 0 |
| 0.25MZ | 20±0.6 | 8±1.2 | 0 |
| 0.05MZ | 12±0.4 | 2±0.3 | 0 |
| 0.1MZ | 8±0.3 | 0 | 0 |
| 0.2MZ | 6±0.5 | 0 | 0 |
| 0.4MZ | 2±0.7 | 0 | 0 |
| Negative control | 38.33±2.51 | 53.66±3.51 | 88.3±6.5 |
| Statistical comparison of groups | P<0.05 | ||
MTT assay
The toxicity of the combination V. thapsus and G. officinale ethanol extract at concentrations (25, 50, 100, 200, 400, 800 μg/ml) in three times (12, 24, 48 h) were obtained using the MTT assay (Table 2). After 12h the combination V. thapsus and G. officinale ethanol extract in 25–800 μg/ml concentrations the fatality percent was between 0.2–0.50. It was between 0.51–1 and 1.01–1.98 after 24 and 48h respectively. Toxicity of the combination V. thapsus and the G. officinale ethanol extract at the highest concentration (800 μg/ml) and time (48 h) was 1.98% in macrophage cells (Table 2).
Table 2:
Comparison of the fatality percent alcoholic extract of combination V. thapsus and G. officinale on macrophages isolated from mice, 12 h, 24 h and 48 h after exposure
| Concentrations (μg/ml) | Percentage of toxicity (%) | ||
|---|---|---|---|
| 12h | 24h | 48h | |
| 25 | 0.20 | 0.51 | 1.01 |
| 50 | 0.25 | 0.66 | 1.12 |
| 100 | 0.33 | 0.78 | 1.21 |
| 200 | 0.38 | 0.87 | 1.31 |
| 400 | 0.45 | 0.99 | 1.46 |
| 800 | 0.50 | 1 | 1.98 |
Flow Cytometry Analysis
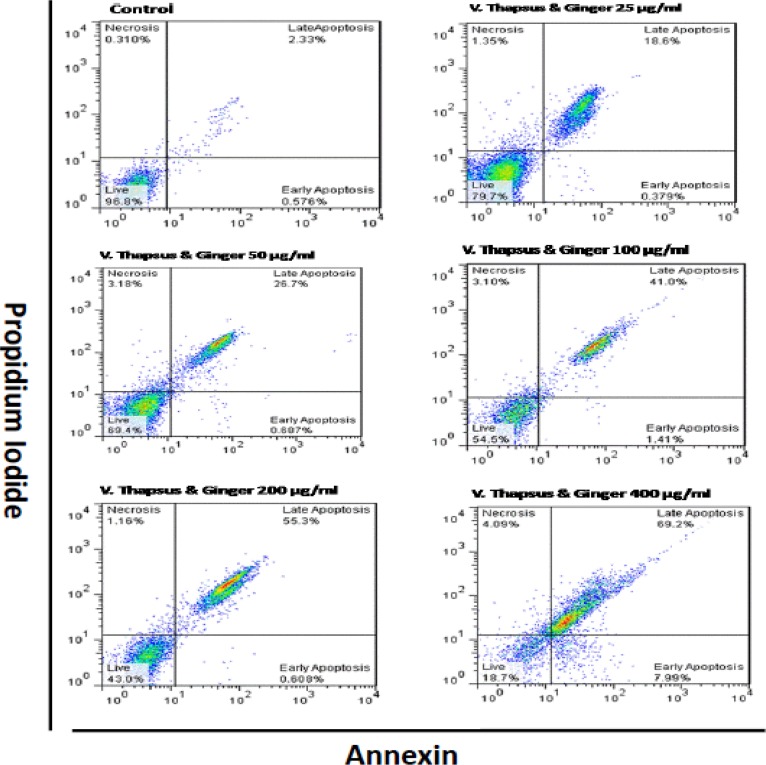
An alcoholic extract of combination V. thapsus and G. officinale induce apoptosis in trophozoites. Necrotic and apoptotic effects of alcoholic extract of combination V. thapsus and G. officinale on the trophozoites have been shown in (Fig. 1). Induced apoptosis (Early and Late) after 48 h in 25,50,100,200 and 400 μg/ml concentrations of the extract was 18.97 to 77.19 and necrosis was calculated 1.35%, 3.18%, 3.10%, 1.16% and 4.09% respectively (Fig. 1). Because the number of parasites after 48h at concentrations 800 is zero, Flow cytometry analysis was not done in this concentration.
