Abstract
Metabolomic studies of body fluids show that immune-mediated inflammatory diseases such as rheumatoid arthritis (RA) are associated with metabolic disruption. This is likely to reflect the increased bioenergetic and biosynthetic demands of sustained inflammation and changes to nutrient and oxygen availability in damaged tissue. The synovial membrane lining layer is the principle site of inflammation in RA. Here the resident cells are the fibroblast-like synoviocytes (FLS) and the synovial tissue macrophages (STM), which are transformed toward overproduction of enzymes which degrade cartilage and bone, and cytokines which promote immune cell infiltration. Recent studies have shown metabolic changes in both FLS and macrophages from RA patients and these may be therapeutically targetable. However, as the origins and subset specific functions of synoviocytes are poorly understood and the signaling modules which control metabolic deviation in RA synovial cells are yet to be explored, significant additional research is needed to translate these findings toward clinical application. Furthermore, in many inflamed tissues, different cell types can forge metabolic collaborations through solute carriers (SLC) in their membranes, to meet a high demand for energy or biomolecules. Such relationships are likely to exist in the synovium and are yet to be explored. Finally, it is not yet known whether metabolic change is a consequence of disease or if primary changes to cellular metabolism might underlie or contribute to early stage disease pathogenesis. This article collates what is known about metabolism in synovial tissue cells and highlights future research directions in this area.
Introduction
Rheumatoid arthritis (RA) is a systemic chronic inflammatory disease which principally manifests in the articular joints. Recent research has yielded biological therapies and small molecules to target signaling pathways and pathogenic components involved in inflammation and immunity, but in spite of these reasonably successful treatments, very few RA patients are able to achieve and stay in a state of drug-free remission. Innovative strategies are needed to obtain new insights into mechanisms which underlie disease pathogenesis and to identify potential new treatments.
In fields such as oncology, the concept of undermining or reprogramming metabolism to improve patient outcomes is established and we and others believe the potential is great to adopt similar strategies in immune-mediated inflammatory diseases (IMID) (1, 2). For three decades researchers have hypothesized an intermediate role for metabolic alterations and local hypoxia in RA pathology (3, 4). Indeed, RA and related IMID are associated with systemically measurable metabolic disruption. This is likely to reflect the increased bioenergetic and biosynthetic demand placed on immune and stromal cells in a chronically activated state. We now have the tools to better understand and translate past observations and to explore our hypothesis that metabolic deviation in synovial cells has a role in early pathogenesis rather than being a consequence of tissue damage. Qualitative changes to cellular metabolism are indeed essential to support physiological and pathological responses seen in the RA synovium and we describe these changes below. RA-associated characteristics include proliferation, migration and invasion that are hallmarks of activated fibroblast-like synoviocyte (FLS) behavior, and also proinflammatory mediator production characteristic of activated synovial tissue macrophages (STM) (5–7). The phenotypic transformation of FLS from a quiescent cell to an aggressive, metabolically active cell, the activation of STM, and the increasingly hypoxic and nutrient deprived microenvironment which develops in the RA joint are characteristics which closely resemble those seen in solid tumors.
Immunometabolism research has recently expanded in the footsteps of the more advanced cancer metabolism literature and this has led to the identification of potential new drug targets for immune-mediated pathologies(1, 8). Limitation of metabolic substrate availability, modulation of signaling pathways which control metabolism and targeting of channels through which metabolic intermediates are shared are exciting new strategies for treatment. Since there is now a widespread acceptance that the future of RA treatment may lie in targeting the synovial tissue and in particular the stromal microenvironment, it is logical to apply these new therapies here (9, 10). In this review, we describe our current knowledge of synovial metabolism and the therapeutic opportunities this field might present. We focus upon the FLS and STM which together form a destructive frontier and whose aberrant behavior is underpinned by pathological metabolic changes.
A systemic metabolic phenotype in RA
Metabolic perturbations have long been associated with RA and the hallmark ‘calor’ (heat) observed in the rheumatoid joint is widely considered a consequence of metabolic activity. Daily resting whole body energy expenditure is 8% higher in RA than in healthy individuals suggesting these metabolic changes are significant and systemic (11). RA patients have an increased susceptibility to cardiovascular comorbidity and metabolic syndrome during the progression of their disease, associated with disruption of lipid and glucose metabolism (12). Furthermore, the catabolic condition ‘cachexia’ occurs in RA, with muscle atrophy and gain in fat associated with systemically elevated pro-inflammatory cytokines such as TNFα, IL-1β, LIF, IFNγ and IL-6 (13). Untargeted metabolomic studies of body fluids are helping to characterize the systemic observations alluded to above. These involve analysis of small molecules (<3kDa) usually using one-dimensional nuclear magnetic resonance (1D NMR) spectroscopy or mass spectrometry coupled to gas or liquid phase separation techniques, technologies which we have recently compared and evaluated (14). This approach has highlighted urinary metabolite signatures which can identify the six most prevalent immune-mediated inflammatory diseases (15), and a serum metabolite signature which correlates with C-reactive protein (CRP) level and associates metabolism with underlying inflammatory mechanisms (16). Serum (17) and synovial fluid (SF) (18, 19) metabolomic profiles have also demonstrated the potential to distinguish RA from psoriatic arthritis and other diseases (14). Furthermore, our data and that of others show that patient responses to biological therapies including etanercept and rituximab can be predicted from urine, serum and plasma metabolic profiles, highlighting the power of metabolomics in stratifying patients and directing RA treatment (20–22).
Since immune and stromal cells within the inflamed joints are known to produce inflammatory cytokines it is likely that they are contributing to the global metabolic phenotype in RA. However, the metabolic profiles of individual cell types involved in chronicity or resolution of inflammation have sparsely been elucidated. This will be important to link diseases associated metabolites to pathogenic processes and to gain a full understanding of RA pathogenesis.
The rheumatoid synovium
Though RA is a systemic disease, the major manifestation is joint pain and loss of function. The normal diarthrodial joint is lined with a thin, soft-tissue membrane, the synovium, which comprises a sublining and a thin intimal lining layer, and which produces and encapsulates a lubricating hyaluronic acid-rich fluid (23). Resident to the tissue are two heterogeneous and inadequately characterized cell types a) the STM; a mixed population of prenatally seeded cells and those which have differentiated from circulating monocytes (24, 25), and b) the FLS, mesenchymal-derived cells, which compared with fibroblasts in other anatomical locations, are characterized by the expression of UDP-glucose 6-dehydrogenase, an enzyme required for the synthesis of hyaluronic acid, and of complement decay-accelerating factor (also known as CD55) (26). Collaborative networks such as the National Institutes for Health Accelerating Medicines Partnership are facilitating the effective digestion of tissue to obtain pure populations of FLS and STM. This combined with a growing profile of soluble and surface markers and revolutions in fate mapping and single cell analysis techniques is improving our ability to study and understand the functions of these important cells (27, 28).
The hallmark of RA is macroscopically visible change to the synovial lining layer which becomes inflamed, hyperplastic and invasive of local cartilage and bone (29). This is driven by a complex interaction between chronically activated and epigenetically transformed synoviocytes and infiltrating cells of the innate and adaptive immune system (5, 30). Both FLS and STM contribute to synovial inflammation by production of mediators which recruit and activate immune cells. Importantly, these cells also drive each other’s activation and survival by paracrine production of cytokines such as TNFα and GM-CSF (30). However, it is the FLS in the intimal lining which form the aggressive pannus and are the major effectors of tissue damage through production of extracellular matrix degrading enzymes such as matrix metalloproteinases and cathepsins (30).
Though the joint exists at an oxygen tension as low as 8% even in health, the microenvironment in RA is characterized by severe hypoxia at oxygen tensions which fall to below 1% (31). Nutrient availability is also low as immune cells and activated synoviocytes consume available resources at a rate which exceeds their delivery. Synovial angiogenesis, which is mediated by factors released by both FLS and STM and enhances the ingress of leukocytes into the synovial tissue, is insufficient, and the aggressive front formed by the hyperplastic synovial lining increases the distance between blood vessels and synoviocytes (32, 33). RA FLS are transformed from a quiescent state to an aggressive, invasive phenotype and they persist despite enrichment of apoptosis-inducing factors such as oxygen radicals, nitric oxide and cytokines supplied by activated STM. As such the synovium bears resemblance to tumor tissue and cells are likely to be subject to similarly elevated bioenergetic and biosynthetic demands to those seen in cancer. This comparison is summarized in Figure 1.
Figure 1. Similarities between the rheumatoid arthritis synovium and the solid tumor microenvironment.
In both tissues, fibroblasts and macrophages (MØ) reside in close proximity and within an oxygen and nutrient deprived, cytokine rich environment. Here they sustain mitochondrial damage and take on a chronically activated phenotype supported by increased glycolytic metabolism. In RA, the fibroblasts themselves are proliferative, invasive and migratory while in cancer fibroblasts support proliferation, invasion and metastasis of tumor cells. Adaptive immune cell-fibroblast interactions differ in tumor and RA microenvironments. Activated T (green) and B (blue) cells are present in the synovium in RA but are suppressed in the tumor microenvironment. Little is known about fibroblast or macrophage metabolism in early RA and the metabolic changes which take place during the transition from health to disease. (ROS, reactive oxygen species).
It is likely that adaptation of mitochondria and cytoplasmic metabolic pathways is needed to meet the requirements of chronic inflammation in RA. Indeed, of the few studies which profile the metabolome of FLS, profound metabolic differences were identified in end stage RA when compared with OA and many of these are described below (34, 35). Our unpublished data shows a correlation between systemic inflammation (measured as CRP) and the metabolic profile of FLS from very early RA. However, studies comparing to healthy FLS or assessing changes during the transformation in early disease are lacking. Consequently, we are currently unable to determine if metabolic adaptation of fibroblasts is a normal response to chronic inflammation, or if primary changes to cellular metabolism might themselves underlie or contribute to the disease pathogenesis. Studies of STM are also few and most of what is known about RA macrophage metabolism is from animal models or study of cells differentiated in vitro from SF or peripheral blood monocytes (36). Since macrophages in healthy synovium are thought to be largely yolk sac-derived and cues leading to monocyte differentiation in tissue are poorly defined, there is a pressing need for better characterization of synovial macrophage subsets and their metabolism (37).
