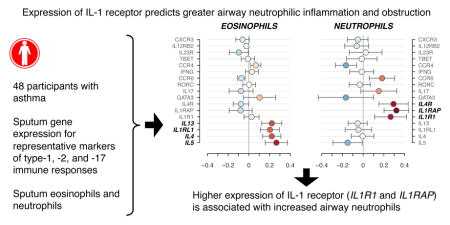
Abstract
Background
Various clinical, biologic or physiologic markers of asthma have been used to identify patient clusters and potential targets for therapy. However, these identifiers frequently overlap among the different asthma groups. For instance, both eosinophils and neutrophils are often increased in the airway of asthma patients despite their typical association with type-2 and type-17 immune response, respectively.
Objectives
To determine whether inflammatory gene expressions are related to patterns of airway inflammation and lung function, and identify molecular markers for neutrophilic asthma.
Methods
Expression levels of 17 genes characterizing type-1, type-2 and type-17 lymphocytes were measured in sputum samples from 48 participants with asthma. The relationships between gene expression levels and sputum cell differentials or measures of pulmonary function were examined using partial least squares regression.
Results
Gene expression levels were strongly associated with cell differentials, explaining 71% of variation in eosinophil counts and 64% of variation in neutrophil counts. The three genes with the strongest relationships to sputum neutrophils were IL1R1 (standardized regression coefficient β=+0.27, p=0.005), IL1RAP (β=+0.32, p=0.0004), and IL4R (β=+0.29, p=0.002). Higher expression levels of IL1R1, IL1RAP, and IL4R were associated with reduced FEV1/FVC (β= −0.11, −0.08, −0.10; p=0.005, 0.07, 0.05).
Conclusion
IL-1 receptor appears to be a marker of neutrophilic inflammation and airflow obstruction in patients with asthma that have a wide range of disease severity. The IL-1 pathway may contribute to airway neutrophilia, and is a potential therapeutic target in neutrophilic asthma.
Keywords: Asthma, sputum samples, type-1, type-2, type-17, lymphocyte, partial least squares (PLS), neutrophil, IL-1 receptor, pulmonary function
Graphical Abstract
Introduction
Asthma is a chronic inflammatory disease with airway inflammation marked by increased eosinophils, basophils, mast cells, innate lymphoid cells, and CD4+ T lymphocytes, which produce type-2 cytokines (IL-4, IL-5, and IL-13). In addition to type-2 cytokines, more severe asthma is often associated with an enhanced type-17 immune response, with increased IL-17A, IL-8, and airway neutrophils1–3. While type-2 asthma generally responds to corticosteroid (CS) therapy, asthma patients with low type-2 markers may not respond well to current therapies and lack CS sensitivity4. Several new biological therapeutics have been developed, targeting type-2 pathways, and have been shown to improve outcomes in patients with a predominantly eosinophilic phenotype. However, there is a critical need for new therapies to treat low type-2, neutrophilic asthma. Recent studies with large cohorts have aimed at phenotyping asthma in specific clusters. In the Severe Asthma Research Project (SARP) cohort, Moore et al used clinical and physiological characteristics as well as sputum and blood eosinophil and neutrophil counts to identify four clusters of asthma patients; two clusters which exhibited substantially higher sputum neutrophils also showed increased health care utilization, greater use of high dose inhaled CS and oral and systemic CS, and reduced lung function5. In addition, in this analysis, ~1/3 of both clusters with neutrophilic predominance also displayed high eosinophilia. In another report, mixed granulocytic sputum samples (high neutrophils + high eosinophils) were found in ~15 % of the asthmatic population6. Therefore, these studies indicate that the characteristics and biomarkers associated specifically with sputum neutrophils are difficult to identify.
The immune responses (type-2 and type-17) directing the accumulation of granulocytes (eosinophils and neutrophils) in the airway are complex. The type-2 and type-17 pathways can reciprocally down-regulate each other7, and it has been recently shown that highly polarized type-2 and type-17 pathways did not co-exist in asthmatic individuals8. Conversely, it has been reported that IL-17A and IL-4 were co-produced in T lymphocytes, and these type-17/type-2 CD4+ T lymphocytes are increased in asthma9. Also, airway dual-positive type-2/type-17 lymphocytes have been found in patients with severe asthma10. Studies by Liu et al and Seys et al have described the expression of high levels of either IL-4 or IL-5 along with IL-17 in asthmatic subjects 11–13. In addition, type-17 (IL-17A) and type-1 (IFN-γ) lymphocytes are concomitantly produced in circulating blood cells from asthmatic patients with steroid resistance14. Surprisingly enough, studies analyzing the expression of the three pathways, type-1, type-2 and type-17 in asthma are lacking. Although type-1, type-2 cytokines and the IL-17A protein have been concurrently measured in sputum samples in relation to airway remodeling, the association with inflammatory cells, particularly granulocytes, was not reported15.
