Abstract
The ability to prevent disease is the holy grail of medicine. For decades, efforts have been made to extend the successes seen with vaccination against infectious diseases to cancer. In some instances, preventive vaccination against viruses (prototypically HPV) has successfully prevented tumorigenesis and will make a major impact on public health in the decades to come. However, the majority of cancers that arise are a result of genetic mutation within the host, or non-viral environmental exposures. We present compelling evidence that vaccination against an overexpressed self-tumor oncoprotein has the potential to prevent tumor development. Vaccination against the Epidermal Growth Factor Receptor (EGFR) using a multipeptide vaccine in a preventive setting decreased EGFR-driven lung carcinogenesis by 76.4% in a mouse model of EGFR-driven lung cancer. We also demonstrate that anti-EGFR vaccination primes the development of a robust immune response in vivo. This study provides proof of concept for the first time that targeting tumor drivers in a preventive setting in lung cancer using peptide vaccination can inhibit tumorigenesis and may provide useful clinical insights into the development of strategies to vaccinate against EGFR in populations that where EGFR-mutant disease is highly prevalent.
Keywords: Cancer Prevention, Lung Cancer vaccine, Lung Cancer, Peptide Vaccine
INTRODUCTION
Lung cancer remains a disease with high morbidity and mortality. Most patients present with advanced disease, not amenable to surgical resection that responds poorly to chemotherapeutics [1]. Strategies such as vaccination which are capable of either regressing early-stage disease or preventing recurrence would be useful. In addition, such an approach may prove applicable in the setting of primary prevention among populations where the preponderance of lung adenocarcinomas are associated with EGFR mutation which is the clinical picture in East Asia [2]. Vaccines could also be a useful strategy for patients with a familial predisposition to lung cancer [3], pre-existing lung diseases that predispose to malignant transformation, such as chronic obstructive pulmonary disorder [4] and those exposed to environmental pollutants [5–7], as well as those with a history of heavy smoking. To date, therapeutic vaccination using peptides has shown only modest clinical efficacy, likely due to the significant immunosuppressive mechanisms that evolve over the course of tumor development. We aimed to try an entirely different approach- vaccinating with tumor antigen peptides before cancer development and the development of tumor-associated immunosuppression. The distinction between preventive versus therapeutic vaccines is an important one that cannot be stressed enough- a number of studies have established that preventive vaccination may be more effective than therapeutic vaccination in cancer (reviewed in detail in [8]).
The vast majority of clinical trials have been conducted in patients with advanced disease (Stage III/IV). Targeted antigens have included epidermal growth factor (EGF--reviewed in [9]), as well as MUC-1 (reviewed in [10]) and telomerase [11], among others. These studies have shown that a subset of patients benefit from vaccination, with clinical benefit being confined to those who respond most robustly in immunologic terms. The overarching lesson from these trials is that responses to therapeutic vaccines can be significant, but are generally limited to a subset of patients [12]. We hypothesized that using a vaccine as a preventive strategy could enable the polarization of an immune response capable of successfully eliminating early lesions and preventing tumor development in the lung.
In non-small cell lung cancer (NSCLC), mutations in the Epidermal Growth Factor Receptor (EGFR) are associated with tumor development in a large subset of patients. Common mutations include EGFR L858R and small in frame deletions in exon 19 of the EGFR protein with EGFR overexpression or mutation detected in 43–89% of lung adenocarcinoma samples evaluated [13]. In addition, certain populations are known to express high levels of EGFR mutations in the context of NSCLC. In particular, over 50% of NSCLC presenting in East Asians or patients of East Asian descent harbor EGFR mutations [2]. Thus, vaccinating against EGFR as a preventive approach may be a useful strategy in this population. In addition, vaccinating against EGFR following targeted anti-EGFR therapies may represent a means to repurpose vaccination into another preventive context to prevent recurrent disease, based on the same hypothesis that vaccination functions better when tumor-mediated immunosuppression is minimal.
To model the ability of EGFR vaccination to prevent primary NSCLC, we used a murine model engineered to inducibly express full-length human EGFR with the L858R mutation. These animals rapidly develop lung adenocarcinomas visible by magnetic resonance imaging (MRI) of the lungs within 10 weeks of continuous dietary doxycycline administration, with a tumor incidence of 100% and expression of the transgene limited to the lung [14].