Fig. 1:
Flow cytometry of results showed the extract had considerable induced apoptosis and also low necrotic effects on T. vaginalis trophozoites
Discussion
The number of the parasites at 25 and 100 μg/ml concentrations of extract reduced after 12 and 24h respectively, and IC50 was calculated 73.80 μg/ml after 24h. In this study, IC50 for metronidazole was calculated 0.0326 μg/ml, that is in similar to results of recently published data (IC50:0.0326) (14). The alcoholic and hydroalcoholic extract of Pelargonium has anti-Trichomonas effect; the anti-Trichomonas properties of alcoholic extract or more than its aqueous extract and the IC50 of the aqueous and alcoholic extracts of Pelargonium after 24h were 54.67, 27.63μg/ml respectively. In another study, the alcoholic extracts of Allium cepa, Oliveria decumbens Vent, and Muscari neglectum had an inhibitory effect on in vitro growth of T. vaginalis. The IC50 rate was calculated 101.8μg/ml for Olivera documents Vent, 572.3μg/ml for Allium cepa and 329.4μg/ml for Muscari neglectum after 24 h (15, 16). Lavender oil in all concentrations has an inhibitory effect on the T. vaginalis (17). Extract, Papaya and Cocos have powerful anti-trichomonal effect and extract Bocconia frutescens, Geranium mexicanum, Lygodium venustum have effective anti-trichomonal activity (5). Flavonoids, Saponins, Tannins, Terpenoids, Glycoside, Carbohydrates, Proteins, Fats, and oils that have effective anti-Giardia lamblia (18), anti-Trichomonas galina (19), anti-T.vaginalis (20), anti-Acanthamoeba castellani (21) and anti L. infantum activity (22). Ginger had higher anti-Schistosoma mansoni, Angiostrongylus cantonensis and Anisakis larvae activity (23, 24).
According to the results of flow cytometry in the present study, in the control group, 96.8% of cell was alive and percentages of necrosis, early apoptosis, and late apoptosis were 0.310%, 0.576% and 2.33% respectively. Induced apoptosis (Early and Late) by 25, 50, 100, 200 and 400 μg/ml of extract was 18.97%, 27.3%, 42.41%, 55.9% and 77.19%, respectively.
The results of flow cytometry showed that combination V. thapsus and G. officinale ethanol extract after 48h induction of apoptosis. Treatment of T. vaginalis with metronidazole does not lead to necrosis and causes cell death by apoptosis (25). The effect of anti-T. vaginalis Sapindus saponins were examined and the results showed the extract induced apoptosis (26). IC50 of V. thapsus ethanol extract after 24h was 39.17 μg/ml and the effect of the Verba scumthapsus ethanol extract on induced apoptosis in T. vaginalis was determined by Flow Cytometry and toxicity of V. thapsus alcoholic extract on mice macrophages was observed between 0.17–0.25 after 12 h and they were between 0.25–0.42 and 0.45–0.95 after 24 and 48h respectively (27). Toxicity of alcoholic extract of combination V. thapsus and G. officinale at the highest concentration (800μg/ml) and time (48h) was 1.98% in macrophage cells. Therefore, these extract as no toxicity in BALB/c mice peritoneal macrophages. Alcohol extract, Ginger was evaluated on liver cells and was IC50:2500μg/ml and without any toxic effect (28).
Conclusion
Alcoholic extract of combination V. thapsus and G. officinale induces programmed death in T. vaginalis. No toxicity on infected macrophages was observed. More comprehensive studies are needed to survey anti-Trichomonas activity of alcoholic extract of combination V. thapsus and G. officinale in vivo conditions.
