Abstract
Animal models are essential for understanding the biological factors that contribute to drug and alcohol addiction and discovering new pharmacotherapies to treat these disorders. Alcohol (ethanol) is the most commonly abused drug in the world, and as the prevalence of alcohol use disorder (AUD) increases, so does the need for effective pharmacotherapies. In particular, treatments with high efficacy in the growing number of female AUD sufferers are needed. Female animals remain underrepresented in biomedical research and sex differences in the brain’s response to alcohol are poorly understood. To help bridge the gender gap in addiction research, this review discusses strategies that researchers can use to examine sex differences in the context of several common animal models of AUD. Self-administration, two-bottle choice, drinking in the dark, and conditioned place preference are discussed, with a focus on the role of estrogen as a mediator of sex differences in alcohol-related behaviors.
Keywords: addiction, alcohol, ethanol, estradiol, estrogen, female
Animal models are an essential component of biomedical research. They are often our best tool for understanding biological phenomena and are routinely employed in the development of pharmacological treatments for disease. In recent years, the utility of animal models in translational research has been called into question1, 2 because of issues with between-study reproducibility in preclinical research and the fact that only 10-20% of new therapies show efficacy in clinical trials, despite prior success in animal studies1, 3. While this has led some to question the validity of animal models altogether, the consensus is that improved study design will greatly enhance the reproducibility and translational value of animal studies in the biomedical sciences2-4. One crucial and long-overlooked aspect of experimental design is the sex of laboratory animals—specifically, the need to include both males and females5-7.
Females are severely underrepresented in biomedical research6, 8. Since most available prescription medications were developed exclusively in male animals, it is not surprising that women experience higher incidence of adverse drug events9, 10 and tend to have poorer health outcomes compared with men11. Although sociological factors such as financial resources, living conditions, and access to care play an important role in health outcomes, biological factors should not be overlooked11.
One particularly pressing need is the development of new therapies for the treatment of drug addiction, a chronic, relapsing condition characterized by compulsive drug seeking, difficulty limiting drug intake, and the emergence of a “negative emotional state (e.g., dysphoria, anxiety, irritability) reflecting a motivational withdrawal syndrome when access to the drug is prevented”12. For some individuals, recreational use of alcohol and/or other drugs can lead to both acute and chronic health problems, as well as social problems, drug tolerance, craving, and withdrawal, and/or repeated, unsuccessful attempts to quit or otherwise control drug use. If a person meets some of these criteria, as listed in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), that individual is considered to have a substance use disorder (SUD)13.
Incidence of drug overdose and related mortality in the United States have more than doubled since the year 200014, and the National Survey on Drug Use and Health (NSDUH) reported that 20.2 million Americans aged 18 or older met the criteria for SUD15 in 2014. Of those, nearly 81% had an alcohol use disorder (AUD), making alcohol by far the most commonly abused drug in the U.S. Although there are currently three FDA-approved pharmacotherapies used to treat AUD (disulfuram, naltrexone, and acamprosate), they are not universally effective, demonstrating the need to identify new targets and compounds to treat this disorder. In order to develop the most effective therapies for those suffering from addiction, both male and female animals must be tested. This review will discuss the most common animal models used to study AUD and methods that addiction researchers can use to examine sex differences in these models. We will focus on studies that illustrate sex differences in behaviors related to AUD with particular emphasis on the role of estrogen as a mediator of such differences. While progesterone and testosterone are also known to influence some measures of AUD-like behavior, discussion of these is beyond the scope of this review.
Sex Differences in AUD
As of 2015, the lifetime prevalence of AUD in American women was just under 23%, compared to 36% in men16. Across the globe, men consume significantly more alcohol than women, drink more often, and are more likely to be heavy drinkers16, 17. Women are more likely to abstain from alcohol use altogether and have a lower overall risk of developing AUD16, 17. The reasons for this gender gap in alcohol use (and misuse) are complex, as drinking behavior is heavily influenced by cultural and socio-economic factors18-20. Recent cohort analyses demonstrate that this gap is rapidly closing, with younger generations of women consuming more alcohol and having a higher incidence of AUD than women in previous generations 17, 21-23. In light of these data, it seems unlikely that women are simply less prone to AUD than men by virtue of biological sex. In fact, women who develop AUD (and SUD in general) tend to exhibit a so-called “telescoping” pattern of addiction24-26. These women progress more rapidly from initiation of substance use to onset of physical and psychological health complications and, despite seeking treatment sooner, tend to report equal or more severe symptoms of dependence at the time of treatment entry than those reported by male users24. This is particularly concerning since the physiological effects of alcohol abuse are more severe in females than in males27. Women develop comparable or more pronounced alcohol-related liver and cardiovascular disease at lower levels of alcohol consumption than their male counterparts and are also more vulnerable to alcoholic brain damage and related cognitive impairment 27.
Psychological reasons for alcohol use also differ between the sexes. Notably, women are more likely than men to engage in heavy alcohol use as a way to alleviate psychological distress, and female alcoholics are more likely to cite negative emotions and stressful life experiences as reasons for substance use and relapse28-30. Women who binge drink—defined as the consumption of any quantity of alcohol that generates a blood ethanol concentration (BEC) of 80 mg/dL or greater, usually ≥4 standard drinks for a woman or ≥5 standard drinks for a man31—report more mentally unhealthy days (dealing with stress, depression, and emotional problems) and physically unhealthy days than their male counterparts at both low (≥4 drinks) and high (≥7 drinks) intensities of binge drinking32. Adolescent girls take longer to recover from high-dose drinking than boys do, experiencing negative affective states for longer periods after heavy drinking episodes33. Women with AUD are also more likely to have comorbid psychiatric disorders, particularly anxiety and/or depression.30, 34 On the other hand, men tend to report drinking to enhance positive emotions or in response to peer pressure, and male alcoholics are more likely to cite external temptations as reasons for relapse28-30. This is not to say that women do not enjoy the experience of alcohol intoxication or that men never drink to alleviate negative affective states. In fact, the subjective alcohol experience seems to be quite similar between men and women, and some have even reported higher ratings of mental and physical wellbeing (“feeling good”) in females than in males who were given alcohol in a laboratory setting35. Furthermore, while women suffering from AUD are more likely to have a comorbid mood disorder, men certainly experience anxiety and depressive disorders in conjunction with AUD, especially in vulnerable populations such as veterans of military service36-38.
Use of oral contraceptives containing estrogens is positively correlated with increased ethanol intake, especially if contraceptive use begins at an early age (< 20 years)39, and increased serum levels of 17β-estradiol (E2), the primary circulating form of estrogen, have been associated with higher levels of ethanol consumption in premenopausal women40, 41. Some researchers have also reported subtle differences in subjective response to ethanol across the menstrual cycle. For example, increases in negative mood during the luteal phase are more pronounced in women with a family history of alcoholism (a prominent risk factor for AUD development42), particularly after drinking ethanol43. Most studies have been unable to detect subjective differences in ethanol response across menstrual cycle phase, however, perhaps due to the confounding effects of expectation and learned associations from previous ethanol experience44.
Sex Differences Defined
When designing experiments to study sex differences in the laboratory, it is useful to have an understanding of the types of sex differences that one may find in nature. Two excellent articles have put forth some useful terms and definitions45, 46. McCarthy et al. describe three basic ways to categorize differences between males and females: sexual dimorphism, sex differences, and sex convergence/divergence45. Examples of sexual dimorphism (referred to as qualitative sex differences by Becker and Koob46) include sex-specific copulatory behavior and courtship displays. In these cases, the behavioral or physiological measure has two distinct forms, such as lordosis in the female and mounting/intromission in the male. The term sex differences (or quantitative differences46) applies when a behavioral or physiological measure exists on a continuum, present in both sexes to varying degrees. Examples of this second type include: pain thresholds, food preferences and intake, baseline anxiety levels, stress responses, and responses to various drugs of abuse45. The term sex convergence or divergence refers to situations when the endpoint manifests in the same way/to the same degree in males and females but is brought about by different biological mechanisms in one sex versus the other, such as pair bonding in prairie voles, which is mediated by different neural mechanisms in females than in males47. Finally, Becker and Koob include a fourth category, population differences, in which the incidence or distribution of individual traits varies between males and females. An example of this kind of difference would be the relatively greater frequency of AUD in human males than in females.
