Abstract
Abnormal pigmentation is commonly seen in the wound scar. Despite advancements in the research of wound healing, little is known about the repopulation of melanocytes in the healed skin. Previous studies have demonstrated the capacity of melanocyte stem cells (McSCs) in the hair follicle to contribute skin epidermal melanocytes following injury in mice and humans. Here, we focused on the Wnt pathway, known to be a vital regulator of McSCs in efforts to better understand the regulation of follicle-derived epidermal melanocytes during wound healing. We showed that transgenic expression of Wnt inhibitor, Dkk1 in melanocytes reduced epidermal melanocytes in the wound scar. Conversely, forced activation of Wnt signaling by genetically stabilizing β-catenin in melanocytes increases epidermal melanocytes. Furthermore, we reveal that deletion of Wntless, a gene required for Wnt ligand secretion, within epithelial cells, results in failure in activating Wnt signaling in adjacent epidermal melanocytes. These results reveal the essential function of extrinsic Wnt ligands to initiate Wnt signaling in follicle-derived epidermal melanocytes during wound healing. Collectively, our results suggest the potential for Wnt signal regulation to promote melanocyte regeneration and provide a potential molecular window to promote proper melanocyte regeneration following wounding as well as in conditions such as vitiligo.
Introduction
Upon injury, migration and proliferation of epithelial cells result in re-epithelialization of the wound area, a process that occurs in association with the formation of underlying granulation tissues. However, the new skin tissue replenished in the wound area typically leaves an appearance distinct from the original skin, partly due to imperfect skin pigmentation. Skin hypopigmention is a common complication, particularly following excisional surgical procedures or burn injuries (Massaki et al., 2012). On the other hand, wound injuries or inflammatory processes can also lead to skin hyperpigmentation (Nordlund and Abdel-Malek, 1988). In fact, abnormal pigmentation is one of the most visible changes caused by wounding or inflammation, and therefore, a common source of cosmetic and psychosocial concerns to patients (Wisely et al., 2007). Currently, there are no treatment options to permanently and completely overcome this complication (Chadwick et al., 2012). This is partly rooted in our incomplete understanding of how melanocytes are recruited to the wound site and how they modulate pigment production during wound healing.
Stem cells are responsible for replenishing cells that are lost to homeostatic cellular turn over, injury or diseases. In humans, melanocytes are located in the basal layer of the epidermis and in the hair follicles. Stem cells in the melanocytic lineage residing within the skin epidermis have not yet been identified. Therefore, the presence, identity and function of epidermal melanocyte stem cells (McSCs) remain elusive. In adult mice, melanocytes are not detectable in the inter-follicular epidermis, but are located in the bulge/secondary hair germ (sHG, also referred to as sub-bulge) area and the bulb area of the hair follicle (Nishimura et al., 2002, Nishimura 2011). The bulge/sHG area of the hair follicle harbors McSCs throughout the distinct regenerative phases of the hair follicle cycle (Muller-Rover et al., 2001, Nishimura et al., 2002). Hair bulb melanocytes that produce pigment for the hair during the hair follicle cycle are progeny of McSCs and present only during the growth phase of the hair cycle. Previous studies established that McSCs in the hair follicle also possess the potential to migrate to the skin epidermis to give rise to functional epidermal melanocytes. This function can be clearly observed in the recovery process of vitiligo in humans, a disease characterized by loss of epidermal melanocytes (Birlea et al., 2017, Picardo et al., 2015). Clinical observations and experimental data with vitiligo skin suggest that repigmentation of the de-pigmented skin occurs from hair follicle melanocytes either spontaneously or following narrow band UVB treatment (Cui et al., 1991, Goldstein et al., 2015). Furthermore, lineage tracing of McSCs with a label retaining technique definitively demonstrated the ability of follicular McSCs to undergo epidermal melanocyte fate in mice following injuries or UVB treatment (Chou et al., 2013). When epidermal melanocytes are present, they may be primary contributors to the regeneration of melanocytes following injury, however, a recent study showed that follicular McSCs can populate epidermal melanocytes in human skin following epidermal ablation, regulated by endothelin (Edn) signaling (Takeo et al., 2016). These studies suggest that follicular McSCs serve as additional reservoirs for melanocytes that are kept in a relatively protected area away from the skin surface.
Wnt/β-catenin signaling is a central pathway in melanocyte biology. Binding of Wnt ligand(s) to the cell surface receptors Frizzled and their co-receptor, LRP, leads to the stabilization of cytosolic β-catenin, and its subsequent translocation to the nucleus, where it interacts with LEF/TCF transcription factors to regulate the transcription of downstream genes (Barker, 2008, van Amerongen and Nusse, 2009). Genes targeted by Wnt signaling include those vital for pigmentation such as MITF, Dct and Tyrosinase (Dorsky et al., 2000, Takeda et al., 2000, Wang et al., 2015, Yasumoto et al., 2002). Consistently, this pathway plays a critical role in regulating McSC behavior. Forced activation of this pathway in McSCs leads to their premature differentiation, whereas loss of β-catenin in melanocytes results in defective differentiation of McSCs into hair bulb melanocytes (Rabbani et al., 2011). Moreover, UV irradiation induces McSCs to activate Wnt signaling and abrogation of Wnt leads to defects in melanocyte migration from the hair follicle to the epidermis (Yamada et al., 2013). These studies prompted us to dissect the function and regulation of this pathway in McSCs and their progeny during skin wound healing.
In this study, we utilized multiple genetic mouse models to investigate the impact of the gain and loss of function of Wnt/β-catenin signaling in hair follicle-derived melanocytes following skin excisional wounds in mice. Taking advantage of the tissue-specific promoters that target melanocytes or epithelial cells, we demonstrate 1) the vital function of Wnt signaling in recruitment of follicle-derived epidermal melanocytes to the wound area; and 2) the essential role for epithelium-derived Wnt ligands in the Wnt activation of follicle-derived epidermal melanocytes in the healed area of the adult skin.