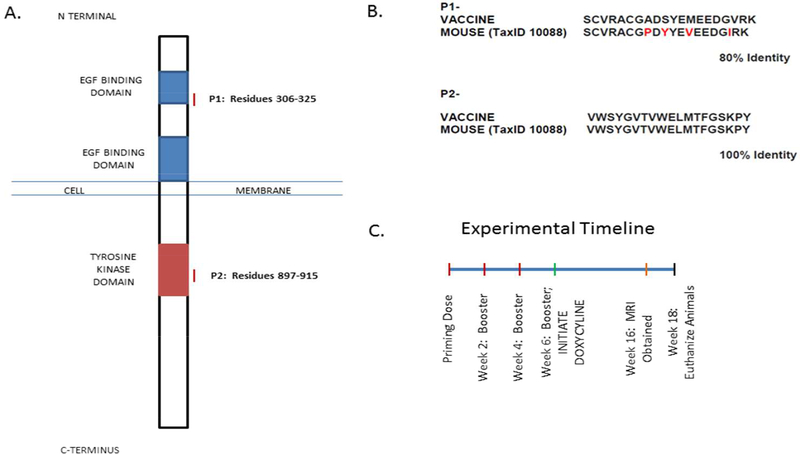
The vaccine studies that we describe in this paper are conducted in a model of primary prevention, although they may offer important lessons for the conduct of future studies in animals with early lesions or in the prevention of disease recurrence. Our vaccine is comprised of two separate peptides (Figure 1A) and an adjuvant (Freund’s adjuvant). Specifically, one peptide corresponds to residues 306-325 of human EGFR and represents an extracellular region of the receptor. The other peptide contains residues 897–915, an intracellular portion of the EGFR protein located within the tyrosine kinase domain. Neither peptide encompasses the specific region of the activating mutation at position 858. Each of these peptide sequences has a homology of 80% (p1) or 100% (p2) between humans and mice (Figure 1B). Highly homologous target sequences for the vaccine were selected to ensure that the reaction to the vaccine was not merely xenogenic in nature. In this model system, it is important to note that the full-length human EGFR, containing the L858R mutation is inducibly expressed, which has100% homology with the target sequences chosen as part of our vaccine. Freund’s adjuvant was selected due to previous literature establishing this adjuvant as a potent inducer of Th1immune responses in vivo [15]. In these studies, we found that 1) the vaccine was highly effective in preventing tumor development; 2) vaccination elicited a strong and specific immune response as measured by ELISpot and was associated with changes in immune composition. We also used MRI and demonstrated that this technique is capable of monitoring responses to this vaccine in vivo.
Figure 1. Vaccine Design & Experimental Strategy.
A. Schematic of the EGFR protein. Red lines indicate the peptide epitopes selected as part of our vaccine.
B. Representation of the homology between the peptides composing the vaccine and corresponding mouse sequences.
C. Schematic of the experimental design outlining timing of vaccine administration, induction of the oncogenic transgene, imaging time points and experimental endpoint.
MATERIAL & METHODS
Peptides
Two peptides comprising residues 306-325 (SCVRACGADSYEMEEDGVRK) and residues 897-915 (VWSYGVTVWELMTFGSKPY) of human EGFR were identified using previously published methods [16]. Peptides were synthesized by GeneMed Synthesis (San Antonio, TX). Purity of >95% was required for peptides to be used in vivo.
Mice
Transgenic mice expressing human EGFR containing the L858R mutation on a C57bl/6 background were a generous gift from Drs. William Pao (Vanderbilt University) and Katerina Politi (Yale). EGFR L858R mice (C57bl/6 strain) were crossed with mice expressing the Tet-on Clara Cell Secreted Protein (CCSP) on the A/J background to permit tissue specific inducible expression of the transgene. For all experiments reported herein, only the F1 generation that harbor both EGFR L858R and CCSP were used. All mice were housed in the Biomedical Resource Center at the Medical College of Wisconsin, Milwaukee, WI. All procedures were approved by the institutional animal care and use committee (IACUC).
Vaccine Preparation
Mice were vaccinated with 50 μg of each peptide. Phosphate buffered saline (PBS) was added to bring the total volume to 50 μL/mouse. An equal amount of adjuvant (Complete Freund’s Adjuvant or Incomplete Freund’s Adjuvant) was added to bring the total volume to 100 μL/mouse. Mice were injected subcutaneously near the scapula, as indicated in Figure 1C. The data presented in this manuscript are pooled results obtained from two separate bioassays conducted under experimentally identical conditions, to the extent possible.
ELISPOT Assay
Cell suspensions from whole spleens were filtered through a 70 μm cell strainer (BD) and subjected to red blood cell lysis using ACK lysis buffer. 3.0 × 104 cells were plated into individual wells of a MAIPS4510 Multiscreen 96 well plate previously coated with anti-interferon γ detection antibody and containing media with either peptide, positive control (conclavin A) or negative control (no antigen). After 72 hours, plates were washed and a secondary antibody (BD) was added and incubated on the plate overnight at 4 degrees Celsius. Wells were then washed with PBS and HRP streptavidin was added. Following a 1 h incubation, the plate was developed using AEC substrate for between 5–25 minutes. The plate was subsequently gently washed under cold running tap water. When dry, an automated plate reader system (CTL Technologies) was used to image the plates and quantify spot number. ELISPOT assays were performed on samples from the first of the two vaccine bioassays.