Sex differences are primarily brought about by organizational and/or activational effects of sex hormones. The traditional theory of sexual differentiation, both of the brain and of other bodily tissues, revolves around the notion that sex genotype (XX vs. XY) guides embryonic differentiation of the gonads (testes or ovaries), which then produce hormones that organize bodily tissues into male- or female-typical patterns of development48, 49. According to this theory, the appropriately organized brain of an individual will be activated by gonadal hormones later in life (i.e. after puberty) and respond by producing either male- or female-typical patterns of behavior48, 49. In the simplest of terms, this theory is a fairly accurate description of the sexual differentiation process. However, we now understand that the endpoints of sexual differentiation are determined by numerous factors, including genes on the X and Y chromosomes that may promote sex differences independently of the sex hormones 49-51. It is important to remember that, though gonadal hormones are certainly crucial mediators of the process, they are not the only factors influencing sexual differentiation in either humans or laboratory animals.
Determining if Sex Differences Exist
The simplest way to look for sex differences in an animal model is to compare gonadally intact males and females across measures of interest. Many researchers assume that any study of sex differences must involve tracking the female estrous or menstrual cycle (in rodents and non-human primates, respectively) and looking for effects of ovarian hormones48. It is true that many traits, both behavioral and physiological, can be influenced by the cyclic hormone fluctuations experienced by females of reproductive age. However, recently published meta-analyses demonstrate that female rats and mice are not more inherently variable than males across a range of measures52, 53. Therefore, it is often unnecessary to track estrous or menstrual cycle when comparing males and females in the laboratory solely to determine if there is a sex difference in a particular measure. That said, tracking the estrous cycle in rats and mice, which are commonly used to model a wide range of disease states (including AUD), is quite simple and inexpensive54, 55. It is useful to have such data available for analysis, especially if sex differences are known to exist in the parameters being studied.
Determining if Sex Hormones Are Responsible for Sex Differences
Once a sex difference has been discovered by a straightforward male-female comparison, researchers can then determine what factors may be responsible for the difference observed (Figure 1). The most obvious factors are sex hormones, such as the estrogens and progesterone produced by the ovaries or the androgens (e.g. testosterone) produced by the testes. To determine if sex hormones are responsible for sex differences, two complementary methods are used. The first is to examine the reproductive cycle in females and the second is to remove the gonads from males and females, known as gonadectomy (GDX).
Figure 1.
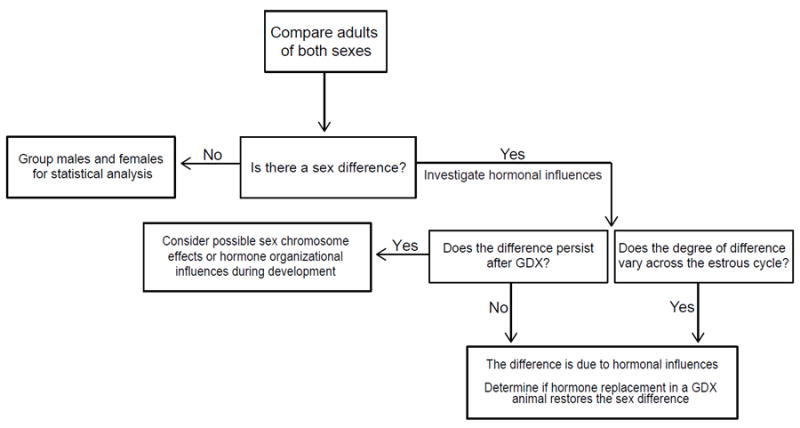
Flow chart showing the process for studying sex differences in behavioral or physiological measures in rodents. The starting point is to examine first if there are sex differences by comparing adult animals and then investigating in more detail if hormones, sex chromosome complement, or organizational differences during development are responsible for sex differences in a particular measure.
In rats and mice, each of the four phases of the estrous cycle (proestrus, estrus, metestrus, and diestrus) is associated with distinctive changes in vaginal cell types (nucleated epithelial, cornified epithelial, and leukocytes, respectively). Therefore, cycle phase can be determined by analyzing vaginal cellular content by light microscopy 54, 55. This is easily done using readily available supplies: cotton swabs or fine-tipped plastic pipettes, water or saline solution, and microscope slides. The vagina is either gently swabbed with a pointed, moistened cotton applicator tip and the collected cells smeared onto pre-cleaned glass microscope slides, or a fine-tipped disposable pipette is used to flush the vagina with saline solution that is then transferred to microscope slides for viewing. By collecting vaginal samples over a period of several days and examining cell types, estrous cycle phase can be determined for a given day. Correlations can then be made between cycle phase and the behavior (or other parameter) measured on that day. This method has pros and cons. It is affordable and easy to perform, requiring neither specialized equipment nor intensive training. On the other hand, it can be difficult to draw conclusions from estrous cycle data for a number of reasons. There is individual variation in the length of the reproductive cycle in female mice and rats, especially in young adult mice, making it difficult to coordinate experiments so that all the animals are in the desired cycle phase when measuring parameters of interest. An alternative method is to synchronize the estrous cycle by treating animals with a gonadotropin-releasing hormone (GnRH) receptor agonist, which regulates estrous cycle progression by inducing release of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) from the anterior pituitary56. This ensures that animals will be in the same cycle phase on a given day, up to about 4 days (the length of a “standard” estrous cycle in mice and rats). For shorter experiments, this method is useful. However, there can still be individual variability in circulating levels of ovarian hormones and animals will eventually diverge in their estrous cycle phase over time.
In some cases, researchers may want to control hormone levels in their experimental animals. This allows for a more direct determination of whether specific hormones are responsible for the phenotype of interest. GDX allows researchers to control circulating levels of androgens, progesterone, and estrogens by surgically removing the primary endogenous source of sex hormones (i.e. testes or ovaries). While this does not eliminate local hormone synthesis (for instance in the brain), it does remove circulating hormones. GDX animals can then be compared to intact controls and/or those treated with hormone receptor ligands—either exogenous forms of naturally occurring hormones or synthetic analogues that are selective for receptor(s) of interest. One challenge associated with this kind of experimental design is choosing the correct type, treatment schedule, delivery method, and dose of ligand(s). For example, E2 has been administered exogenously in numerous behavioral studies. Optimally, E2 treatments would result in physiological levels of circulating E2, to approximate hormone levels in the intact animal. However, E2 levels in mice are very low and are thus difficult to measure accurately. E2 peaks at ~8 pg/mL in mice during proestrus, compared with ~35 pg/mL in rats at this stage57. The lower limit of detection of commercially available E2 immunoassay kits ranges from 2.5-9 pg/ml E2 58. The gold standard method for measuring E2 levels is gas chromatography-tandem mass spectrometry, but this method is more expensive and requires specialized equipment that may not be readily available. Many studies have used different methods to administer E2 with doses ranging from near-physiological to extremely supraphysiological, and research shows that the dose, administration method, and length of time between GDX and the start of hormone replacement can all impact the outcome of behavioral studies59-61. Therefore, these factors must be considered carefully when designing experiments involving hormone replacement.
Determining if Sex Chromosomes Are Responsible for Sex Differences
As discussed previously, sex hormones are not the only factors that cause sex differences49. One useful tool for researchers who wish to dissociate the effects of sex chromosome genes from the effects of gonadal hormones is the four core genotypes (FCG) mouse model51, 62, 63. This model separates animals into four “core genotypes”—XX animals with female-typical gonads (ovaries), XX animals with male-typical gonads (testes), XY animals with female-typical gonads, and XY animals with male-typical gonads—by moving the sex-determining region (Sry) of the Y chromosome to an autosomal chromosome. Since Sry causes masculinization of the genitalia, this helps researchers determine which sex differences result from gonadal hormones and which are produced by genes on the X and Y chromosomes. Barker et al. used these mice to examine sex differences in ethanol consumption and habit formation in the form of operant responding64. In the absence of reinforcement (no ethanol received) or in the case of reinforcer devaluation (ethanol adulterated with lithium chloride) sex chromosome complement, not gonadal phenotype, determined levels of habit-like nose poke responding. On the other hand, voluntary ethanol consumption was determined by gonadal phenotype, with gonadal females consuming more than gonadal males, consistent with findings from other rodent models.