Results
Dkk1 expression inhibits the generation of epidermal melanocytes after wounding
To determine the impact of loss of function of Wnt signaling in melanocytes during wound healing, we sought to overexpress a potent Wnt inhibitor, Dickkopf1 (Dkk1) in melanocytes. Members of the Dkk family of secreted Wnt inhibitors specifically inhibit Wnt/LRP signaling by forming a complex with LRP that is internalized, removing LRP from the cell surface (Bafico et al., 2001, Mao et al., 2001, Semenov et al., 2001). This approach specifically inhibits Wnt signaling unlike the deletion of β-catenin that possesses a Wnt independent function in cell-cell adhesion (Rabbani et al., 2011). We generated Dct-rtTA; tetO-Dkk1; tetO-H2B-GFP transgenic mice (Dct:Dkk1:GFP mice) (Chu et al., 2004, Zaidi et al., 2011) in which Dkk1 and GFP reporter can be inducibly expressed in Dct+ melanocytes upon doxycycline (Dox) treatment. Dct is expressed by undifferentiated McSCs and their differentiated melanocyte progeny (Nishimura et al., 2002). We created 1 cm2 excisional wounds (Chou et al., 2013) on the back skin of 8 week old Dct:Dkk1:GFP mice and fed them with Dox containing diet until harvesting the wound tissues (Figure 1a). As a control experiment, we utilized Dct-rtTA; tetO-H2B-GFP mice in which only GFP reporter is expressed in Dct+ melanocytes upon Dox treatment. At 8 weeks old, the only population of Dct+ melanocytes in the back skin is McSCs that are in the bulge/sHG areas of hair follicles (Nishimura et al., 2002) (Figure 1b). Previous studies showed that McSCs of the hair follicles in the wound periphery migrate to the epidermis and establish epidermal melanocytes in the wound (Chou et al., 2013). Consistent with the past observations, examination of whole mount skin epidermis of control wound tissues showed GFP+ melanocytes in the wound epidermis by 44 days after wounding and these melanocytes were sporadically located without being uniformly localized in the wound area (Figure 1c). In contrast, mice with Dkk1 expression in melanocytes generated significantly reduced numbers of GFP+ melanocytes in the wound epidermis compared to the control mice, suggesting that Wnt signaling is required for the generation of epidermal melanocytes (Figure 1d–e). These results show that Wnt signaling is required for follicular McSCs to generate epidermal melanocytes during skin wound healing (Figure 1p).
Figure 1. Overexpression of Dkk1 in melanocytes inhibits the wound-induced generation of epidermal melanocytes.
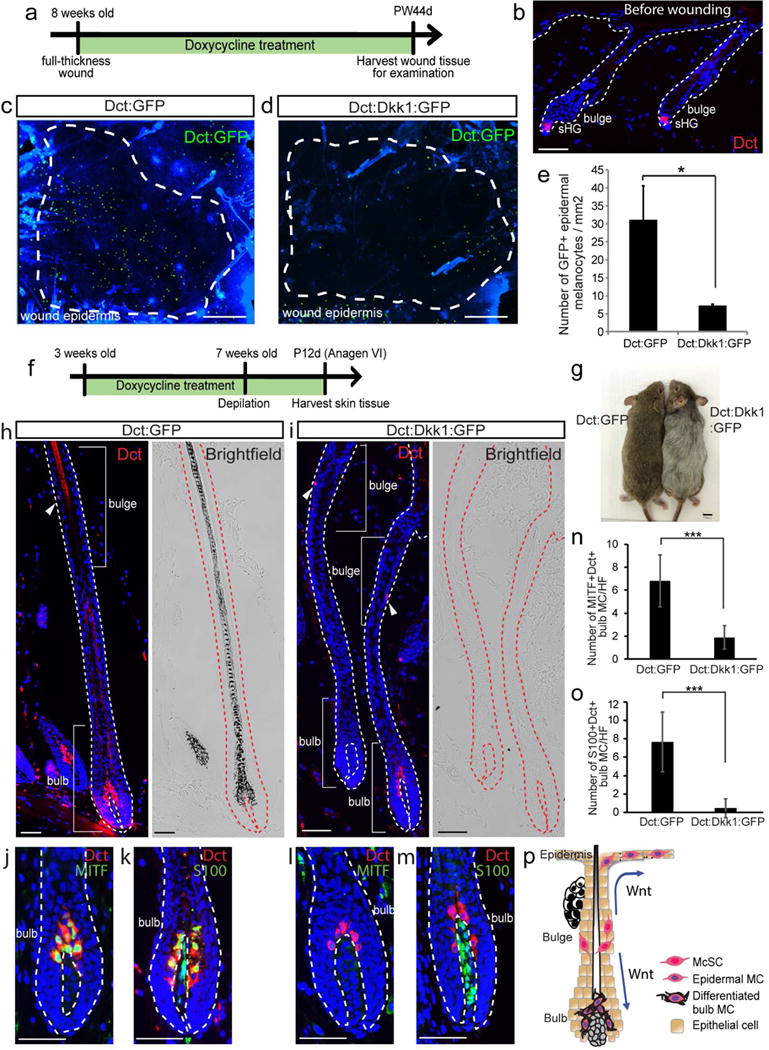
(a-e) Dct-rtTA; tetO-Dkk1; tetO-H2B-GFP (Dct:Dkk1:GFP) and Dct-rtTA; tetO-H2B-GFP (Dct:GFP) mice were wounded and treated with doxycycline for 44 days. (a) Experimental scheme. (b) Immunofluorescence of Dct before wounding. (c, d) Dct:GFP signals in whole mount wound epidermis at PW44d. (e) Quantification of (c-d). (f-o) Dct:Dkk1:GFP and Dct:GFP mice were treated with doxycycline from 3 weeks old and depilated at 7 weeks old. Tissues were harvested at P12d (12 days after depilation). (f) Experimental scheme. (g) Image of mouse at P12d. (h-m) Immunofluorescence of indicated markers and corresponding brightfield images. (n-o) Quantification of (j-m). S100+/Dct− cells in (k, m) are dermal papilla cells. (p) Schematic summary. Dashed lines in (c-d): boundary between wound and intact areas. Dashed lines in (b, h-m) boundary of epithelium and dermis. Arrowheads: melanocyte stem cell (McSC). PW: post wound. MC: melanocytes. HF: hair follicle. sHG: secondary hair germ. Data are represented as mean ± SEM; * p<0.05, *** p<0.001. Scale bar: 50um in (b, h-m), 500um in (c-d), 1cm in (g).
It is noteworthy that the intact area of Dkk1-expressing mice verified an apparent defect in hair pigmentation (Figure 1f–p), which is known to rely on Wnt/β-catenin signaling (Rabbani et al., 2011). In normal skin without wounding, McSCs regenerate differentiated melanocytes in the hair bulb that are responsible for hair pigmentation in association with hair follicle cycle. We thus examined hair bulb melanocytes in these mice. Compared to control (Figure 1h), Dct:Dkk1:GFP mice had a significantly reduced number of hair bulb melanocytes (Figure 1i, left hair follicle). Even when bulb melanocytes were detected in Dkk1-expressing mice, they often lacked pigment (Figure 1i, right hair follicle) and failed to display immunoreactivity for melanocyte differentiation markers, MITF and S100, that are typically up-regulated in hair bulb melanocytes (Figure 1j–o) (Rabbani et al., 2011). The Dkk1-expressing melanocytes maintained expression of Sox10 (Harris et al., 2013) similar to control mice (Figure S1). The overall phenotype seen in Dkk1-expressing hair melanocytes (Figure 1p) is very similar to those reported in β-catenin-deficient mice (Rabbani et al., 2011), verifying that the role of β-catenin in melanocyte differentiation is mainly mediated by Wnt signaling, instead of the cell-adhesion role of β-catenin.
