Abstract
Developmental programming by reduced maternal nutrition alters function in multiple offspring physiological systems, including lipid metabolism. We have shown that intrauterine growth restriction (IUGR) leads to offspring cardiovascular dysfunction with an accelerated aging phenotype in our nonhuman primate, baboon model. We hypothesized age-advanced pericardial fat and blood lipid changes. In pregnancy and lactation, pregnant baboons ate ad lib (control) or 70% ad lib diet (IUGR). We studied baboon offspring pericardial lipid deposition with MRI at 5-6 years (human equivalent 20-24 years), skinfold thickness, and serum lipid profile at 8-9 years (human equivalent 32-36 years), comparing values with a normative life-course baboon cohort, 4-23 years. Increased pericardial fat deposition occurred in IUGR males but not females. Female but not male total cholesterol, low-density lipoprotein, and subcutaneous fat were increased with a trend of triglycerides increase. When comparing IUGR changes to values in normal older baboons, the increase in male apical pericardial fat was equivalent to advancing age by 6 years and the increase in female LDL to an increase of 3 years. We conclude that reduced maternal diet accelerates offspring lipid changes in a sex-dimorphic manner. The interaction between programming and accelerated lipogenesis warrants further investigation.
Keywords: Baboon, Developmental Programming, Lipogenesis, Intrauterine Growth Restriction (IUGR), Maternal Nutrient Reduction, Nonhuman Primates, Pericardial Fat, Serum Lipid, Aging
Introduction
Human and animal studies indicate nutritional challenges in pregnancy result in lifelong changes in offspring physiology, termed “developmental programming.” Intrauterine growth restriction (IUGR) leads to multi-system offspring changes, including metabolic alterations.1 Even though the overall body size and body mass index (BMI) are often not overtly increased, an obesogenic phenotype is frequently suggested by abnormal circulatory lipid concentrations and increased regional fat deposition. In the Dutch Hunger Winter study, female but not male offspring of mothers pregnant during the famine had elevated total and low-density lipoprotein (LDL) cholesterol.2 Increased epicardial fat thickness, recently identified as a cardiovascular risk factor, has been documented in extremely low birth weight preterm human young adults, suggestive of altered lipid metabolism originating from early development.4 Epigenetic studies in very young children revealed association between total and high-density lipoprotein (HDL) cholesterol concentrations and DNA methylation of key metabolic regulatory foci, such as tumor necrosis factor alpha (TNF-α) and leptin (LEP), lending further support for roles of perinatal development in later life metabolic health.3 We have established a model of developmental programming by moderate maternal nutrition reduction (MNR, 30% reduction from ad lib) during pregnancy and lactation in baboons,5 which leads to IUGR offspring with biventricular cardiac programming changes by 5-6 years.6,7 A subset of these animals demonstrated signs of metabolic derangement as evidenced by decreased glucose disposal rate by hyperinsulinemic euglycemic clamp by 3 years of age.8 We hypothesized that MNR induced IUGR in our primate model accelerates offspring pericardial fat deposition, increases subcutaneous fat, and elevates serum lipids, thereby contributing to cardiovascular dysfunction.
Materials and Methods
Ethical Approval
All procedures were approved by the Texas Biomedical Research Institute Institutional Animal Care and Use Committee (IACUC) and conducted in facilities approved by the Association for Assessment and Accreditation of Laboratory Animal Care.
Animal Model
Baboons (Papio species) were housed and maintained in a group social environment and fed using an individual feeding system. Healthy gravid female baboons of similar age and weight were randomized to an ad lib diet during pregnancy and lactation (control) or MNR by reducing diet to 70% globally of feed eaten by controls from 0.16 gestation to end of lactation.9 Offspring baboons were fully weaned at 9 months of age and moved to juvenile group housing, where ad lib diet was given. Feed was Monkey Diet 5038 (Purina LabDiets, St Louis, MO), containing 13% calories from fat, 18% calories from protein, 69% calories from carbohydrates, mineral and vitamin additives, and a metabolizable energy content of 3.22 kcal/g.