Modeling Behaviors Related to AUD in Animals
The addiction cycle is conceptualized as having three stages: binge/intoxication, withdrawal/negative affect, and preoccupation/anticipation 65. The interplay between positive and negative reinforcement—in the forms of achieving the drug “high” and alleviating aversive withdrawal symptoms, respectively—is thought to drive this cycle. While it is impossible to capture every aspect of human addiction behavior in an animal model, there are several useful ways to model various stages of the addiction cycle. Many of the studies comparing sexes in animal models have involved behavioral tests that model the binge/intoxication or preoccupation/anticipation stage. These include operant self-administration, the conditioned place preference (CPP) test, and voluntary ethanol drinking behavior. Figure 2 illustrates these behavioral tests.
Figure 2.
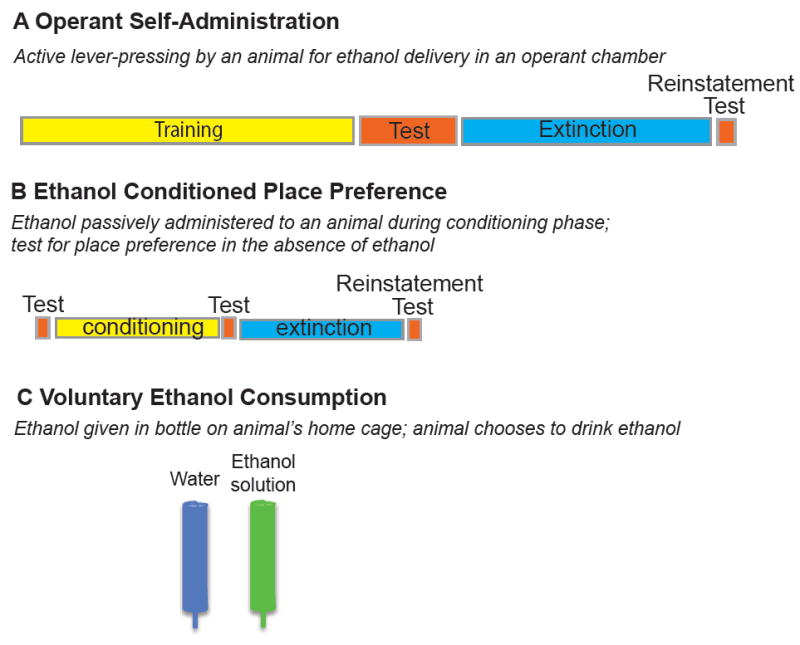
Behavioral tests used in rats and mice to model aspects of alcohol use disorder. (A) Operant self-administration test, in which animals are trained to press a lever to obtain access to an ethanol solution. This test consists of the training phase, in which animals learn to press the lever for ethanol delivery, followed by the testing phase, in which they actively press the lever to obtain ethanol. In the extinction phase, animals press the lever but are not rewarded by ethanol delivery, so they learn to stop pressing the lever. The reinstatement test measures relapse-like behavior and is triggered by a priming injection of ethanol, cues that predict ethanol delivery, or by stress. Animals will begin to actively press the lever again in expectation of obtaining the ethanol reward. (B) The conditioned place preference (CPP) test, which measures the rewarding properties of ethanol. In this associative conditioning experiment, animals are first tested for preference for a particular context. They are then conditioned with injections of ethanol in one context, or saline in a different context. Animals learn to associate the ethanol injection with the context. During the post-conditioning test, animals will spend more time in the ethanol-paired context in the absence of ethanol. Similar to the operant self-administration test, extinction is done by placing the animals back into the context but not giving the ethanol injection. Reinstatement is induced by an ethanol injection, exposure to an ethanol cue, or by a stressor. (C) Voluntary ethanol consumption, which measures the amount of ethanol that an animal drinks in the home cage. Generally, a choice is given between ethanol and water over a 24-hour period, but one bottle limited access procedures such as drinking in the dark are also used to measure binge-like ethanol drinking. Ethanol concentrations can range from 3-20%.
Operant Self-Administration
Self-administration is one of the methods used to study addiction-like behavior that most closely models the motivation to consume drugs. This method emphasizes the action of drugs of abuse as positive reinforcers, meaning that if an animal receives a dose of drug after performing a certain action (e.g. pressing a lever), then the animal is more likely to perform that action again 66. The most common routes of drug administration in these studies are intravenous and oral, but many other routes are possible, including: intracerebroventricular, intracranial, inhalation, intragastric, and intramuscular66.
Most studies conducted in non-human primates rely on self-administration techniques, although ad libitum drinking is also common67. In primates, self-administration has typically been done intravenously (i.v.), intragastrically (directly into the stomach), or through a tube from which the animals are able to consume the alcohol by mouth (p.o)67. Since ethanol naïve primates will generally drink only small quantities of alcohol, p.o. self-administration generally requires some kind of induction procedure (e.g. flavoring the alcohol with palatable fruit juice). The earliest published study on alcohol consumption in non-human primates was conducted in two rhesus monkeys (one male and one female) in 196068. While monkeys are the most common type of non-human primate used in alcohol research, at least one other study from the 1960s examined alcohol drinking behavior in great apes (chimpanzees and orangutans)69. In this study, males of both species drank more than females, on average—though the range of individual differences in quantity of alcohol consumed was large. It has been noted that such variability in baseline alcohol consumption is common among non-human primates, which can prove useful in translational research by allowing for study of risk factors that make certain individuals more susceptible than others to heavy/risky drinking behavior67. In general, sex differences in alcohol drinking among non-human primates are similar to humans, though very few primate studies have examined sex differences67. When given long-term, unlimited access to alcohol, male cynomolgus monkeys drink more than females and attain higher blood alcohol levels70. Male rhesus monkeys also drink more than females of their species when given limited access to sweetened ethanol solution71. Furthermore, drinking in female monkeys may be influenced by ovarian hormones, as female macaque monkeys self-administered significantly more alcohol at mid-cycle, when circulating E2 levels are high, than during menstruation, when E2 levels are low72.
In contrast to what has been observed in human and non-human primates, female rodents tend to consume more alcohol than males across a range of measures46, 73-75. Operant ethanol self-administration generally uses oral alcohol delivery and is most often performed in rats because they are easier to train, although one study found that it is possible to induce operant responding for ethanol vapor in male C57BL/6J mice76. Oral consumption is generally preferred in alcohol studies because this is the route of administration used by humans. Rats will not readily consume unsweetened alcohol (except in strains selectively bred for high alcohol consumption, such as the alcohol preferring “P” rats, or in rats that have been made dependent on ethanol), so sucrose fading procedures are used to induce alcohol drinking, similar to the induction procedures used in non-human primates77.
Moore and Lynch found that female “P” rats administered more alcohol than males during the first 10 days of testing, but males increased responding to levels that equaled female self-administration after the initial 10-day period78. More recently, Priddy et al. found that while females drank more alcohol than males when given access to alcohol in their home cages, no sex differences in consumption were found under operant conditions in either Wistar or Long-Evans rats75. Others have reported higher levels of operant responding for alcohol by females79, 80. Although Randall et al. reported that male Long-Evans rats tend to have greater numbers of alcohol-reinforced responses throughout self-administration training, females show similar or greater alcohol intake after correcting for differences in body weight81. After a period of forced abstinence or extinction, Male Long-Evans rats showed greater reinstatement (i.e. relapse) responding to alcohol cues than females81, but in another set of studies in Sprague Dawley rats, females responded more for alcohol in response to the combination of cues and a stressor79, 80. Increased stress plus cue-induced reinstatement of alcohol seeking in females is consistent with evidence showing that women are more likely than men to relapse to drinking in response to stress30.