The dynamic metabolic response in health
The function of the metabolic machinery within a cell is to provide the energy and biomolecules necessary to perform resting and activated functions, while managing the production of potentially damaging byproducts such as lactate and reactive oxygen species (ROS). Recent studies of immune cells have identified important roles for metabolism in supporting and even directing cell differentiation and fate. These studies have characterized the extent of metabolic plasticity required for cellular responses to stimulation (8). There are 6 main pathways which are involved in these functions (summarized in Figure 2). Of these, oxidative phosphorylation (comprising the tricarboxylic acid TCA cycle and the mitochondrial electron transport chain) and fatty acid oxidation are oxygen dependent and take place in the mitochondria, while utilizing substrates taken up from the cytoplasm. Glycolysis, the pentose phosphate pathway (PPP), amino acid metabolism and fatty acid synthesis are oxygen independent and largely take place in the cytoplasm. However, fatty acid and amino acid intermediates are also shared with mitochondrial processes and use of the PPP to maintain redox balance does require oxygen (8).
Figure 2. Major pathways important in synoviocyte metabolism.
FLS and monocyte derived macrophages are heavily reliant upon glucose metabolism and regulate glucose transporter member 1 (Glut1) in response to inflammatory and stress stimuli. This fuels adenosine triphosphate (ATP) production in conditions of high energetic demand. Glucose is utilized in the pentose phosphate pathway to synthesize building blocks for nucleic acids, and to generate NADPH to control redox status and support lipid synthesis. Alternatively glucose is metabolized via glycolysis to pyruvate which is either transported into the mitochondria to contribute to tricarboxylic acid (TCA) cycle flux or is converted to lactate in the cytoplasm and removed from the cell via monocarboxylate transporter 4 (MCT4). TCA cycle flux contributes to ATP production via oxidative and substrate level phosphorylation. When matrix citrate levels rise, citrate is transported to the cytoplasm and yields acetyl-CoA, the starting material for synthesis of fatty acids, cholesterol and lipids. Some such lipids are exported from the cell as bioactive metabolites such as sphingosine 1 phosphate (S1P), free fatty acids (FFA), phospholipids and eicosanoids. Acetyl-CoA as well as succinate generated from the TCA cycle can be utilized in production chromatin modifying enzymes (CME) and cofactors. Choline is taken up via choline transporter-like (CTL) 1/2 is an important substrate in FLS biology. Choline can be converted to betaine which is used in production CME and cofactors or converted to glycine for use in protein synthesis. Alternatively, choline is phosphorylated to phosphocholine and utilized in membrane phospholipid and bioactive lipid synthesis. A number of signalling molecules have been identified which control the described metabolic pathways but have sparsely been explored in FLS. AKT, protein kinase B; AMPK, 5′ adenosine monophosphate-activated protein kinase; G6P, glucose-6-phosphate; HIF-1α, hypoxia-inducible factor 1α; Myc, Myc proto-oncogene protein; p53, cellular tumor antigen p53; PI3K, phosphatidylinositol 4,5-bisphosphate 3-kinase; R5P, ribose-5-phosphate; S1P, sphingosine-1-phosphate; SREBP, sterol regulatory element-binding protein; mTOR, mechanistic target of rapamycin; PPARγ, peroxisome proliferator-activated receptor gamma; NF-κB, nuclear factor κB pathway; uPFK, 2phosphofructokinase 2.
Normal physiological metabolism and metabolic responses to inflammatory events are poorly studied in tissue resident populations such as fibroblasts and embryonically seeded macrophages(25). This is largely due to the practicalities of disentwining cells from the extracellular matrix, a challenge which researchers are making great efforts to address (38). Indeed there has been limited progress since the stromal metabolism field was thoroughly reviewed in 2014 (39). One study defines what is known about resting, healthy fibroblast metabolism, showing that fore-skin derived cells are predominantly glycolytic and dependent upon the PPP even in quiescence (40). This supports the anabolic processes associated with extracellular matrix production and is likely to also apply to FLS which maintain SF and produce it in excess in RA.
Reversible regulation in the balance of cytoplasmic and mitochondrial metabolism is crucial for all cells to respond and adapt to changing microenvironments. Metabolic change is orchestrated by signaling pathways responding to nutrient, energy and oxygen levels as well as growth factor, pattern recognition, and cytokine receptors. The pathways involved have been reviewed elsewhere (41, 42) and are summarized in figure 2. These include the master regulators mechanistic target of rapamycin (mTOR) and 5′ adenosine monophosphate activated protein kinase (AMPK) pathways which work in opposition to one another. The mTOR network is a nutrient sensing system. mTORC1 or mTORC2 complex formation downstream of PI3K or MAPK pathways induces activation of AKT and transcription factors HIF1α and MYC, which in turn activate glycolytic pathway genes upregulating aerobic glycolysis. Sterol regulatory element binding proteins (SREBP) and peroxisome proliferator-activated receptor gamma (PPARγ) acting downstream of mTORC1 also activate genes which upregulate fatty acid synthesis. Other pathways have also been shown to activate glycolysis in a HIF1α independent manner, including the NF-κB pathway involving ubiquitous phosphofructokinase 2 (uPFK2). AMPK is an energy sensing system often considered a metabolic checkpoint as it can inhibit glycolysis, control cell proliferation and promote mitochondrial biogenesis when activated under energetic stress (43, 44). Downstream nuclear respiratory factor-1 (NRF-1), NRF-2 and PPARγ coactivator-1α (PGC-1α), as well as SIRT1 or STAT6 activated PGC-1β, induce mitochondrial biogenesis and fusion (discussed below), induce protective antioxidant enzymes, and promote oxidative metabolism. The mTOR pathway has also been shown to be important in polarization of macrophages to pro-inflammatory (M1) or pro-resolving (M2) phenotypes and in activation of both macrophage subtypes, and this has been thoroughly reviewed (45). AMPK activation in macrophages is associated with suppression of IL-6 production, anti-inflammatory M2 macrophage differentiation from SF monocytes and suppressed inflammation in K/BxN serum induced arthritis (46, 47). The signaling pathways which instruct stromal cell behavior are less well defined. mTOR activation has been linked to invasive properties in arthritic rat FLS (48). Furthermore, mice deficient in NRF2 which acts downstream of AMPK, show more severe cartilage injuries and more oxidative damage in a murine model of arthritis (49). However, no link between metabolism and cell signaling has yet been made in human FLS or STM and it is unknown whether signaling pathways controlling metabolic phenotype are dysregulated in RA.
Mitochondrial responses in the RA synovium
Under normoxic conditions mitochondrial oxidative phosphorylation is the most efficient source of ATP (50). In addition, mitochondria integrate various metabolic pathways and through this process produce intermediates needed for the synthesis of lipids, steroid hormones, and heme. Other more specialized mitochondrial functions include maintenance of Ca2+ homeostasis, regulation of apoptosis, and production of the physiological levels of ROS which act as signaling molecules (50). Importantly, mitochondria have mechanisms to respond spatially and temporally to heterogeneous nutrient and oxygen concentrations, increased ATP demands and increased stress signals including oxidative stress, for continued support of cellular functions and survival. These mechanisms include the overexpression of antioxidant enzymes and remodeling of respiratory complex subunits, changes in substrate choice (utilizing glutamine, pyruvate, fatty acids and ketone bodies) or switching toward increased glycolysis when energetic demand outpaces oxygen delivery (50).
Changes in mitochondrial biogenesis, mitochondrial-selective autophagy (mitophagy) and in the equilibrium between mitochondrial fusion and fission are also critical to maintain normal mitochondrial and cellular function (Table 1 and Figure 3). Mitochondrial biogenesis is induced through the signaling pathways described above, not only in association with cell division but also in response to oxidative stimuli and increased energy requirements. The flux between fusion and fission of mitochondria in response to stress is also crucial to maintaining the metabolic capacity of the mitochondria as well as protecting genetic stability. Mitophagy plays a pivotal role in the maintenance of mitochondrial homeostasis, regulating the size and quality of the mitochondrial population. In addition, mitophagy eliminates damaged mitochondria under diverse stress conditions, which is critical as mitochondrial DNA (mtDNA) is a damage-associated molecular pattern (DAMP) that contributes to systemic inflammatory responses (51). Excess or inadequacy in mitochondrial dynamics, biogenesis and mitophagy are deleterious in mice and are associated with aging and multiple serious human diseases, highlighting their importance in cell phenotype and function (51, 52). Furthermore, mitochondrial surveillance and quality control mechanisms including mitochondrial biogenesis and mitophagy decline with age causing progressive deterioration of mitochondrial function. This may have a role in diseases such as RA and suggests that targeting mitochondrial processes could be beneficial to restore cell function (53).