Constitutive activation of Wnt signaling promotes the generation of epidermal melanocytes after wounding
Next, we asked whether constitutive activation of Wnt/β-catenin signaling in melanocytes affects the generation of epidermal melanocytes. For this, we generated Tyr-CreER; β-catenin fl(ex3)/+; Dct-LacZ mice (β-catenin-STA mice) (Bosenberg et al., 2006, Harada et al., 1999, Zhao and Overbeek, 1999) that specifically express a stabilized mutant form of β-catenin in melanocytes upon tamoxifen (TAM) treatment. These mice contain a Dct-LacZ reporter that allows us to detect Dct+ melanocytes by LacZ expression. We created 1 cm2 excisional wounds (Chou et al., 2013) as described above, using β-catenin-STA mice and control mice (Dct-LacZ mice) and then treated them with TAM for 21 days (Figure 2a). By 44 days after wounding, forced Wnt activation in melanocytes resulted in epidermal melanocytes that were distributed over the entire wound scar area (Figure 2c). This was in clear contrast to the control samples in which melanocytes were sparsely distributed and largely concentrated in the peripheral area of the wound scar (Figure 2b). In quantifications, we observed a considerably higher number of melanocytes in the wound area of β-catenin-STA mice compared to control mice (Figure 2d). Tissue section analysis showed the presence of Dct+ melanocyte in the wound epidermis, but not dermis of control and β-catenin-STA mice (Figure 2e–f). There was significant increase of the percentage of nuclear β-catenin+ epidermal melanocytes in β-catenin-STA mice (90%) compared to control (55%), as well as increased expression of nuclear β-catenin in McSCs in the hair follicles of β-catenin-STA mice, confirming the successful forced Wnt activation in melanocytes of these mice (Figure 2e–g). These results show that forced activation of Wnt signaling via β-catenin stabilization enhances the number and distribution of melanocytes within the wound epidermis.
Figure 2. Overexpression of β-catenin promotes the generation of epidermal melanocytes following wounding.
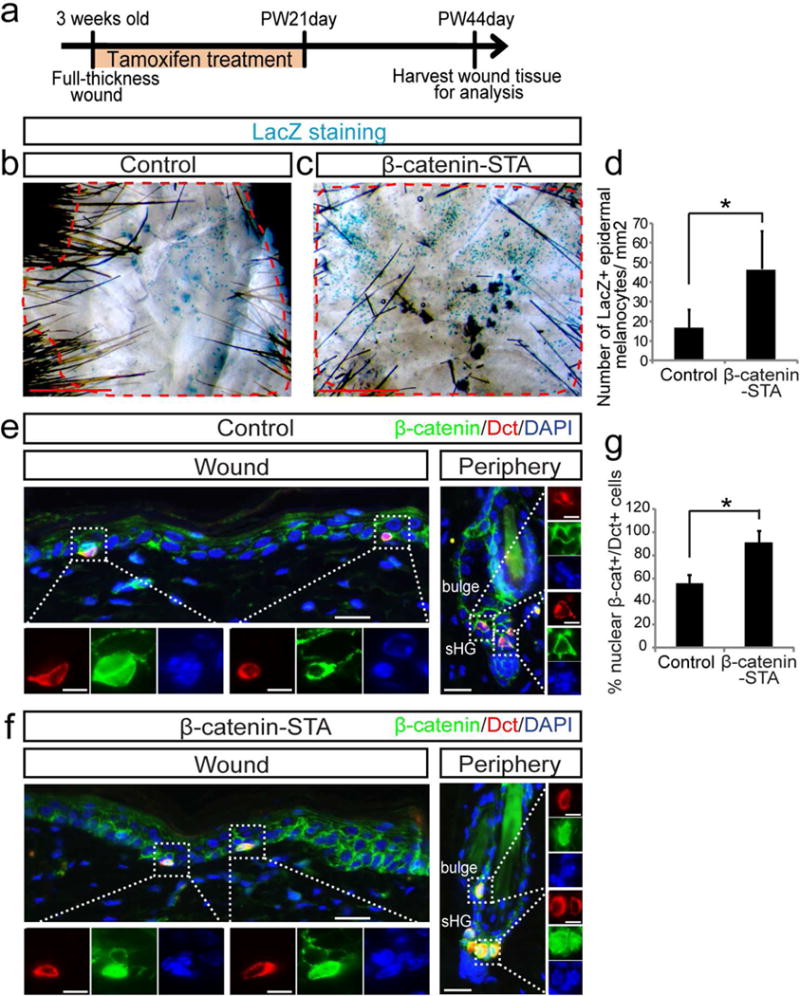
Tyr-CreER; β-catenin fl(ex3)/+; Dct-LacZ (β-catenin-STA) and Dct-LacZ control mice were wounded and treated with tamoxifen for 21 days. Wound tissues were harvested at PW44day. (a) Experimental scheme. (b, c) β-galactosidase staining of whole mount wound epidermis. (d) Quantification of (b, c). (e, f) Immunofluorescence of indicated markers in wound center and hair follicles in wound periphery. (g) Quantification of the percentage of nuclear β-catenin+ melanocytes in wound epidermis of (e, f). Dashed lines outline the boundary between wound and intact areas. Dotted boxes outline magnified regions in separate fluorescent channels. PW: post wound. Data are represented as mean ± SEM; * p<0.05. Scale bar: 1000um in (b, c), 25um in (e, f) and 10um in magnified images in (e, f).
Wnt ligands secreted by epithelial cells are essential for the activation of Wnt/β-catenin signaling in epidermal melanocytes
Next, we asked what is the source of Wnt ligands that drive Wnt activation in epidermal melanocytes during wound healing. For this, we isolated epidermal melanocytes (Kawaguchi et al., 2008) and epithelial cells from healed wound at 17ds after wounding (Figure S2) and determined the expression of Wnt ligands in both populations. We found that epithelial cells significantly upregulate several Wnt ligands, including Wnt3, 4, 10a and 16 as compared to epidermal melanocytes, suggesting that epithelial cells may be the major source of Wnt ligands in the wound area (Figure 3a).
Figure 3. Wnt ligands provided by epithelial cells are required for Wnt activation and differentiation of epidermal melanocytes.