A small number of studies have also looked for estrous cycle effects on alcohol self-administration. Three studies found no effect of estrous cycle on operant responding for ethanol in freely cycling female rats 56, 75, 80, but Roberts et al. reported a modest effect of cycle phase in animals whose cycles were synchronized with a GnRH receptor agonist, with highest intake levels occurring in diestrus, when E2 levels are rising, suggesting that it may be possible to unmask estrous cycle effects under certain testing conditions56. Evidence that E2 promotes ethanol self-administration was recently demonstrated in ovariectomized (OVX) rats that had been treated for several weeks with E2. The E2-treated rats lever-pressed at a higher rate and drank more ethanol compared with control OVX and gonadally intact female rats80. Interestingly, in this same study, E2 treatment had no effect on the combination of cue- and stress-induced reinstatement of operant responding80, suggesting that the sex difference observed in reinstatement may primarily be driven by organizational and/or sex chromosome effects.
Conditioned Place Preference
Place conditioning tests are a well-established method of measuring the “rewarding” (pleasurable or appetitive) or aversive effects of a given stimulus in laboratory animals82-85. Place conditioning tests use a classical conditioning paradigm to form an association between a stimulus of interest (e.g. ethanol) and a contextually distinct environment. After the conditioning procedure, the subject animal can choose to spend time in or choose to avoid the stimulus-paired environment. If the animal chooses to spend more time in the stimulus-paired environment than it did before conditioning, then the stimulus is considered to be rewarding; this is called conditioned place preference (CPP). Conversely, if the animal chooses to avoid the stimulus-paired environment, the stimulus is described as aversive. In this case, the phenomenon would be called conditioned place aversion (CPA).
Mice and rats are the most commonly used animals in CPP testing, although some researchers have used zebrafish84. Establishing CPP for ethanol in male rats that have not been specifically bred for high alcohol consumption is difficult. The Wistar rat strain may be more amenable to developing ethanol CPP because several researchers have reported significant ethanol CPP in males of this strain86-90. Interestingly, however, obtaining ethanol CPP in male rats seems to depend on pretreatment with low-dose ethanol for an extended period (~15 days) before the start of the actual conditioning procedure, suggesting that a sensitization period is necessary for males to find alcohol rewarding in this test89. This is not commonly done for other drugs in the CPP test.
Very few studies have examined ethanol CPP in female rats, but Torres et al. demonstrated that female Wistar rats are more sensitive to ethanol reward than males across a range of doses91. In this study, which did not use a pre-conditioning sensitization period, neither adult nor adolescent males developed ethanol CPP. On the other hand, adult females developed CPP at both low (0.5 g/kg) and moderate (1.0 g/kg) doses of ethanol, and adolescent females developed CPP at the moderate dose91. When adult females were ovariectomized (OVX), their preference did not differ from males, suggesting that ovarian hormones play an important role in increasing ethanol reward in female rats. We have found that E2 treatment of OVX female mice enhances ethanol CPP (Hilderbrand and Lasek, manuscript under review).
In addition to its usefulness as a measure of drug reward, CPP, like operant self-administration, can also be used to assess vulnerability to a relapse-like state in animal models (Figure 2)92, known as reinstatement. A typical reinstatement model will begin with a normal CPP conditioning procedure, followed by a period of “extinction” during which the animal is exposed to the drug-paired environment in the absence of drug. CPP is extinguished when the time spent in the drug-paired environment is roughly equal to the time spent in that environment before the conditioning period. Reinstatement of CPP is induced by re-exposure to the drug or by exposure to a stressor. Little is known about sex differences in reinstatement of ethanol CPP, as few studies have tested for such differences, but the available data suggest that sex differences in this behavior do exist. For example, one study found that, while early adolescent female mice required higher doses of ethanol to induce CPP than early adolescent males, females continued to be responsive to ethanol reward into late adolescence93. Late adolescent males, on the other hand, did not develop ethanol CPP at any doses tested. This same study found that reinstatement of CPP occurred in early adolescent males and both early and late adolescent females.
Two-Bottle Choice Ethanol Consumption
The two-bottle choice test of ethanol consumption is a method of measuring voluntary ethanol drinking in the home cage and is one of the simplest tests to perform. In this test, animals are given access to two drinking bottles, one filled with normal drinking water and the other filled with an ethanol solution that ranges from 3-20%. As mentioned above, most rat strains do not readily consume alcohol without the addition of sweeteners, so sucrose or saccharin is generally used at the start of these experiments to encourage drinking. “Fading” procedures, in which the amount of sweetener is gradually decreased and alcohol concentration is gradually increased, can be used to transition rats to higher levels of alcohol consumption. Mice, especially the C57BL/6 inbred strain, will readily consume ethanol in this procedure without the addition of sweetener. For a comparison of two-bottle choice drinking behavior by different inbred mouse lines, see Belknap et al. and Yoneyama et al. 94, 95. By providing animals with a choice between water and alcohol, this method also allows researchers to measure preference for one liquid over the other. Typically, two-bottle choice studies in rats have been used to examine preference for alcohol over a 24-hour period. One drawback of this model is that animals tend to consume relatively low (sub-intoxicating) quantities of alcohol in two-bottle choice tests, unless strains specifically bred for high alcohol consumption (e.g. “P” rats) or ethanol-dependent animals are used. However, Long-Evans and Wistar rats will consume large amounts of ethanol without sucrose fading using a 24-hr intermittent access procedure pioneered by Wise in the 1970s96, 97.
Females tend to drink more than males and show higher preference for alcohol over water in two-bottle choice tests95, 98-107. Some studies have reported equal consumption between males and females, however78, 108, 109. This may be related to animal strain differences; CD (derived from Sprague-Dawley) or alcohol-preferring “P” rats were used in these studies, and strain is known to be an important determining factor of voluntary alcohol consumption in both rats and mice73, 95, 100. Some have reported different findings even within the same strain. Vetter-O’Hagan reported that adolescent males of the Sprague-Dawley strain consume more alcohol relative to their body weights than adolescent females and adults of both sexes, whereas adult females generally consume more than adult males110. In contrast, Lancaster et al. found that adolescent Sprague-Dawley females drank more than males, although their consumption decreased up until puberty101. However, it was noted by Lancaster et al. that the type of alcohol (beer vs. ethanol in water) and the delivery method (graduated drinking vial vs. standard water sipper) differed from other studies101. These inconsistencies likely explain the different results obtained from this study. After puberty, females drank more than males, especially when animals were exposed to stress by pair-feeding, suggesting that ovarian hormones may partly contribute higher levels of drinking in females. Marco et al. also found that female Wistar rats subjected to chronic mild stress (CMS), a widely accepted animal model for depression, showed significantly higher ethanol consumption and preference for ethanol over water, compared to CMS males111. This is consistent with results from other animal studies and with evidence from the human literature, which shows that women are more susceptible than men to stress-induced drinking behavior. Several studies have demonstrated that OVX, which depletes circulating ovarian hormones, reduces ethanol intake in female rats and mice to levels similar to those seen in males112-114. This effect is not universal, as others have reported unaltered ethanol consumption after OVX in females103, 115, 116. Several factors—such as the timing of OVX (adolescence vs. adulthood), strain of animal used, degree of ethanol availability (i.e. limited vs. continuous access), and correction for baseline levels of consumption prior to OVX—may explain these discrepancies. The hypothesis that ovarian hormones promote ethanol consumption in females is also supported by studies that have used supplemental E2 treatment in OVX animals. For example, Ford et al. demonstrated a positive correlation between E2 dose and ethanol consumption in the two-bottle choice test117.
The drinking in the dark (DID) test is a variation on the two-bottle choice procedure and models binge-like alcohol consumption118, 119. In this test, mice are given limited access (2-4 hours) to a single bottle of alcohol during the dark portion of the light-dark cycle. This is when mice, being nocturnal animals, are most likely to be awake and naturally engaging in feeding and drinking behavior. One advantage of this procedure over others (such as 24 hour two-bottle access) is that mice will routinely drink to intoxication and achieve blood EtOH concentrations (BECs) greater than 100 mg% 119. Because of the high levels of drinking and pharmacologically relevant BECs obtained by this test, DID has become a popular model in alcohol research and is commonly used to test for effects of genetic and pharmacological manipulations on binge-like drinking118. Similar to other alcohol consumption models, females consume more than males in the DID test73 and we recently found that E2 promotes higher levels of drinking in the DID test120.