Multivariate statistical methods are needed to explore these complex associations between molecular immune markers and the types of airway granulocytes in asthma. In this study, we used partial least squares (PLS) regression to analyze the relative expression of a group of genes characterizing the three T-cell immune response pathways (type-1, type-2 and type-17) in relation to cell differential in sputum samples and pulmonary functions in asthmatic patients. The objective was to identify gene predictors specifically of neutrophilic asthma and, therefore, to identify potential molecular pathways to target in developing new treatments for neutrophilic asthma.
Methods
Subjects
The study protocol was approved by the University of Wisconsin–Madison Health Sciences Institutional Review Board. Informed written consent was obtained from subjects before participation. Induced sputum was obtained by using a standard method as previously described16, from 48 subjects enrolled in SARP I&II at the University of Wisconsin. The subjects included in the current study are a subset of our recently published study, which included 56 subjects17. Of the 56 subjects, seven did not have cell differential performed in their sputum samples and an additional subject was excluded due to low expression level of the housekeeping gene (Ct>29) in the sputum sample, while the average Ct level of the housekeeping gene (GUSB) for the 48 samples was <23. Current smokers and patients with more than five pack-years of smoking history were ineligible to enroll in the study. All sputum induction visits were required to be at least four weeks after resolution of any asthma exacerbation. More detailed methods including acquirement of subjects’ characteristics and pulmonary functions were previously reported17.
RNA and reverse transcription quantitative PCR
Total RNA was prepared and PCR was performed as previously described17. Briefly, expression levels of mRNA were determined by qPCR with SYBR Green Master Mix (SA Biosciences) or TaqMan primers and probe (Life Technologies). Primers used in this study are shown Table E1. Standard curves were performed, and efficiencies were determined for each set of primers. Efficiencies ranged between 91% and 99%. Data are expressed as minus ΔCt using the reference gene β-glucuronidase (GUSB) as described previously18. Expressions of 17 different genes in 48 sputum samples from 48 subjects were analyzed, and 18 of 816 observations were missing. Out of the 18 missing observations, 7 did not reach sufficient quality as determined by the dissociation curves (3 IL4, 2 RORC, 1 IFNG, 1 IL1RAP), and 11 were lacking sufficient remaining sputum quantity to measure IL5 expression. More details are in the Supplemental Information.
Statistical analysis
Analyses were conducted using R version 3.2.319. Expression levels for each gene were centered and scaled to have zero mean and unit variance and normalized using the empirical normal quantile transformation20. Cell differential percentages and lung function measurements were centered and scaled (following log transformation for eosinophils, lymphocytes, FEV1 reversibility, and FVC reversibility) to have zero mean and unit variance. Missing gene expression values (18 out of 816 observations) were addressed using a regularized iterative PCA imputation algorithm using the R package missMDA version 1.1021. The correlation matrix of genes, cells, and pulmonary functions was displayed using the R package corrplot version 0.7722; genes, cells, and pulmonary functions are each arranged by the angular order of their correlation matrix eigenvectors23 which places similar variables adjacently.
Because the genes measured are relatively numerous and highly correlated with one another, PLS (partial least squares / projection to latent structures) regression models24, 25 were used to examine the relationships between gene expression and cell responses or lung function responses. Briefly, the goal of PLS regression is, given a set of numerous and/or correlated predictor variables, to extract a smaller set of orthogonal (uncorrelated) latent variables, called components, which best predict the response variable(s). For the cell differential PLS model, a multi-response (neutrophils, eosinophils, macrophages, lymphocytes) model was fit with 17 genes as predictors and a three-component model was selected (Figure E1). To interpret the latent variables, the X (gene predictors) loadings and Y (cell responses) loadings for each latent variable were examined. Jackknife variance estimates were used to construct confidence intervals for the regression coefficients for each gene. For pulmonary function responses, similar PLS regression models were fit and two-component models were selected in each case. The R package pls version 2.5-026 was used to fit and examine the PLS regression models. The optimal number of latent variables was chosen using 8-fold cross-validation.
Results
The subjects’ characteristics are described in Table 1. The 48 subjects had either severe asthma (n = 17) or non-severe asthma (n = 31), as defined by the American Thoracic Society criteria27. Fourteen subjects out of 48 were not using any corticosteroids, 16 were using low or medium doses of inhaled corticosteroids, 17 were taking high doses of inhaled corticosteroids, and one severe asthmatic subject took oral corticosteroids. Forty-three of the 48 subjects (90%) displayed an allergic reaction by skin prick test to at least one of 12 common aeroallergens.