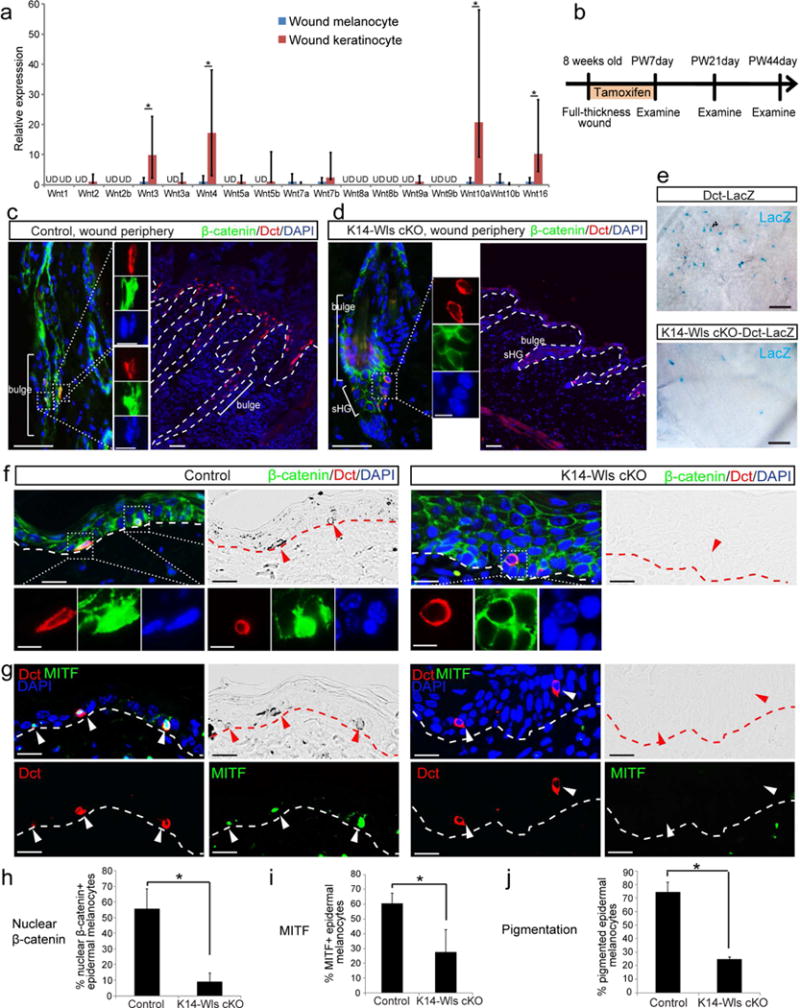
(a) Wnt ligand qPCR of epidermal melanocytes and keratinocytes isolated from wound epidermis 17 days after wounding. UD: undetected. (b-j) K14-CreER; Wls fl/fl (K14-Wls cKO) or K14-CreER; Wls fl/fl ; Dct-LacZ (K14-Wls cKO-Dct-LacZ) mice and control littermates were wounded and immediately treated with tamoxifen for 7 days. Wound tissues were harvested at indicated time points. (b) Experimental scheme. Immunofluorescence of indicated markers and corresponding brightfield images at PW7day (c, d) and PW21day (f, g). (h-j) Quantification of (f, g). (e) β-galactosidase staining of whole mount wound tissue at PW44day. Dashed lines outline the boundary of epithelium and dermis. Dotted boxes outline magnified regions in separate fluorescent channels. Arrowheads point to epidermal melanocytes. PW: post wound. Data are represented as mean ± SEM; * p<0.05. Scale bar: 50um in (c-e), 25um in (f, g), 10um in magnified images in (c, d, f).
Although several studies suggest the potential function for epithelial Wnt ligands to influence McSC behavior (Rabbani et al., 2011, Yamada et al., 2013), the necessity for these extrinsic ligands has never been addressed. To determine whether Wnt ligands derived from epithelial cells are required for Wnt signaling in melanocytes during wound healing, we generated K14-CreER; Wls fl/fl; Dct-LacZ mice (K14-Wls cKO mice) in which Wntless (Wls), which is essential for Wnt ligand secretion (Banziger et al., 2006, Bartscherer et al., 2006, Goodman et al., 2006), was ablated in Keratin 14+ basal epithelial cells following TAM treatment (Carpenter et al., 2010, Vasioukhin et al., 1999). These mice contain the Dct-LacZ reporter to visualize Dct+ melanocytes. We created 1 cm2 excisional wounds as above, using K14-Wls cKO mice and control mice (Dct-LacZ) and treated them with TAM for 7 days (Figure 3b). At 7 days after wounding, we observed substantial expansion of Dct+ McSCs in the hair follicle of wound periphery area, and they migrate upward into the epidermis, consistent with previous study (Chou et al., 2013), and some of these Dct+ melanocytes are Wnt active, as evidenced by nuclear β-catenin signals (Figure 3c). In contrast, loss of epithelial Wnt ligands in K14-Wls cKO mice inhibited Wnt activation in Dct+ McSCs and their expansion into the epidermis (Figure 3d). As a result, at 44 days after wounding, the examination of whole mount wound samples revealed a significant reduction of epidermal melanocytes in the wound area of K14-Wls KO mice compared to control mice (Figure 3e). This phenotype was reminiscent of what was seen upon Wnt inhibition within melanocytes in Dkk1 mice (Figure 1a–e). Indeed, the analyses of wound tissue sections from K14-Wls cKO mice revealed a clear defect in Wnt signal activation in epidermal melanocytes, shown by the lack of nuclear β-catenin signals (Figure 3f). They also down-regulated another marker of Wnt activation, Tcf1 in comparison with epidermal melanocytes of control mice (Figure S3). In control wounds, about 55%–60% epidermal melanocytes demonstrated Wnt activation with nuclear β-catenin expression (Figure 3h). Moreover, epidermal melanocytes in K14-Wls cKO mice showed defect in pigment production, and they failed to express melanocyte differentiation marker MITF that is expressed by normal epidermal melanocytes (Figure 3f–g, i–j). These results showed that epithelium-derived Wnt ligands are essential for Wnt activation of McSCs and epidermal melanocytes, which is required to recruit functional melanocytes to the wound area.
Inhibition of Wnt ligand secretion from melanocytes does not affect their Wnt activation
If Wnt signaling in epidermal melanocytes is controlled by epithelial Wnt ligands, Wnt ligand secretion from melanocytes themselves may not influence their regenerative behavior. To verify this, we generated Tyr-CreER; Wls fl/fl (Tyr-Wls cKO) mice in which Wntless can be deleted in melanocytes (Bosenberg et al., 2006, Carpenter et al., 2010) following TAM treatment. Following wounding and subsequent TAM treatment on Tyr-Wls cKO and control mice (Figure 4a), we found no difference in the pigmentation and number of epidermal melanocytes between these mice (Figure 4b, d–e). Additionally, we did not detect a difference in Wnt activation of epidermal melanocytes between melanocytes of control and Tyr-Wls cKO mice assessed by expression of nuclear β-catenin between melanocytes of control and Tyr-Wls cKO mice (Figure 4b–c). These results show that although melanocytes have the potential to express Wnt ligands in some conditions (Figure 3a) (Ye et al., 2013), such intrinsic Wnt ligands are dispensable for Wnt signal activation of epidermal melanocytes during skin wound healing.