CONCLUSIONS
Several studies have determined that sex differences in AUD exist during different phases of the addiction cycle. In humans, more men abuse alcohol than women, but the number of women with problematic drinking has increased in recent years. Women also experience higher comorbidity of mood disorders and AUD and may be more vulnerable to developing an AUD. Studies in rodents have demonstrated a potential role for E2 in promoting behaviors during the binge/intoxication phase of the addiction cycle, indicating that this hormone may be involved in increasing the risk for women to develop AUD. More preclinical and clinical studies are clearly needed to determine sex differences and the role of sex hormones in the withdrawal/negative affect and preoccupation/anticipation (“craving”) aspects of AUD so that effective pharmacotherapies can be developed to treat AUD in both sexes.
Acknowledgments
Funding
This work was supported by the National Institute on Alcohol Abuse and Alcoholism (grant numbers P50 AA022538 and U01 AA020912 to AWL, and F31 AA024344 to ERH) and the National Institute on Drug Abuse (R01 DA033429 to AWL).
Footnotes
Author contributions
E.R.H. and A.W.L wrote the manuscript.
The authors declare no conflicts of interest.
ORCID
0000-0002-7099-2442
References
- 1.van der Worp HB, Howells DW, Sena ES, Porritt MJ, Rewell S, O’Collins V, Macleod MR. Can animal models of disease reliably inform human studies? PLoS Med. 2010;7:e1000245. doi: 10.1371/journal.pmed.1000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collins FS, Tabak LA. Policy: NIH plans to enhance reproducibility. Nature. 2014;505:612–613. doi: 10.1038/505612a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perrin S. Preclinical research: Make mouse studies work. Nature. 2014;507:423–425. doi: 10.1038/507423a. [DOI] [PubMed] [Google Scholar]
- 4.Landis SC, Amara SG, Asadullah K, Austin CP, Blumenstein R, Bradley EW, Crystal RG, Darnell RB, Ferrante RJ, Fillit H, Finkelstein R, Fisher M, Gendelman HE, Golub RM, Goudreau JL, Gross RA, Gubitz AK, Hesterlee SE, Howells DW, Huguenard J, Kelner K, Koroshetz W, Krainc D, Lazic SE, Levine MS, Macleod MR, McCall JM, Moxley RT, 3rd, Narasimhan K, Noble LJ, Perrin S, Porter JD, Steward O, Unger E, Utz U, Silberberg SD. A call for transparent reporting to optimize the predictive value of preclinical research. Nature. 2012;490:187–191. doi: 10.1038/nature11556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beery AK, Zucker I. Sex bias in neuroscience and biomedical research. Neurosci Biobehav Rev. 2011;35:565–572. doi: 10.1016/j.neubiorev.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zucker I, Beery AK. Males still dominate animal studies. Nature. 2010;465:690. doi: 10.1038/465690a. [DOI] [PubMed] [Google Scholar]
- 7.Shansky RM, Woolley CS. Considering Sex as a Biological Variable Will Be Valuable for Neuroscience Research. J Neurosci. 2016;36:11817–11822. doi: 10.1523/JNEUROSCI.1390-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mazure CM, Jones DP. Twenty years and still counting: including women as participants and studying sex and gender in biomedical research. BMC Womens Health. 2015;15:94. doi: 10.1186/s12905-015-0251-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson GD. Gender differences in pharmacological response. Int Rev Neurobiol. 2008;83:1–10. doi: 10.1016/S0074-7742(08)00001-9. [DOI] [PubMed] [Google Scholar]
- 10.Miller MA. Gender-based differences in the toxicity of pharmaceuticals--the Food and Drug Administration’s perspective. Int J Toxicol. 2001;20:149–152. doi: 10.1080/109158101317097728. [DOI] [PubMed] [Google Scholar]
- 11.Vlassoff C. Gender differences in determinants and consequences of health and illness. J Health Popul Nutr. 2007;25:47–61. [PMC free article] [PubMed] [Google Scholar]
- 12.Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 2016;3:760–773. doi: 10.1016/S2215-0366(16)00104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hasin DS, O’Brien CP, Auriacombe M, Borges G, Bucholz K, Budney A, Compton WM, Crowley T, Ling W, Petry NM, Schuckit M, Grant BF. DSM-5 criteria for substance use disorders: recommendations and rationale. Am J Psychiatry. 2013;170:834–851. doi: 10.1176/appi.ajp.2013.12060782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rudd RA, Seth P, David F, Scholl L. Increases in Drug and Opioid-Involved Overdose Deaths - United States, 2010-2015. MMWR Morbidity and mortality weekly report. 2016;65:1445–1452. doi: 10.15585/mmwr.mm655051e1. [DOI] [PubMed] [Google Scholar]
- 15.Lipari RN, Van Horn SL. The CBHSQ Report. Rockville (MD): 2013. Trends in Substance Use Disorders Among Adults Aged 18 or Older. [PubMed] [Google Scholar]
- 16.Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, Pickering RP, Ruan WJ, Smith SM, Huang B, Hasin DS. Epidemiology of DSM-5 Alcohol Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72:757–766. doi: 10.1001/jamapsychiatry.2015.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilsnack SC. The GENACIS project: a review of findings and some implications for global needs in women-focused substance abuse prevention and intervention. Subst Abuse Rehabil. 2012;3:5–15. doi: 10.2147/SAR.S21343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holmila M, Raitasalo K. Gender differences in drinking: why do they still exist? Addiction. 2005;100:1763–1769. doi: 10.1111/j.1360-0443.2005.01249.x. [DOI] [PubMed] [Google Scholar]
- 19.Wells JE, Haro JM, Karam E, Lee S, Lepine JP, Medina-Mora ME, Nakane H, Posada J, Anthony JC, Cheng H, Degenhardt L, Angermeyer M, Bruffaerts R, de Girolamo G, de Graaf R, Glantz M, Gureje O. Cross-national comparisons of sex differences in opportunities to use alcohol or drugs, and the transitions to use. Subst Use Misuse. 2011;46:1169–1178. doi: 10.3109/10826084.2011.553659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Becker JB, McClellan M, Reed BG. Sociocultural context for sex differences in addiction. Addiction biology. 2016;21:1052–1059. doi: 10.1111/adb.12383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keyes KM, Grant BF, Hasin DS. Evidence for a closing gender gap in alcohol use, abuse, and dependence in the United States population. Drug and alcohol dependence. 2008;93:21–29. doi: 10.1016/j.drugalcdep.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colell E, Sanchez-Niubo A, Domingo-Salvany A. Sex differences in the cumulative incidence of substance use by birth cohort. Int J Drug Policy. 2013;24:319–325. doi: 10.1016/j.drugpo.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 23.White A, Castle IJ, Chen CM, Shirley M, Roach D, Hingson R. Converging Patterns of Alcohol Use and Related Outcomes Among Females and Males in the United States, 2002 to 2012. Alcoholism, clinical and experimental research. 2015;39:1712–1726. doi: 10.1111/acer.12815. [DOI] [PubMed] [Google Scholar]
- 24.Hernandez-Avila CA, Rounsaville BJ, Kranzler HR. Opioid-, cannabis- and alcohol-dependent women show more rapid progression to substance abuse treatment. Drug and alcohol dependence. 2004;74:265–272. doi: 10.1016/j.drugalcdep.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 25.Becker JB. Sex differences in addiction. Dialogues in clinical neuroscience. 2016;18:395–402. doi: 10.31887/DCNS.2016.18.4/jbecker. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agabio R, Pisanu C, Gessa GL, Franconi F. Sex Differences in Alcohol Use Disorder. Curr Med Chem. 2017;24:2661–2670. doi: 10.2174/0929867323666161202092908. [DOI] [PubMed] [Google Scholar]
- 27.Wilsnack SC, Wilsnack RW, Kantor LW. Focus on: women and the costs of alcohol use. Alcohol research : current reviews. 2013;35:219–228. [PMC free article] [PubMed] [Google Scholar]