Table 1.
Subject Characteristics (n=48)
| Age (y) | 28 [21, 45] |
| Sex | 21 F, 27 M |
| Race | 38 C, 6 AA, 2 H, 1 AsA, 1 NtA |
| BMI (kg/m2) | 27 [25, 32] |
| Asthma severity | 31 Non-severe, 17 Severe |
| Inhaled Corticosteroid Dose | 14 none, 6 low, 10 medium, 17 high |
| Oral Corticosteroid | 1 severe asthmatic subject |
| Total IgE (IU/mL) | 125 [44, 296] |
| Positive skin prick test | 90% (43/48) |
| FEV1 PP | 81 [66, 99] |
| FEV1/FVC PP | 87 [78, 98] |
| FEV1/FVC MX PP | 96 [88, 103] |
| FEV1 % reversibility | 13 [7, 24] |
| FVC % reversibility | 4 [1, 11] |
| RV/TLC PP | 117 [107, 132] |
| Sputum PMN % | 49 [33, 66] |
| Sputum EOS % | 0.8 [0.1, 3.0] |
| Sputum MAC % | 43 [26, 61] |
| Sputum LYM % | 1.6 [1.0, 2.8] |
Continuous measures summarized as median [25th, 75th].
C, Caucasian; AA, African American; H, Hispanic; AsA, Asian American; NtA, Native American; BMI, body mass index; IgE, immunoglobulin E; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; PP, percent of predicted; MX, maximum bronchodilation; reversibility, reversibility after β-agonist; RV, residual volume; TLC, total lung capacity; PMN, neutrophils; EOS, eosinophils; MAC, macrophages; LYM, lymphocytes.
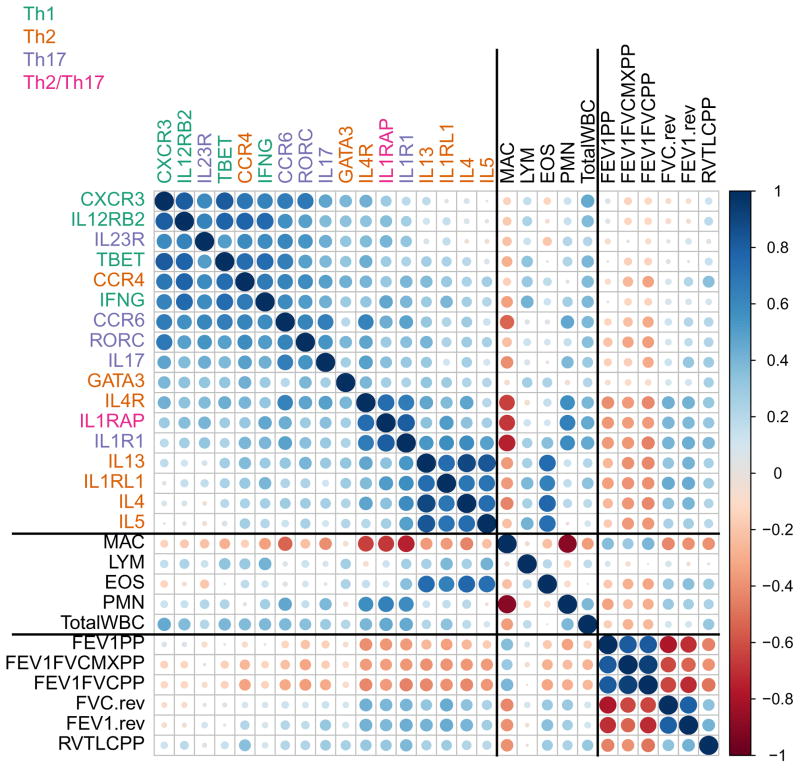
Expression levels of representative markers of the type-1, type-2, and type-17 immune response were measured by RT-qPCR using 17 genes. Genes coding for cytokines, chemokine receptors, differentiating receptors, and transcription factors were used to define each of the three immune response groups. The type-2 immune response was analyzed using IL4, IL5, IL13, CCR4, IL4R, IL1RL1 (a subunit of the IL-33 receptor), and GATA3; the type-17 response was analyzed using IL17A, CCR6, IL1R1 (a subunit of the IL-1 receptor), IL23R, and RORC; the type-1 response was characterized using IFNG, CXCR3, IL12RB2 (a subunit of the IL-12 receptor), and TBET. IL1RAP, the co-receptor with IL1RL1 or IL1R1 required for intracellular signaling of both IL-1 and IL-33, may be included in both the type-17 and type-2 group of genes. Figure 1 depicts the correlations among genes, cell differentials and total white blood cells, and pulmonary function measures. There was a tendency for positive correlation among all genes, indicating that some subjects have generally higher expression than others across all markers, independent of sample quality. There were also several genes that were highly correlated with each other. For instance, IL4, IL5, IL13, and IL1RL1, or IL17A, CCR6, and RORC, formed 2 groups that may well-define the type-2 or type-17 immune response, respectively. Another interesting noticeable set of genes is IL1R1, IL1RAP, and IL4R. While the strong association between IL1R1 and IL1RAP (the two subunits of the IL-1 receptor) might be expected, the presence of IL4R in this small cluster was surprising. Interestingly, IL1RAP was more closely associated with its co-receptor IL1R1 (type-17) than with IL1RL1 (type-2). There was a negative correlation between PMN and MAC percentages, as expected, and