Abstract
Introduction
Musculoskeletal manifestations are well-recognized side effects of treatment with statins. New advances in this field have appeared in recent years. This review focuses on the diagnosis of these conditions and their underlying pathogenesis, in particular immune-mediated necrotizing myopathy.
Areas covered
Clinical phenotypes including rhabdomyolysis, myalgia and/or mild hyperCKemia, self-limited toxin statin myopathy, and immune-mediated necrotizing myopathy are herein described. Therapeutic recommendations and a diagnostic algorithm in statin-associated myopathy are also proposed. The etiology and pathogenesis of statin-induced myopathy has mainly focused on the anti-HMGCR antibodies and the responsibility of the immune-mediated necrotizing myopathy is discussed. The fact that patients who have not been exposed to statins may develop statin-associated autoimmune myopathy with anti-HMGCR antibodies is also addressed. The literature search strategy included terms identified by searches of PubMed between 1969 and December 2017. The search terms ‘myositis’, ‘statin-induced autoimmune myopathy’, ‘immunemediate necrotizing myopathy’, ‘statins’, ‘muscular manifestations’, and ‘anti-HMGCR antibodies’ were used.
Expert commentary
Full characterization of the known phenotypes of statin toxicity and the specific role of the anti-HMGCR in those exposed and not exposed (i.e. juvenile forms) to statins and in some types of neoplasms is of paramount relevance.
Keywords: Myositis, statin-induced autoimmune myopathy, immune-mediate necrotizing myopathy, statins, muscular manifestations, anti-HMGCR antibodies
1. Introduction
Drugs that inhibit the enzyme, 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR), known as statins, are one of the most widely used medications [1]. Statin use is associated with reductions in cardiovascular risk and mortality due to cardiovascular disease [2,3]. However, a well-recognized adverse effect of these drugs is muscle toxicity. Although data from randomized clinical trials suggest that this would not be a significant reason for discontinuing these drugs, patients may show poor adherence to statin regimens because of myalgia and other muscle complaints [4,5].
Currently there is no consensus regarding the terminology physicians should use for the muscle involvement occurring in patients receiving statins. Hence, myalgia, myositis, and myopathy are used interchangeably, and this adds some confusion to the topic [6–8]. There is some epidemiological evidence that adverse effects of statins on muscle are more common in patients with predisposing factors, such as a history of increased creatine kinase (CK) levels, a family history of myopathy, or a previous diagnosis of neuromuscular diseases or hypothyroidism [8]. Moreover, certain genetic factors may increase the risk of experiencing statin-induced muscle toxicity. One of these is a common single-nucleotide polymorphism in the SLCO1B1 gene, which is associated with a higher risk of myopathy in patients taking simvastatin, although it is uncertain whether the risk also applies to other statins (e.g. rosuvastatin or atorvastatin) [9].
Several scoring systems have been designed to quantitate the risk of developing statin-associated muscle symptoms and to manage goal-inhibiting statin intolerance [6,10], but in clinical practice, it seems reasonable to work with four separate clinical scenarios that have different diagnostic or treatment implications: rhabdomyolysis, myalgia and mild hyperCKemia, self-limited toxic statin myopathy, and the recently described immune-mediated necrotizing myopathy. This review describes these conditions, the challenges of their diagnosis, and their pathogenesis, with a particular focus on immune-mediated necrotizing myopathy. Recommendations for clinicians and suggested approaches for managing these patients are discussed.
2. Defining clinical phenotypes
From the clinical viewpoint, patients with statin-associated myopathy can be divided into four groups [11,12]: those with rhabdomyolysis, those with myalgia or mild hyperCKemia (<5.0 times the upper limit of normal), those having self-limited toxic statin myopathy, and those with myositis (i.e. the recently described immune-mediated necrotizing myopathy with anti-HMGCR antibodies).
2.1. Rhabdomyolysis
Rhabdomyolysis, the most severe form of acute muscle disease associated with statins, is fortunately, very rare, affecting less than 1 patient per 100,000 treated per year with these drugs [13]. High CK concentrations (>100-fold the upper limit of normality) are characteristic of rhabdomyolysis, but what defines the syndrome is evidence of myoglobinuria and renal impairment, due to acute tubular necrosis caused by myoglobin precipitation in the renal tubules [14]. Rhabdomyolysis is a life-threatening condition; statins must be discontinued and in most cases, avoided in the future for safety reasons. Dark urine with a positive dipstick test may be mistaken for hematuria. The absence of red cells in the urine sediment supports the diagnosis of myoglobinuria. In severe cases of rhabdomyolysis, muscle weakness may be a cardinal manifestation, but it is usually transitory, disappearing a few days after stopping the drug. Muscle biopsy is not generally indicated, and when it is necessary for diagnostic purposes, it should be performed several weeks or months after the clinical event.
2.2. Myalgia and/or mild hyperCKemia
This mild form of statin-induced muscle toxicity is often seen in clinical practice. Myalgia or muscle pain is the most common symptom and a frequent cause of statin discontinuation. However, in a Cochrane Foundation analysis of 37,939 patients receiving statins in 9 clinical trials [15], only 9.4% (3551 patients) developed symptoms of myalgia, a rate similar to that found in patients receiving a placebo. It has been argued that these studies may be biased, as patients who had developed some type of muscle disease in the past were excluded. Supporting this concept, the prevalence of myalgia in observational studies is higher, close to 20% [8,16–18]. On the other hand, clinicians have to take into account the nocebo effect, meaning the negative expectations of the patient and their doctors regarding muscular manifestations attributed to statins. This nocebo effect may partially explain the muscle aching in absence of biochemical substrate that is detected in observational studies and not in randomized double-blind placebo-controlled trials [19].
Clinically, the pain experienced is usually in the calves and thighs, but it is sometimes diffuse, affecting all muscles. Although myalgia is a cause of statin discontinuation, it is not a life-threatening situation. It can be accompanied by a mild CK elevation, usually reaching less than 1000 IU/L (<5-fold the upper limit of normal), which in itself, is not a mandatory reason for discontinuing statin treatment. Moreover, the outcome of this clinical syndrome is highly variable; it can improve or not after withdrawing the offending drug. Some patients continue experiencing myalgia and/or mild CK elevations even after statins have been stopped, and others improve despite continuation of the drug [20,21].
2.3. Self-limited toxic statin myopathy
Some patients develop toxic myopathy during treatment with statins, a condition that is not immune-mediated. The severity of the presentation varies, with proximal muscle weakness preventing the basic activities of daily living in more severe forms and high CK levels, usually between 10 and 100 times the upper limit of normality (2000–20,000 IU/L). Myalgia is also a common symptom.