Table 1.
| Term | Definition in the context of this review |
|---|---|
| Aerobic glycolysis | The metabolic pathway which utilizes glucose to generate ATP in the presence of oxygen. End products of this process are pyruvate which can be imported into mitochondria for use in the TCA cycle or lactate which is expelled from the cell as waste. |
| Anabolism | The enzymatic synthesis of molecules from smaller components. |
| Anaplerosis | The replenishment of metabolic intermediates into the TCA cycle as substrates for biosynthesis and the generation of ATP. |
| Bioenergetics | The study of energy production by cells. Often associated with use of the Seahorse analyzer to assess glycolysis and oxidative phosphorylation rates in real time. |
| Catabolism | The enzymatic degradation of molecules into smaller products. |
| Cataplerosis | The removal of TCA cycle intermediates for use in biosynthesis or to prevent build up within mitochondria. |
| Electron transport chain | A set of complexes of the inner mitochondrial membrane that shuttle electrons from NADH and FADH2 to oxygen. The redox reactions of the chain produce an electrochemical gradient of protons across the membrane which drives synthesis of ATP by oxidative phosphorylation. |
| Hypoxia | Oxygen deficiency in tissues, such that oxygen tension is below that of healthy physiological conditions. Normal oxygen levels differ between tissues and therefore the level at which a tissue is considered to be hypoxic is variable. The RA joint has an oxygen tension of 8% in health and <3% in RA. |
| Imaging mass spectrometry | A mass spectrometry based technique which allows the spatial distribution of the metabolome to be visualized in a tissue section. This offers insights into where pathogenic metabolic changes are taking place in tissues and is yet to be applied to the synovium. |
| Immunometabolism | The research field which investigates metabolism in the context of immunity and inflammation. As stromal cells are crucial to both induction and resolution of these processes, we consider the study of their metabolic processes to be embedded in this field. |
| Metabolic coupling | The transfer of metabolites between cells in a manner which benefits the biosynthetic and bioenergetic requirements of the recipient cell. |
| Metabolic flux analysis | Also known as ‘stable isotope metabolic tracer analysis’, this is a 13C isotope tracing methodology involving incubation of cells with a stable isotope, quantitation of metabolite labeling using mass spectrometry or NMR spectroscopy, and computational fitting of the data to a model allowing estimates of pathway-specific flux. |
| Metabolic memory | Imprinting of a metabolic phenotype on a cell by cues within its microenvironment, such that the phenotype is maintained after the cues are removed or the cells is removed from the tissue. |
| Metabolome | All substrates, intermediates and products of metabolism associated with a given system or compartment. These may be intracellular or extracellular. |
| Metabolomics | Used synonymously with the term ‘metabolic profiling’, this is the identification and measurement of all or a targeted set of metabolites within a body fluid, cell population or tissue conducted by NMR spectroscopy or mass spectrometry. |
| Mitochondrial biogenesis | The generation of greater mitochondrial mass to increase the capacity for mitochondrial function and ATP production within a cell; this process is important to health but is uninvestigated in RA. |
| Mitochondrial dynamics | The balance and transition between mitochondrial fusion and fission, movement and degradation; processes which are important to health but in uninvestigated in RA. |
| Mitochondrial fission | The division of mitochondrial networks into individual, punctate organelles, principally controlled by outer membrane proteins DRP1 and FIS1. |
| Mitochondrial fusion | The formation of tubular mitochondrial networks through MFN1, MFN2 and OPA1 mediated joining of individual organelle membranes. This process is associated with increased ATP production and protection of mitochondrial DNA from reactive oxygen species. |
| Mitophagy | ‘Mitochondrial-selective autophagy’. The selective degradation of defective mitochondria without the release of inflammatory mitochondria- associated DAMPS. |
| Oxidative phosphorylation | Generation of ATP by the mitochondrial ATP synthase and driven by the electrochemical gradient of protons generated by the electron transport chain. |
| Pentose phosphate pathway | The pathway which oxidizes glucose to generate NADPH for the maintenance of the cellular redox balance and 5 carbon sugars utilized in anabolic processes such as nucleic acid synthesis. |
| Positron emission tomography | Use of a radioactive tracer isotope incorporated into a metabolic substrate to visualize metabolizing cells in a whole organism. Commonly, labelled glucose is used to identify tumors but is also useful in highlighting sites of inflammation such as the RA joint. |
| Reverse Warburg effect – | The production of high energy metabolic intermediates by one cell to anaplerotically feed ATP production by a neighboring cell. Currently characterized only in epithelial tumors where cancer-associated fibroblast feed lactate and other metabolites to tumor cells through channels such as the monocarboxylate transporters. |
| TCA cycle | The tricarboxylic acid cycle is a series of chemical reactions which take place in the mitochondrial matrix generating ATP by substrate level phosphorylation and NADH and FADH2 by oxidation of fuel molecules. The NADH and FADH2 are further oxidized by the electron transport chain. |
| Warburg effect | Pathological increase in glycolysis associated with reduced oxidative phosphorylation despite the presence of oxygen. This describes a cellular bioenergetic phenotype classically associated with tumor cells but now also associated with activated immune cells. |
Figure 3. Mitochondrial dynamics; the cycle of fusion and fission.
Mitochondrial morphology changes dynamically in response to stress and changing energetic demand. This is under the control of signalling molecules including sirtuin 1 (SIRT1), signal transducer and activator of transcription 6 (STAT6), nuclear respiratory factors 1 and 2 (NRF1/2) and peroxisome proliferator-activated receptor gamma coactivators 1α and β (PGC-1α/β). Mitochondria fuse to make tubular networks under the control of mitofusins (MFN) 1 and 2 and optic atrophy (OPA1), a mechanism which is thought to increase ATP production by oxidative phosphorylation, protect mitochondrial DNA from damage in the presence of elevated reactive oxygen species (ROS) and leads to mitochondrial biogenesis and increased mitochondrial mass. Mitochondrial fission occurs under the control of dynamin related protein 1 (DRP1) and mitochondrial fission 1 (FIS1) and produces increased numbers of punctate mitochondria. Fission usually corresponds with reduced oxidative phosphorylation and increased aerobic glycolysis and can predispose to mitochondrial-selective autophagy (mitophagy) to regulate mitochondrial mass or remove damaged organelles. The box shows a number of mitochondrial observations made in FLS cultured from RA patients both in a resting state and after stimulation with proinflammatory cytokines, alluding to possible but as yet uninvestigated changes in mitochondrial dynamics.
Surprisingly little is known about mitochondrial dynamics and function in RA synoviocytes and there is a particular absence of observations in STM. However, a growing literature describes mitochondrial metabolism downstream of glycolytic glucose consumption and its role in differentiation and activation of pro-inflammatory and pro-resolving monocyte-derived macrophages subtypes. These model systems might inform research in the synovium. For example, stimulation of macrophages with lipopolysaccharide (LPS) and interferon gamma (IFNγ) (so-called M1-polarising conditions) produces proinflammatory cells which may resemble those which populate the RA synovium. This leads to inhibition of the TCA cycle and the mitochondrial oxidative phosphorylation pathway to which it is coupled and results in upregulation of glucose transporter 1 (Glut1) to facilitate efficient uptake of glucose. Glucose is consumed through upregulation of aerobic glycolysis and gives rise to production of copious lactate which must be extruded from the cell to prevent lactic acidosis (54). Production of reactive oxygen is increased, partly as a consequence of reversed electron transport by mitochondria and certain intermediates of the TCA cycle accumulate, notably succinate. This promotes expression of the pro-inflammatory cytokine IL-1β by inhibiting prolyl hydroxylases and activating the transcription factor HIF-1a. Succinate has also been linked to changes in methylation of DNA and associated histone proteins to alter gene expression (55). Furthermore, isocitrate is diverted from the Krebs cycle and metabolised to itaconic acid, another more recently identified TCA cycle inhibitor (56). RA synovial macrophages express HIF-1a, consistent with a switch to glycolytic metabolism but efforts are required to characterize mitochondrial metabolism and dynamics in resident and infiltrating STM (57).
Several studies have observed mitochondria in late stage RA FLS. Baseline reductions in respiration and membrane potential (58) and changes to mitochondrial morphology (59) have been shown when compared with OA. Further changes suggestive of mitochondrial dysfunction such as perinuclear clustering of mitochondria, abnormally dark cristae, and autophagosome formation have been associated with lower basal mitochondrial membrane potential, as well as lower basal, maximum and ATP-linked mitochondrial respiratory rates (58). In a complementary study also in comparison with OA, we recently showed RA FLS had a higher baseline glycolytic rate to respiratory rate ratio, implying a shift toward reliance on glycolysis to meet the energy demand of the cells (60). Changes in mitochondrial metabolism can be induced in FLS by cytokines and growth factors including IL-17, TNF and PDGF which are associated with RA and related inflammatory conditions (58, 61). The reported consequences, though incompletely understood, include reduced ATP production by oxidative phosphorylation, production of excessive reactive oxygen and nitrogen species, dysregulation of Ca2+, opening of the permeability transition pore and initiation of cell death in vitro. Significant increases in mtDNA mutation frequency have been demonstrated in inflamed synovial tissue and were positively correlated with macroscopic synovitis, vascularity and SF levels of TNF and IFNγ (62). A further study showed that exposure of RA synovial tissue to 1% oxygen in vivo induced mtDNA mutations suggesting that the inflamed and hypoxic joint microenvironment may be eliciting the mitochondrial changes observed in RA FLS and likely to be occurring in STM (31). Hypoxia also altered the bioenergetics of cultured FLS by promoting a switch to glycolysis while attenuating mitochondrial respiration and ATP synthesis. This supported abnormal angiogenesis, cellular invasion and pannus formation (59). Further in vitro studies have indicated higher mtDNA mutations and ROS levels in RA compared to OA FLS (63), correlating with elevated matrix metalloproteinase (MMP) expression and an invasive phenotype in RA (61, 64).
The above findings suggest that there are mitochondrial changes in late stage RA FLS which are maintained in in vitro culture, yet we lack understanding of which changes are a normal response to meet metabolic demands of inflammation, which might play a role in driving the pathology of chronic disease and which are the result of damage and an increasingly hypoxic environment. In fact, resistance to induction of programmed cell death (apoptosis) by apoptotic signals abundant in the inflamed joint, is a prominent characteristic of the RA synovium (65) and sustains the synovial hyperplasia that characterizes the rheumatoid pannus. This would suggest that mitochondrial responses induced by hypoxia and inflammation in synoviocytes are able to repurpose the mitochondrion as a biosynthetic hub similar to that of tumor cells (66) and with conserved mechanisms for limiting oxidative stress and supporting effector functions and proliferation (5). Finally, although we lack information regarding mitophagy in the synovium, it has been suggested that TNF significantly induces mitophagy and mitochondrial antigen presentation in mouse macrophages with implications for RA (67). Several reports have shown altered autophagy in RA FLS, which could also contribute to synovial hyperplasia. RA FLS show an increase of genes involved in autophagy such as beclin-1 and LC3, which inversely correlate with their apoptosis rate (68, 69). In addition, RA FLS under endoplasmic reticulum stress may increase autophagy while becoming resistant to apoptotic death (70). Further studies are needed to understand the tangled relationship between metabolism, apoptosis and autophagy in synoviocytes and to identify whether restoring normal metabolism and mitochondrial function might have therapeutic potential in RA.