relatively strong correlations among spirometric measures. Finally, Figure 1 shows correlations between genes, cells, and pulmonary functions. Notable findings include 1) high correlations between EOS and IL4, IL5, IL13, and IL1RL1; 2) high correlations between PMN and IL1R1, IL1RAP, and IL4R, among others; 3) correlations between pulmonary functions and IL4, IL5, IL13, IL1RL1, IL1R1, IL1RAP, and IL4R; and 4) high EOS and PMN are each associated with worse pulmonary function.
Figure 1. Correlation plot of sputum gene expression, sputum cell differentials, and pulmonary function measures.
Visualization of the correlations between each pair of variables. Variables are grouped by: gene expression levels, sputum inflammatory cells, and pulmonary function measurements. Similar variables are placed adjacently using correlation-based variable ordering. Stronger correlations are represented by darker colors and larger circles. Blue indicates positive correlation and red indicates negative correlation.
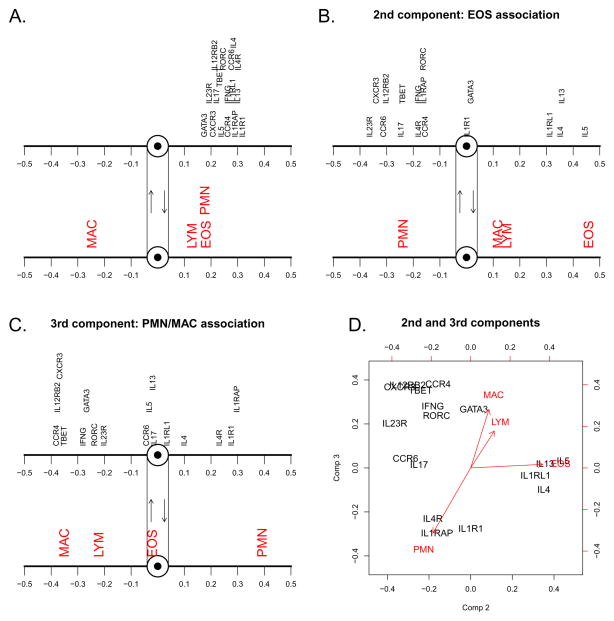
To examine the relationship between expression of these 17 genes and sputum cell differentials, we fit a PLS regression model. A summary of the model fit is shown in Table E2, and predicted versus actual cell differentials are shown in Figure E2. The three orthogonal latent variable components which form the PLS model account for 72% of the total variation across all 17 genes, and explain 71% of eosinophil variation, 64% of neutrophil variation, and 68% of macrophage variation, though only 26% of lymphocyte variation. Visual representations of the X loadings (gene predictors) and Y loadings (cell responses) for the three components of the PLS model are shown in Figure 2. The first component (Figure 2A) explains 42% of the total variation across all 17 genes, and may be interpreted as a latent variable representing subjects’ general level of gene expression. Higher expression in general tends to tilt a subject’s balance in favor of increased eosinophils, increased neutrophils, and decreased macrophages. The second component (Figure 2B), orthogonal to the first component and thus independent of one’s general tendency toward high or low expression, identifies genes whose expression is most strongly associated with eosinophils. High expression of IL4, IL5, IL13, and IL1RL1 (type-2) is associated with higher eosinophils, while elevated expression of CXCR3, IL12RB2 (type-1), and CCR6, IL23R (type-17) favors lower eosinophils. The third component (Figure 2C), which is uncorrelated with both the first and second components, identifies genes whose expression is associated with elevated neutrophils and decreased macrophages. High expression of IL1RAP, IL1R1, and IL4R corresponds to higher neutrophils and lower macrophages. Higher expression of genes such as CCR4, GATA3 (type-2), and TBET, IL12RB2, CXCR3, IFNG (type-1) is associated with reduced neutrophils and increased macrophages. The X and Y loadings for the second and third components are plotted together in Figure 2D.