Acknowledgments
This study was financially supported through grant No. 9265 offered by the Research Affairs of Kashan University of Medical Sciences, Kashan, Iran.
Footnotes
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Gutierrez Yezid. Diagnostic Pathology of Parasitic Infection. Second Edition; Oxford University press; 2000:52–58. [Google Scholar]
- 2.Moodley P, Wilkinson D, Connolly C, et al. Trichomonas vaginalis Is Associated with Pelvic Inflammatory Disease in Women Infected with Human Immunodeficiency Virus. Clin Infect Dis. 2002; 34:519–522. [DOI] [PubMed] [Google Scholar]
- 3.Chaves Vilela R, Benchimol M. Trichomonas vaginalis and Trichomonas foetus: interaction with fibroblasts and muscle cells-new insights into parasite-mediated host cell cytotoxicity. Mem Inst Oswaldo Cruz. 107(6):720–7. [DOI] [PubMed] [Google Scholar]
- 4.Hezarjaribi HZ, Fakhar M, Shokri A, et al. Trichomonas vaginalis infection among Iranian general population of women: a systematic review and meta-analysis. Parasitol Res. 114(4):1291–300. [DOI] [PubMed] [Google Scholar]
- 5.Calzada F, Yépez-Mulia L, Tapia-Contreras A. Effect of mexican medicinal plant used to treat trichomoniasis on Trichomonas vaginalis trophozoites. J Ethnopharmacol. 2007;113(2):248–51. [DOI] [PubMed] [Google Scholar]
- 6.Schwebke JR, Barrientes FJ, Barrientes FJ. Prevalence of Trichomonas vaginalis isolates with resistance to metronidazole and tinidazole. Antimicrob Agents Chemother. 2006; 50(12):4209–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Semnani MK, Saeidi M, Mahdavi MR, Rahimi F. Antimicrobial effects of methanolic extracts of some species of Stachys and Phlomis. J Mazandaran Univ Med Sci. 2007; 17(57): 57–66. [Google Scholar]
- 8.Kavita V, Sanjay G. Herbal medicines for sexually transmitted disease and AIDS. J Ethnopharmacol. 2002;80(1):49–66. [DOI] [PubMed] [Google Scholar]
- 9.Kupeli E, Tatli II, Akdemir ZS, Yesilada E. Biossay-guided isolation of anti-inflammatory & anti nociceptive glycoterpenoids from the folwer of Verbascum lasianthum Boiss. ex Bentham. J Ethnopharmacol. 2007;110(3):444–50. [DOI] [PubMed] [Google Scholar]
- 10.Mirhaidar H. Plant sciences. Nashre Farhange Eslami. 2005; 418–423. [Google Scholar]
- 11.Chang JS, Wang KC, Yeh CF, et al. Fresh ginger (Zingiber officinale) has anti-viral activity against human respiratory syncytial virus in human respiratory tract cell lines. J Ethnopharmacol. 2013;145(1):146–51. [DOI] [PubMed] [Google Scholar]
- 12.Diamond LS, Harlow DR, Cunnick CC. A new medium for the axenic cultivation of Entamoeba histolytica and other Entamoeba. Trans R Soc Trop Med Hyg. 1978;72(4):431–2. [DOI] [PubMed] [Google Scholar]
- 13.Foster S, Tyler VE. A sensible guide to the use of herbs and related remedies. 4th ed New York: The Havorth Herbal Press; 1999;98–102. [Google Scholar]
- 14.Calzada F, Yepez-Mulia L, Tapia-Contreras A. Effect of Mexican medicinal plant used to treat trichomoniasis on Trichomonas vaginalis trophozoites. J Ethnopharmacol. 2007;113(2):248–251. [DOI] [PubMed] [Google Scholar]