- 28.Kerr-Correa F, Igami TZ, Hiroce V, Tucci AM. Patterns of alcohol use between genders: a cross-cultural evaluation. J Affect Disord. 2007;102:265–275. doi: 10.1016/j.jad.2006.09.031. [DOI] [PubMed] [Google Scholar]
- 29.Erol A, Karpyak VM. Sex and gender-related differences in alcohol use and its consequences: Contemporary knowledge and future research considerations. Drug and alcohol dependence. 2015;156:1–13. doi: 10.1016/j.drugalcdep.2015.08.023. [DOI] [PubMed] [Google Scholar]
- 30.Greenfield SF, Back SE, Lawson K, Brady KT. Substance abuse in women. Psychiatr Clin North Am. 2010;33:339–355. doi: 10.1016/j.psc.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.NIAAA council approves definition of binge drinking. Council NIoAAaAA NIAAA Newsletter 2004 [Google Scholar]
- 32.Wen XJ, Kanny D, Thompson WW, Okoro CA, Town M, Balluz LS. Binge drinking intensity and health-related quality of life among US adult binge drinkers. Preventing chronic disease. 2012;9:E86. doi: 10.5888/pcd9.110204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bekman NM, Winward JL, Lau LL, Wagner CC, Brown SA. The impact of adolescent binge drinking and sustained abstinence on affective state. Alcoholism, clinical and experimental research. 2013;37:1432–1439. doi: 10.1111/acer.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Conway KP, Compton W, Stinson FS, Grant BF. Lifetime comorbidity of DSM-IV mood and anxiety disorders and specific drug use disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2006;67:247–257. doi: 10.4088/jcp.v67n0211. [DOI] [PubMed] [Google Scholar]
- 35.McCance-Katz EF, Hart CL, Boyarsky B, Kosten T, Jatlow P. Gender effects following repeated administration of cocaine and alcohol in humans. Subst Use Misuse. 2005;40:511–528. doi: 10.1081/ja-200030693. [DOI] [PubMed] [Google Scholar]
- 36.Carter AC, Capone C, Short EE. Co-occurring Posttraumatic Stress Disorder and Alcohol Use Disorders in Veteran Populations. J Dual Diagn. 2011;7:285–299. doi: 10.1080/15504263.2011.620453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoon G, Petrakis IL, Rosenheck RA. Correlates of major depressive disorder with and without comorbid alcohol use disorder nationally in the veterans health administration. The American journal on addictions / American Academy of Psychiatrists in Alcoholism and Addictions. 2015;24:419–426. doi: 10.1111/ajad.12219. [DOI] [PubMed] [Google Scholar]
- 38.Fuehrlein BS, Mota N, Arias AJ, Trevisan LA, Kachadourian LK, Krystal JH, Southwick SM, Pietrzak RH. The burden of alcohol use disorders in US military veterans: results from the National Health and Resilience in Veterans Study. Addiction. 2016;111:1786–1794. doi: 10.1111/add.13423. [DOI] [PubMed] [Google Scholar]
- 39.Lund E, Jacobsen BK. Use of oral contraceptives in relation to dietary habits and alcohol consumption. Contraception. 1990;42:171–177. doi: 10.1016/0010-7824(90)90100-a. [DOI] [PubMed] [Google Scholar]
- 40.Muti P, Trevisan M, Micheli A, Krogh V, Bolelli G, Sciajno R, Schunemann HJ, Berrino F. Alcohol consumption and total estradiol in premenopausal women. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 1998;7:189–193. [PubMed] [Google Scholar]
- 41.Martin CA, Mainous AG, 3rd, Curry T, Martin D. Alcohol use in adolescent females: correlates with estradiol and testosterone. The American journal on addictions / American Academy of Psychiatrists in Alcoholism and Addictions. 1999;8:9–14. doi: 10.1080/105504999306036. [DOI] [PubMed] [Google Scholar]
- 42.Uhart M, Oswald L, McCaul ME, Chong R, Wand GS. Hormonal responses to psychological stress and family history of alcoholism. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2006;31:2255–2263. doi: 10.1038/sj.npp.1301063. [DOI] [PubMed] [Google Scholar]
- 43.Evans SM, Levin FR. Response to alcohol in women: role of the menstrual cycle and a family history of alcoholism. Drug and alcohol dependence. 2011;114:18–30. doi: 10.1016/j.drugalcdep.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Terner JM, de Wit H. Menstrual cycle phase and responses to drugs of abuse in humans. Drug and alcohol dependence. 2006;84:1–13. doi: 10.1016/j.drugalcdep.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 45.McCarthy MM, Arnold AP, Ball GF, Blaustein JD, De Vries GJ. Sex differences in the brain: the not so inconvenient truth. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:2241–2247. doi: 10.1523/JNEUROSCI.5372-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Becker JB, Koob GF. Sex Differences in Animal Models: Focus on Addiction. Pharmacological reviews. 2016;68:242–263. doi: 10.1124/pr.115.011163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hammock EA, Young LJ. Oxytocin, vasopressin and pair bonding: implications for autism. Philos Trans R Soc Lond B Biol Sci. 2006;361:2187–2198. doi: 10.1098/rstb.2006.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sanchis-Segura C, Becker JB. Why we should consider sex (and study sex differences) in addiction research. Addiction biology. 2016;21:995–1006. doi: 10.1111/adb.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arnold AP. The end of gonad-centric sex determination in mammals. Trends Genet. 2012;28:55–61. doi: 10.1016/j.tig.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arnold AP, Chen X, Link JC, Itoh Y, Reue K. Cell-autonomous sex determination outside of the gonad. Dev Dyn. 2013;242:371–379. doi: 10.1002/dvdy.23936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arnold AP, Chen X. What does the “four core genotypes” mouse model tell us about sex differences in the brain and other tissues? Frontiers in neuroendocrinology. 2009;30:1–9. doi: 10.1016/j.yfrne.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prendergast BJ, Onishi KG, Zucker I. Female mice liberated for inclusion in neuroscience and biomedical research. Neurosci Biobehav Rev. 2014;40:1–5. doi: 10.1016/j.neubiorev.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 53.Becker JB, Prendergast BJ, Liang JW. Female rats are not more variable than male rats: a meta-analysis of neuroscience studies. Biol Sex Differ. 2016;7:34. doi: 10.1186/s13293-016-0087-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Byers SL, Wiles MV, Dunn SL, Taft RA. Mouse estrous cycle identification tool and images. PloS one. 2012;7:e35538. doi: 10.1371/journal.pone.0035538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Caligioni CS. Assessing reproductive status/stages in mice. Current protocols in neuroscience / editorial board, Jacqueline N Crawley … [et al] Appendix 4. 2009 doi: 10.1002/0471142301.nsa04is48. Appendix 4I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roberts AJ, Smith AD, Weiss F, Rivier C, Koob GF. Estrous cycle effects on operant responding for ethanol in female rats. Alcoholism, clinical and experimental research. 1998;22:1564–1569. [PubMed] [Google Scholar]
- 57.Nilsson ME, Vandenput L, Tivesten A, Norlen AK, Lagerquist MK, Windahl SH, Borjesson AE, Farman HH, Poutanen M, Benrick A, Maliqueo M, Stener-Victorin E, Ryberg H, Ohlsson C. Measurement of a Comprehensive Sex Steroid Profile in Rodent Serum by High-Sensitive Gas Chromatography-Tandem Mass Spectrometry. Endocrinology. 2015;156:2492–2502. doi: 10.1210/en.2014-1890. [DOI] [PubMed] [Google Scholar]
- 58.Haisenleder DJ, Schoenfelder AH, Marcinko ES, Geddis LM, Marshall JC. Estimation of estradiol in mouse serum samples: evaluation of commercial estradiol immunoassays. Endocrinology. 2011;152:4443–4447. doi: 10.1210/en.2011-1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Strom JO, Theodorsson E, Theodorsson A. Order of magnitude differences between methods for maintaining physiological 17beta-oestradiol concentrations in ovariectomized rats. Scand J Clin Lab Invest. 2008;68:814–822. doi: 10.1080/00365510802409703. [DOI] [PubMed] [Google Scholar]