Figure 2. Loadings plot for the three orthogonal latent components.
Structure of the three independent components of the PLS regression analysis of gene expression levels vis-à-vis cell differentiation in sputum A. X loadings (genes) and the corresponding Y loadings (cells) for the first component. B. X and Y loadings for the second component. C. X and Y loadings for the third component. D. X and Y loadings for the second component (horizontal axis) and third component (vertical axis).
The combined effects of these three components constitute the full PLS regression model. Figure 3 shows the regression coefficients with approximate 95% confidence intervals. IL4, IL5, IL13, and IL1RL1 expression levels are most strongly associated with higher eosinophil percentage. IL1R1, IL1RAP, and IL4R are most strongly associated with higher neutrophils and lower macrophages. To a lesser extent, CCR6 and IL17A appear to be associated with higher neutrophils, and GATA3 and CCR4 with lower neutrophils. For lymphocytes, it appears that the genes associated with higher eosinophils also tend to associate with lymphocytes, though the estimated effects are more modest in size. These results may be contrasted with those obtained from ordinary multiple linear regression, shown in Figure E3, where, due to the numerous and correlated predictors, the regression coefficients are unstable and have large standard errors. Additional PLS regression models that examine the potential influence of asthma severity, age, and corticosteroid use on these relationships were also considered; the gene expression and cell differential relationships described above were not altered in these models (Figure E5, E6, E7). Notably, older subjects tended toward higher eosinophil percentage, and severe subjects tended toward higher neutrophil percentage (Figure E5).
Figure 3. Regression coefficients for gene expression level and sputum cell PLS model with approximate 95% confidence intervals.
Standardized regression coefficients; for example, a 1 standard deviation (SD) increase in IL1RAP expression is associated with a 0.33 SD increase in neutrophils. Coefficient direction is represented by color, with blue and red indicating positive and negative relationships, respectively. Larger coefficients are represented by darker colors. The ordering of the genes is carried over from Figure 1.
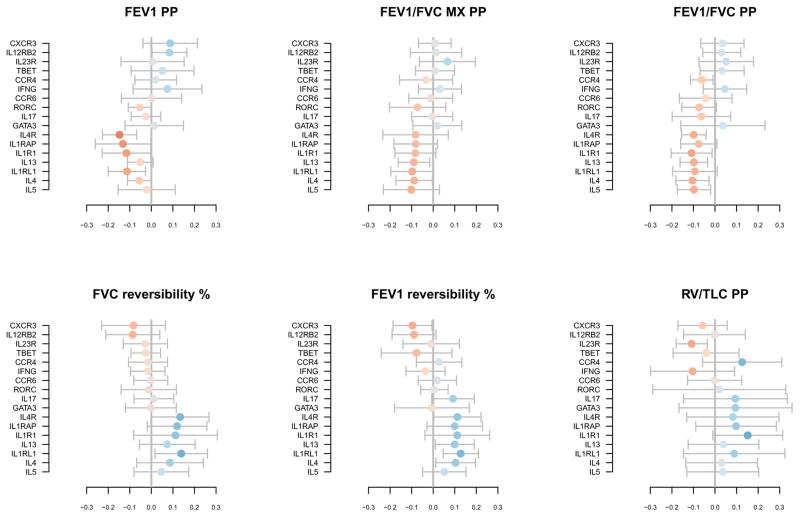
The expression levels of the 17 genes were further analyzed in relation to pulmonary function measurements using separate PLS regression models. Two-component models were selected in each case; summaries of the model fits are shown in Table E3. Whereas the gene expression components in the cell differential model accounted for 71% and 64% of EOS and PMN variation, their explanatory power for pulmonary function is more modest, accounting for between 24% (RV/TLC) and 34% (FEV1 reversibility) of pulmonary function variation. Loadings of genes and pulmonary functions on the first and second components of each model are shown in Figure E4. The first components of each model again may represent the general level of gene expression, with high expression favoring worse lung function, and the second components indicate genes whose relative balance favors better or worse lung function. Figure 4 shows the regression coefficients for each model. Importantly, IL1RAP, IL4R, and IL1R1 (best predictors of neutrophilia) and IL4, IL5, IL13, and IL1RL1 (best predictors of eosinophilia) displayed the strongest associations with worse pulmonary outcomes across multiple measures.