Muscle biopsy is usually performed in these patients, although it can be deferred for 4–6 weeks after statin discontinuation while monitoring the patients’ clinical evolution. If the clinical complaints progressively improve, muscle biopsy is not necessary. Pathological study shows necrosis and regenerating muscle fibers, but none of the other characteristic immune-mediated features. Nevertheless, it is well-recognized that regenerating muscle fibers can express major histocompatibility complex (MHC) class I, and this may make it difficult to establish whether the pathological findings indicate an immune-mediated phenomenon. Determination of antibodies against 3-hydroxy-3-methylglutaryl-CoA reductase (anti-HMGCR antibodies) [22] is essential to confirm an immune-mediated condition and promptly start immunosuppressive therapy, as patients with self-limited statin toxic myopathy generally test negative to these antibodies [23].
2.4. Immune-mediated necrotizing myopathy and anti-HMGCR antibodies
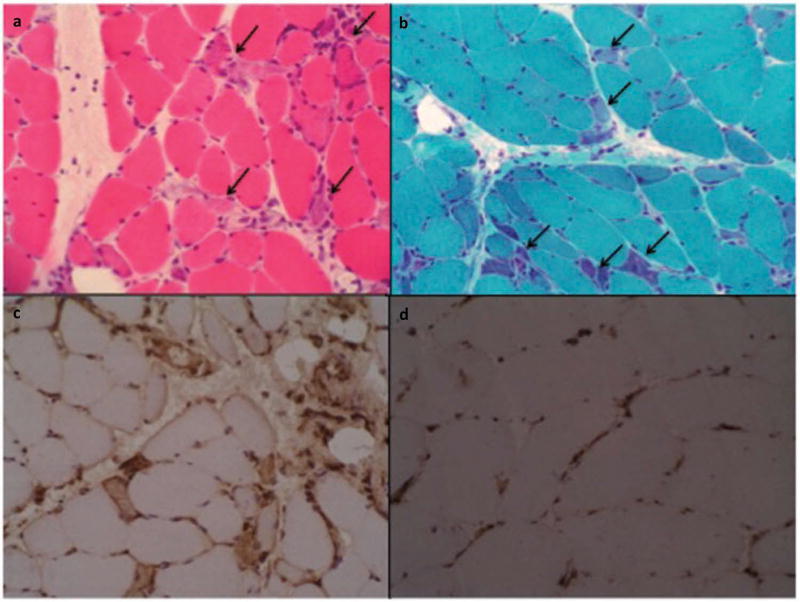
The last of the four muscle conditions associated with statin treatment, immune-mediated necrotizing myopathy (IMNM), should be considered a true autoimmune myopathy. Affected patients show progressive proximal muscle weakness involving both limb girdles, CK values between 10 and 100 times the upper limit (2000 and 20,000 IU/L), myopathic electromyography findings, and muscle biopsy usually showing necrosis with regeneration of muscle fibers and scarce inflammation, mainly composed of macrophages [24,25]. Certain immune-mediated features, such as endothelial membrane attack complex (MAC) deposition in non-necrotic fibers and MHC class I expression are additional pathologic features of this condition (Figure 1). One differential characteristic is that statin withdrawal does not usually improve the patient’s symptoms. Immunosuppressive treatment is required to obtain a clinical response.
Figure 1.
Immune-mediated necrotizing myopathy. Necrotic muscle fiber cells (arrows). (a) Hematoxylin-eosin, (b) Numerous regenerating fibers are seen (arrows). Masson Trichrome. (c) Universal sarcolemmal MHC class I positive (stronger in regenerating cells). (d) MHC class I negative control (positivity only in endothelial cells). All samples are frozen tissue. Published with permission of Elsevier. Original source: Alvarado Cárdenas et al. Med Clin (Barc) [63]. Copyright © 2015 Elsevier España, S.L.U. All rights reserved.
IMNM was first described by a group of researchers from the Johns Hopkins Myositis Center in Baltimore. The clinical picture was associated with a new autoantibody against a protein doublet of 100–200 kDa detected by immunoprecipitation techniques [24]. Later, the same group identified the protein, HMGCR, as the rate-limiting enzyme in cholesterol synthesis [26]. This form of autoimmune myopathy is now recognized worldwide, with authors from several countries, from Asia to Europe and from the United States to New Zealand, reporting their experience [27–32].
This autoimmune myopathy is rare, with an estimated incidence of 2–3 new cases in every 100,000 patients exposed to statins [33]. In addition, it is associated with a specific immunogenetic background, in which adults often show the human leukocyte antigen (HLA) DRB1*11:01 and children DRB1*07:01 [34,35]. Most patients from the population exposed to statins, and even those with familial hypercholesterolemia receiving high doses of the drug do not develop this clinical picture, and test negative to anti-HMGCR antibodies when screened [36].
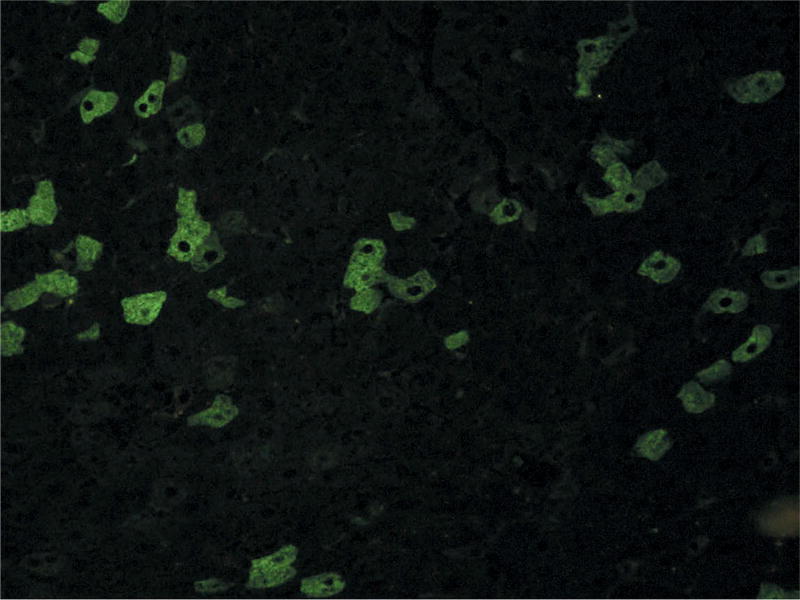
Over the years, new and more efficient techniques to detect anti-HMGCR autoantibodies have been developed to replace the expensive and time-consuming immunoprecipitation method [37]. Commercial ELISA or blot tests are easy to perform and work acceptably well. A complementary and even less expensive test to screen for these antibodies is the detection of a characteristic HALIP (HMGCR-Associated Liver Indirect immunofluorescence Pattern), using cryostat triple tissue sections (rat liver, kidney, and stomach), which is subsequently confirmed by ELISA or blotting (Figure 2) [28]. Although immunoprecipitation remains the gold standard technique for detecting anti-HMGCR antibodies, it is now used only for confirmation purposes in rare cases with inconclusive results on other tests.