Morphometric Measurements, Magnetic Resonance Imaging, and Serum Lipid Assessment
Morphometric measurements of the IUGR (8M/8F, age = 7.7 ± 1.2 yrs; mean ± SD) and control (CTL) baboons (12M/12F, age = 8.0 ± 1.4 yrs) were obtained. Body weight and length were measured as well as skinfold thickness at the biceps and triceps at mid humerus, anterior quadriceps at the level of the mid femur, and lateral abdomen in the suprailiac region in a blinded fashion.
Magnetic resonance imaging was performed on the IUGR baboons (8M/8F, age = 5.7 ± 1.3 yrs) and age-matched controls (8M/8F, age = 5.6 ± 1.3 yrs) using a 3.0 Tesla MRI scanner (TIM Trio, Siemens Healthcare, Malvern, PA). T1 weighted inversion recovery long-axis cardiac images were obtained through the ventricular apex (TE/TR/TI 1.75/934/480 ms). MRI studies were conducted under anesthesia, induced by ketamine hydrochloride (10 mg kg -1, I.M.) and maintained by isoflurane (0.8–1.0%, INH).
To remove potential diurnal and prandial effects, all studies were conducted in the morning (9 am-11 am) after an overnight fast. Blood samples were taken from the IUGR (8M/8F, age = 8.8 ± 1.2 yrs) and CTL baboons (10M/9F, age = 9.0 ± 1.6 yrs). Serum total cholesterol, HDL, triglyceride, glucose, and whole blood hemoglobin A1c (HbA1c) concentrations were measured using an ACE™ Chemistry Analyzer (Alfa Wassermann, Woerden, Netherlands) in a blinded fashion.
Data Processing and Analysis
The body mass index (BMI) was calculated as
The CMR42 image analysis package (Circle Cardiovascular, Calgary, AB) was used for assessment of pericardial fat with blinding during the analysis. Using long axis images, the thickness of apical pericardial fat was measured. Normalization to body surface area (BSA) was performed, with BSA estimated using previously established formulas.10 For females,
and in males,
Serum LDL was estimated using the formula,
Data were analyzed using GraphPad Prism 7 (GraphPad Software, La Jolla, CA). Grubbs’ test (extreme Studentized deviate) was used to evaluate for statistical outliers. Normality of distribution is assessed by the d’Agostino-Pearson test. One tailed Student’s t-test of equal variance was used for comparison between groups in a sex-specific manner. The measured male pericardial fat deposit thickness and female serum LDL concentration were compared to similar measurements of the life-course cohort (LC, age = 4-23 yrs) to enable calculation of biological age against control chronological age.
Results
The obtained data are shown in Table 1.
Table 1.
Measurements
| Measurement | CTL | IUGR | t-Test | ||
|---|---|---|---|---|---|
| M | F | M | F | ||
| Morphometric Data | |||||
|
| |||||
| Number | 12 | 12 | 8 | 8 | - |
|
| |||||
| Weight (kg) | 30.5 ± 1.4 | 17.0 ± 0.8 | 27.6 ± 0.9 | 16.9 ± 0.9 | NS |
|
| |||||
| Length (cm) | 126 ± 2 | 106 ± 1 | 120 ± 2 | 106 ± 2 | M: CTL > IUGR* |
| F: NS | |||||
|
| |||||
| BMI | 19.3 ± 0.8 | 15.4 ± 1.0 | 19.2 ± 0.8 | 15.1 ± 0.7 | NS |
|
| |||||
| Biceps Skinfold (cm) | 2.6 ± 0.1 | 2.1 ± 0.2 | 2.8 ± 0.2 | 2.4 ± 0.2 | NS |