Figure 4. Melanocytes do not produce Wnt ligands to drive their Wnt activation and differentiation.
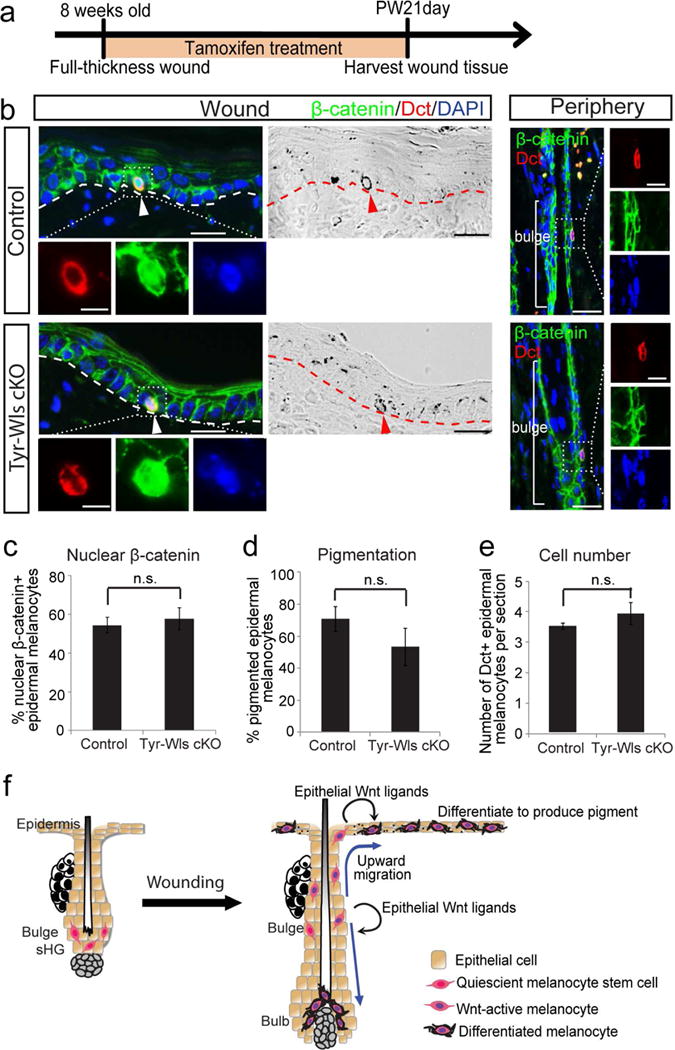
(a-e) Tyr-CreER; Wls fl/fl (Tyr-Wls cKO) mice and control littermates were wounded and immediately treated with tamoxifen for 21 days. Wound tissues were harvested 21 days after wounding. (a) Experimental scheme. (b) Immunofluorescence of indicated markers and corresponding brightfield images in wound center and hair follicles in wound periphery. (c-e) Quantification of (b). (f) Schematic model: Upon wounding, epithelial cells generate Wnt ligands to drive the Wnt activation and upward migration of quiescent McSCs. Once in the epidermis, Wnt ligands from epithelial cells continue to induce Wnt activation in epidermal melanocytes, which differentiate and produce pigment. Wnt activation is also required for McSCs to generate hair bulb melanocytes. Dashed lines outline the boundary of epithelium and dermis. Dotted boxes outline magnified regions in separate fluorescent channels. Arrowheads: epidermal melanocytes. PW: post wound. Data are represented as mean ± SEM; n.s.: not significant. Scale bar: 25um and 10um in magnified images.
Discussion
Previous studies have noted the distinct behaviors in melanocytic lineage following global modification of Wnt signaling in adult skin through various approaches including injection/topical application of Wnt inhibitors, agonists or siRNA. For example, intradermal injection of Wnt3a and Wnt10b in mouse skin promoted melanocyte differentiation in the hair follicle, while injection of Wnt inhibitor, sFRP4 inhibited this process (Guo et al., 2017, Guo et al., 2016, Ye et al., 2013). Topical application of GSK3β-inhibitor, LiCl promoted the regeneration of pigmented hair in wound scar (Yuriguchi et al., 2016). Intradermal injection of Wnt inhibitor, IWR-1 and siRNA against Wnt7a in mouse skin inhibited Wnt activation in McSCs and their migration into the epidermis following UVB irradiation (Yamada et al., 2013). This study specifically inhibit and activate Wnt signaling in melanocytes using genetic mouse models during wound healing to definitively demonstrate the vital role for the Wnt signaling in recruitment of melanocytes to the injured skin (Figure 4f).
Our results suggest that modulation of Wnt signaling in melanocytes may promote the recovery phase of vitiligo, or conversely, prevent undesirable hyperpigmentation caused by UV irradiation, a theory previously proposed by other studies that used non-tissue specific approaches (Yamada et al., 2013). Further, our study extended these findings to also show that Wnt signaling is vital for regeneration of hair melanocytes through analyses of transgenic overexpression of Wnt inhibitor, Dkk1 in melanocytes. Thus, the differential fate choice for McSCs to become epidermal melanocytes versus hair melanocytes may not be necessarily determined by Wnt signaling. Instead, we propose that Wnt signaling may function to enhance proliferation and differentiation of McSCs thereby reinforcing recruitment of melanocytes to the wound site, which is similar to the effect of Edn signaling (Takeo et al., 2016). Such effect of Wnt signaling is in contrast to that of Melanocortin 1 receptor (Mc1R), whose inhibition specifically blocks epidermal melanocyte fate without affecting hair melanocyte production (Chou et al., 2013, Takeo et al., 2016). How the Wnt and Mc1R pathways cooperatively regulate epidermal melanocyte generation during wound healing or during the recovery process of vitiligo will be an important subject for future investigation. More broadly, understanding the crosstalk between Wnt-driven melanocyte regeneration and mechanisms of wound healing (e.g. inflammatory signals) may illuminate the mechanism behind the fate decision of McSCs.