Tumor Growth Evaluation
Magnetic Resonance Imaging
Ten weeks post initiation of doxycycline, mice were imaged using a 9.4T MRI (Bruker, Billerica, MA) with a custom birdcage style quadrature coil (Doty Scientific, Columbia, SC). Mice were anesthesized with isoflurane for the duration of the imaging procedure. Mice were induced at 2.5% isoflurane and maintained at 1.0–1.5%. Mouse heart rate, body temperature and respiratory rate were continuously monitored throughout imaging. Both respiratory and cardiac gating using an electrocardiogram were used to ensure that images were consistently acquired during latent periods of the respiratory cycle and at a consistent point during the cardiac cycle. Tumors were imaged using a multi-slice, multi-echo acquisition (MSME). Images were acquired using the following parameters; TE=8.07 ms, TR≥400 ms (variable), matrix=128 × 128, 1 average, 20 axial slices.
Coronal slice images were obtained from each animal and processed in ImageJ, enabling the identification and definition of regions of interest (ROI). The average pixel intensity was then calculated within the ROI. This average pixel intensity was multiplied by the area of the ROI, and the sum was obtained across multiple slices and ROIs. This sum was divided by the total area covered by the ROIs to reach an average lung image intensity value, in accordance with the accepted methodology [17].
Immunohistochemistry
All mouse lung samples were inflation and formalin fixed and processed (Sakura Tissue Tek VIP5) for paraffin embedding. After paraffin embedding, samples were sectioned at 4um (Microm HMS355S) onto poly-l-lysine coated slides and air dried at 45°C overnight for prior to immunohistochemistry or H&E staining.
For H&E staining, 20 slides were cut per lung with 20 μm spacing between slides.
H&E stained slides were scanned using the NanoZoomer slide scanner (Hamamatsu). Subsequently, NanoZoomer software was used and tumor regions were specifically highlighted by and measured. Three slides were selected per mouse for analysis, corresponding to a ventral, midline and dorsal region of the lung. Slides stained with EGFR were visualized at 100x total magnification on a Nikon Eclipse 50i microscope. Using the NIS Elements BR 3.2 software a threshold for positive staining was established. This threshold was then applied to 10 randomly selected regions of each slide and a percentage of positive area calculated using this defined ROI.
Flow Cytometry
Splenocytes were evaluated using flow cytometry for expression of surface markers CD4, CD8, CD44, CD62L and CD25 (eBioscience), as well as intracellular staining for FoxP3 (eBioscience). Stained cells were fixed in 1% paraformaldehyde and were permeabilized following the manufacturer’s instructions to evaluate the expression of intracellular targets (FoxP3). Flow cytometry was conducted using an LSR-II flow cytometer (BD). Data was analyzed using FlowJo software (Tree Star). Due to sample availability, flow cytometry was performed on samples from the second of the two vaccine bioassays only.
Results
Preventive Vaccination Inhibits Lung Tumor Formation
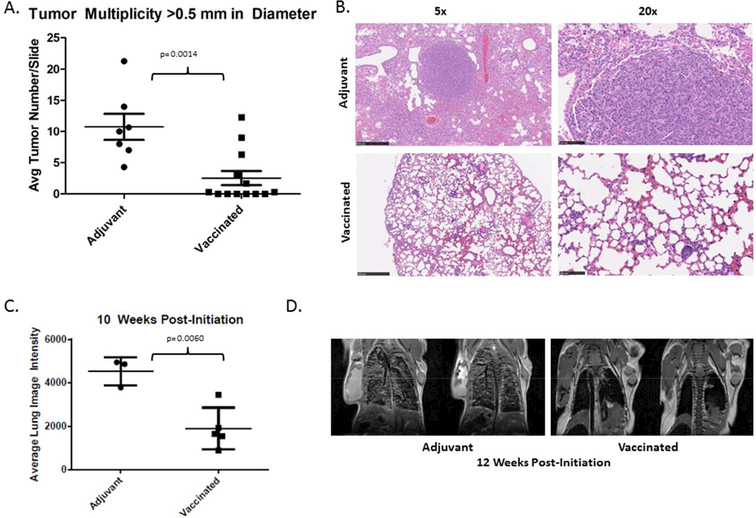
Using the vaccine outlined in Figure 1A, which was developed against human EGFR but has high homology to mouse EGFR (Figure 1B) (Experimental timeline; Figure 1C), we demonstrated that tumor development can be significantly inhibited in a preventive setting using a multi-peptide vaccine targeting EGFR. Specifically, vaccination resulted in decreased tumor multiplicity in vaccinated animals twelve weeks after induction of the EGFR transgene. Vaccinated animals demonstrated an average of ~2.5 tumors/slide with a diameter of greater than 0.5 mm, versus an average of ~10.76 tumors/slide found in animals not receiving vaccine, equating to a 76.4% reduction in tumor multiplicity (p= 0.0014, Figure 2A). Tumors in these transgenic animals are located within the lung parenchyma and are not easily quantifiable on the surface of the lung. As a result, analysis of slides from different anatomic regions and averaging of the tumor multiplicity within each animal was conducted as outlined in the Methods section in order to rigorously evaluate changes in tumor multiplicity. Representative histology from vaccinated versus adjuvant treated animals also reveals changes within the lung parenchyma. Non-vaccinated animals demonstrate a hypercellular lung parenchyma with few air spaces; by contrast, vaccinated animals exhibit grossly normal alveolar structures with limited evidence of lung disease, and some evidence of aggregates with a lymphoid character (Figure 2B). Imaging conducted on a subset of animals also reveals that these differences could be detected prior to the experimental endpoint. MRI at 10 weeks revealed significantly increased image intensity within the lungs in adjuvant treated animals versus vaccinated animals (p= 0.0060; Figure 2C). This technique has been rigorously validated as a means to evaluate tumor growth within the lungs using MRI in small animals [17]. In addition, the ability to serially monitor tumors non-invasively may be a useful technique for future studies evaluating vaccines in prevention of recurrence models. Final magnetic resonance images were obtained from representative animals just prior to euthanization at the 12 week experimental endpoint, also demonstrating significant qualitative differences within the lung parenchyma between non- vaccinated and vaccinated mice, which are also readily perceptible with this imaging modality (Figure 2D).