Glucose metabolism in the RA synovium
Glucose and other metabolites such as glutamine, fatty acids, and ketone bodies can be metabolized through the mitochondrial tricarboxylic acid (TCA) cycle in metabolically active tissues, but a shift away from oxidative phosphorylation towards aerobic glycolysis often occurs in response to cellular activation and in inflamed tissues (71). This supports various biosynthetic pathways and, consequently, the metabolic requirements for proliferation and cytokine production. Accelerated glucose metabolism is a hallmark of proliferative and activated cells (72) and can be observed with clinical imaging. Several studies have used fluoro-2-deoxyglucose (FDG) which is taken up by glycolytic cells to form FDG-phosphate, and can be shown by positron emission tomography (PET) to accumulate in swollen joints (73). Indeed, glycolytic inhibition by BrPa administered in a serum transfer animal model significantly decreased arthritis severity, highlighting the importance of glucose metabolism in fueling pathological processes and making it a promising target for therapeutic intervention in RA (60). However, as discussed in a recent review by Weyand et al comparing macrophages and T cells, cells can coexist in the same microenvironment and utilize metabolites differently(74). This highlights the need to dissect glucose utilization in different synoviocytes since any successful treatment will necessarily be cell type specific.
Metabolic profiling of synovial tissue has revealed that FLS consistently show altered basal glucose metabolism in RA (34, 35, 75), and we and others have found that stimuli such as PDGF or TNF increase in vitro glucose metabolism by both glycolysis and mitochondrial respiration (60). Furthermore, the glucose channel Glut1 is upregulated in response to hypoxia and cytokines and correlates with phenotypic perturbations in RA FLS (59, 60). Glucose deprivation or glycolytic inhibitors such as 2-deoxy-D-glucose (2-DG), bromopyruvate (BrPa) (60) and 3-(3-Pyridinyl)-1-(4-pyridinyl)-2-propen-1-one (59) have been shown to impair cytokine secretion, proliferation, migration and invasion in RA FLS. Although, glucose metabolism is enhanced in activated macrophages, no data in STM is available yet.
Diversion of glucose metabolism away from glycolysis and toward the PPP is important to support biosynthetic role of some cells. A role for this pathway in RA was highlighted by work on T cells which showed impaired glycolytic flux due to elevated pentose-shunt activity and up-regulation of phosphofructokinase (76). In light of RA synoviocyte mitochondrial responses and elevated ROS production described above, increased flux of glucose through the PPP might also be expected in these cells to produce cytoplasmic NADPH, and drive the reducing power of ROS detoxifying enzyme systems in both the cytoplasm and the mitochondrial compartments. Indeed, a global increase of glucose metabolism (both the PPP and glycolysis) was observed in total synoviocytes (4), and recent metabolite profiling of FLS (77) showed both metabolites from glycolysis and PPP were significantly increased in RA and compared to OA, together with other metabolites that belonged to the amine, fatty acid, phosphate, and organic acid classes. Although this hints toward the importance of the PPP, its role and the importance of NADPH production and other antioxidant mechanisms such as the glutathione oxidation pathway have not yet been dissected in FLS and resident STM populations and such research might yield new opportunities for therapeutic intervention in RA.
Secondary roles have emerged for glucose metabolites, metabolic enzymes and TCA cycle intermediates outside of metabolism. For instance, succinate stabilizes the transcription factor HIF-1α in activated macrophages and also rat synovial fibroblasts promoting glycolysis (55, 78). Succinate and other metabolites including α-ketoglutarate, fumarate and acetyl-CoA might be expected to accumulate in macrophages and FLS under hypoxic conditions and are involved in eliciting important epigenetic changes, with unexplored potential for driving chronic inflammation (55, 78). Also, essential glycolytic enzymes have been reported to translocate to the nucleus or mitochondria where they function independently of their canonical metabolic roles in regulation of cytokines and anti-apoptotic responses (79, 80). For example, PKM2 also stabilizes HIF-1α promoting inflammatory M1 macrophage differentiation. Use of a small molecule modulator of PKM2 to prevent nuclear translocation has potential for driving a shift toward an M2 phenotype and restoration of tolerance in diseases such as RA. The hexokinase (HK) enzymes are also important regulators of metabolism. HK1 is known to drive cleavage and activation of pro-IL-1β in macrophages via the NLPR3 inflammasome and the downstream activation of caspase 1 (81), though this has not been studied in STM. HK2 also binds to the mitochondrial membrane via its interaction with the outer membrane porin protein (also termed the voltage-dependent anion channel (VDAC)) and this interaction inhibits the release of intermembrane pro-apoptotic proteins, thereby protecting cells from apoptosis. Importantly, the expression of HK2 is increased in RA FLS compared to OA FLS, and might provide an important link between metabolism and apoptosis resistance in the RA synovium (60).
Lipid metabolism in the RA synovium
Though a complex and incomplete picture, lipids are known to be important in fueling adaptive immunity and in resolution of inflammation (8, 82). A few studies have described lipid changes in RA FLS, although this has not been studied in STM. Metabolomic profiling has shown perturbation of lipid metabolism in RA FLS versus OA cells (34), and recent studies have identified important roles for molecules which interact with lipids such as choline, an important component of membrane phospholipids which may be limiting in proliferating cells such as RA FLS. Choline C-11 PET scanning, which is already in clinical use to identify prostate cancer metastasis, showed increased uptake in inflammatory arthritis joints (83) and choline is present at elevated levels in RA FLS and synovium (34, 75). Of interest, choline like transporter (CTL)1 (high-affinity) and CTL2 (low-affinity) are also highly expressed in RA FLS and the functional inhibition of choline transporters promoted apoptotic FLS cell death (84). We have also shown possible therapeutic benefit in targeting choline kinase (ChoKα), the enzyme that catalyzes the first step in the cytidine diphosphate-choline pathway and which is essential for phosphatidylcholine (PC) biosynthesis. Its inhibition suppressing migration and enhancing apoptosis in cultured RA FLS, and significantly decreasing experimental arthritis in pre-treatment protocols as well as in established disease (35). Related to PC metabolism, phospholipase D (PLD) enzymes specifically cleave PC producing phosphatidic acid (PA) and choline. Agonist-induced PLD activation results in PA synthesis thought to be involved in a variety of rapid cellular responses such as cytokine secretion (85). In RA FLS and RA synovial biopsy explants, PLD isoform-specific inhibitors significantly reduced constitutive secretion of IL-6 and IL-8, further highlighting the importance of phospholipid metabolism in inflammation (86). Another study showed that activated inflammatory arthritis FLS from humans and animal models express significant quantities of autotaxin, which catalyzes the conversion of lysophosphatidylcholines (LPC) to lysophosphatidic acid (LPA). Notably, high levels of LPC and low PC/LPC ratios in plasma were shown to represent a reliable measure of inflammation (87). TNF induced autotaxin expression from FLS and LPA, in turn, induced an activated FLS phenotype in synergy with TNF (88, 89). Conditional genetic ablation of autoxin in mesenchymal cells, including FLS, resulted in disease attenuation in animal models of arthritis (90).
Metabolic coupling of cells in the RA synovium
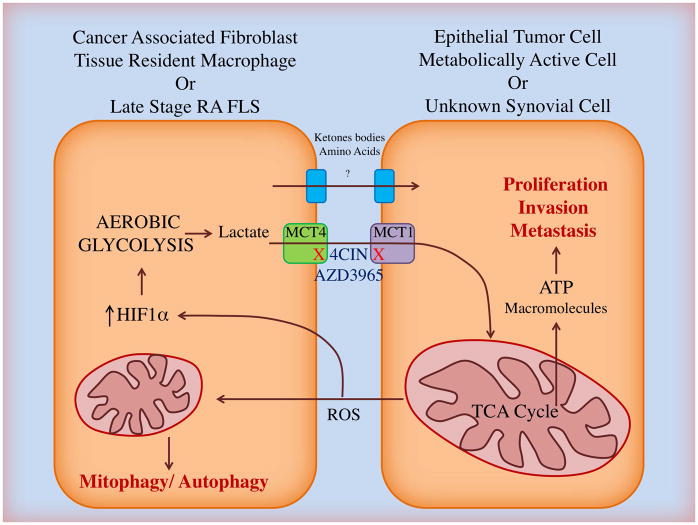
The problem with many of the studies described above is that they tend to treat FLS and STM as if they exist in isolation. But metabolism is not a private function, and metabolites produced by one cell can have profound effects on the biology of another. As described above, metabolites such as succinate, citrate and isocitrate accumulate in inflammatory macrophages and control metabolism in a feedback loop. It is possible that such metabolites derived from STM might have a role in influencing metabolism and function of proximal FLS, but this is yet to be investigated. Indeed, metabolite exchange between stromal and parenchymal cells is an essential function common to numerous metabolically active tissues including muscle, nerve, kidney, liver and testicle. SLC including the monocarboxylate transporters (MCT) are employed as a means of physiological metabolic coupling between different cell types, as extensively reviewed (91). Here macrophage-like cells commonly provide high-energy metabolic intermediates such as lactate and pyruvate to bioenergetically demanding partner cells where they can anaplerotically fuel the TCA cycle. A similar mechanism has been identified in epithelial cancers where both Warburg and so called ‘Reverse-Warburg’ metabolisms have been demonstrated and highlighted as therapeutic targets (92). The Warburg effect describes upregulation of aerobic glycolysis in the tumor cell itself and in the presence of ample oxygen. The ‘Reverse-Warburg effect’ describes a situation in which cancer-associated fibroblasts (CAF) provide lactate, ketone bodies and amino acids passed through MCT to fuel tumor cell proliferative behavior (93), as well as local angiogenesis associated with metastasis (94). Due to commonalities in cell behavior and microenvironment, one could hypothesize that similar symbiotic relationships might exist in the synovium to fuel immune cell effector function and FLS pathogenic behavior (Figure 4). Fujii et al showed in 2015 that late stage RA FLS have elevated levels of MCT4 compared to OA and that siRNA knockdown of MCT4 reduced arthritis severity in the murine collagen-induced arthritis model, linking their findings to apoptosis resistance and synovial acidification (95). Expulsion via MCT4 is likely to be a necessary mechanism to protect cells from damaging effects of lactate accumulation. However, whether the lactate can be taken up and utilized by other cells is unknown. It is very likely that the role of MCTs and the sharing of metabolites between cells will differ during the course of RA as a reflection of mitochondrial health and changes to the joint microenvironment. Consequently, it will be important to study metabolic coupling in normal tissue and in the acute phase of disease to determine whether such relationships exist and if so, exploit their therapeutic potential, as is showing promise in cancer (96).