- 15.Fakhrie-Kashan Z, Arbabi M, Delavari M, et al. The effect of aqueous and alcoholic extracts of Pelarqonium roseumon the growth of Trichomonas vaginalis in vitro. Feyz, Journal of Kashan University of Medical Sciences 2014;18(4):369–375. [Google Scholar]
- 16.Fakhrieh-Kashan Z, Arbabi M, Delavari M, et al. In-vitro Therapeutic Effect of Allium Cepa, Oliveria Decumbens Vent and Muscari Neglectum against Trichomonas vaginalis. Journal of Isfahan Medical Sciences. 2015; 32(310):1985–1992. [Google Scholar]
- 17.Ezatpur B, Badparva E, Ahmadi Sh, et al. Investigation of Anti Trichomonas vaginalis Activity of Lavandula angyustifolia Essential Oil in in vitro Media. Sci J Ilam Med Univ. 2009;16(4):31–37. [Google Scholar]
- 18.Barbosa E, Calzada F, Campos R. In vivo antigiardial activity of three flavonoids isolated of some medicinal plants used in Mexican traditional medicine for the treatment of diarrhea. J Ethnopharmacol. 2007;109(3):552–4. [DOI] [PubMed] [Google Scholar]
- 19.Adebajo AC, Ayoola OF, Iwalewa EO, et al. Anti-trichomonal, biochemical and toxicological activities of methanolic extract and some carbazole alkaloids isolated from the leaves of Murraya koenigii growing in Nigeria. Phytomedicine. 2006;13(4):246–54. [DOI] [PubMed] [Google Scholar]
- 20.Arthan D, Sithiprom S, Thima K, et al. Inhibitory effects of Thai plants beta-glycosides on Trichomonas vaginalis. Parasitol Res. 2008;103(2):443–8. [DOI] [PubMed] [Google Scholar]
- 21.Ródio C, da Rocha VD, Kowalski K, et al. In vitro evaluation of the amebicidal activity of Pterocaulon polystachyum (Asteraceae) against trophozoites of Acanthamoeba castellanii. Parasitol Res. 2008;104(1):191–4. [DOI] [PubMed] [Google Scholar]
- 22.González-Coloma A, Reina M, Sáenz C, et al. Anti leishmanial, anti trypanosomal, and cytotoxic screening of ethnopharmacologically selected Peruvian plants. Parasitol Res. 2012;110(4):1381–92. [DOI] [PubMed] [Google Scholar]
- 23.Mostafa OM, EidR A, Adly MA. Antischistosomal activity of ginger (Zingiber officinale) against Schistosoma mansoni harbored in C57 mice. Parasitol Res. 2011;109(2):395–403. [DOI] [PubMed] [Google Scholar]
- 24.Lin RJ, Chen CY, Chung LY, Yen CM. Larvicidal activities of ginger (Zingiber officinale) against Angiostrongylus cantonensis. Acta Trop. 2010;115(1–2):69–76. [DOI] [PubMed] [Google Scholar]
- 25.Chose O, Noël C, Gerbod D, et al. A form of cell death with some features resembling apoptosis in the a mitochondrial unicellular organism Trichomonas vaginalis. Exp Cell Res. 2002;276(1):32–9. [DOI] [PubMed] [Google Scholar]
- 26.Tiwari P, Singh D, Singh MM. Anti-Trichomonas activity of Sapindus saponins, a candidate for development as microbicidal contraceptive. J Antimicrob Chemother. 2008;62(3):526–34. [DOI] [PubMed] [Google Scholar]
- 27.Fakhrieh Kashan Z, Arbabi M, Delavari M, et al. Effect of Verbascum thapsus Ethanol Extract on Induction of Apoptosis in Trichomonas vaginalis in vitro. Infect Disord Drug Targets. 2015;15(2):125–30. [DOI] [PubMed] [Google Scholar]
- 28.Tavakol Afshari J, Moheghi N, Brook A. Ethanolic Extract Cytotoxic Effect of Zingiber officinale in Hepatocellular Carcinoma (HEPG2) Cell Line. Sci J Hamadan Univ Med Sci 2010;17(3): 52–56. [Google Scholar]