Figure 2. Vaccination Effectively Prevents Tumor Initiation.
A. Quantification of tumor/lesions by histology. Each of the lobes of the lung were examined and tumors quantified (left). Representative lung slices stained with hematoxylin and eosin (5x and 20x magnification) from a representative adjuvant treated and a vaccinated mouse. Data is pooled between two independent runs of the experimental protocol.
B. Quantification of tumor burden by MRI in adjuvant versus vaccinated animals. Average lung intensity is defined as mean pixel intensity x area/total lung area quantified (left). Representative images (2 slices) from an adjuvant treated and vaccinated animal (middle, right) obtained at 10 weeks post-EGFRL858R transgene induction. Data represents a subset of animals selected for imaging from the second run of the bioassay.
Vaccination Results in a Decrease in EGFR-Expressing Cells within the Lungs
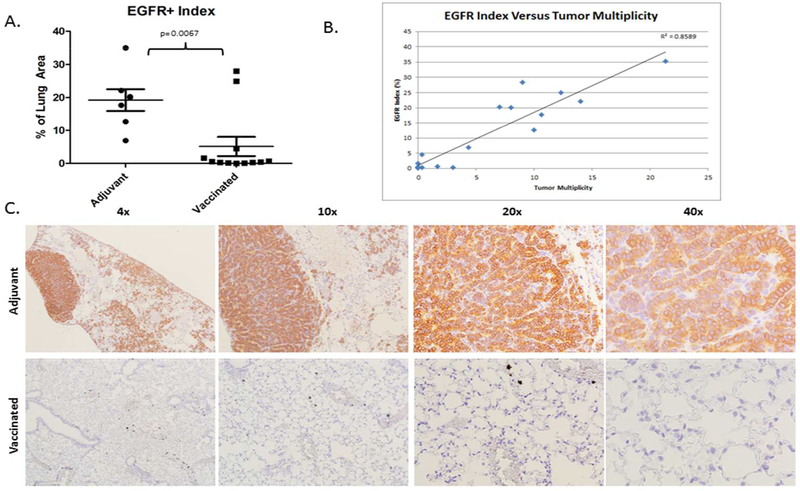
Non-vaccinated mice exhibit high levels of EGFR expression within the lung parenchyma, averaging nearly 19% of the lung area. By contrast, vaccinated mice exhibit decreased EGFR expression, with positive EGFR staining cells occupying an average of 5.64% of the total lung area (Figure 3A). This represents a 70.8% decrease in EGFR+ cells occupying the lung area in vaccinated animals versus controls (p=0.0067). Representative images of sections obtained from non-vaccinated controls and vaccinated animals (Figure 3C) corroborate from hematoxylin and eosin stained sections presented in Figure 2B. EGFR positivity is highly correlated with tumor lesions greater than 0.5 mm in diameter observed in hematoxylin and eosin stained sections (Figure 3B; r2= 0.86, p<0.001). Non-vaccinated animals demonstrate grossly hypercellular lungs pocked with EGFR positive cells clustered into tumors and spread diffusely throughout the parenchyma. Analysis at higher magnification reveals clearly abnormal cellular morphology in EGFR+ cells which is generally absent in the lungs of the majority of vaccinated animals. Staining for EGFR was conducted on all animals involved in the study with the exception of two animals for which sufficient tissue was not available.
Figure 3. Vaccination Results in a Decrease in EGFR Expressing Cells in the Lungs.
A. Quantification of EGFR expression as a percentage of the total lung area imaged. These data are pooled from two independent runs of the experimental protocol.