|
| |||||
| Triceps Skinfold (cm) | 2.4 ± 0.2 | 2.1 ± 0.2 | 2.7 ± 0.2 | 2.6 ± 0.2 | M: NS |
| F: IUGR > CTL* | |||||
|
| |||||
| Quadriceps Skinfold (cm) | 2.7 ± 0.2 | 2.1 ± 0.1 | 2.7 ± 0.2 | 3.0 ± 0.5 | M: NS |
| F: IUGR > CTL* | |||||
|
| |||||
| Suprailiac Skinfold (cm) | 3.8 ± 0.5 | 3.2 ± 0.2 | 3.9 ± 0.5 | 4.3 ± 0.7 | M: NS |
| F: IUGR > CTL* | |||||
| Pericardial Fat Data | |||||
|
| |||||
| Number | 8 | 8 | 8 | 8 | - |
|
| |||||
| Periapical Fat Thickness (mm) | 6.33 ± 0.75 | 9.00 ± 1.59 | 12.01 ± 2.16 | 9.31 ± 0.61 | M: IUGR > CTL* |
| F: NS | |||||
|
| |||||
| Periapical Fat Thickness/BSA (mm/m2) | 12.2 ± 1.5 | 19.9 ± 3.3 | 20.0 ± 3.6 | 21.6 ± 1.4 | M: IUGR > CTL* |
| F: NS | |||||
|
| |||||
| Serum Data | |||||
|
| |||||
| Number | 10 | 9 | 8 | 8 | - |
|
| |||||
| Total Cholesterol (mg/dL) | 88.8 ± 3.0 | 90.0 ± 6.4 | 85.0 ± 6.4 | 106.6 ± 5.8 | M: NS |
| F: IUGR > CTL* | |||||
|
| |||||
| LDL Cholesterol (mg/dL) | 27.1 ± 2.5 | 31.4 ± 3.6 | 24.5 ± 3.0 | 42.4 ± 2.4 | M: NS |
| F: IUGR > CTL* | |||||
|
| |||||
| HDL Cholesterol (mg/dL) | 52.3 ± 1.7 | 47.8 ± 3.5 | 52.3 ± 3.4 | 51.0 ± 3.8 | NS |
|
| |||||
| Triglycerides (mg/dL) | 49.2 ± 5.8 | 56.2 ± 3.5 | 43.4 ± 4.5 | 67.5 ± 7.0 | NS |
|
| |||||
| Glucose (mg/dL) | 101.8 ± 8.3 | 102.7 ± 8.6 | 109.4 ± 8.4 | 117.0 ± 13.1 | NS |
|
| |||||
| HbA1c (%) | 4.4 ± 0.1 | 4.2 ± 0.1 | 4.3 ± 0.1 | 4.2 ± 0.1 | NS |
Mean ± SEM.
p < 0.05;
NS- not significant.
Morphometric Measurements
There was a near significant decrease in weight of the IUGR male baboons (p = 0.07) with concurrent decrease in body length (p = 0.02) and no difference in BMI (p = 0.47). The female IUGR baboons were similar in weight (p = 0.44), length (p = 0.48), and BMI (p = 0.42) compared to CTL. The IUGR male baboons had similar skinfold thickness at biceps (p = 0.18), quadriceps (p = 0.46), and suprailiac region (p = 0.44) with near significant increase in triceps skinfold thickness (p = 0.07). The IUGR female baboons had increased skinfold thickness at triceps (p = 0.03), quadriceps (p = 0.02), and suprailiac region (p = 0.04) without change in biceps skinfold thickness (p = 0.19).
Apical Pericardial Fat Deposition
Apical pericardial fat thickness adjusted for BSA was increased in male IUGR baboons vs. CTL (Figure 1 A, p = 0.04), but not in females (p = 0.32). Similar changes were noted prior to normalization to BSA (male p = 0.02, female p = 0.42). Sex-specific regression analysis of the life-course cohort (LC) revealed positive correlation between age and normalized apical pericardial fat thickness in both males (Figure 1B, p < 0.01, r = 0.66) and females (Figure 1C, p < 0.01, r = 0.65). Overlay of the IUGR data visually demonstrated increased apical pericardial fat thickness in the IUGR males but not females. Using the regression data obtained from the life-course cohort, the effect of IUGR is equivalent to advancing male baboon age by approximately 6 years.
Figure 1. Apical Pericardial Adiposity and Serum Lipid Measurements.