Figure 4. Regression coefficients for gene expression level and pulmonary function PLS models with approximate 95% confidence intervals.
Standardized regression coefficients. Coefficient direction is represented by color, with blue and red indicating positive and negative relationships, respectively. Larger coefficients are represented by darker colors. The ordering of the genes is carried over from Figure 1.
Discussion
In this study, using PLS regression analysis of inflammatory gene expression in sputum samples from asthma patients, we identified the IL-1 receptor as a predictor of neutrophilic asthma. Despite general expression level correlations between all 17 (type-1, type-2, and type-17) genes, and unlike the results when using an ordinary multiple linear regression model, the PLS analysis clearly distinguished the type-17 from the type-2 immune responses in relation to the percentages of sputum neutrophils or eosinophils. The validity of this approach is supported by its confirmation of a strong association of the type-2 markers, IL4, IL5, IL13, and IL1RL1, with eosinophil percentage, as would be anticipated. As expected from prior studies1, 28, the neutrophil percentage was predicted by IL17A and CCR6, 2 major hallmarks of the type-17 lymphocytes29, 30. Yet, our analyses showed that sputum neutrophilia in asthma was more strongly predicted by the 2 subunits of the functional receptor for IL-1, IL1R1 and IL1RAP.
IL1R1 has already been suggested as a potential therapeutic target in asthma31, probably due to allergic animal models where the lack of IL-1 receptor impairs the development of the type-2 immune airway response32, and reduced airway eosinophilia and goblet cell hyperplasia33. The importance of the IL-1/IL-1R1 pathway in the airway type-2 inflammation was further confirmed using dual IL-1α and IL-1β or IL-1RA knock-out mice34. In humans, IL-1β is increased in both atopic and non-atopic asthma, is increased at night in nocturnal asthma, is associated with asthma symptoms35-37, and is elevated in bronchoalveolar lavage and sputum samples in asthmatic subjects38, 39. We have recently reported that IL1R1 and IL17A expression in sputum samples were positively correlated to systemic stress markers in asthmatic subjects40, suggesting that severe and uncontrolled asthma symptoms often seen in individuals with depression or anxiety41 may be partially due to activation of the IL-1/IL-17 pathway. Interestingly, canakinumab is a humanized therapeutic monoclonal IL-1β antibody, which has been evaluated in phase 1/2 in mild asthma in a randomized double-blind clinical study with good safety and anti-inflammatory outcomes42–44. However, no further therapeutic anti-IL-1 studies in asthma using canakinumab have been reported. In addition to canakinumab, two other drugs can block IL-1 action: anakinra, which is an IL-1 receptor antagonist protein, and the dimeric fusion protein, rilonacept, which binds both IL-1R1 and IL1RAP. Interestingly, bronchoalveolar lavage cells from asthma patients, treated, in vitro, with anakinra displayed reduced numbers of IL-4+/IL-17+ cells11. In a phase I clinical investigation, anakinra was injected in subjects before an inhaled endotoxin challenge. In this study, anakinra significantly reduced airway neutrophilia and the drug was well tolerated45. However, no trials using these two drugs have been reported in asthma.
The inclusion of IL1R1 among the markers of type-17 lymphocytes in our study was driven by compelling evidence from the literature. The IL-1 receptor is present on type-17 lymphocytes29 and IL-1β alone can induce the production of RORC into naïve CD4+ T cells leading to type-17 differentiation46. In addition, IL1β increases IL-17 production by activated Th17+ memory T cells30. Supporting these findings, we showed that the release of IL-1β by eosinophils was essential to enhance IL-17 production by activated CD45RO+ CD4+ T cells in vitro18. Of note, IL-1 receptor upregulation on T cells can be reached by either a common γ-chain cytokine (IL-7 or 15) plus TGF-β47, or a cocktail closely similar to the IL-17-induced cocktail including IL-2, IL-1β, IL-23 and TGF-β48. However, considering that previous studies have demonstrated increased airway neutrophils through IL-17-induced IL-8 production in the airway1,2,49,50, our data further support that the IL-1 receptor is critical for neutrophil recruitment possibly via IL-17 production. Although, it is unclear why the expression level of the IL-1 receptor was the utmost molecule associated with neutrophilia compared to the other type-17 markers (IL17A and CCR6).