B. EGFR Index versus tumor multiplicity was plotted for animals from which tumor multiplicity and EGFR staining data were available.
C. Representative images of immunohistochemistry for EGFR in a representative adjuvant treated animal (top) and vaccinated animal (bottom) at 4x, 10x, 20x and 40x magnification. Quantification of EGFR expression as a percentage of the total lung area imaged.
Anti-EGFR Vaccination Induces an Immune Response
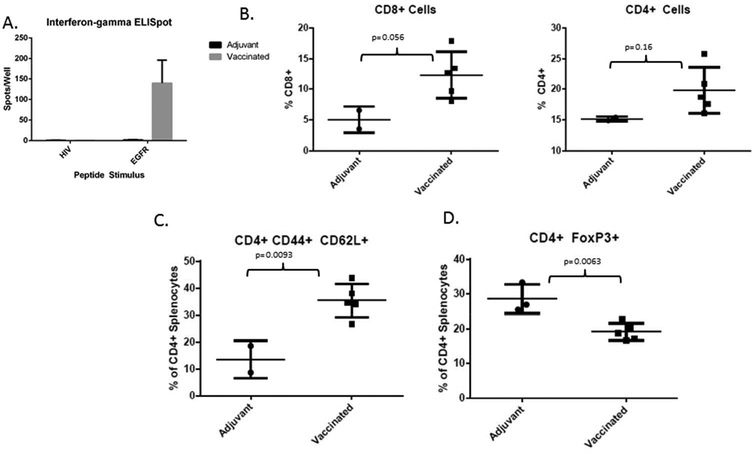
An interferon-gamma ELISpot of splenocytes isolated from control and vaccinated animals at the experimental endpoint revealed that vaccinated animals expressed a significant interferon- gamma-associated immune response upon peptide re-stimulation (Figure 4A). This reactivity to the vaccine EGFR peptides was highly specific, as demonstrated by the lack of response present in non-vaccinated animals. In addition, the response is not the result of general immune hyperactivity- stimulation with an irrelevant antigen that the mice are naive to (a peptide derived from the human immunodeficiency virus) reveals no reactivity in either the vaccinated or control mice. Flow cytometry analysis of cell surface markers in a subset of mice reveals differences in the character of the CD4+ and CD8+ lymphoid populations present in the spleen. Specifically, the overall percentage of both CD4+ and CD8+ cells within the spleens of vaccinated animals are trending upward (p=0.16 and 0.056, respectively; Figure 4B). Within the CD4+ population, other changes are also apparent. The percentage of CD4+ lymphocytes expressing CD44 and CD62L is significantly elevated in vaccinated animals versus controls (p= 0.0093; Figure 4B). In addition, the percentage of CD4+ cells expressing the FoxP3 transcription factor is significantly attenuated in vaccinated animals versus controls (p=0.0063; Figure 4C).
Figure 4. Immunologic Consequences of Vaccination.
A. Interferon-gamma ELISpot conducted on splenocytes isolated from adjuvant treated and vaccinated animals at the completion of the 18 week experiment, demonstrating a robust and specific immune response to the EGFR peptides in vaccinated animals. This assay was conducted with the splenocytes from animals in the first bioassay.
B. CD8+ cells expressed as a percentage of total live splenocytes isolated from the spleens of adjuvant treated (n=2) and vaccinated animals (n=5) at the experimental endpoint (left); CD4+ cells expressed as a percentage of total live splenocytes isolated from the spleens of adjuvant treated and vaccinated animals at the experimental endpoint (right). All flow cytometry experiments were performed on splenocytes isolated from animals in the second bioassay (panels B-D) due to sample availability.
C. CD44+ CD62L+ (double positive) cells expressed as a percentage of the total live CD4+ pool isolated from the spleens of adjuvant treated (n=2) and vaccinated animals (n=5) at the experimental endpoint.
D. FoxP3+ cells expressed as a percentage of the total live CD4+ pool isolated from the spleens of adjuvant treated (n=3) and vaccinated animals (n=5) at the experimental endpoint.
Discussion
Therapeutic use of peptide vaccines has had limited efficacy in clinical trials. This is likely the result of profound immune suppression mediated by advanced tumors. However, the role for peptide vaccines in the setting of prevention, where such factors are limited has not been extensively studied. We tested an EGFR peptide vaccine in a defined, EGFR mutant transgenic mouse model of human lung adenocarcinoma. We present compelling data that supports our hypothesis that prophylactic vaccination against EGFR using a multivalent peptide vaccine can prevent tumorigenesis driven by mutant EGFR.
The prevalence of EGFR mutation is relatively low within Caucasian smokers, although recent clinical and epidemiological studies have revealed that greater than half of all lung adenocarcinomas in East Asia and in patients of East Asian descent are associated with EGFR mutation [2]. In addition, many tumors that do not necessarily harbor EGFR mutations may exhibit EGFR overexpression [13]. EGFR inhibitors such as gefitinib, erlotinib and others have proven highly effective patients with EGFR-mutant disease. However, these treatments, while prolonging progression-free survival do not significantly increase overall survival as a result of the development of resistance to targeted therapies [18].