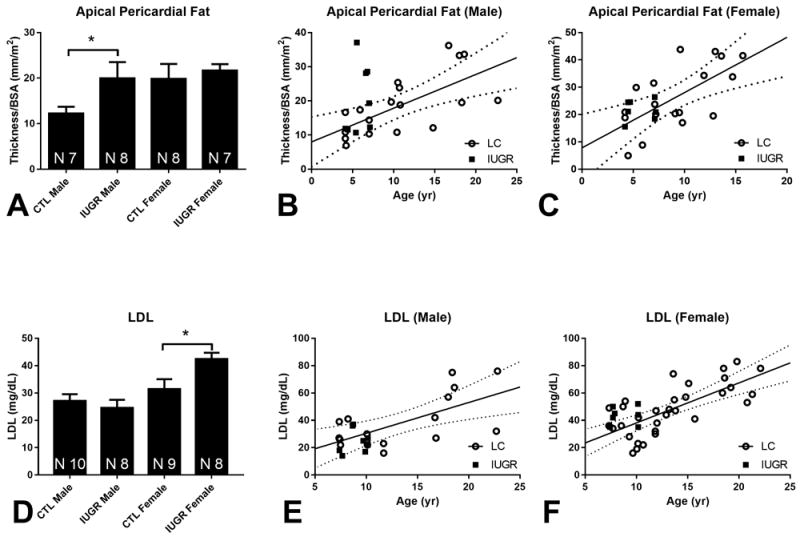
(A) Apical pericardial fat normalized for BSA at 5-6 years, mean + SEM, * p < 0.05. One statistical outlier was removed from the male CTL group and one from the female IUGR group. (B) Normalized apical pericardial fat thickness of the IUGR males (closed squares, N = 8) against the life course cohort (open circle, N = 18) with solid regression line for the life course cohort and dotted 95% confidence interval bands. (C) Corresponding graph for females with life course cohort (open circle, N = 18) and female IUGR group (closed squares, N = 7). (D) LDL concentration at 8-9 years, mean + SEM, * p < 0.05. (E) IUGR male baboon (closed squares, N = 8) LDL concentration against the life course cohort (open circle, N = 17) with solid regression line for the life course cohort and dotted 95% confidence interval bands. (F) Female IUGR (closed squares, N = 8) LDL concentration against life course cohort (open circle, N = 32) with solid regression line for the life course cohort and dotted 95% confidence interval bands.
Serum Lipid Assessments
Increased total cholesterol concentration was seen in IUGR females compared to CTL (p = 0.04), but not in males (p = 0.29). LDL cholesterol was higher in IUGR females compared to CTL (Figure 1 D, p = 0.01) while male values were similar (p = 0.25). HDL cholesterol was not different between groups in males (p = 0.50) or females (p = 0.27). A trend of increased triglyceride was seen in females (p = 0.08) but not in males (p = 0.23). Glucose concentrations were not different between groups in males (p = 0.27) or females (p = 0.18). HbA1c concentrations were not different between groups in males (p = 0.29) or females (p = 0.26). When female serum LDL concentration was compared against the life-course cohort, LDL was increased by an amount equivalent to approximately 3 years (Figure 1 F).
Discussion
In this study, we show sexually-dimorphic changes in lipid metabolism in young adult IUGR offspring of baboon mothers that received 70% ad lib diet during pregnancy and lactation. We detected an increase in IUGR male but not female pericardial adiposity at 5-6 years (human equivalent 20 yrs), which prompted investigation into serum lipids. An increase in IUGR female but not male serum total cholesterol was observed at 8-9 years (human equivalent 35 yrs) accompanied by an increase in LDL and a trend of increase in serum triglyceride. Serum HDL, glucose, and HbA1c (a long-term marker of serum glucose concentration) were similar in both groups and in both sexes. Increased skinfold thickness in the triceps, quadriceps, and suprailiac region in the females is indicative of increased subcutaneous fat deposition, not seen in the males. While BMI is unchanged in both sexes, these changes advocate a change in body composition and a phenotype akin to obesity with IUGR, contributing to the early cardiac dysfunction that we have previously reported.6,7 A recent systemic review of studies in humans found a significant association between epicardial fat accumulation and metabolic syndrome.11 Increased cardiac fat deposition was reported in MNR induced IUGR male sheep12 and protein restriction induced IUGR rats.13
The aberrant lipid changes seen in this study may be a result of programming of key endocrine systems during development.1 For example, early-life stressors can result in lasting changes of the hypothalamic-pituitary-adrenal axis, leading to glucocorticoid signaling dysregulation and altered lipid handling and metabolic homeostasis.14 In a nutrient-deprived environment, these altered processes ensure adequate substrate uptake in critical organs. However, when nutrient availability is no longer limited postnatally, persistence of these adaptations in conjunction with abnormal energy utilization likely results in over-accumulation of fat.