Our results definitively demonstrate the essential role for epithelial Wnt ligands in the activation of Wnt signaling and subsequent melanocyte functions in the wound epidermis (Figure 4f). Supporting this, previous studies detected Wnt 10b protein in regenerating epithelial cells up to 3 day and Wnt4 protein up to 5 day after wounding (Okuse et al., 2005), and Wnt7a is upregulated in epidermal cells and hair follicle stem cells upon UV irradiation that promote generation of epidermal melanocytes (Yamada et al., 2013). It is intriguing that epithelial Wnt ligands are the primary source for Wnt activation in melanocytes and the lack of epithelial Wnt ligands cannot be compensated by other sources. Interactions between epithelial cells and melanocytes have been well delineated in melanocyte biology. Ligands of multiple signaling pathways including Edns (Hara et al., 1995, Imokawa et al., 1992), α-Melanocyte-stimulating hormone (αMSH) (De Luca et al., 1993, Schauer et al., 1994), transforming growth factor β (TGFβ) (Lee et al., 1997, Nishimura et al., 2010) are secreted from epithelial cells, which are thought to paracrinely influence adjacent melanocytes in the skin. Our experiments exemplify the strikingly passive nature of melanocytes and this knowledge will help direct and formulate strategies to manipulate melanocytes in efforts to enhance their regeneration or modulate their pigment production.
Materials and Methods
Mice
All animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at NYU School of Medicine. Tyr-CreER, K14-CreER, Wntless fl/fl mice were obtained from Jackson Laboratories. tetO-Dkk1 mouse was from Dr. Sarah Millar at University of Pennsylvania. β-catenin fl(ex3)/+ mice were from Dr. M. Mark Taketo. Dct-lacZ mouse was from Dr. Paul Overbeek. Dct-rtTA and tetO-H2B-GFP mice were from NCI Mouse Repository. Both male and female mice were used in all experiments. To induce Cre recombination, tamoxifen (Sigma-Aldrich) treatment was performed by i.p. injection (0.1 mg/g body weight) of a 20 mg/ml solution in corn oil per day (Ito et al., 2007). For tetracycline-inducible mouse models, mice were given doxycycline-containing chow (20g/kg, Bio-Serv, NJ) for indicated time period.
Wound Experiment
Wound experiments were performed as described with minor modification (Chou et al., 2013). Briefly, mice were anesthetized with isoflurane. The dorsal fur was clipped and 1 cm2 area of full-thickness skin was excised with scissors.
For whole-mount wound epidermis analysis, wound scar tissues were harvested at indicated time points. The connective tissues were carefully removed with forceps. The wound tissue was incubated in 20mM EDTA for 4 hours to separate the wound epidermis from dermis. The epidermal tissue was carefully removed from the dermal tissue with forceps and then fixed in 4% paraformaldehyde (PFA) for 10 min at room temperature.
Immunofluorescence
Immunofluorescence was performed as published (Rabbani et al., 2011). Briefly, skin tissues were embedded in paraffin and cut into 6um sections. Tissue sections were incubated with primary antibodies listed below for 2 hours at room temperature or overnight at 4°C, followed by incubation with Alexa 488/594-conjugated secondary antibodies (1:200; Invitrogen, Carlsbad) for 1 hr at room temperature. Sections were counterstained with 4′,6-diamidino-2-phenylindole (DAPI, Vector Laboratories, Burlingame). The primary antibodies used were: mouse anti-β-catenin (1:400, Sigma-Aldrich, c7207), mouse anti-MITF (1:100, Vector Laboratories, VP-M650) (used in Figure 3–4), mouse anti-MITF (1:100, Abcam, ab12039) (used in Figure 1), goat anti-sox10 (1:100, Santa Cruz Biotechnology, sc-17342), goat anti-Dct (1:100, Santa Cruz Biotechnology, sc-10451), rabbit anti-S100 (1:100, DAKO, Z0311) and rabbit anti-Tcf1 (1:100, Cell Signaling Technology, C63D9). Images were taken with an inversed Eclipse Ti microscope (Nikon, Japan) or an upright Axioplan microscope (Zeiss, Germany).
Whole mount β-galactosidase staining
Whole mount β-galactosidase staining was performed as published (Chou et al., 2013, Ito et al., 2007) using wound epidermis or whole wound tissue.
FACS Isolation of melanocytes and keratinocytes from wound epidermis
Wound scar tissue was harvested at day 17 after wounding. Wound tissue (lack hair follicles) was carefully cut out from wound periphery with scissors. The connective tissues were carefully removed with forceps. The wound tissue was incubated in 0.25% Trypsin (Invitrogen) for 2 hours at 37° C. Wound epidermis was separated from the dermis using forceps and scalpel blades and the epidermis was chopped finely and transferred into Media A (DMEM, 10% FBS, 1x penicillin/streptomycin). The epidermal melanocyte and keratinocyte mixture was stirred at RT for 20 min to generate single cells. The obtained single cell suspension was filtered through a 70μm nylon filter and centrifuged at 200 g for 7 min and re-suspended in PBS+10% FBS. The cell suspension was incubated with PE-Cy7-anti-mouse CD117 (1:300, BD Pharmingen) for 15 minutes at room temperature. Cells were pelleted and washed twice in PBS+10% FBS then incubated with APC-CY7-anti-mouse CD45 (1:300, BD Pharmingen) for 15 minutes at room temperature. Cells were washed and sorted on a SY3200 cell sorter (Sony Biotechnology Inc.). Epidermal melanocytes were collected as PE-Cy7 positive and APC-Cy7 negative population (Kawaguchi et al., 2008). Epidermal keratinocytes were collected as PE-Cy7 negative and APC-Cy7 negative population.
Quantitative RT-PCR
RNA was obtained from FACS-sorted cells and prepared using RNeasy micro kit (Qiagen) following manufacture’s protocol. RNA was reverse transcribed with Superscript III First Strand Synthesis System (Invitrogen) for cDNA synthesis. cDNA was pre-amplified using TaqMan PreAmp Master Mix Kit (Applied Biosystems). Real-time amplification was carried out using the StepOnePlus Real-Time PCR System (Applied Biosystems). The target gene transcripts were quantified relative to the housekeeping gene, GAPDH. Data was analyzed using ΔΔCT method. For each Wnt ligand, the expression level in keratinocytes is calculated relative to that of melanocytes. For Wnt ligands that were not detected in melanocytes, the relative expression level in keratinocytes was considered as 1. Table S1 shows the list of primers used.
Quantification and statistical analyses
For whole wound tissue (epidermis) analysis, at least whole wound tissues from 3 mice were analyzed. For tissue section analysis, 20 epidermal melanocytes or hair follicles were analyzed in tissues from at least 2 mice. Quantification was done in a blinded manner by at least two investigators. Data was analyzed using Student’s t-test (two-tailed). A value of p<0.05 was deemed significant. Statistical analyses were performed using Microsoft Excel.
Supplementary Material
Acknowledgments
We thank the NYU Langone’s Cytometry and Cell Sorting Laboratory (supported in part by grant P30CA016087 from the NIH/NCI) for cell sorting. M.I is grateful for the supports from NIH/NIAMS grants R01 AR059768, R01 AR066022, and the Arnold and Mabel Beckman Foundation. Q.S. and M.T. were supported by NYSTEM institutional training grant (Contract #C026880). C.H.L was supported by NYU Cutaneous Biology and Skin Disease Training Program (NIAMS T32 AR064184).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare no conflict of interest.