Previous work by one of the co-authors of this manuscript has demonstrated that vaccination against wildtype Her2/Neu can result in prevention or regression of early stage breast cancers. Based on this work, we aimed to employ a similar vaccine approach in the setting of EGFR- driven disease. Immunization was not targeted against a specific EGFR mutation in order to allow us to evaluate the potential ability of this vaccination approach to function across EGFR overexpressing tumor subtypes, regardless of the specific mutation present. We chose to evaluate the use of the vaccine first in primary preventive setting, based on the observation that EGFR mutation can lead to overexpression of immune inhibitory proteins such as PD-L1 [19]. If we were unable to see efficacy in a primary preventive model where immune suppression is much less likely to be a factor, it is unlikely that efficacy could be observed in the presence of lesions. Our work in the preventive setting may now lead to future studies to evaluate the use of vaccination against early lesions or in the setting of recurrence. This early work and the subsequent studies may have significant clinical relevance given that EGFR mutation represents the single most prevalent mutation in cases of lung adenocarcinoma in East Asia [2]. The selection of an appropriate preclinical animal model was vital to the work conducted here. The transgenic model employed is one of the standard models of EGFR mutant lung adenocarcinoma. The model expresses full-length mutant EGFR L858R upon transcriptional activation of the CCSP promoter by doxycycline resulting in expression in restricted lung tissues of the mutant EGFR transgene. The resulting tumors are dependent on continued mutant EGFR expression and rapidly regress upon withdrawal of doxycycline, or treatment with EGFR inhibitors- closely paralleling the clinical scenario observed in human patients [14]. The expression of inducible full-length human EGFR with a point mutation at position 858 may lead to concerns about potential xenogenic rejection, however, this concern is mitigated by several points. Both human and mouse EGFR are highly homologous; a comparison of the two using NCBI Protein Blast reveals that human and mouse EGFR have 95% query coverage and 88% identity (UNIPROTQ504U8 human versus Mus TaxID 10088). In addition, the ability of tumor to grow rapidly within the mouse suggests that any endogenous immune response to the tumor antigen is minimal. Strikingly, pulsing splenocytes derived from mice that were not vaccinated with our vaccine peptides that have 80 and 100% homology with the mouse sequences at the experimental endpoint revealed no reactivity to either peptide. This strongly suggests that there is not an endogenous immune response against EGFR that is present in the animals prior to vaccination and supports the contention that the mice are tolerant to the transgenic construct. If the transgenic construct itself were immunogenic, we might expect to observe an immune response against EGFR-derived peptides in diseased animals; this is not the case in the present study. This suggests that prophylactic vaccination with EGFR peptides used in this study is capable of breaking self-tolerance without the development of systemic autoimmunity, resulting in inhibition of tumor development. This is the situation most likely to be present in the clinic in human patients overexpressing EGFR. The model used in this study enabled us to examine whether immunization against EGFR could induce an effective immune response and to evaluate immune parameters in a relatively sensitive setting. These studies could lay the foundation for future preclinical work to evaluate the use of this vaccine in animals with lesions, or in animals with minimal residual disease following treatment.
In the present study, we found no evidence of off-target autoimmunity against native EGFR. This is potentially a serious concern when using self-antigens that are overexpressed by tumors as a vaccine target. In fact, skin-related toxicities are frequently seen in human patients when small molecule EGFR inhibitors are used. We observed none of this overt toxicity in this model system. Interestingly, other studies using peptide vaccines have reported similar results in both animal and human models. Her2/neu is another example of a commonly overexpressed tumor protein, which is also a self-antigen expressed at a basal level in several different tissues. Numerous clinical trials using Her2/neu peptide vaccines in breast cancer patients have been conducted over the course of the past decade with no evidence of autoimmunity or targeting of tissues with only basal expression of the Her2/neu self protein (reviewed in [20]). The underlying mechanism that governs this phenomenon has not been fully elucidated, but it is possible that the dramatic overexpression of tumor antigens such as Her2/neu and EGFR in tumor tissue specifically may allow for tumor specific targeting with little autoimmunity. Other studies have also pointed out that a subset of patients with advanced disease are capable of developing detectable antibodies and T cell mediated responses to tumor overexpressed self-antigens, yet these patients also do not exhibit overt signs of autoimmune pathology, although the T cell responses may in some cases bear a less Th1 related immune signature [21]. The presence of circulating antibodies directed against self-tumor antigens without evidence of autoimmune pathologies or targeting of non-tumor tissues by the immune system also suggests that there may be a thresholding effect or intricate immune regulation that prevents the development of autoimmunity [22]. Our result in this preclinical system as well as the plethora of self-antigen peptide vaccine clinical trials and natural development of anti-self-tumor associated antigen reactivity in cancer patients without autoimmune toxicities suggest that there is much that we have yet to learn about the intricate regulation of immune responses.