Glucose is the principal energy substrate for the placenta and the fetal myocardium, whereas the adult myocardium primarily relies on fatty acids.15 A report of overexpression of the fatty acid transporters in IUGR rat myocardium suggests a metabolic shift in utero, which may have consequences in adult life.16 Similarly, increased serum cholesterol concentration has been reported in IUGR rats induced by MNR17 and protein restriction.18 Increased concentrations of total and LDL cholesterols are established risk factors of cardiovascular mortality.19
Altogether, our findings indicate while metabolism is altered in both sexes with IUGR, underlying pathophysiology is likely sexually dimorphic. Sex-dimorphic lipid handling is a well-documented phenomenon, with prior reports showing subcutaneous predominant storage of fat in females versus visceral fat deposition in males,20 which may partially explain our findings. Our results show the need for future studies in both sexes on the programming of altered metabolism and cardiovascular function in this cohort of nonhuman primates to enable comparison with information from rodents.
Acknowledgments
The authors thank Dr. Robert Lanford and the Southwest National Primate Center staff for their ongoing support of the baboon research program described in this article. The authors also acknowledge the technical support of Steven Rios, Sam Vega, Susan Jenkins, and McKenna Considine, as well as the administrative support of Karen Moore.
Funding
This work was supported by the National Institutes of Health 5P01HD021350- PWN, 5R24OD011183- PWN, 5K25DK089012- GDC, and 1R25EB016631- AHK. NIH grant OD P51 OD011133 was from the Office of Research Infrastructure Programs/Office of the Director. This work was supported in part by funding from the EU FP 7/HEALTH/GA No.: 279281: BrainAge- Impact of Prenatal Stress on BRAINAGEing as well as Julio C. Palmaz Endowment for Excellence in Radiology Research Pilot Grant- AHK.
Abbreviations
- BMI
body mass index
- BSA
body surface area
- HDL
high-density lipoprotein
- HbA1c
hemoglobin A1c
- IUGR
intrauterine growth restriction
- LDL
low-density lipoprotein
- LEP
leptin
- MNR
maternal nutrient reduction
- MRI
magnetic resonance imaging
- NS
not significant
- TNF-α
tumor necrosis factor alpha
Footnotes
Competing interests
The authors have no potential conflict of interest, financial or otherwise, to disclose.
References
- 1.McMillen IC, Robinson JS. Developmental origins of the metabolic syndrome: Prediction, plasticity, and programming. Physiol Rev. 2005;85(2):571–633. doi: 10.1152/physrev.00053.2003. [DOI] [PubMed] [Google Scholar]
- 2.Lumey LH, Stein AD, Kahn HS, Romijn JA. Lipid profiles in middle-aged men and women after famine exposure during gestation: The dutch hunger winter families study. Am J Clin Nutr. 2009;89(6):1737–1743. doi: 10.3945/ajcn.2008.27038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wijnands K, Obermann-Borst S, Steegers-Theunissen R. Early life lipid profile and metabolic programming in very young children. Nutr Metab Cardiovasc Dis. 2015;25(6):608–614. doi: 10.1016/j.numecd.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Bassareo P, Fanos V, Puddu M, Marras S, Mercuro G. Epicardial fat thickness, an emerging cardiometabolic risk factor, is increased in young adults born preterm. J Dev Orig Health Dis. 2016;7(4):369–373. doi: 10.1017/S2040174416000234. [DOI] [PubMed] [Google Scholar]