Figure 4. The ‘reverse Warburg’ effect in cancer and rheumatoid arthritis?
In epithelial tumours, the reactive oxygen species (ROS) produced by metabolically active cancer cells causes mitochondria-selective autophagy (mitophagy) and activate hypoxia inducible factor 1α (HIF1α) in local cancer-associated fibroblasts (CAF). As a result the CAF upregulate aerobic glycolysis, producing copious lactate which is expelled from the cell via monocarboxylate transporter 4 (MCT4) and taken up by the cancer cell via MCT1. Lactate, pyruvate and other metabolic intermediates such as amino acids and ketone bodies can feed the mitochondrial TCA cycle in cancer cells or indeed local endothelium to increase ATP and biomolecule synthesis and drive pathogenic proliferation, invasion and metastasis. MCT 1 and 4 can be blocked in vitro using the small molecule inhibitor α-cyano-4-hydroxycinnamic acid (4CIN) and in vivo using AZD3965, which is in early phase clinical trials for treatment of small cell lung cancer. In RA it is known that mitochondrial damage, HIF1α activation and upregulation of MCT4 can be induced in late stage disease FLS by the pathogenic microenvironment but the metabolic relationships between these cells and other cells within the joint have yet to be elucidated.
Expanding knowledge of fibroblast metabolism
The study of metabolism in cultured synovial cells has been important in understanding RA biology. Such studies have illustrated a ‘metabolic memory’ which is epigenetically imprinted upon cells in an inflammatory microenvironment and is lasting in in vitro culture. Metabolomic analysis continues to be a useful strategy for providing a metabolic snapshot on the status of such cells and to hint toward pathways worthy of further study. Use of technologies such as the Seahorse analyzer to observe bioenergetic responses, small molecule metabolic inhibitors and animal models have also played a role in expanding our current understanding of the field. However, for capturing the dynamic processes that regulate cellular metabolism, stable isotope metabolic tracer analysis is a powerful technique not yet applied to synoviocytes (97). This technique provides a comprehensive biological overview, allowing simulation and reconstruction of metabolism, and can provide insight at the compartment level (e.g. mitochondria versus cytoplasm, depending upon the tracer) (98). The increased concentration of a metabolite can be associated to either the upregulation of the enzyme responsible for synthesis or the downregulation of the one consuming it and metabolic tracer analysis allows these processes to be deconvoluted where traditional metabolomics is less informative (99, 100). Several heavy isotopes are available and include deuterium (2H), nitrogen (15N), oxygen (18O) but the most commonly used is carbon (13C). The most common isotopically labeled tracers are 13C-glucose and 13C-glutamine as these metabolites are main energy sources in many mammalian systems (101). Recent development of approaches to apply tandem mass spectrometry (MS/MS) to isotope tracing has offered the opportunity to determine positional labeling based on mass fragmentation (102).
As alluded to above, studying metabolic relationships of synoviocytes after treatment (103), and between FLS and STM within the RA synovium is an important future direction. Stable isotope analysis in concert with small molecule inhibitors will allow us to trace metabolite exchange between cells in culture. There is also a pressing need for conditional knock out animals to observe the importance of metabolite channels, transporters and enzymes in inflammatory diseases as an area of great therapeutic potential. Finally, better histological markers are improving our understanding of FLS subsets, resident and infiltrating STM and the tissue organization of these cells. Imaging mass spectrometry can provide a metabolomic snapshot on a per pixel basis and may reveal spatially distinct metabolic signatures and important metabolic heterogeneity within the synovial environment (104).
Metabolic pathways as therapeutic targets in rheumatoid arthritis
The metabolic rewiring of immune cells has been viewed as a promising source of novel drug targets (8, 14, 105) but resetting metabolism in tissues central to RA pathogenesis offers additional opportunities for disease modulation and restoration of homeostasis in RA. In fact, rheumatologists already use the antimetabolites methotrexate (MTX) and leflunomide for the treatment of patients with inflammatory arthritis. Both drugs inhibit the reproduction of rapidly dividing cells such as lymphocytes, but have also been shown to inhibit FLS functions (106, 107). Of interest, sulfasalazine, which was initially developed as an anti-inflammatory drug to treat rheumatoid arthritis, was subsequently found to inhibit XCT, a cystine–glutamate exchange transporter (108).
Targeted approaches to metabolic inhibition are required to inhibit aggressive behavior in pathological cell types and leave those which aid resolution intact. Potentiation or restoration of protective mechanisms such as mitochondrial fusion and biogenesis and mechanisms which promote resolution of inflammation may also be favorable. Indeed, small molecules such as Mdivi1 which potentiate mitochondrial fusion have already shown potential in animal models of sepsis and may have further reaching applications (109). In addition, metformin is used in clinical management of type II diabetes and has shown promise in mouse models of arthritis (110). Though its effects on FLS are unknown, it is thought to act upon immune cells both by inhibiting complex I of the mitochondrial electron transport chain and through effects on the signaling molecule AMPK. Other AMPK activators, which will control cell proliferation and promote mitochondrial biogenesis might be another approach (43, 44).
Inhibitors of the signaling molecule PI3K which regulates glycolysis upstream of mTOR are in early stage clinical trials for cancer and show potential in RA (111). Furthermore, blockers of key glycolytic enzymes including phosphofructokinase-2/fructose-2,6-bisphosphatase 3 (PFKFB3) are showing promise to work in synergy with other inhibitors that target the angiogenic factor VEGF (112). Inhibiting glycolytic intermediates including succinate or lactate production could be of interest if proven to fuel synovial cell activation. Use of a small molecule modulator of PKM2 to prevent nuclear translocation has also potential for driving a shift toward an M2 phenotype and restoration of tolerance in diseases such as RA. In FLS, HK2 release from the mitochondrial membrane could potentially trigger FLS apoptosis. However, a better understanding of the signaling pathways which dictate the metabolic phenotype of immune and stromal cells in RA is required to capitalize on this area.
Blockers of the MCT which are central to metabolic coupling have been used in as yet unpublished first-in-man trials for epithelial cancers, and the effects of their knockdown in experimental arthritis suggest they offer potential in RA (95). Other solute carrier transporters, including Glut1, aminoacids or choline are potential therapeutic targets yet to be explored in arthritis. Furthermore, better stratification of patients through prognostic metabolomic analysis (16) and techniques such as choline C-11 PET scanning (83) may improve treatment of non-responders to existing therapies. Overall, whether targeting metabolism truly presents an option to increase the drug armamentarium in rheumatic diseases remains to be determined.
Summary
Systemic metabolism, immunometabolism and stromal metabolism are altered in RA and a growing body of publications in this field offers novel biomarkers for patient stratification and avenues for treatment. However, we are far from an exhaustive understanding of the pathways which discern the normal or pathogenic phenotypes of cells resident to the synovium in order to capitalize on these therapeutic opportunities. It will be important to better understand mitochondrial metabolism and dynamics, which are sparsely studied in the synovium, with a view to harnessing fusion and biogenesis pathways to restore mitochondrial health and tip the balance away from aerobic glycolysis in RA. Future directions toward single cell transcriptomic analysis and use of cells sorted directly from synovial tissue promises to improve our understanding of FLS and STM biology and there is a need for metabolic profiling of these cells prior to dedifferentiation associated with cell culture. Growing evidence suggests that multiple fibroblast and macrophage subsets are present in the inflamed synovium and their characterization will aid in directing new treatment toward those with a pathogenic and not a protective phenotype. Furthermore, the metabolic interactions between FLS, STM and infiltrating immune cells are unexplored and lessons from cancer biology suggest this will provide further avenues for therapeutic intervention. There is a pressing need for techniques such as stable isotope-based metabolic tracer analysis to track these interactions. Finally, a strategic window of opportunity exists such that RA patients receiving disease modifying treatments within 3 months of symptom development show a much improved prognosis when compared to those treated after this time point (113). To date, all studies of FLS metabolism have been conducted in cells derived from the joints of late stage disease patients after arthroplasty and studies of macrophages have utilized cells differentiated from monocytes in vitro. As we begin to understand that transient metabolic responses in acute inflammation may differ significantly from metabolic adaptation to damage in chronic inflammation, characterization of cells from uninflamed and earliest stages of human disease is needed to inform appropriate future therapeutic strategies with the ultimate goal of drug-free remission or cure for RA.
Acknowledgments
M.G. was supported by NIH 1K08AR064834, R03AR068094 and Rheumatology Research Foundation. This work was also supported by grants from the NIHR/Welcome Trust Clinical Research Facility, University Hospitals Birmingham NHS Foundation Trust and from Arthritis Research UK Grants: Targeting fibroblasts in the treatment of inflammatory arthritis (19791), Rheumatoid Arthritis Pathogenesis Centre of Excellence grant (20298) and Arthritis Research UK Experimental Arthritis Treatment Centre (20015).