Figure 2.
HALIP indirect immunofluorescence pattern on rat liver cryostat section. Confocal microscope FV (Olympus × 200)27.
IMNM has a heterogeneous clinical picture with widely varying degrees of muscle involvement. Occasionally patients may improve after statin withdrawal, but usually they require immunosuppressive treatment. The standard therapy includes various immunosuppressants. Azathioprine and methotrexate are the most common, but other drugs such as calcineurin antagonists (cyclosporine or tacrolimus) and mycophenolate mofetil are being used increasingly more often. A recent study has reported that intravenous immunoglobulin, the only agent with proven effectiveness for treating inflammatory myopathies in a randomized controlled clinical trial [38], seems to be the best option for treating statin-associated immune-mediated necrotizing myopathy [33,39].
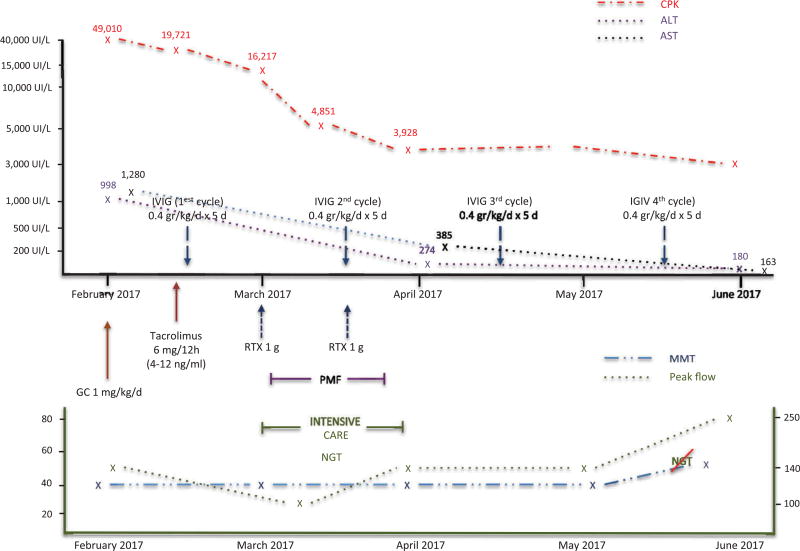
Clinicians sometimes encounter patients with aggressive forms of the disease (Figure 3), in which glucocorticoids, intravenous immunoglobulins, and a single immunosuppressive drug do not suffice to improve the condition. In this situation, other drugs such as rituximab or even plasmapheresis may be required for treatment. Patients who do not respond well to conventional therapy may develop extremely severe muscle manifestations, which can include severe dysphagia requiring nasogastric tube feeding and dyspnea resulting from diaphragmatic muscle involvement that can lead to respiratory failure.
Figure 3.
Clinical, functional, and biological evolution of a severe form of IMNM (see description in the text). GC: glucocorticoids, IVIG: intravenous immunoglobulin, MMT: manual muscle test, NGT: nasogastric tube, PMF: plasmapheresis, RTX: rituximab.
In muscle biopsy specimens of patients with anti-HMGCR-induced myopathy, the pathological findings predominantly show necrosis with scarce inflammatory infiltrate, as in other immune-mediated necrotizing myopathies. However, in some rare cases, there is a pathological picture of marked inflammation [29,31,40–43]. Furthermore, an unknown myopathy of genetic or metabolic origin is seen in some muscle biopsy analyses of patients with suspected statin-related myopathy, suggesting that the drug may act as a ‘trigger’ for the condition (unpublished personal observations).
There is a well-known association between cancer and some types of idiopathic inflammatory myositis, including immune-mediated necrotizing myopathy. In some studies, the presence of anti-HMGCR autoantibodies was marginally associated with a higher risk of developing cancer, although the risk was lower than in patients with autoantibody-negative immune-mediated necrotizing myopathy [44]. The risk doubles with anti-HMGCR antibodies, but in seronegative patients, it is more than eightfold higher than in the general population. Although studies in Japan seem to confirm the association between cancer and anti-HMGCR [45], it is not supported in all publications [31,46].
In the original report describing anti-HMGCR antibodies, most, but not all patients with anti-HMGCR-associated myopathy had been exposed to statins (72.7% of the total) [24]. The percentage of unexposed patients is even higher in other series, such as the largest cohort of European patients, in which 45.6% of patients with anti-HMGCR-associated myopathy were statin-naïve [32]. Furthermore, in recent reports including children with inflammatory myopathy, some of them misdiagnosed as having dystrophy, none of the children had received statins [34,47,48]. Therefore, a relevant question remains: Is the clinical management of anti-HMGCR-associated myopathy the same in patients who have been exposed to statins and those who have not?
3. Etiology and pathogenesis
Statin-induced myopathy encompasses a heterogeneous group of muscle manifestations that have not yet been well characterized. Mitochondrial dysfunction, oxidative stress, and several mechanisms derived from impaired mevalonate metabolism, such as isoprenylation of small G-proteins, have been implicated in the mechanism of statin toxicity [49–51].
Genetic susceptibility related to the pharmacokinetics and pharmacodynamics of the drug is also of paramount relevance in statin-related myopathy. Polymorphisms of the SLCO1B1 gene are the best-known pharmacokinetic-related alteration, although polymorphisms of other genes have also been described [9]. Regarding pharmacodynamics, certain genetic factors can increase the risk of statin-induced myopathy. Some examples are those associated with plasma membrane calcium transporting ATPase or with mitochondrial energy production regulated by the CoQ2 gene. This gene codes for the coenzyme Q10, known as ubiquinone, which is located in the phospholipid bilayer of mitochondria and is essential for obtaining energy, especially in muscle tissue [8,52].