Hastie et al have reported increased expression and production of IL-1β in a group of asthmatic subjects with high sputum neutrophilia51. Although, IL1B expression level was not measured in the present study, the induction of IL-1β can possibly lead to up-regulation of the IL-1 receptor52, 53. The production and release of mature and functional IL-1β requires more than just IL1B expression and is largely dependent on the inflammasome activation54,55. IL-1β production and release generally depends on the activations of both a toll-like receptor and adenosine triphosphate (ATP), which consequently activate the inflammasome (NLRP3 and caspase-1)56. Toll-like receptor ligands are microbial agents, while ATP originates from local cell death and signals through P2X756. Interestingly, P2X7 pore activity has been linked to virus-induced asthma symptoms and asthma exacerbations57, 58. Furthermore, in agreement with the implication of the inflammasome in asthma, elevated levels of both NLRP3 and IL-1β have been reported in neutrophilic asthma59. Therefore, excess inflammasome activity due to viral or bacterial inflammation could be the upstream mechanism leading to enhanced IL-1 receptor production observed in our current study. A recent semi-biased study identified a cluster of asthma patients with high sputum neutrophil counts60. The 39 genes characterizing this cluster did not contain the receptor for IL-1, yet these genes were associated with inflammasome and pattern recognition proteins. Analysis of the inflammasome activity and its link to the IL-1 pathway in our asthmatic population will require further investigations.
In addition to airway neutrophilia, we also found that IL1R1 was one of the best predictors of worse pulmonary functions. IL1R1 was associated with FEV1, airway obstruction (FEV1/FVC), and air trapping (RV/TLC), all indications of severe asthma61. The high predictive value of IL1R1 for these pulmonary functions is possibly due to its presence on both type-17 and type-2 lymphocytes. In fact, the possible presence of IL1R1 on type-2 lymphocytes and its implication in the development of the type-2 airway inflammation32,62 may explain why IL1R1 was not inversely associated with sputum eosinophils. The IL-1 receptor is also expressed on non-T cells such as fibroblasts, epithelial cells and airway smooth muscle cells53,63,64. Interestingly also, IL-1β and IL-17 have synergistic functions on epithelial cells and airway smooth muscle cells enhancing mucin production and neutrophil chemoattractant, and thus they may synergistically enhance airway obstruction65,66. Even though sputum samples with high amounts of non-inflammatory cells were excluded, we cannot rule out that epithelial cells are part of the IL1R1-expressing cells in our analysis.
Some of the lymphocytes-characterizing genes used in the present study did not display the expected prediction value for neutrophils or eosinophils. For instance, IL4R, a common subunit receptor for IL-4 and IL-13, was highly correlated with IL1R1 and IL1RAP. IL4R is expressed on B and T lymphocytes and is upregulated by its ligand IL-4 during the T lymphocyte differentiation into type-2 lymphocytes70,71. While a mechanistic explanation for the strong association between IL4R and the IL-1 receptor remains uncertain, recent high-throughput gene expression analyses have clustered IL4R with IL1R1 in viral infection models and in patients with urticaria or periodontitis72–74.
Of note, the high allergy rate (90%) and the relatively young age of our population is a possible limitation of the current study. Indeed, some previous studies have demonstrated a higher percentage of non-allergic asthmatic patients in older asthmatic populations75,76. However, although an older more neutrophilic population may have been underrepresented in our study, the analysis clearly distinguished eosinophilic and neutrophilic asthma in relation to the gene expression profile. We also note that, while the 17 genes included in this study were carefully chosen to characterize the type-1, type-2, and type-17 immune response pathways, that measuring a larger number of genes using microarray or next-generation sequencing approaches will provide a fuller understanding of these relationships and is an important topic for further studies.
In conclusion, by using a multivariate statistical approach, we have identified IL-1 receptor as an important predictor of both neutrophilic asthma and worse pulmonary functions. A treatment targeting the IL-1 pathway may be a reasonable consideration in neutrophilic severe asthma.
Supplementary Material
Figure E1. Cross-validation (black solid line) and bias-adjusted (red, dashed line) cross-validation root mean squared error of prediction (RMSEP) estimators used to select the number of components.
Figure E2. Actual versus predicted cell differentials (centered and scaled) from the three-component PLS model.
Figure E3. Regression coefficients with 95% confidence intervals for ordinary multiple linear regression models of sputum cells.
Figure E4. Loadings plots for the first component (horizontal axis) and second component (vertical axis) of each pulmonary function PLS model.
Figure E5. Regression coefficients with 95% confidence intervals for PLS model of gene expression and sputum cells including additional predictors for age, steroid dose level, and asthma severity.
Figure E6. Regression coefficients with 95% confidence intervals for PLS model of gene expression and sputum cells in 31 non-severe asthma subjects.
Figure E7. Regression coefficients with 95% confidence intervals for PLS model of gene expression and sputum cells in 17 severe asthma subjects.