References
- 1.Bettencourt IA, Powell JD. Targeting Metabolism as a Novel Therapeutic Approach to Autoimmunity, Inflammation, and Transplantation. J Immunol. 2017;198(3):999–1005. doi: 10.4049/jimmunol.1601318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel CH, Powell JD. Targeting T cell metabolism to regulate T cell activation, differentiation and function in disease. Curr Opin Immunol. 2017;46:82–8. doi: 10.1016/j.coi.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahlqvist J. A hypothesis on the pathogenesis of rheumatoid and other non-specific synovitides. IV A. The possible intermediate role of local hypoxia and metabolic alterations. Med Hypotheses. 1984;13(3):257–302. doi: 10.1016/0306-9877(84)90162-2. [DOI] [PubMed] [Google Scholar]
- 4.Henderson B, Bitensky L, Chayen J. Glycolytic activity in human synovial lining cells in rheumatoid arthritis. Ann Rheum Dis. 1979;38(1):63–7. doi: 10.1136/ard.38.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bottini N, Firestein GS. Duality of fibroblast-like synoviocytes in RA: passive responders and imprinted aggressors. Nat Rev Rheumatol. 2013;9(1):24–33. doi: 10.1038/nrrheum.2012.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bustamante MF, Garcia-Carbonell R, Whisenant KD, Guma M. Fibroblast-like synoviocyte metabolism in the pathogenesis of rheumatoid arthritis. Arthritis Res Ther. 2017;19(1):110. doi: 10.1186/s13075-017-1303-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naylor AJ, Filer A, Buckley CD. The role of stromal cells in the persistence of chronic inflammation. Clin Exp Immunol. 2013;171(1):30–5. doi: 10.1111/j.1365-2249.2012.04634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Neill LAJ, Kishton RJ, Rathmell J. A guide to immunometabolism for immunologists. Nat Rev Immunol. 2016;16(9):553–65. doi: 10.1038/nri.2016.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel R, Filer A, Barone F, Buckley CD. Stroma: fertile soil for inflammation. Best practice & research Clinical rheumatology. 2014;28(4):565–76. doi: 10.1016/j.berh.2014.10.022. [DOI] [PubMed] [Google Scholar]
- 10.Juarez M, Filer A, Buckley CD. Fibroblasts as therapeutic targets in rheumatoid arthritis and cancer. Swiss Med Wkly. 2012;142:w13529. doi: 10.4414/smw.2012.13529. [DOI] [PubMed] [Google Scholar]
- 11.Metsios GS, Stavropoulos-Kalinoglou A, Nevill AM, Douglas KM, Koutedakis Y, Kitas GD. Cigarette smoking significantly increases basal metabolic rate in patients with rheumatoid arthritis. Ann Rheum Dis. 2008;67(1):70–3. doi: 10.1136/ard.2006.068403. [DOI] [PubMed] [Google Scholar]
- 12.Cojocaru M, Cojocaru IM, Silosi I, Vrabie CD. Metabolic Syndrome in Rheumatoid Arthritis. Mædica. 2012;7(2):148–52. [PMC free article] [PubMed] [Google Scholar]
- 13.Cederholm T, Wretlind B, Hellstrom K, Andersson B, Engstrom L, Brismar K, et al. Enhanced generation of interleukins 1 beta and 6 may contribute to the cachexia of chronic disease. Am J Clin Nutr. 1997;65(3):876–82. doi: 10.1093/ajcn/65.3.876. [DOI] [PubMed] [Google Scholar]
- 14.Guma M, Tiziani S, Firestein GS. Metabolomics in rheumatic diseases: desperately seeking biomarkers. Nat Rev Rheumatol. 2016;12(5):269–81. doi: 10.1038/nrrheum.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alonso A, Julià A, Vinaixa M, Domènech E, Fernández-Nebro A, Cañete JD, et al. Urine metabolome profiling of immune-mediated inflammatory diseases. BMC Medicine. 2016;14(1):133. doi: 10.1186/s12916-016-0681-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Young SP, Kapoor SR, Viant MR, Byrne JJ, Filer A, Buckley CD, et al. The impact of inflammation on metabolomic profiles in patients with arthritis. Arthritis Rheum. 2013;65(8):2015–23. doi: 10.1002/art.38021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Madsen RK, Lundstedt T, Gabrielsson J, Sennbro CJ, Alenius GM, Moritz T, et al. Diagnostic properties of metabolic perturbations in rheumatoid arthritis. Arthritis research & therapy. 2011;13(1):R19. doi: 10.1186/ar3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hugle T, Kovacs H, Heijnen IA, Daikeler T, Baisch U, Hicks JM, et al. Synovial fluid metabolomics in different forms of arthritis assessed by nuclear magnetic resonance spectroscopy. Clinical and experimental rheumatology. 2012;30(2):240–5. [PubMed] [Google Scholar]
- 19.Kim S, Hwang J, Xuan J, Jung YH, Cha H-S, Kim KH. Global Metabolite Profiling of Synovial Fluid for the Specific Diagnosis of Rheumatoid Arthritis from Other Inflammatory Arthritis. PLOS ONE. 2014;9(6):e97501. doi: 10.1371/journal.pone.0097501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cuppen BVJ, Fu J, van Wietmarschen HA, Harms AC, Koval S, Marijnissen ACA, et al. Exploring the Inflammatory Metabolomic Profile to Predict Response to TNF-α Inhibitors in Rheumatoid Arthritis. PLOS ONE. 2016;11(9):e0163087. doi: 10.1371/journal.pone.0163087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sweeney SR, Kavanaugh A, Lodi A, Wang B, Boyle D, Tiziani S, et al. Metabolomic profiling predicts outcome of rituximab therapy in rheumatoid arthritis. RMD Open. 2016;2(2):e000289. doi: 10.1136/rmdopen-2016-000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tatar Z, Migne C, Petera M, Gaudin P, Lequerre T, Marotte H, et al. Variations in the metabolome in response to disease activity of rheumatoid arthritis. BMC Musculoskeletal Disorders. 2016;17(1):353. doi: 10.1186/s12891-016-1214-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith MD. The Normal Synovium. The Open Rheumatology Journal. 2011;5:100–6. doi: 10.2174/1874312901105010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Misharin AV, Cuda CM, Saber R, Turner JD, Gierut AK, Haines GK, 3rd, et al. Nonclassical Ly6C(−) monocytes drive the development of inflammatory arthritis in mice. Cell Rep. 2014;9(2):591–604. doi: 10.1016/j.celrep.2014.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Udalova IA, Mantovani A, Feldmann M. Macrophage heterogeneity in the context of rheumatoid arthritis. Nat Rev Rheumatol. 2016;12(8):472–85. doi: 10.1038/nrrheum.2016.91. [DOI] [PubMed] [Google Scholar]
- 26.Bottini N, Firestein GS. Duality of fibroblast-like synoviocytes in RA: passive responders and imprinted aggressors. Nat Rev Rheumatol. 2013;9(1):24–33. doi: 10.1038/nrrheum.2012.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Croft AP, Naylor AJ, Marshall JL, Hardie DL, Zimmermann B, Turner J, et al. Rheumatoid synovial fibroblasts differentiate into distinct subsets in the presence of cytokines and cartilage. Arthritis research & therapy. 2016;18:270. doi: 10.1186/s13075-016-1156-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mizoguchi FSK, Chang SK, Rao DA, Nguyen H, Noss EH, Earp BE, Blazar PE, Wright J, Simmons BP, Hacohen N, Nigrovic PA, Raychaudhuri S, Brenner MB. Identification of Synovial Fibroblast Subsets That Define Pathology in Rheumatoid Arthritis [abstract] Arthritis & Rheumatology. 2015;67(supplement 10) [Google Scholar]
- 29.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423(6937):356–61. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 30.Bartok B, Firestein GS. Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunol Rev. 2010;233(1):233–55. doi: 10.1111/j.0105-2896.2009.00859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Biniecka M, Fox E, Gao W, Ng CT, Veale DJ, Fearon U, et al. Hypoxia induces mitochondrial mutagenesis and dysfunction in inflammatory arthritis. Arthritis Rheum. 2011;63(8):2172–82. doi: 10.1002/art.30395. [DOI] [PubMed] [Google Scholar]
- 32.Gaber T, Dziurla R, Tripmacher R, Burmester GR, Buttgereit F. Hypoxia inducible factor (HIF) in rheumatology: low O2! See what HIF can do! Ann Rheum Dis. 2005;64(7):971–80. doi: 10.1136/ard.2004.031641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Szekanecz Z, Besenyei T, Paragh G, Koch AE. Angiogenesis in rheumatoid arthritis. Autoimmunity. 2009;42(7):563–73. doi: 10.1080/08916930903143083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahn JK, Kim S, Hwang J, Kim J, Kim KH, Cha HS. Joint Bone Spine. 2016. GC/TOF-MS-based metabolomic profiling in cultured fibroblast-like synoviocytes from rheumatoid arthritis. [DOI] [PubMed] [Google Scholar]
- 35.Guma M, Sanchez-Lopez E, Lodi A, Garcia-Carbonell R, Tiziani S, Karin M, et al. Choline kinase inhibition in rheumatoid arthritis. Ann Rheum Dis. 2015;74(7):1399–407. doi: 10.1136/annrheumdis-2014-205696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kurowska-Stolarska M, Alivernini S. Synovial tissue macrophages: friend or foe? RMD Open. 2017;3(2) doi: 10.1136/rmdopen-2017-000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weyand CM, Zeisbrich M, Goronzy JJ. Metabolic signatures of T-cells and macrophages in rheumatoid arthritis. Current Opinion in Immunology. 2017;46:112–20. doi: 10.1016/j.coi.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nayar S, Campos J, Steinthal N, Barone F. Tissue Digestion for Stromal Cell and Leukocyte Isolation. Methods in molecular biology (Clifton, NJ) 2017;1591:225–34. doi: 10.1007/978-1-4939-6931-9_16. [DOI] [PubMed] [Google Scholar]
- 39.Ghesquiere B, Wong BW, Kuchnio A, Carmeliet P. Metabolism of stromal and immune cells in health and disease. Nature. 2014;511(7508):167–76. doi: 10.1038/nature13312. [DOI] [PubMed] [Google Scholar]