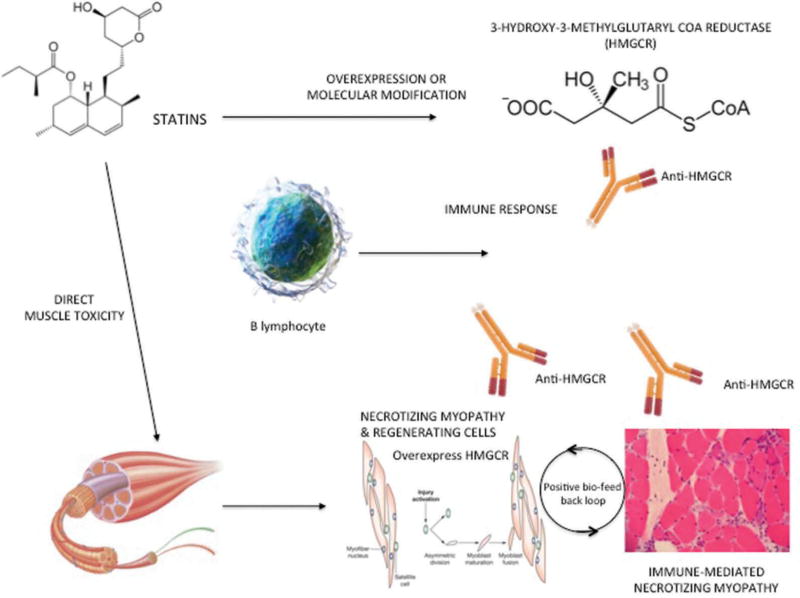
Recently, a new etiopathogenetic mechanism has been proposed, in which the immune system also plays a role. This is the case of immune-mediated necrotizing myopathy and antibodies against HMGCR, the enzyme that is usually upregulated by statins. Statins upregulate HMGCR and overexpression of this enzyme likely facilitates presentation of highly immunogenic HMGCR peptides by the HLA DRB1*11:01, thus triggering the autoimmune disease [35,53].
In other words, a new paradigm of disease mechanism is elicited. In susceptible patients who have a characteristic HLA or an appropriate genetic background, HMGCR, the molecular target of statins, may trigger the autoimmune phenomenon after some type of interaction with the drug. Once the autoimmune process is started, HMGCR overexpression by statins is not needed to maintain the disease, as regenerating cells naturally express HMGCR [54,55] and will maintain the feed-forward loop of autoimmunity. This pathogenetic model is shown in Figure 4. Anti-HMGCR can be found in muscle cells, and some authors have reported that levels of this antibody correlate with the CK concentration and clinical disease activity, thus suggesting a possible role of these antibodies in the pathogenesis of the disease [26,56]. In addition, experimental studies in muscle biopsies of patients with anti-HMGCR have shown that these autoantibodies may impair muscle regeneration and induce muscle atrophy [57].
Figure 4.
Etiopathogenesis of statin-induced immune-mediated necrotizing myopathy. EXPERT REVIEW OF CLINICAL IMMUNOLOGY 219
A challenging scenario is one in which patients who have not been exposed to statins show a full-blown clinical syndrome of immune-mediated necrotizing myopathy and anti-HMGCR antibodies. These patients are usually younger and more difficult to treat [46]. Some authors have proposed that consumption of red yeast rice, Puerh tea, or food containing certain types of oyster mushrooms, all natural sources of statins, may explain this association. Monascus purpureus, a mold traditionally used in East Asia to dye and conserve food, participates in the fermentation of red yeast rice, and gives it the characteristic red color. This mold produces lovastatin in its natural form, the so-called monacolin K, its pharmacologic active compound, and a well-known type of statin [58]. After long-term storage, Pu-erh tea is rich in Aspergillus terreus, which plays a role in its maturation and specific flavor and is also a source of lovastatin [59]. Of note, the first statin synthesized in 1976 by Akira Endo, a Japanese biochemist, was derived from the fungus species Penicillium citrinum [60]. Hence, it is reasonable to suggest that statin-naive patients with anti-HMGCR-associated myositis have somehow been inadvertently exposed to natural sources of statins. The fact that Asian cohorts, where the diet is rich in statin-containing food, show a higher prevalence of statin-naïve anti-HMGCR patients [29,30] also supports this theory, although this could be due in part to the pharmacokinetics differences observed in distinct ethnicities. Epidemiologic studies focused on clarifying this issue would undoubtedly be of great interest.
Also of interest, an indirect immunofluorescence study reported that the characteristic HMGCR-associated immunofluorescence pattern, HALIP, was recognized in rat liver tissue and confirmed by immunoabsorption with human purified HMGCR antigen [28]. This finding prompted us to speculate that the same mechanism of toxicity and immunity could also be the cause of autoimmune hepatitis associated with statin-induced liver toxicity, a rare but well-described condition. This disorder occasionally courses as a self-sustained autoimmune hepatitis that persists even after statins have been stopped, in the same manner as immune-mediated necrotizing myopathy [61–64]. However, on analysis of 30 patients with autoimmune hepatitis and 30 others with primary biliary cholangitis in our center, as well as 1 patient with autoimmune hepatitis showing a tight temporal relationship with initiation of statins (personal observation), anti-HMGCR antibodies tested negative in all cases. To the best of our knowledge only one such a case, which combine IMNM and autoimmune hepatitis, positive to anti-HMGCR, has been published until now [65].
4. Clinical recommendations
A practical approach to establish clinical recommendations regarding the muscle complaints related to statin use takes into account which of the abovementioned phenotypes best fits a given patient. Nevertheless, as a set of general rules, it is recommended to first review the indication for statin use in terms of cardiovascular risk, exclude secondary causes of myopathy such as hypothyroidism or the presence of any well-defined myopathy (e.g. McArdle disease or Steinert myotonic dystrophy), and explore whether a high level of physical activity was related with the patient’s signs and symptoms.
In a patient with myalgia, serumCK below 1000 IU/L (<5 times the upper limit of normal), and no muscle weakness on physical examination, it is probably safe to maintain the drug and monitor CK levels, which should be stable or have normalized at the next follow-up visit. If CK values rise to more than 10 times the upper limit, the clinician should assess the risk/benefits of drug withdrawal, although it is probably warranted. As a general rule, in a high-risk patient (e.g. previous myocardial infarction) who has myalgia with normal serum CK values and no muscle weakness, we do not recommend withdrawing statin treatment.
When statin administration is associated with rhabdomyolysis, a severe condition, the advice is clear: withdraw the statin and do not administer it in the future. If such as patient is at high cardiovascular risk, we suggest the use of other, recently approved effective drugs, such as PCSK9 inhibitors [66]. These monoclonal antibodies (e.g. evolocumab, alirocumab, and bococizumab), which act against the proprotein convertase, have proven effective in reducing cardiovascular events and could be an alternative to statin therapy in severe cases of drug toxicity, such as rhabdomyolysis.