- 60.Strom JO, Theodorsson A, Ingberg E, Isaksson IM, Theodorsson E. Ovariectomy and 17beta-estradiol replacement in rats and mice: a visual demonstration. J Vis Exp. 2012:e4013. doi: 10.3791/4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mosquera L, Shepherd L, Torrado AI, Torres-Diaz YM, Miranda JD, Segarra AC. Comparison of Two Methods of Estradiol Replacement: their Physiological and Behavioral Outcomes. J Vet Sci Technol. 2015;6:276. doi: 10.4172/2157-7579.1000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.De Vries GJ, Rissman EF, Simerly RB, Yang LY, Scordalakes EM, Auger CJ, Swain A, Lovell-Badge R, Burgoyne PS, Arnold AP. A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:9005–9014. doi: 10.1523/JNEUROSCI.22-20-09005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cox KH, Bonthuis PJ, Rissman EF. Mouse model systems to study sex chromosome genes and behavior: relevance to humans. Frontiers in neuroendocrinology. 2014;35:405–419. doi: 10.1016/j.yfrne.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barker JM, Torregrossa MM, Arnold AP, Taylor JR. Dissociation of genetic and hormonal influences on sex differences in alcoholism-related behaviors. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:9140–9144. doi: 10.1523/JNEUROSCI.0548-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Koob GF. Addiction is a Reward Deficit and Stress Surfeit Disorder. Front Psychiatry. 2013;4:72. doi: 10.3389/fpsyt.2013.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lynch WJ, Nicholson KL, Dance ME, Morgan RW, Foley PL. Animal models of substance abuse and addiction: implications for science, animal welfare, and society. Comp Med. 2010;60:177–188. [PMC free article] [PubMed] [Google Scholar]
- 67.Grant KA, Bennett AJ. Advances in nonhuman primate alcohol abuse and alcoholism research. Pharmacol Ther. 2003;100:235–255. doi: 10.1016/j.pharmthera.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 68.Clark R, Polish E. Avoidance Conditioning and Alcohol Consumption in Rhesus Monkeys. Science. 1960;132:223–224. doi: 10.1126/science.132.3421.223. [DOI] [PubMed] [Google Scholar]
- 69.Fitz-Gerald FL, Barfield MA, Warrington RJ. Voluntary alcohol consumption in chimpanzees and orangutans. Q J Stud Alcohol. 1968;29:330–336. [PubMed] [Google Scholar]
- 70.Vivian JA, Green HL, Young JE, Majerksy LS, Thomas BW, Shively CA, Tobin JR, Nader MA, Grant KA. Induction and maintenance of ethanol self-administration in cynomolgus monkeys (Macaca fascicularis): long-term characterization of sex and individual differences. Alcoholism, clinical and experimental research. 2001;25:1087–1097. [PubMed] [Google Scholar]
- 71.Fahlke C, Lorenz JG, Long J, Champoux M, Suomi SJ, Higley JD. Rearing experiences and stress-induced plasma cortisol as early risk factors for excessive alcohol consumption in nonhuman primates. Alcoholism, clinical and experimental research. 2000;24:644–650. [PubMed] [Google Scholar]
- 72.Mello NK, Bree MP, Mendelson JH. Alcohol and food self-administration by female Macaque monkeys as a function of menstrual cycle phase. Physiology & behavior. 1986;36:959–966. doi: 10.1016/0031-9384(86)90460-9. [DOI] [PubMed] [Google Scholar]
- 73.Rhodes JS, Ford MM, Yu CH, Brown LL, Finn DA, Garland T, Jr, Crabbe JC. Mouse inbred strain differences in ethanol drinking to intoxication. Genes, brain, and behavior. 2007;6:1–18. doi: 10.1111/j.1601-183X.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- 74.Jury NJ, DiBerto JF, Kash TL, Holmes A. Sex differences in the behavioral sequelae of chronic ethanol exposure. Alcohol. 2017;58:53–60. doi: 10.1016/j.alcohol.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Priddy BM, Carmack SA, Thomas LC, Vendruscolo JC, Koob GF, Vendruscolo LF. Sex, strain, and estrous cycle influences on alcohol drinking in rats. Pharmacology, biochemistry, and behavior. 2017;152:61–67. doi: 10.1016/j.pbb.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kantak KM, Luzzo C. Ethanol vapor self-administration in adult C57BL/6J male mice. Drug and alcohol dependence. 2007;86:123–131. doi: 10.1016/j.drugalcdep.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 77.Becker JB, Hu M. Sex differences in drug abuse. Frontiers in neuroendocrinology. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Moore CF, Lynch WJ. Alcohol preferring (P) rats as a model for examining sex differences in alcohol use disorder and its treatment. Pharmacology, biochemistry, and behavior. 2015;132:1–9. doi: 10.1016/j.pbb.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bertholomey ML, Nagarajan V, Torregrossa MM. Sex differences in reinstatement of alcohol seeking in response to cues and yohimbine in rats with and without a history of adolescent corticosterone exposure. Psychopharmacology. 2016;233:2277–2287. doi: 10.1007/s00213-016-4278-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bertholomey ML, Torregrossa MM. Gonadal hormones affect alcohol drinking, but not cue+yohimbine-induced alcohol seeking, in male and female rats. Physiology & behavior. 2017 doi: 10.1016/j.physbeh.2017.10.025. published online Oct 26, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Randall PA, Stewart RT, Besheer J. Sex differences in alcohol self-administration and relapse-like behavior in Long-Evans rats. Pharmacology, biochemistry, and behavior. 2017;156:1–9. doi: 10.1016/j.pbb.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Prus AJ, James JR, Rosecrans JA. Conditioned Place Preference. In: Buccafusco JJ, editor. Methods of Behavior Analysis in Neuroscience. 2. Boca Raton (FL): 2009. [Google Scholar]
- 83.Cunningham CL, Gremel CM, Groblewski PA. Drug-induced conditioned place preference and aversion in mice. Nat Protoc. 2006;1:1662–1670. doi: 10.1038/nprot.2006.279. [DOI] [PubMed] [Google Scholar]
- 84.Lucke-Wold B. The Varied Uses of Conditioned Place Preference in Behavioral Neuroscience Research: An Investigation of Alcohol Administration in Model Organisms. Impulse (Columbia) 2011. 2011 [PMC free article] [PubMed] [Google Scholar]
- 85.Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addiction biology. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- 86.Gawel K, Labuz K, Gibula-Bruzda E, Jenda M, Marszalek-Grabska M, Silberring J, Kotlinska JH. Acquisition and reinstatement of ethanol-induced conditioned place preference in rats: Effects of the cholinesterase inhibitors donepezil and rivastigmine. J Psychopharmacol. 2016;30:676–687. doi: 10.1177/0269881116642539. [DOI] [PubMed] [Google Scholar]
- 87.Kotlinska J, Pachuta A, Dylag T, Silberring J. Neuropeptide FF (NPFF) reduces the expression of morphine- but not of ethanol-induced conditioned place preference in rats. Peptides. 2007;28:2235–2242. doi: 10.1016/j.peptides.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 88.Kotlinska JH, Bochenski M, Danysz W. The role of group I mGlu receptors in the expression of ethanol-induced conditioned place preference and ethanol withdrawal seizures in rats. Eur J Pharmacol. 2011;670:154–161. doi: 10.1016/j.ejphar.2011.09.025. [DOI] [PubMed] [Google Scholar]
- 89.Bienkowski P, Kuca P, Piasecki J, Kostowski W. Low dose of ethanol induces conditioned place preference in rats after repeated exposures to ethanol or saline injections. Alcohol Alcohol. 1996;31:547–553. doi: 10.1093/oxfordjournals.alcalc.a008190. [DOI] [PubMed] [Google Scholar]
- 90.Gibula-Bruzda E, Marszalek-Grabska M, Gawel K, Witkowska E, Izdebski J, Kotlinska JH. The influence of the new enkephalin derivative, cyclo[N(epsilon),N(beta)-carbonyl-d-Lys(2),Dap(5)] enkephalinamide (cUENK6), on reinstatement of ethanol-induced conditioned place preference in rats. Physiology & behavior. 2015;145:50–56. doi: 10.1016/j.physbeh.2015.03.031. [DOI] [PubMed] [Google Scholar]