- 40.Lemons JM, Feng XJ, Bennett BD, Legesse-Miller A, Johnson EL, Raitman I, et al. Quiescent fibroblasts exhibit high metabolic activity. PLoS Biol. 2010;8(10):e1000514. doi: 10.1371/journal.pbio.1000514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O’Neill LAJ, Hardie DG. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature. 2013;493(7432):346–55. doi: 10.1038/nature11862. [DOI] [PubMed] [Google Scholar]
- 42.Weichhart T, Hengstschlager M, Linke M. Regulation of innate immune cell function by mTOR. Nat Rev Immunol. 2015;15(10):599–614. doi: 10.1038/nri3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yuan H-X, Xiong Y, Guan K-L. Nutrient sensing, metabolism, and cell growth control. Molecular cell. 2013;49(3):379–87. doi: 10.1016/j.molcel.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vincent EE, Coelho PP, Blagih J, Griss T, Viollet B, Jones RG. Differential effects of AMPK agonists on cell growth and metabolism. Oncogene. 2015;34(28):3627–39. doi: 10.1038/onc.2014.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Covarrubias AJ, Aksoylar HI, Horng T. Control of macrophage metabolism and activation by mTOR and Akt signaling. Seminars in immunology. 2015;27(4):286–96. doi: 10.1016/j.smim.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guma M, Wang Y, Viollet B, Liu-Bryan R. AMPK Activation by A-769662 Controls IL-6 Expression in Inflammatory Arthritis. PLoS ONE. 2015;10(10):e0140452. doi: 10.1371/journal.pone.0140452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park SY, Lee SW, Lee SY, Hong KW, Bae SS, Kim K, et al. SIRT1/Adenosine Monophosphate-Activated Protein Kinase α Signaling Enhances Macrophage Polarization to an Anti-inflammatory Phenotype in Rheumatoid Arthritis. Front Immunol. 2017;8:1135. doi: 10.3389/fimmu.2017.01135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wruck CJ, Fragoulis A, Gurzynski A, Brandenburg LO, Kan YW, Chan K, et al. Role of oxidative stress in rheumatoid arthritis: insights from the Nrf2-knockout mice. Ann Rheum Dis. 2011;70(5):844–50. doi: 10.1136/ard.2010.132720. [DOI] [PubMed] [Google Scholar]
- 49.Jornayvaz FR, Shulman GI. Regulation of mitochondrial biogenesis. Essays Biochem. 2010;47:69–84. doi: 10.1042/bse0470069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Osellame LD, Blacker TS, Duchen MR. Cellular and molecular mechanisms of mitochondrial function. Best Pract Res Clin Endocrinol Metab. 2012;26(6):711–23. doi: 10.1016/j.beem.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krysko DV, Agostinis P, Krysko O, Garg AD, Bachert C, Lambrecht BN, et al. Emerging role of damage-associated molecular patterns derived from mitochondria in inflammation. Trends Immunol. 2011;32(4):157–64. doi: 10.1016/j.it.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 52.Wai T, Langer T. Mitochondrial Dynamics and Metabolic Regulation. Trends in Endocrinology & Metabolism. 27(2):105–17. doi: 10.1016/j.tem.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 53.Seo AY, Joseph AM, Dutta D, Hwang JC, Aris JP, Leeuwenburgh C. New insights into the role of mitochondria in aging: mitochondrial dynamics and more. Journal of cell science. 2010;123(Pt 15):2533–42. doi: 10.1242/jcs.070490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Corcoran SE, O’Neill LA. HIF1alpha and metabolic reprogramming in inflammation. J Clin Invest. 2016;126(10):3699–707. doi: 10.1172/JCI84431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mills E, O’Neill LA. Succinate: a metabolic signal in inflammation. Trends Cell Biol. 2014;24(5):313–20. doi: 10.1016/j.tcb.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 56.Lampropoulou V, Sergushichev A, Bambouskova M, Nair S, Vincent EE, Loginicheva E, et al. Itaconate Links Inhibition of Succinate Dehydrogenase with Macrophage Metabolic Remodeling and Regulation of Inflammation. Cell Metab. 2016;24(1):158–66. doi: 10.1016/j.cmet.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hollander AP, Corke KP, Freemont AJ, Lewis CE. Expression of hypoxia-inducible factor 1alpha by macrophages in the rheumatoid synovium: implications for targeting of therapeutic genes to the inflamed joint. Arthritis Rheum. 2001;44(7):1540–4. doi: 10.1002/1529-0131(200107)44:7<1540::AID-ART277>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 58.Kim EK, Kwon JE, Lee SY, Lee EJ, Kim DS, Moon SJ, et al. IL-17-mediated mitochondrial dysfunction impairs apoptosis in rheumatoid arthritis synovial fibroblasts through activation of autophagy. Cell Death Dis. 2017;8(1):e2565. doi: 10.1038/cddis.2016.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Biniecka M, Canavan M, McGarry T, Gao W, McCormick J, Cregan S, et al. Dysregulated bioenergetics: a key regulator of joint inflammation. Ann Rheum Dis. 2016 doi: 10.1136/annrheumdis-2015-208476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Garcia-Carbonell R, Divakaruni AS, Lodi A, Vicente-Suarez I, Saha A, Cheroutre H, et al. Critical role of fibroblast-like synoviocytes glycolytic metabolism in rheumatoid arthritis. Arthritis Rheumatol. 2016 doi: 10.1002/art.39608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fearon U, Canavan M, Biniecka M, Veale DJ. Hypoxia, mitochondrial dysfunction and synovial invasiveness in rheumatoid arthritis. Nat Rev Rheumatol. 2016 doi: 10.1038/nrrheum.2016.69. [DOI] [PubMed] [Google Scholar]
- 62.Ng CT, Biniecka M, Kennedy A, McCormick J, FitzGerald O, Bresnihan B, et al. Synovial tissue hypoxia and inflammation in vivo. Annals of the Rheumatic Diseases. 2010 doi: 10.1136/ard.2009.119776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Da Sylva TR, Connor A, Mburu Y, Keystone E, Wu GE. Somatic mutations in the mitochondria of rheumatoid arthritis synoviocytes. Arthritis research & therapy. 2005;7(4):R844–51. doi: 10.1186/ar1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Harty LC, Biniecka M, O’Sullivan J, Fox E, Mulhall K, Veale DJ, et al. Mitochondrial mutagenesis correlates with the local inflammatory environment in arthritis. Ann Rheum Dis. 2012;71(4):582–8. doi: 10.1136/annrheumdis-2011-200245. [DOI] [PubMed] [Google Scholar]
- 65.Pope RM. Apoptosis as a therapeutic tool in rheumatoid arthritis. Nature Reviews Immunology. 2002;2:527. doi: 10.1038/nri846. [DOI] [PubMed] [Google Scholar]
- 66.Mayers JR, Vander Heiden MG. Famine versus feast: understanding the metabolism of tumors in vivo. Trends in biochemical sciences. 2015;40(3):130–40. doi: 10.1016/j.tibs.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bell C, English L, Boulais J, Chemali M, Caron-Lizotte O, Desjardins M, et al. Quantitative Proteomics Reveals the Induction of Mitophagy in Tumor Necrosis Factor-α-activated (TNFα) Macrophages. Molecular & Cellular Proteomics: MCP. 2013;12(9):2394–407. doi: 10.1074/mcp.M112.025775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xu K, Xu P, Yao JF, Zhang YG, Hou WK, Lu SM. Reduced apoptosis correlates with enhanced autophagy in synovial tissues of rheumatoid arthritis. Inflammation research: official journal of the European Histamine Research Society [et al] 2013;62(2):229–37. doi: 10.1007/s00011-012-0572-1. [DOI] [PubMed] [Google Scholar]
- 69.Zhang J, Song X, Cao W, Lu J, Wang X, Wang G, et al. Autophagy and mitochondrial dysfunction in adjuvant-arthritis rats treatment with resveratrol. Sci Rep. 2016;6:32928. doi: 10.1038/srep32928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shin YJ, Han SH, Kim DS, Lee GH, Yoo WH, Kang YM, et al. Autophagy induction and CHOP under-expression promotes survival of fibroblasts from rheumatoid arthritis patients under endoplasmic reticulum stress. Arthritis Res Ther. 2010;12(1):R19. doi: 10.1186/ar2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–33. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4(11):891–9. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 73.Kubota K, Ito K, Morooka M, Mitsumoto T, Kurihara K, Yamashita H, et al. Whole-body FDG-PET/CT on rheumatoid arthritis of large joints. Annals of nuclear medicine. 2009;23(9):783–91. doi: 10.1007/s12149-009-0305-x. [DOI] [PubMed] [Google Scholar]
- 74.Weyand CM, Goronzy JJ. Immunometabolism in early and late stages of rheumatoid arthritis. Nat Rev Rheumatol. 2017;13(5):291–301. doi: 10.1038/nrrheum.2017.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Volchenkov R, Dung Cao M, Elgstoen KB, Goll GL, Eikvar K, Bjorneboe O, et al. Metabolic profiling of synovial tissue shows altered glucose and choline metabolism in rheumatoid arthritis samples. Scand J Rheumatol. 2016:1–2. doi: 10.3109/03009742.2016.1164242. [DOI] [PubMed] [Google Scholar]
- 76.Yang Z, Fujii H, Mohan SV, Goronzy JJ, Weyand CM. Phosphofructokinase deficiency impairs ATP generation, autophagy, and redox balance in rheumatoid arthritis T cells. J Exp Med. 2013;210(10):2119–34. doi: 10.1084/jem.20130252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ahn JK, Kim S, Hwang J, Kim J, Kim KH, Cha HS. GC/TOF-MS-based metabolomic profiling in cultured fibroblast-like synoviocytes from rheumatoid arthritis. Joint Bone Spine. 2016;83(6):707–13. doi: 10.1016/j.jbspin.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 78.Li Y, Zheng JY, Liu JQ, Yang J, Liu Y, Wang C, et al. Succinate/NLRP3 Inflammasome Induces Synovial Fibroblast Activation: Therapeutical Effects of Clematichinenoside AR on Arthritis. Front Immunol. 2016;7:532. doi: 10.3389/fimmu.2016.00532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chang CH, Curtis JD, Maggi LB, Jr, Faubert B, Villarino AV, O’Sullivan D, et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell. 2013;153(6):1239–51. doi: 10.1016/j.cell.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yu X, Li S. Non-metabolic functions of glycolytic enzymes in tumorigenesis. Oncogene. 2016 doi: 10.1038/onc.2016.410. [DOI] [PubMed] [Google Scholar]