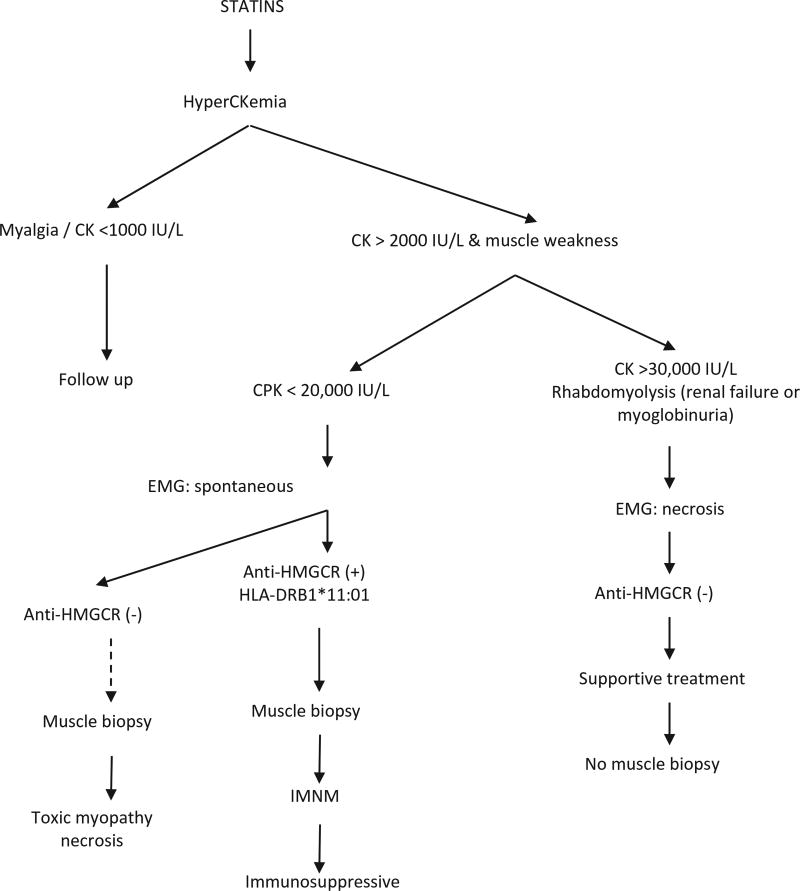
It is more difficult to establish clinical recommendations in the other two phenotypes: self-limited toxic myopathy and statin-induced autoimmune myopathy. In the first case, drug withdrawal usually suffices. Muscle biopsy can sometimes be misleading in these patients, and it is important to test for anti-HMGCR autoantibodies to differentiate between the two conditions. If the patient does not improve within 2 weeks after discontinuing the drug and anti-HMGCR antibodies test positive, the diagnosis of immune-mediated necrotizing myopathy is supported and immunosuppressive therapy should be considered. If such a patient is at high cardiovascular risk, clinicians may try to rechallenge the drug, using another statin, a lower dose, or an every-other-day schedule, with strict monitoring of the patient’s CK levels and muscle strength. PCSK9 inhibitors may be an alternative option, as their mechanism of action is radically different from that of statins, but there is no experience yet in this clinical scenario. Most of the related recommendations are included in Table 1 and the diagnostic algorithm (Figure 5).
Table 1.
A comparison of different statin-induced muscle manifestations.
| Myalgia and/or mild elevated CK |
Self-limited toxic myopathy | IMNM | Rhabdomyolysis | |
|---|---|---|---|---|
| Symptoms | Myalgia (++) | Proximal muscle weakness (++) | Proximal muscle weakness (+++) | Myoglobinuria |
| Muscle weakness (−) | Myalgia (++) | Renal failure | ||
| Myalgia (+) | Dysphagia (+/++) | Muscle weakness (++) | ||
| Ventilatory failure (+/−) | Myalgia (++) | |||
| Maximum CK (IU/L) | <5 × UNL [<1000 IU/L] | <100 × UNL [<20,000 IU/L] | >10 < 100 × UNL [>2000 < 20,000 IU/L] | >100 × UNL [>20,000 IU/L] |
| Muscle biopsy | Not mandatorya | Myofiber necrosis with no immune findings | Myofiber necrosis MHC class I upregulation MAC deposition on non-necrotic fibers | Massive necrosisb |
| Genetic Background | SNP in SLCO1B1 gene | HLA-DRB1*11:01 | SNP in SLCO1B1 gene | |
| Anti-HMGCR antibody | Negative | Negative | Positive | Negative |
| Clinical course after withdrawal | Unpredictable (but good outcome) | Improvement spontaneously | Persistent/progressive weakness | Total recoveryc |
| Recommended therapy | None | Statin withdrawal (or dose reduction) | Statin withdrawal Immunosuppression | Support and avoid statins in the future |
Minimal changes include cytochrome oxidase (COX) negative fibers, vacuolization, and type 2 fiber atrophy, which are nonspecific findings.
For diagnostic purposes, muscle biopsy should be performed several weeks or months after the clinical event.
Damage could remain.
IMNM: immune-mediated necrotizing myopathy; MAC: membrane attack complex; MHC: major histocompatibility complex; SNP: single-nucleotide polymorphism (for simvastatin); UNL: upper normal limit.
Figure 5.
Diagnostic algorithm in statin-associated myopathy. CK: creatine kinase (values are intended to be approximate); EMG: electromyography; HMGCR: 3-hydroxy-3-methylglutaryl coenzyme A reductase; IMNM: immune-mediated necrotizing myopathy. Dotted line indicates that muscle biopsy is not always mandatory (see text).
No guidelines are available for the management of statin-induced muscle disorders. The recommendations reported here are based on our personal experience and are fundamentally in accordance with guidelines on statin use endorsed by scientific societies or working groups [6,8].
5. Expert commentary
Statin-induced muscle disorders are not rare. It is important to fully characterize the known phenotypes of statin toxicity as well as any new ones that may appear to delineate proper management of each type.
Several questions that are now unanswered will be resolved over the next few years. Epidemiologic studies investigating the role of other sources of statins in patients with anti-HMGCR associated myopathy who have not been exposed to medical statins will help to elucidate the pathophysiologic mechanisms causing this condition. Research will also be done in juvenile and pediatric forms of anti-HMGCR myopathy for this same purpose. The hypothetical relationship with cancer and the appropriate genetic background that contributes to explain why some patients and not others develop the syndrome will be clarified in the near future, perhaps with genome-wide association studies, next-generation sequencing studies, or even epigenetic studies.
Data on the novel toxic-immune disease-causing mechanism will contribute to our understanding of statin-induced muscle disorders and may be applicable to other clinical phenomena and diseases, such as a drug-related liver toxicity and autoimmune hepatitis, and other immune-mediated diseases in which the trigger can be a drug or toxic agent.
Therapeutic guidelines based on evidence-based medicine are lacking for this condition. Randomized, controlled clinical trials are needed to establish appropriate recommendations. Given the rarity of some of these diseases, international initiatives are advocated to join efforts. In the interim, observational studies reporting the experience with different therapeutic strategies are also endorsed.
We should remember that new drugs such as PCSK9 inhibitors have shown great potential in the treatment of high LDL-cholesterol levels and may be a good option for reducing cardiovascular events in patients formerly receiving statins. Time will tell whether statins can be easily substituted by these drugs, and whether both types of treatment can be safely used together to improve the prognosis of patients at high cardiovascular risk.