The development of an expanded CD4+ central memory pool (CD4+, CD44+, CD62L+) in the vaccinated animals provides encouraging evidence that vaccination results in the development of long-term memory. The ability to induce long-term memory is an important consideration in the development of preventive vaccines. The unique association of this expanded pool with vaccination strongly suggests that these cells are specific to vaccine peptides or to tumor antigens; however, additional work will be required to definitively determine whether this central memory population is specific to the vaccination.
The dramatic reduction in EGFR positive cells in the lungs of vaccinated animals at the experimental endpoint, despite continuous transgene induction with doxycycline suggests that the multipeptide vaccination primed an anti-EGFR immune response that eliminated EGFR expressing cells. This is ultimately reflected in the dramatic reduction in tumor multiplicity, which is a function of tumor initiation. The close correlation between tumor multiplicity and EGFR expression (Figure 3B) affirms that the tumors in this model system are in fact driven by EGFR and suggests that vaccination eliminates these tumor cells. It should be noted that this work draws on two separate experimental runs conducted under identical experimental conditions (to the best of our knowledge and the degree possible) due to limits in the availability of transgenic mice. Although this is not ideal, decreased tumorigenesis in each study and the pooled data provides additional confidence that the preventive efficacy that we observed is repeatable.
We present a proof of concept study indicating that vaccination against a known tumor antigen is capable of inhibiting tumorigenesis driven by that antigen. In view of the advent of molecular medicine, it has become increasingly feasible to successfully identify tumor specific antigens, including tumor specific antigens that play an important role early in the tumorigenic process. Although EGFR mutant protein is a driver in approximately 10% of US lung cancer cases, it is a much larger contributor to lung cancer cases around the world, with some studies indicating that over 50% of non-small cell lung cancer cases in East Asia exhibit EGFR mutations [2]. In addition, a large percentage of non-EGFR mutant lung cancer cases also overexpress EGFR, which represents another group where vaccinating against wild type, rather than mutant EGFR could be applicable [13]. From a clinical perspective, this approach could have immediate utility in at-risk populations that can be identified within these subgroups.
The rationale that underpins prophylactic vaccination of high-risk groups may also have implications in the prevention of disease recurrence. The central hypothesis that context— specifically timing—matters in anti-tumor vaccination is significantly bolstered by this work. In minimal residual disease states that occur after treatment of patients with EGFR-mutant disease with targeted agents, vaccination could be a powerful adjunctive treatment that could conceivably prevent the outgrowth of resistant disease and make responses to targeted agents durable, based on the reasoning that low levels of tumor-mediated immune suppression create an environment in which vaccination can effectively function.
More importantly, this work provides a blueprint for future studies using tumor antigens as targets for vaccination in pre-clinical transgenic models for the purpose of tumor prevention. Conceivably, certain risk groups could be targeted in the future with vaccines designed against antigens that match their risk profiles. For patients of East Asian descent, such a vaccine may include peptides derived from EGFR. Significant additional work will be required to unlock the clinical potential of these approaches; however, this study reveals that in the instance of lung cancer, prophylactic vaccination against tumor oncoproteins is a strategy that is efficacious at the pre-clinical level.
Acknowledgements
We thank Drs. Matthew Riese, MD, PhD, Bryon Johnson, PhD and Michael James, PhD as well as Yongik Lee and Rachel Lieberman for their critical review of this manuscript. We also acknowledge Matthew Runquist of the MCW Small Animal Imaging Core and the histology core at the Children’s Research Institute, Milwaukee, WI for their assistance. We also thank Erin Geissler and Dr. Riese for their assistance with the flow cytometry assays described in this report, as well as Drs. Katerini Politi, William Pao and the National Cancer Institute Mouse Repository for the generous gift of EGFR L858R mice that made this work possible.
Grant Support: M.L. Disis and E Gad were supported by NCI contract HHSN261200433001C, the Athena Distinguished Professorship of Breast Cancer Research, and DOD Breast Cancer Program # W81XWH-11–1-0760. M You and J Ebben were supported by NCI contract HHSN261201200013I.
ABBREVIATIONS
- CCSP
Clara Cell Secreted Protein
- EGFR
Epidermal Growth Factor Receptor
- EGF
Epidermal Growth Factor
- ELISpot
Enzyme-Linked Immunosorbent Spot Assay
- MRI
Magnetic Resonance Imaging
- MUC-1
Mucin-1
- NSCLC
Non-small cell lung cancer
- ELISpot
Enzyme-Linked Immunosorbent Spot Assay
Footnotes
Authors’ Contributions
Conception and design: J Ebben, R.A. Lubet, M You
Development of methodology: E Gad, M.L. Disis, J Ebben
Acquisition of data: J Ebben
Analysis and interpretation of data: J Ebben, R.A. Lubet, M You, M.L. Disis
Writing, review and/or revision of the manuscript: J Ebben, R.A. Lubet, E Gad, M.L. Disis, M You
Study Supervision: M You, R.A. Lubet, M.L. Disis
Conflicts of Interest Dr. Mary L. Disis is a stockholder in Epithany, a vaccine-related company. She is also an inventor on a number of patents related to peptide vaccines.