- 91.Torres OV, Walker EM, Beas BS, O’Dell LE. Female rats display enhanced rewarding effects of ethanol that are hormone dependent. Alcoholism, clinical and experimental research. 2014;38:108–115. doi: 10.1111/acer.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Katz JL, Higgins ST. The validity of the reinstatement model of craving and relapse to drug use. Psychopharmacology. 2003;168:21–30. doi: 10.1007/s00213-003-1441-y. [DOI] [PubMed] [Google Scholar]
- 93.Roger-Sanchez C, Aguilar MA, Rodriguez-Arias M, Aragon CM, Minarro J. Age- and sex-related differences in the acquisition and reinstatement of ethanol CPP in mice. Neurotoxicology and teratology. 2012;34:108–115. doi: 10.1016/j.ntt.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 94.Belknap JK, Crabbe JC, Young ER. Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology. 1993;112:503–510. doi: 10.1007/BF02244901. [DOI] [PubMed] [Google Scholar]
- 95.Yoneyama N, Crabbe JC, Ford MM, Murillo A, Finn DA. Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol. 2008;42:149–160. doi: 10.1016/j.alcohol.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wise RA. Voluntary ethanol intake in rats following exposure to ethanol on various schedules. Psychopharmacologia. 1973;29:203–210. doi: 10.1007/BF00414034. [DOI] [PubMed] [Google Scholar]
- 97.Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res. 2008;32:1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Varlinskaya EI, Truxell EM, Spear LP. Ethanol intake under social circumstances or alone in sprague-dawley rats: impact of age, sex, social activity, and social anxiety-like behavior. Alcoholism, clinical and experimental research. 2015;39:117–125. doi: 10.1111/acer.12604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sluyter F, Hof M, Ellenbroek BA, Degen SB, Cools AR. Genetic, sex, and early environmental effects on the voluntary alcohol intake in Wistar rats. Pharmacology, biochemistry, and behavior. 2000;67:801–808. doi: 10.1016/s0091-3057(00)00425-1. [DOI] [PubMed] [Google Scholar]
- 100.Li TK, Lumeng L. Alcohol preference and voluntary alcohol intakes of inbred rat strains and the National Institutes of Health heterogeneous stock of rats. Alcoholism, clinical and experimental research. 1984;8:485–486. doi: 10.1111/j.1530-0277.1984.tb05708.x. [DOI] [PubMed] [Google Scholar]
- 101.Lancaster FE, Brown TD, Coker KL, Elliott JA, Wren SB. Sex differences in alcohol preference and drinking patterns emerge during the early postpubertal period. Alcoholism, clinical and experimental research. 1996;20:1043–1049. doi: 10.1111/j.1530-0277.1996.tb01945.x. [DOI] [PubMed] [Google Scholar]
- 102.Lancaster FE, Spiegel KS. Sex differences in pattern of drinking. Alcohol. 1992;9:415–420. doi: 10.1016/0741-8329(92)90041-8. [DOI] [PubMed] [Google Scholar]
- 103.Almeida OF, Shoaib M, Deicke J, Fischer D, Darwish MH, Patchev VK. Gender differences in ethanol preference and ingestion in rats. The role of the gonadal steroid environment. The Journal of clinical investigation. 1998;101:2677–2685. doi: 10.1172/JCI1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Juarez J, Barrios de Tomasi E. Sex differences in alcohol drinking patterns during forced and voluntary consumption in rats. Alcohol. 1999;19:15–22. doi: 10.1016/s0741-8329(99)00010-5. [DOI] [PubMed] [Google Scholar]
- 105.Vendruscolo LF, Terenina-Rigaldie E, Raba F, Ramos A, Takahashi RN, Mormede P. Evidence for a female-specific effect of a chromosome 4 locus on anxiety-related behaviors and ethanol drinking in rats. Genes, brain, and behavior. 2006;5:441–450. doi: 10.1111/j.1601-183X.2005.00177.x. [DOI] [PubMed] [Google Scholar]
- 106.Vendruscolo LF, Izidio GS, Takahashi RN, Ramos A. Chronic methylphenidate treatment during adolescence increases anxiety-related behaviors and ethanol drinking in adult spontaneously hypertensive rats. Behav Pharmacol. 2008;19:21–27. doi: 10.1097/FBP.0b013e3282f3cfbe. [DOI] [PubMed] [Google Scholar]
- 107.de la Torre ML, Escarabajal MD, Aguero A. Sex differences in adult Wistar rats in the voluntary consumption of ethanol after pre-exposure to ethanol-induced flavor avoidance learning. Pharmacology, biochemistry, and behavior. 2015;137:7–15. doi: 10.1016/j.pbb.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 108.Bell RL, Rodd ZA, Sable HJ, Schultz JA, Hsu CC, Lumeng L, Murphy JM, McBride WJ. Daily patterns of ethanol drinking in peri-adolescent and adult alcohol-preferring (P) rats. Pharmacology, biochemistry, and behavior. 2006;83:35–46. doi: 10.1016/j.pbb.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 109.Schramm-Sapyta NL, Francis R, MacDonald A, Keistler C, O’Neill L, Kuhn CM. Effect of sex on ethanol consumption and conditioned taste aversion in adolescent and adult rats. Psychopharmacology. 2014;231:1831–1839. doi: 10.1007/s00213-013-3319-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vetter-O’Hagen C, Varlinskaya E, Spear L. Sex differences in ethanol intake and sensitivity to aversive effects during adolescence and adulthood. Alcohol Alcohol. 2009;44:547–554. doi: 10.1093/alcalc/agp048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Marco EM, Ballesta JA, Irala C, Hernandez MD, Serrano ME, Mela V, Lopez-Gallardo M, Viveros MP. Sex-dependent influence of chronic mild stress (CMS) on voluntary alcohol consumption; study of neurobiological consequences. Pharmacology, biochemistry, and behavior. 2017;152:68–80. doi: 10.1016/j.pbb.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 112.Ford MM, Eldridge JC, Samson HH. Ethanol consumption in the female Long-Evans rat: a modulatory role of estradiol. Alcohol. 2002;26:103–113. doi: 10.1016/s0741-8329(01)00203-8. [DOI] [PubMed] [Google Scholar]
- 113.Forger NG, Morin LP. Reproductive state modulates ethanol intake in rats: effects of ovariectomy, ethanol concentration, estrous cycle and pregnancy. Pharmacology, biochemistry, and behavior. 1982;17:323–331. doi: 10.1016/0091-3057(82)90087-9. [DOI] [PubMed] [Google Scholar]
- 114.Becker HC, Anton RF, De Trana C, Randall CL. Sensitivity to ethanol in female mice: effects of ovariectomy and strain. Life Sci. 1985;37:1293–1300. doi: 10.1016/0024-3205(85)90244-9. [DOI] [PubMed] [Google Scholar]
- 115.Vetter-O’Hagen CS, Spear LP. The effects of gonadectomy on age- and sex-typical patterns of ethanol consumption in Sprague-Dawley rats. Alcoholism, clinical and experimental research. 2011;35:2039–2049. doi: 10.1111/j.1530-0277.2011.01555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hilakivi-Clarke L. Role of estradiol in alcohol intake and alcohol-related behaviors. Journal of studies on alcohol. 1996;57:162–170. doi: 10.15288/jsa.1996.57.162. [DOI] [PubMed] [Google Scholar]
- 117.Ford MM, Eldridge JC, Samson HH. Determination of an estradiol dose-response relationship in the modulation of ethanol intake. Alcoholism, clinical and experimental research. 2004;28:20–28. doi: 10.1097/01.ALC.0000108647.62718.5A. [DOI] [PubMed] [Google Scholar]
- 118.Thiele TE, Navarro M. “Drinking in the dark” (DID) procedures: a model of binge-like ethanol drinking in non-dependent mice. Alcohol. 2014;48:235–241. doi: 10.1016/j.alcohol.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiology & behavior. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 120.Satta R, Hilderbrand ER, Lasek AW. Ovarian Hormones Contribute to High Levels of Binge-Like Drinking by Female Mice. Alcoholism, clinical and experimental research. 2018 doi: 10.1111/acer.13571. published online Dec 5, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]