6. Five-year view
Over the next 5 years, research will help to unravel the epidemiology and the mechanisms that underline those patients with statin-induced autoimmune myopathies not apparently exposed to statins, and the case of children with autoimmune myopathy with anti-HMGCR antibodies. Analysis of the feasibility of different serological test or provocation test to ascertain the role of Aspergillum sp. or others yeast that could be involved in theses disease may be useful ways to address this issue. Whether these autoantibodies are pathogenic or not will be established over the next years. The new paradigm that considers the disease as the conjoint of toxicity and autoimmunity could be applied to other autoimmune disorders.
Key issues.
Patients with autoimmune myopathy associated with statin use and anti-HMGCR antibodies show a pathological picture on muscle biopsy that fits with immune-mediated necrotizing myopathy. Occasionally, a typical inflammatory infiltrate can also be seen. The term statin-induced autoimmune myopathy encompasses this clinical spectrum.
Statin-naïve patients with anti-HMGCR antibodies and autoimmune myopathy have been described, including children. Other sources of possible statin exposure produced by Aspergillus spp., Penicillium spp., and other molds should be investigated.
The clinical presentation of statin-induced autoimmune myopathy is heterogeneous. There are mild forms that do not need immunosuppressants and severe forms that are refractory to intense immunosuppression. Clinicians should be aware of this heterogeneity.
Intravenous immunoglobulin seems to be the best treatment for patients diagnosed with immune-mediated necrotizing myopathy and anti-HMGCR antibodies, and should be included in all therapeutic schemes.
Immune-mediated conditions such as statin-associated myopathy establish a new paradigm in which drug-related muscle toxicity may modify the HMGCR molecule and trigger an autonomous, autoimmune phenomenon.
Acknowledgments
Funding
This work was funded by the Instituto de Salud Carlos III, grants PI12-01320 and PI15-02100, co-financed by the European Regional Development Fund (ERDF).
Footnotes
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Statins for millions more? Lancet. 2014;383:669. doi: 10.1016/S0140-6736(14)60240-3. [DOI] [PubMed] [Google Scholar]
- 2.Stone NJ, Robinson JG, Lichtenstein AH, et al. American College of Cardiology/American Heart Association task force on practice guidelines, 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2889–2934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388:2532–2561. doi: 10.1016/S0140-6736(16)31357-5. [DOI] [PubMed] [Google Scholar]
- 4.Fernandez G, Spatz ES, Jablecki C, et al. Statin myopathy: a common dilemma not reflected in clinical trials. Cleve Clin J Med. 2011;78:393–403. doi: 10.3949/ccjm.78a.10073. [DOI] [PubMed] [Google Scholar]
- 5.Ganga HV, Slim HB, Thompson PD. A systematic review of statin-induced muscle problems in clinical trials. Am Heart J. 2014;168:6–15. doi: 10.1016/j.ahj.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 6.Mancini GB, Baker S, Bergeron J, et al. Diagnosis, prevention, and management of statin adverse effects and intolerance: Canadian Consensus Working Group update. Can J Cardiol. 2016;32(7 Suppl):S35–65. doi: 10.1016/j.cjca.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Pasternak RC, Smith SC, Jr, Bairey Merz CN, et al. American College of Cardiology; American Heart Association; National Heart, Lung and Blood Institute, ACC/AHA/NHLBI clinical advisory on the use and safety of statins. J Am Coll Cardiol. 2002;40:56. doi: 10.1016/s0735-1097(02)02030-2. [DOI] [PubMed] [Google Scholar]
- 8.Stroes ES, Thompson PD, Corsini A, et al. European Atherosclerosis Society Consensus Panel. Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel statement on assessment, aetiology and management. Eur Heart J. 2015;36:1012–1022. doi: 10.1093/eurheartj/ehv043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9•.Link E, Parish S, Armitage J, et al. SEARCH Collaborative Group. SLCO1B1 variants and statin-induced myopathy genome wide study. N Engl J Med. 2008;359:789–799. doi: 10.1056/NEJMoa0801936. In this genomewide association study (GWAS), the authors found a strong association between rs4363657 single-nucleotide polymorphism located in SLCO1B1 and the risk of simvastatin-induced myopathy. [DOI] [PubMed] [Google Scholar]
- 10.Rosenson RS, Baker SK, Jacobson TA, et al. An assessment by the Statin Muscle Safety Task Force: 2014 update. J Clin Lipidol. 2014;8:S58–71. doi: 10.1016/j.jacl.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Mohassel P, Mammen AL. The spectrum of statin myopathy. Curr Opin Rheumatol. 2013;25:747–752. doi: 10.1097/01.bor.0000434673.85515.89. [DOI] [PubMed] [Google Scholar]
- 12.Alfirevic A, Neely D, Armitage J, et al. Phenotype standardization for statin-induced myotoxicity. Clin Pharmacol Ther. 2014;96:470–476. doi: 10.1038/clpt.2014.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Law M, Rudnicka AR. Statin safety: a systematic review. Am J Cardiol. 2006;97:52C–60C. doi: 10.1016/j.amjcard.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 14•.Bosch X, Poch E, Grau JM. Rhabdomyolysis and acute kidney injury. N Engl JmMed. 2009;361:62–72. doi: 10.1056/NEJMra0801327. A comprehensive review focused in rhabdomyolysis and one of its main complications, that is, the renal failure. [DOI] [PubMed] [Google Scholar]
- 15.Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013:CD004816. doi: 10.1002/14651858.CD004816.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bruckert E, Hayem G, Dejager S, et al. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients - the PRIMO study. Cardiovasc Drugs Ther. 2005;19:403–414. doi: 10.1007/s10557-005-5686-z. [DOI] [PubMed] [Google Scholar]
- 17.El-Salem K, Ababneh B, Rudnicki S, et al. Prevalence and risk factors of muscle complications secondary to statins. Muscle Nerve. 2011;44:877–881. doi: 10.1002/mus.22205. [DOI] [PubMed] [Google Scholar]
- 18.Parker BA, Capizzi JA, Grimaldi AS, et al. Effect of statins on skeletal muscle function. Circulation. 2013;127:96–103. doi: 10.1161/CIRCULATIONAHA.112.136101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta A, Thompson D, Whitehouse A, et al. ASCOT Investigators. Adverse events associated with unblinded, but not with blinded, statin therapy in the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid-Lowering Arm (ASCOT-LLA): a randomised double-blind placebo-controlled trial and its non-randomised non-blind extension phase. Lancet. 2017 Jun;24(389):2473–2481. doi: 10.1016/S0140-6736(17)31075-9. [DOI] [PubMed] [Google Scholar]