- 5.Schlabritz-Loutsevitch N, Ballesteros B, Dudley C, et al. Moderate maternal nutrient restriction, but not glucocorticoid administration, leads to placental morphological changes in the baboon (papio sp.) Placenta. 2007;28(8):783–793. doi: 10.1016/j.placenta.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuo AH, Li C, Li J, Huber HF, Nathanielsz PW, Clarke GD. Cardiac remodelling in a baboon model of intrauterine growth restriction mimics accelerated ageing. J Physiol (Lond) 2016;595(4):1093–1110. doi: 10.1113/JP272908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuo AH, Li C, Huber HF, Schwab M, Nathanielsz PW, Clarke GD. Maternal nutrient restriction during pregnancy and lactation leads to impaired right ventricular function in young adult baboons. J Physiol (Lond) 2017;595(13):4245–4260. doi: 10.1113/JP273928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi J, Li C, McDonald TJ, Comuzzie A, Mattern V, Nathanielsz PW. Emergence of insulin resistance in juvenile baboon offspring of mothers exposed to moderate maternal nutrient reduction. Am J Physiol Regul Integr Comp Physiol. 2011;301(3):R757–62. doi: 10.1152/ajpregu.00051.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li C, Jenkins S, Mattern V, et al. Effect of moderate, 30 percent global maternal nutrient reduction on fetal and postnatal baboon phenotype. J Med Primatol. 2017 doi: 10.1111/jmp.12290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.VandeBerg JL, Williams-Blangero S, Tardif SD. The baboon in biomedical research. Springer Science & Business Media; 2009. [Google Scholar]
- 11.Rabkin SW. The relationship between epicardial fat and indices of obesity and the metabolic syndrome: A systematic review and meta-analysis. Metab Syndr Relat Disord. 2014;12(1):31–42. doi: 10.1089/met.2013.0107. [DOI] [PubMed] [Google Scholar]
- 12.Chan LL, Sebert SP, Hyatt MA, et al. Effect of maternal nutrient restriction from early to midgestation on cardiac function and metabolism after adolescent-onset obesity. Am J Physiol Regul Integr Comp Physiol. 2009;296(5):R1455–63. doi: 10.1152/ajpregu.91019.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slater-Jefferies J, Lillycrop K, Townsend P, et al. Feeding a protein-restricted diet during pregnancy induces altered epigenetic regulation of peroxisomal proliferator-activated receptor-α in the heart of the offspring. J Dev Orig Health Dis. 2011;2(04):250–255. doi: 10.1017/S2040174410000425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maniam J, Antoniadis C, Morris MJ. Early-life stress, HPA axis adaptation, and mechanisms contributing to later health outcomes. Front Endocrinol (Lausanne) 2014;5:73. doi: 10.3389/fendo.2014.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Szablewski L. Glucose transporters in healthy heart and in cardiac disease. Int J Cardiol. 2017;230:70–75. doi: 10.1016/j.ijcard.2016.12.083. [DOI] [PubMed] [Google Scholar]
- 16.Abbasi A, Thamotharan M, Shin BC, et al. Myocardial macronutrient transporter adaptations in the adult pregestational female intrauterine and postnatal growth-restricted offspring. Am J Physiol Endocrinol Metab. 2012;302(11):E1352–62. doi: 10.1152/ajpendo.00539.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Desai M, Gayle D, Babu J, Ross MG. The timing of nutrient restriction during rat pregnancy/lactation alters metabolic syndrome phenotype. Obstet Gynecol. 2007;196(6):555. e1-555.e7. doi: 10.1016/j.ajog.2006.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sohi G, Marchand K, Revesz A, Arany E, Hardy DB. Maternal protein restriction elevates cholesterol in adult rat offspring due to repressive changes in histone modifications at the cholesterol 7 α-hydroxylase promoter. Mol Endocrinol. 2011;25(5):785–798. doi: 10.1210/me.2010-0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pekkanen J, Linn S, Heiss G, et al. Ten-year mortality from cardiovascular disease in relation to cholesterol level among men with and without preexisting cardiovascular disease. N Engl J Med. 1990;322(24):1700–1707. doi: 10.1056/NEJM199006143222403. [DOI] [PubMed] [Google Scholar]
- 20.Power ML, Schulkin J. Sex differences in fat storage, fat metabolism, and the health risks from obesity: Possible evolutionary origins. Br J Nutr. 2008;99(5):931–940. doi: 10.1017/S0007114507853347. [DOI] [PubMed] [Google Scholar]