References
- Bafico A, Liu G, Yaniv A, Gazit A, Aaronson SA. Novel mechanism of Wnt signalling inhibition mediated by Dickkopf-1 interaction with LRP6/Arrow. Nat Cell Biol. 2001;3:683–686. doi: 10.1038/35083081. [DOI] [PubMed] [Google Scholar]
- Banziger C, Soldini D, Schutt C, Zipperlen P, Hausmann G, Basler K. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell. 2006;125:509–522. doi: 10.1016/j.cell.2006.02.049. [DOI] [PubMed] [Google Scholar]
- Barker N. The canonical Wnt/beta-catenin signalling pathway. Methods Mol Biol. 2008;468:5–15. doi: 10.1007/978-1-59745-249-6_1. [DOI] [PubMed] [Google Scholar]
- Bartscherer K, Pelte N, Ingelfinger D, Boutros M. Secretion of Wnt ligands requires Evi, a conserved transmembrane protein. Cell. 2006;125(3):523–533. doi: 10.1016/j.cell.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Birlea SA, Costin GE, Roop DR, Norris DA. Trends in Regenerative Medicine: Repigmentation in Vitiligo Through Melanocyte Stem Cell Mobilization. Med Res Rev. 2017;37(4):907–935. doi: 10.1002/med.21426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosenberg M, Muthusamy V, Curley DP, Wang Z, Hobbs C, Nelson B, et al. Characterization of melanocyte-specific inducible Cre recombinase transgenic mice. Genesis. 2006;44(5):262–267. doi: 10.1002/dvg.20205. [DOI] [PubMed] [Google Scholar]
- Carpenter AC, Rao S, Wells JM, Campbell K, Lang RA. Generation of mice with a conditional null allele for Wntless. Genesis. 2010;48(9):554–558. doi: 10.1002/dvg.20651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadwick S, Heath R, Shah M. Abnormal pigmentation within cutaneous scars: A complication of wound healing. Indian J Plast Surg. 2012;45(2):403–411. doi: 10.4103/0970-0358.101328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou WC, Takeo M, Rabbani P, Hu H, Lee W, Chung YR, et al. Direct migration of follicular melanocyte stem cells to the epidermis after wounding or UVB irradiation is dependent on Mc1r signaling. Nat Med. 2013;19(7):924–929. doi: 10.1038/nm.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu EY, Hens J, Andl T, Kairo A, Yamaguchi TP, Brisken C, et al. Canonical WNT signaling promotes mammary placode development and is essential for initiation of mammary gland morphogenesis. Development. 2004;131(19):4819–4829. doi: 10.1242/dev.01347. [DOI] [PubMed] [Google Scholar]
- Cui J, Shen LY, Wang GC. Role of hair follicles in the repigmentation of vitiligo. J Invest Dermatol. 1991;97(3):410–416. doi: 10.1111/1523-1747.ep12480997. [DOI] [PubMed] [Google Scholar]
- De Luca M, Siegrist W, Bondanza S, Mathor M, Cancedda R, Eberle AN. Alpha melanocyte stimulating hormone (alpha MSH) stimulates normal human melanocyte growth by binding to high-affinity receptors. J Cell Sci. 1993;105(Pt 4):1079–1084. doi: 10.1242/jcs.105.4.1079. [DOI] [PubMed] [Google Scholar]
- Dorsky RI, Raible DW, Moon RT. Direct regulation of nacre, a zebrafish MITF homolog required for pigment cell formation, by the Wnt pathway. Genes Dev. 2000;14(2):158–162. [PMC free article] [PubMed] [Google Scholar]
- Goldstein NB, Koster MI, Hoaglin LG, Spoelstra NS, Kechris KJ, Robinson SE, et al. Narrow Band Ultraviolet B Treatment for Human Vitiligo Is Associated with Proliferation, Migration, and Differentiation of Melanocyte Precursors. J Invest Dermatol. 2015;135(8):2068–2076. doi: 10.1038/jid.2015.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman RM, Thombre S, Firtina Z, Gray D, Betts D, Roebuck J, et al. Sprinter: a novel transmembrane protein required for Wg secretion and signaling. Development. 2006;133(24):4901–4911. doi: 10.1242/dev.02674. [DOI] [PubMed] [Google Scholar]
- Guo H, Lei M, Li Y, Liu Y, Tang Y, Xing Y, et al. Paracrine Secreted Frizzled-Related Protein 4 Inhibits Melanocytes Differentiation in Hair Follicle. Stem Cells Int. 2017;2017:2857478. doi: 10.1155/2017/2857478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Xing Y, Liu Y, Luo Y, Deng F, Yang T, et al. Wnt/beta-catenin signaling pathway activates melanocyte stem cells in vitro and in vivo. J Dermatol Sci. 2016;83(1):45–51. doi: 10.1016/j.jdermsci.2016.04.005. [DOI] [PubMed] [Google Scholar]
- Hara M, Yaar M, Gilchrest BA. Endothelin-1 of keratinocyte origin is a mediator of melanocyte dendricity. J Invest Dermatol. 1995;105(6):744–748. doi: 10.1111/1523-1747.ep12325522. [DOI] [PubMed] [Google Scholar]
- Harada N, Tamai Y, Ishikawa T, Sauer B, Takaku K, Oshima M, et al. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. EMBO J. 1999;18(21):5931–5942. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris ML, Buac K, Shakhova O, Hakami RM, Wegner M, Sommer L, et al. A dual role for SOX10 in the maintenance of the postnatal melanocyte lineage and the differentiation of melanocyte stem cell progenitors. PLoS Genet. 2013;9(7):e1003644. doi: 10.1371/journal.pgen.1003644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imokawa G, Yada Y, Miyagishi M. Endothelins secreted from human keratinocytes are intrinsic mitogens for human melanocytes. J Biol Chem. 1992;267(34):24675–24680. [PubMed] [Google Scholar]
- Ito M, Yang Z, Andl T, Cui C, Kim N, Millar SE, et al. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature. 2007;447(7142):316–320. doi: 10.1038/nature05766. [DOI] [PubMed] [Google Scholar]
- Kawaguchi A, Chiba K, Tanimura Y, Motohashi T, Aoki H, Takeda T, et al. Isolation and characterization of Kit-independent melanocyte precursors induced in the skin of Steel factor transgenic mice. Dev Growth Differ. 2008;50(2):63–69. doi: 10.1111/j.1440-169X.2007.00976.x. [DOI] [PubMed] [Google Scholar]
- Lee HS, Kooshesh F, Sauder DN, Kondo S. Modulation of TGF-beta 1 production from human keratinocytes by UVB. Exp Dermatol. 1997;6(2):105–110. doi: 10.1111/j.1600-0625.1997.tb00155.x. [DOI] [PubMed] [Google Scholar]