- 20.Hansen KE, Hildebrand JP, Ferguson EE, et al. Outcomes in 45 patients with statin-associated myopathy. Arch Intern Med. 2005;165:2671–2676. doi: 10.1001/archinte.165.22.2671. [DOI] [PubMed] [Google Scholar]
- 21.Armour R, Zhou L. Outcomes of statin myopathy after statin withdrawal. J Clin Neuromuscul Dis. 2013;14:103–109. doi: 10.1097/CND.0b013e3182852558. [DOI] [PubMed] [Google Scholar]
- 22.Selva-O’Callaghan A, Alvarado-Cardenas M, Marin A, et al. Statins and myositis: the role of anti-HMGCR antibodies. Expert Rev Clin Immunol. 2015;11:1277–1279. doi: 10.1586/1744666X.2015.1102632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Floyd JS, Brody JA, Tiniakou E, et al. Absence of anti-HMG-CoA reductase autoantibodies in severe self-limited statin-related myopathy. Muscle Nerve. 2016;54:142–144. doi: 10.1002/mus.25127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Christopher-Stine L, Casciola-Rosen LA, Hong G, et al. A novel autoantibody recognizing 200-kd and 100-kd proteins is associated with an immune-mediated necrotizing myopathy. Arthritis Rheum. 2010;62:2757–2766. doi: 10.1002/art.27572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nazir S, Lohani S, Tachamo N, et al. Statin-associated autoimmune myopathy: a systematic review of 100 cases. J Clin Rheumatol. 2017;23:149–154. doi: 10.1097/RHU.0000000000000497. [DOI] [PubMed] [Google Scholar]
- 26••.Mammen AL, Chung T, Christopher-Stine L, et al. Autoantibodies against 3-hydroxy-3-methylglutaryl-coenzyme A reductase in patients with statin-associated autoimmune myopathy. Arthritis Rheum. 2011;63:713–721. doi: 10.1002/art.30156. This is a seminal research that first identifies the anti-HMGCR antibodies as a cause of immune-mediated necrotizing myopathy in patients treated with statins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kennedy N, Keating P, O’Donnell J. HMGCR-associated myositis: a New Zealand case series and estimate of incidence. Intern Med J. 2016;46:622–625. doi: 10.1111/imj.13023. [DOI] [PubMed] [Google Scholar]
- 28•.Alvarado-Cardenas M, Marin-Sánchez A, Martínez MA, et al. Statin-associated autoimmune myopathy: a distinct new IFL pattern can increase the rate of HMGCR antibody detection by clinical laboratories. Autoimmun Rev. 2016;15:1161–1166. doi: 10.1016/j.autrev.2016.09.005. A characteristic and specific immunofluorescence pattern (HALIP) that would help to identify patients with statin-associated autoimmune myopathy in a standard laboratory setting is described. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ge Y, Lu X, Peng Q, et al. Clinical characteristics of anti-3-hydroxy-3-methylglutaryl coenzyme a reductase antibodies in Chinese patients with idiopathic inflammatory myopathies. PLoS One. 2015;10:e0141616. doi: 10.1371/journal.pone.0141616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watanabe Y, Suzuki S, Nishimura H, et al. Statins and myotoxic effects associated with anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase autoantibodies: an observational study in Japan. Medicine (Baltimore) 2015;94:e416. doi: 10.1097/MD.0000000000000416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Limaye V, Bundell C, Hollingsworth P, et al. Clinical and genetic associations of autoantibodies to 3-hydroxy-3-methylglutaryl-coenzyme a reductase in patients with immunemediated myositis and necrotizing myopathy. Muscle Nerve. 2015;52:196–203. doi: 10.1002/mus.24541. [DOI] [PubMed] [Google Scholar]
- 32.Allenbach Y, Drouot L, Rigolet A, et al. French Myositis Network. Anti-HMGCR autoantibodies in European patients with autoimmune necrotizing myopathies: inconstant exposure to statin. Medicine (Baltimore) 2014;93:150–157. doi: 10.1097/MD.0000000000000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mammen AL. Statin-associated autoimmune myopathy. N Engl J Med. 2016;374:664–669. doi: 10.1056/NEJMra1515161. [DOI] [PubMed] [Google Scholar]
- 34.Kishi T, Rider LG, Pak K, et al. //Childhood Myositis Heterogeneity Study Group. Association of anti-3-hydroxy-3-methylglutaryl-coenzyme a reductase autoantibodies with DRB1*07:01 and severe myositis in juvenile myositis patients. Arthritis Care Res (Hoboken) 2017;69:1088–1094. doi: 10.1002/acr.23113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mammen AL, Gaudet D, Brisson D, et al. Increased frequency of DRB1*11:01 in anti-hydroxymethylglutaryl-coenzyme A reductase-associated autoimmune myopathy. Arthritis Care Res (Hoboken) 2012;64:1233–1237. doi: 10.1002/acr.21671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36•.Mammen AL, Pak K, Williams EK, et al. Rarity of anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase antibodies in statin users, including those with self-limited musculoskeletal side effects. Arthritis Care Res (Hoboken) 2012;64:269–272. doi: 10.1002/acr.20662. This epidemiologic research study addresses the prevalence of anti-HMGCR in patients being treated with statins. The authors demonstrate that most patients receiving statin will not develop anti-HMGCR antibodies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Musset L, Miyara M, Benveniste O, et al. Analysis of autoantibodies to 3-hydroxy-3-methylglutaryl-coenzyme A reductase using different technologies. J Immunol Res. 2014;2014:405956. doi: 10.1155/2014/405956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dalakas MC, Illa I, Dambrosia JM, et al. A controlled trial of high-dose intravenous immune globulin infusions as treatment for dermatomyositis. N Engl J Med. 1993;329:1993–2000. doi: 10.1056/NEJM199312303292704. [DOI] [PubMed] [Google Scholar]
- 39.Mammen AL, Tiniakou E. Intravenous immune globulin for statin-triggered autoimmune myopathy. N Engl J Med. 2015 Oct;373:1680–1682. doi: 10.1056/NEJMc1506163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Komai E, Takemoto M, Yokote K. Atorvastatin-induced dermatomyositis in a 47-year-old woman with Sjogren’s syndrome. Acta Cardiol. 2015;70:373. doi: 10.1080/ac.70.3.3080648. [DOI] [PubMed] [Google Scholar]