References
- 1.Lehto RH, Lung cancer screening guidelines. The nurse's role in patient education and advocacy. Clin J Oncol Nurs, 2014. 18(3): p. 338–42. [DOI] [PubMed] [Google Scholar]
- 2.Shi Y, et al. , A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol, 2014. 9(2): p. 154–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwartz AG and Ruckdeschel JC, Familial lung cancer: genetic susceptibility and relationship to chronic obstructive pulmonary disease. Am J Respir Crit Care Med, 2006. 173(1): p. 16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Putila J and Guo NL, Combining COPD with Clinical, Pathological and Demographic Information Refines Prognosis and Treatment Response Prediction of Non-Small Cell Lung Cancer. PLoS One, 2014. 9(6): p. e100994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hystad P, et al. , Long-term residential exposure to air pollution and lung cancer risk. Epidemiology, 2013. 24(5): p. 762–72. [DOI] [PubMed] [Google Scholar]
- 6.Raaschou-Nielsen O, et al. , Lung cancer incidence and long-term exposure to air pollution from traffic. Environ Health Perspect, 2011. 119(6): p. 860–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lubin JH, Studies of radon and lung cancer in North America and China. Radiat Prot Dosimetry, 2003. 104(4): p. 315–9. [DOI] [PubMed] [Google Scholar]
- 8.Lollini PL, et al. , Vaccines for tumour prevention. Nat Rev Cancer, 2006. 6(3): p. 204–16. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez PC, et al. , Clinical development and perspectives of CIMAvax EGF, Cuban vaccine for non-small-cell lung cancer therapy. MEDICC Rev, 2010. 12(1): p. 17–23. [DOI] [PubMed] [Google Scholar]
- 10.Forde PM, Kelly RJ, and Brahmer JR, New strategies in lung cancer: translating immunotherapy into clinical practice. Clin Cancer Res, 2014. 20(5): p. 1067–73. [DOI] [PubMed] [Google Scholar]
- 11.Brunsvig PF, et al. , Telomerase peptide vaccination in NSCLC: a phase II trial in stage III patients vaccinated after chemoradiotherapy and an 8-year update on a phase I/II trial. Clin Cancer Res, 2011. 17(21): p. 6847–57. [DOI] [PubMed] [Google Scholar]
- 12.Szyszka-Barth K, et al. , Actual status of therapeutic vaccination in non-small cell lung cancer. Contemp Oncol (Pozn), 2014. 18(2): p. 77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bethune G, et al. , Epidermal growth factor receptor (EGFR) in lung cancer: an overview and update. J Thorac Dis, 2010. 2(1): p. 48–51. [PMC free article] [PubMed] [Google Scholar]
- 14.Politi K, et al. , Lung adenocarcinomas induced in mice by mutant EGF receptors found in human lung cancers respond to a tyrosine kinase inhibitor or to down-regulation of the receptors. Genes Dev, 2006. 20(11): p. 1496–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Billiau A and Matthys P, Modes of action of Freund's adjuvants in experimental models of autoimmune diseases. J Leukoc Biol, 2001. 70(6): p. 849–60. [PubMed] [Google Scholar]
- 16.Park KH, et al. , Insulin-like growth factor-binding protein-2 is a target for the immunomodulation of breast cancer. Cancer Res, 2008. 68(20): p. 8400–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krupnick AS, et al. , Quantitative monitoring of mouse lung tumors by magnetic resonance imaging. Nat Protoc, 2012. 7(1): p. 128–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee CK, et al. , Impact of EGFR inhibitor in non-small cell lung cancer on progression-free and overall survival: a meta-analysis. J Natl Cancer Inst, 2013. 105(9): p. 595–605. [DOI] [PubMed] [Google Scholar]
- 19.Akbay EA, et al. , Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov, 2013. 3(12): p. 1355–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baxevanis CN, et al. , HER-2/neu as a target for cancer vaccines. Immunotherapy, 2010. 2(2): p. 213–26. [DOI] [PubMed] [Google Scholar]
- 21.Inokuma M, et al. , Functional T cell responses to tumor antigens in breast cancer patients have a distinct phenotype and cytokine signature. J Immunol, 2007. 179(4): p. 2627–33. [DOI] [PubMed] [Google Scholar]
- 22.Stockert E, et al. , A survey of the humoral immune response of cancer patients to a panel of human tumor antigens. J Exp Med, 1998. 187(8): p. 1349–54. [DOI] [PMC free article] [PubMed] [Google Scholar]