- Mao B, Wu W, Li Y, Hoppe D, Stannek P, Glinka A, et al. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature. 2001;411(6835):321–325. doi: 10.1038/35077108. [DOI] [PubMed] [Google Scholar]
- Massaki AB, Fabi SG, Fitzpatrick R. Repigmentation of hypopigmented scars using an erbium-doped 1,550-nm fractionated laser and topical bimatoprost. Dermatol Surg. 2012;38(7 Pt 1):995–1001. doi: 10.1111/j.1524-4725.2012.02389.x. [DOI] [PubMed] [Google Scholar]
- Muller-Rover S, Handjiski B, van der Veen C, Eichmuller S, Foitzik K, McKay IA, et al. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J Invest Dermatol. 2001;117(1):3–15. doi: 10.1046/j.0022-202x.2001.01377.x. [DOI] [PubMed] [Google Scholar]
- Nishimura EK, Jordan SA, Oshima H, Yoshida H, Osawa M, Moriyama M, et al. Dominant role of the niche in melanocyte stem-cell fate determination. Nature. 2002;416(6883):854–860. doi: 10.1038/416854a. [DOI] [PubMed] [Google Scholar]
- Nishimura EK, Suzuki M, Igras V, Du J, Lonning S, Miyachi Y, et al. Key roles for transforming growth factor beta in melanocyte stem cell maintenance. Cell Stem Cell. 2010;6(2):130–140. doi: 10.1016/j.stem.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura EK. Melanocyte stem cells: a melanocyte reservoir in hair follicles for hair and skin pigmentation. Pigment Cell Melanoma Res. 2011;24(3):401–410. doi: 10.1111/j.1755-148X.2011.00855.x. [DOI] [PubMed] [Google Scholar]
- Nordlund JJ, Abdel-Malek ZA. Mechanisms for post-inflammatory hyperpigmentation and hypopigmentation. Prog Clin Biol Res. 1988;256:219–236. [PubMed] [Google Scholar]
- Okuse T, Chiba T, Katsuumi I, Imai K. Differential expression and localization of WNTs in an animal model of skin wound healing. Wound Repair Regen. 2005;13(5):491–497. doi: 10.1111/j.1067-1927.2005.00069.x. [DOI] [PubMed] [Google Scholar]
- Picardo M, Dell’Anna ML, Ezzedine K, Hamzavi I, Harris JE, Parsad D, et al. Vitiligo. Nat Rev Dis Primers. 2015;1:15011. doi: 10.1038/nrdp.2015.11. [DOI] [PubMed] [Google Scholar]
- Rabbani P, Takeo M, Chou W, Myung P, Bosenberg M, Chin L, et al. Coordinated activation of Wnt in epithelial and melanocyte stem cells initiates pigmented hair regeneration. Cell. 2011;145(6):941–955. doi: 10.1016/j.cell.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer E, Trautinger F, Köck A, Schwarz A, Bhardwaj R, Simon M, et al. Proopiomelanocortin-derived peptides are synthesized and released by human keratinocytes. J Clin Invest. 1994;93(5):2258–2262. doi: 10.1172/JCI117224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenov MV, Tamai K, Brott BK, Kuhl M, Sokol S, He X. Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr Biol. 2001;11(12):951–961. doi: 10.1016/s0960-9822(01)00290-1. [DOI] [PubMed] [Google Scholar]
- Takeda K, Yasumoto K, Takada R, Takada S, Watanabe K, Udono T, et al. Induction of melanocyte-specific microphthalmia-associated transcription factor by Wnt-3a. J Biol Chem. 2000;275(19):14013–14016. doi: 10.1074/jbc.c000113200. [DOI] [PubMed] [Google Scholar]
- Takeo M, Lee W, Rabbani P, Sun Q, Hu H, Lim CH, et al. EdnrB Governs Regenerative Response of Melanocyte Stem Cells by Crosstalk with Wnt Signaling. Cell Rep. 2016;15(6):1291–1302. doi: 10.1016/j.celrep.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Amerongen R, Nusse R. Towards an integrated view of Wnt signaling in development. Development. 2009;136(19):3205–3214. doi: 10.1242/dev.033910. [DOI] [PubMed] [Google Scholar]
- Vasioukhin V, Degenstein L, Wise B, Fuchs E. The magical touch: Genome targeting in epidermal stem cells induced by tamoxifen application to mouse skin. Proc Natl Acad Sci U S A. 1999;96(15):8551–8556. doi: 10.1073/pnas.96.15.8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Liu Y, Chen H, Mei L, He C, Jiang L, et al. LEF-1 Regulates Tyrosinase Gene Transcription In Vitro. PLoS One. 2015;10(11):e0143142. doi: 10.1371/journal.pone.0143142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisely JA, Hoyle E, Tarrier N, Edwards J. Where to start? Attempting to meet the psychological needs of burned patients. Burns. 2007;33(6):736–746. doi: 10.1016/j.burns.2006.10.379. [DOI] [PubMed] [Google Scholar]
- Yamada T, Hasegawa S, Inoue Y, Date Y, Yamamoto N, Mizutani H, et al. Wnt/beta-catenin and kit signaling sequentially regulate melanocyte stem cell differentiation in UVB-induced epidermal pigmentation. J Invest Dermatol. 2013;133(12):2753–2762. doi: 10.1038/jid.2013.235. [DOI] [PubMed] [Google Scholar]
- Yasumoto K, Takeda K, Saito H, Watanabe K, Takahashi K, Shibahara S. Microphthalmia-associated transcription factor interacts with LEF-1, a mediator of Wnt signaling. EMBO J. 2002;21(11):2703–2714. doi: 10.1093/emboj/21.11.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Yang T, Guo H, Tang Y, Deng F, Li Y, et al. Wnt10b promotes differentiation of mouse hair follicle melanocytes. Int J Med Sci. 2013;10(6):691–698. doi: 10.7150/ijms.6170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuriguchi M, Aoki H, Taguchi N, Kunisada T. Pigmentation of regenerated hairs after wounding. J Dermatol Sci. 2016;84(1):80–87. doi: 10.1016/j.jdermsci.2016.07.004. [DOI] [PubMed] [Google Scholar]
- Zaidi MR, Davis S, Noonan FP, Graff-Cherry C, Hawley TS, Walker RL, et al. Interferon-gamma links ultraviolet radiation to melanomagenesis in mice. Nature. 2011;469(7331):548–553. doi: 10.1038/nature09666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Overbeek PA. Tyrosinase-related protein 2 promoter targets transgene expression to ocular and neural crest-derived tissues. Dev Biol. 1999;216(1):154–163. doi: 10.1006/dbio.1999.9480. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