- 41.Giordano N, Senesi M, Mattii G, et al. Polymyositis associated with simvastatin. Lancet. 1997;349:1600–1601. doi: 10.1016/S0140-6736(05)61628-5. [DOI] [PubMed] [Google Scholar]
- 42.Noel B, Cerottini JP, Panizzon RG. Atorvastatin-induced dermatomyositis. Am J Med. 2001;110:670–671. doi: 10.1016/s0002-9343(01)00711-2. [DOI] [PubMed] [Google Scholar]
- 43.Wu Y, Lach B, Provias JP, et al. Statin-associated autoimmune myopathies: a pathophysiologic spectrum. Can J Neurol Sci. 2014;41:638–647. doi: 10.1017/cjn.2014.22. [DOI] [PubMed] [Google Scholar]
- 44•.Allenbach Y, Keraen J, Bouvier AM, et al. High risk of cancer in autoimmune necrotizing myopathies: usefulness of myositis specific antibody. Brain. 2016;139:2131–2135. doi: 10.1093/brain/aww054. An increased incidence of cancer was observed in patients with immune-mediated necrotizing myopathy. Risk of patients with anti-HMGCR persisted but was minor than those without these autoantibodies. [DOI] [PubMed] [Google Scholar]
- 45.Kadoya M, Hida A, Hashimoto Maeda M, et al. Cancer association as a risk factor for anti-HMGCR antibody-positive myopathy. Neurol Neuroimmunol Neuroinflamm. 2016;3:e290. doi: 10.1212/NXI.0000000000000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tiniakou E, Pinal-Fernandez I, Lloyd TE, et al. More severe disease and slower recovery in younger patients with anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase-associated autoimmune myopathy. Rheumatology (Oxford) 2017;56:787–794. doi: 10.1093/rheumatology/kew470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tansley SL, Betteridge ZE, Simou S, et al. Juvenile Dermatomyositis Research Group. Anti-HMGCR autoantibodies in juvenile idiopathic inflammatory myopathies identify a rare but clinically important subset of patients. J Rheumatol. 2017;44:488–492. doi: 10.3899/jrheum.160871. [DOI] [PubMed] [Google Scholar]
- 48•.Liang WC, Uruha A, Suzuki S, et al. Pediatric necrotizing myopathy associated with anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase antibodies. Rheumatology (Oxford) 2017;56:287–293. doi: 10.1093/rheumatology/kew386. Pediatric patients with undiagnosed muscular dystrophy should be tested for anti-HMGCR. Early diagnosis and immunosuppressive treatment can improve the outcome in some patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sakamoto K, Kimura J. Mechanism of statin-induced rhabdomyolysis. J Pharmacol Sci. 2013;123:289–294. doi: 10.1254/jphs.13r06cp. [DOI] [PubMed] [Google Scholar]
- 50.Ramachandran R, Wierzbicki AS. Statins, muscle disease and mitochondria. J Clin Med. 2017;6(8) doi: 10.3390/jcm6080075. pii: E75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Backes JM, Ruisinger JF, Gibson CA, et al. Statin-associated muscle symptoms-managing the highly intolerant. J Clin Lipidol. 2017;11:24–33. doi: 10.1016/j.jacl.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 52.Taylor BA, Thompson PD. Muscle-related side-effects of statins: from mechanisms to evidence-based solutions. Curr Opin Lipidol. 2015;26:221–227. doi: 10.1097/MOL.0000000000000174. [DOI] [PubMed] [Google Scholar]
- 53.Patel J, Superko HR, Martin SS, et al. Genetic and immunologic susceptibility to statin-related myopathy. Atherosclerosis. 2015;240:260–271. doi: 10.1016/j.atherosclerosis.2015.03.025. [DOI] [PubMed] [Google Scholar]
- 54.Trapani L, Segatto M, La Rosa P, et al. 3-Hydroxy 3-methylglutaryl coenzyme A reductase inhibition impairs muscle regeneration. J Cell Biochem. 2012;113:2057–2063. doi: 10.1002/jcb.24077. [DOI] [PubMed] [Google Scholar]
- 55.Martini C, Trapani L, Narciso L, et al. 3-Hydroxy 3-methylglutaryl coenzyme A reductase increase is essential for rat muscle differentiation. J Cell Physiol. 2009;220:524–530. doi: 10.1002/jcp.21810. [DOI] [PubMed] [Google Scholar]
- 56.Werner JL, Christopher-Stine L, Ghazarian SR, et al. Antibody levels correlate with creatine kinase levels and strength in anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase-associated autoimmune myopathy. Arthritis Rheum. 2012;64:4087–4093. doi: 10.1002/art.34673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57•.Arouche-Delaperche L, Allenbach Y, Amelin D, et al. Pathogenic role of antisignal recognition protein and anti-3-hydroxy-3-methylglutaryl-CoA reductase antibodies in necrotizing myopathies: myofiber atrophy and impairment of muscle regeneration in necrotizing autoimmune myopathies. Ann Neurol. 2017;81:538–548. doi: 10.1002/ana.24902. The authors report muscle fibers atrophy induced by anti-HMGCR antibodies and associated with high levels of inflammatory cytokines. This study supports the pathogenic role of anti-HMGCR antibodies. [DOI] [PubMed] [Google Scholar]
- 58.Wang TH, Lin TF. Monascus rice products. Adv Food Nutr Res. 2007;53:123–159. doi: 10.1016/S1043-4526(07)53004-4. [DOI] [PubMed] [Google Scholar]
- 59.Zhao ZJ, Pan YZ, Liu QJ, et al. Exposure assessment of lovastatin in Pu-erh tea. Int J Food Microbiol. 2013;164:26–31. doi: 10.1016/j.ijfoodmicro.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 60.Endo A, Kuroda M, Tsujita Y. ML-236A, ML-236B, and ML-236C, new inhibitors of cholesterogenesis produced by Penicillium citrinium. J Antibiot (Tokyo) 1976;29:1346–1348. doi: 10.7164/antibiotics.29.1346. [DOI] [PubMed] [Google Scholar]
- 61.Pelli N, Setti M, Ceppa P, et al. Autoimmune hepatitis revealed by atorvastatin. Eur J Gastroenterol Hepatol. 2003;15:921–924. doi: 10.1097/00042737-200308000-00014. [DOI] [PubMed] [Google Scholar]
- 62.Wolters LM, Van Buuren HR. Rosuvastatin-associated hepatitis with autoimmune features. Eur J Gastroenterol Hepatol. 2005;17:589–590. doi: 10.1097/00042737-200505000-00019. [DOI] [PubMed] [Google Scholar]
- 63.Alvarado Cárdenas M, Marín Sánchez A, Lima Ruiz J. Statins and autoimmunity. Med Clin (Barc) 2015;145:399–403. doi: 10.1016/j.medcli.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 64.Russo MW, Hoofnagle JH, Gu J, et al. Spectrum of statin hepatotoxicity: experience of the drug-induced liver injury network. Hepatology. 2014;60:679–686. doi: 10.1002/hep.27157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Palamuthusingam D, Mantha M, Dheda S. HMG CoA reductase inhibitor associated myositis and autoimmune hepatitis. Intern Med J. 2017;47:1213–1215. doi: 10.1111/imj.13561. [DOI] [PubMed] [Google Scholar]
- 66.Dullaart RPF. PCSK9 inhibition to reduce cardiovascular events. N Engl J Med. 2017;376:1790–1791. doi: 10.1056/NEJMe1703138. [DOI] [PubMed] [Google Scholar]