- 81.Moon JS, Hisata S, Park MA, DeNicola GM, Ryter SW, Nakahira K, et al. mTORC1-Induced HK1-Dependent Glycolysis Regulates NLRP3 Inflammasome Activation. Cell reports. 2015;12(1):102–15. doi: 10.1016/j.celrep.2015.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 82.Nomura M, Liu J, Rovira II, Gonzalez-Hurtado E, Lee J, Wolfgang MJ, et al. Fatty acid oxidation in macrophage polarization. Nature immunology. 2016;17(3):216–7. doi: 10.1038/ni.3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Roivainen A, Parkkola R, Yli-Kerttula T, Lehikoinen P, Viljanen T, Mottonen T, et al. Use of positron emission tomography with methyl-11C-choline and 2-18F-fluoro-2-deoxy-D-glucose in comparison with magnetic resonance imaging for the assessment of inflammatory proliferation of synovium. Arthritis Rheum. 2003;48(11):3077–84. doi: 10.1002/art.11282. [DOI] [PubMed] [Google Scholar]
- 84.Seki M, Kawai Y, Ishii C, Yamanaka T, Odawara M, Inazu M. Functional analysis of choline transporters in rheumatoid arthritis synovial fibroblasts. Modern rheumatology. 2017:1–9. doi: 10.1080/14397595.2017.1280118. [DOI] [PubMed] [Google Scholar]
- 85.Lim HK, Choi YA, Park W, Lee T, Ryu SH, Kim SY, et al. Phosphatidic acid regulates systemic inflammatory responses by modulating the Akt-mammalian target of rapamycin-p70 S6 kinase 1 pathway. J Biol Chem. 2003;278(46):45117–27. doi: 10.1074/jbc.M303789200. [DOI] [PubMed] [Google Scholar]
- 86.Friday SC, Fox DA. Phospholipase D enzymes facilitate IL-17- and TNFalpha-induced expression of proinflammatory genes in rheumatoid arthritis synovial fibroblasts (RASF) Immunol Lett. 2016;174:9–18. doi: 10.1016/j.imlet.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 87.Fuchs B, Schiller J, Wagner U, Hantzschel H, Arnold K. The phosphatidylcholine/lysophosphatidylcholine ratio in human plasma is an indicator of the severity of rheumatoid arthritis: investigations by 31P NMR and MALDI-TOF MS. Clin Biochem. 2005;38(10):925–33. doi: 10.1016/j.clinbiochem.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 88.Bourgoin SG, Zhao C. Autotaxin and lysophospholipids in rheumatoid arthritis. Curr Opin Investig Drugs. 2010;11(5):515–26. [PubMed] [Google Scholar]
- 89.Miyabe Y, Miyabe C, Iwai Y, Yokoyama W, Sekine C, Sugimoto K, et al. Activation of fibroblast-like synoviocytes derived from rheumatoid arthritis via lysophosphatidic acid-lysophosphatidic acid receptor 1 cascade. Arthritis Res Ther. 2014;16(5):461. doi: 10.1186/s13075-014-0461-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nikitopoulou I, Oikonomou N, Karouzakis E, Sevastou I, Nikolaidou-Katsaridou N, Zhao Z, et al. Autotaxin expression from synovial fibroblasts is essential for the pathogenesis of modeled arthritis. J Exp Med. 2012;209(5):925–33. doi: 10.1084/jem.20112012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Halestrap AP. Monocarboxylic acid transport. Compr Physiol. 2013;3(4):1611–43. doi: 10.1002/cphy.c130008. [DOI] [PubMed] [Google Scholar]
- 92.Pavlides S, Whitaker-Menezes D, Castello-Cros R, Flomenberg N, Witkiewicz AK, Frank PG, et al. The reverse Warburg effect: aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle. 2009;8(23):3984–4001. doi: 10.4161/cc.8.23.10238. [DOI] [PubMed] [Google Scholar]
- 93.Pavlides S, Vera I, Gandara R, Sneddon S, Pestell RG, Mercier I, et al. Warburg meets autophagy: cancer-associated fibroblasts accelerate tumor growth and metastasis via oxidative stress, mitophagy, and aerobic glycolysis. Antioxidants & redox signaling. 2012;16(11):1264–84. doi: 10.1089/ars.2011.4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vegran F, Boidot R, Michiels C, Sonveaux P, Feron O. Lactate influx through the endothelial cell monocarboxylate transporter MCT1 supports an NF-kappaB/IL-8 pathway that drives tumor angiogenesis. Cancer research. 2011;71(7):2550–60. doi: 10.1158/0008-5472.CAN-10-2828. [DOI] [PubMed] [Google Scholar]
- 95.Fujii W, Kawahito Y, Nagahara H, Kukida Y, Seno T, Yamamoto A, et al. Monocarboxylate transporter 4, associated with the acidification of synovial fluid, is a novel therapeutic target for inflammatory arthritis. Arthritis Rheumatol. 2015;67(11):2888–96. doi: 10.1002/art.39270. [DOI] [PubMed] [Google Scholar]
- 96.Sanita P, Capulli M, Teti A, Galatioto GP, Vicentini C, Chiarugi P, et al. Tumor-stroma metabolic relationship based on lactate shuttle can sustain prostate cancer progression. BMC cancer. 2014;14:154. doi: 10.1186/1471-2407-14-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Metallo CM. In: Metabolic analysis using stable isotopes. Metallo CM, editor. United States: Waltham, MA: Elsevier Academic Press; 2015. [Google Scholar]
- 98.Zamboni N. 13 C metabolic flux analysis in complex systems. Current opinion in biotechnology. 2011;22(1):103–8. doi: 10.1016/j.copbio.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 99.Birkemeyer C, Luedemann A, Wagner C, Erban A, Kopka J. Metabolome analysis: the potential of in vivo labeling with stable isotopes for metabolite profiling. Trends in biotechnology. 2005;23(1):28–33. doi: 10.1016/j.tibtech.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 100.Rodrigues TB, Serrao EM, Kennedy BW, Hu D-E, Kettunen MI, Brindle KM. Magnetic resonance imaging of tumor glycolysis using hyperpolarized 13C-labeled glucose. Nature medicine. 2014;20(1):93–7. doi: 10.1038/nm.3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schug ZT, Peck B, Jones DT, Zhang Q, Grosskurth S, Alam IS, et al. Acetyl-CoA Synthetase 2 Promotes Acetate Utilization and Maintains Cancer Cell Growth under Metabolic Stress. Cancer cell. 2015;27(1):57–71. doi: 10.1016/j.ccell.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Choi J, Grossbach MT, Antoniewicz MR. Measuring complete isotopomer distribution of aspartate using gas chromatography/tandem mass spectrometry. Analytical chemistry. 2012;84(10):4628–32. doi: 10.1021/ac300611n. [DOI] [PubMed] [Google Scholar]
- 103.Ahn JK, Kim S, Hwang J, Kim J, Lee YS, Koh EM, et al. Metabolomic Elucidation of the Effects of Curcumin on Fibroblast-Like Synoviocytes in Rheumatoid Arthritis. PLoS One. 2015;10(12):e0145539. doi: 10.1371/journal.pone.0145539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rocha B, Ruiz-Romero C, Blanco FJ. Mass spectrometry imaging: a novel technology in rheumatology. Nat Rev Rheumatol. 2017;13(1):52–63. doi: 10.1038/nrrheum.2016.184. [DOI] [PubMed] [Google Scholar]
- 105.Galluzzi L, Kepp O, Vander Heiden MG, Kroemer G. Metabolic targets for cancer therapy. Nat Rev Drug Discov. 2013;12(11):829–46. doi: 10.1038/nrd4145. [DOI] [PubMed] [Google Scholar]
- 106.Lories RJ, Derese I, De Bari C, Luyten FP. In vitro growth rate of fibroblast-like synovial cells is reduced by methotrexate treatment. Ann Rheum Dis. 2003;62(6):568–71. doi: 10.1136/ard.62.6.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Vergne-Salle P, Leger DY, Bertin P, Treves R, Beneytout JL, Liagre B. Effects of the active metabolite of leflunomide, A77 1726, on cytokine release and the MAPK signalling pathway in human rheumatoid arthritis synoviocytes. Cytokine. 2005;31(5):335–48. doi: 10.1016/j.cyto.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 108.Timmerman LA, Holton T, Yuneva M, Louie RJ, Padro M, Daemen A, et al. Glutamine sensitivity analysis identifies the xCT antiporter as a common triple-negative breast tumor therapeutic target. Cancer Cell. 2013;24(4):450–65. doi: 10.1016/j.ccr.2013.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Buck MD, O’Sullivan D, Klein Geltink RI, Curtis JD, Chang CH, Sanin DE, et al. Mitochondrial Dynamics Controls T Cell Fate through Metabolic Programming. Cell. 2016;166(1):63–76. doi: 10.1016/j.cell.2016.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Son HJ, Lee J. Metformin attenuates experimental autoimmune arthritis through reciprocal regulation of Th17/Treg balance and osteoclastogenesis. 2014;2014:973986. doi: 10.1155/2014/973986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Massacesi C, Di Tomaso E, Urban P, Germa C, Quadt C, Trandafir L, et al. PI3K inhibitors as new cancer therapeutics: implications for clinical trial design. OncoTargets and therapy. 2016;9:203–10. doi: 10.2147/OTT.S89967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schoors S, De Bock K, Cantelmo AR, Georgiadou M, Ghesquiere B, Cauwenberghs S, et al. Partial and transient reduction of glycolysis by PFKFB3 blockade reduces pathological angiogenesis. Cell metabolism. 2014;19(1):37–48. doi: 10.1016/j.cmet.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 113.Raza K, Filer A. The therapeutic window of opportunity in rheumatoid arthritis: does it ever close? Ann Rheum Dis. 2015;74(5):793–4. doi: 10.1136/annrheumdis-2014-206993. [DOI] [PubMed] [Google Scholar]