Table E1. Primer sequences used for real-time PCR
Table E2. Variation explained (%) by cell differential model
Table E3. Variation explained (%) by pulmonary function models
key messages.
In sputum samples from asthmatic subjects, expression levels for type-1, type-2 and type-17 markers are positively correlated with one another
A multivariate analysis identifies the IL-1 receptor as a strong predictor of sputum neutrophilia
IL-1 pathway could contribute to neutrophilic asthma pathobiology
‡.
Funding: This work was supported by the Severe Asthma Research Program Grant R01 HL069116, U10 HL109168, Program Project Grant P01 HL088594, and Clinical and Translational Research Center Grant UL1 RR025011 and UL1 TR000427 from the National Institutes of Health.
The authors thank Erin Billmeyer, Michele Wolff and Holly Eversoll for patient recruitment, screening, and assessments; Gina Crisafi, Katie Popp and Helen Werner for preparing sputum samples; Gina Crisafi for protocol coordination; Larissa DeLain for laboratory technical support. We acknowledge contributions from all investigators, staff, and participants in the SARP study.
Abbreviations
- CS
corticosteroid
- EOS
eosinophils
- FEV1
forced expiratory volume in 1 second
- FVC
forced vital capacity
- ICS
inhaled corticosteroids
- LYM
lymphocytes
- MAC
macrophages
- PLS
partial least squares, PMN, neutrophils
- RV
residual volume
- SARP
Severe Asthma Research Program
- TLC
total lung capacity
Footnotes
Conflicts of interest: none
This article has online Supporting Information
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bullens DM, Truyen E, Coteur L, Dilissen E, Hellings PW, Dupont LJ, et al. IL-17 mRNA in sputum of asthmatic patients: linking T cell driven inflammation and granulocytic influx? Respir. Res. 2006;7:135. doi: 10.1186/1465-9921-7-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barczyk A, Pierzchala W, Sozanska E. Interleukin-17 in sputum correlates with airway hyperresponsiveness to methacholine. Respiratory Medicine. 2003;97:726–33. doi: 10.1053/rmed.2003.1507. [DOI] [PubMed] [Google Scholar]
- 3.Jatakanon A, Uasuf C, Maziak W, Lim S, Chung KF, Barnes PJ. Neutrophilic inflammation in severe persistent asthma. Am J Respir Crit Care Med. 1999;160:1532–9. doi: 10.1164/ajrccm.160.5.9806170. [DOI] [PubMed] [Google Scholar]
- 4.Vazquez-Tello A, Halwani R, Hamid Q, Al-Muhsen S. Glucocorticoid receptor-beta up-regulation and steroid resistance induction by IL-17 and IL-23 cytokine stimulation in peripheral mononuclear cells. J Clin Immunol. 2013;33:466–78. doi: 10.1007/s10875-012-9828-3. [DOI] [PubMed] [Google Scholar]
- 5.Moore WC, Hastie AT, Li X, Li H, Busse WW, Jarjour NN, et al. Sputum neutrophil counts are associated with more severe asthma phenotypes using cluster analysis. J Allergy Clin Immunol. 2014;133:1557–63. e5. doi: 10.1016/j.jaci.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hastie AT, Moore WC, Li H, Rector BM, Ortega VE, Pascual RM, et al. Biomarker surrogates do not accurately predict sputum eosinophil and neutrophil percentages in asthmatic subjects. J Allergy Clin Immunol. 2013;132:72–80. doi: 10.1016/j.jaci.2013.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Newcomb DC, Boswell MG, Zhou W, Huckabee MM, Goleniewska K, Sevin CM, et al. Human TH17 cells express a functional IL-13 receptor and IL-13 attenuates IL-17A production. Journal of Allergy and Clinical Immunology. 2011;127:1006–13. doi: 10.1016/j.jaci.2010.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choy DF, Hart KM, Borthwick LA, Shikotra A, Nagarkar DR, Siddiqui S, et al. TH2 and TH17 inflammatory pathways are reciprocally regulated in asthma. Sci Transl Med. 2015;7:301ra129. doi: 10.1126/scitranslmed.aab3142. [DOI] [PubMed] [Google Scholar]
- 9.Cosmi L, Maggi L, Santarlasci V, Capone M, Cardilicchia E, Frosali F, et al. Identification of a novel subset of human circulating memory CD4(+) T cells that produce both IL-17A and IL-4. Journal of Allergy and Clinical Immunology. 2010;125:222–30. doi: 10.1016/j.jaci.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 10.Irvin C, Zafar I, Good J, Rollins D, Christianson C, Gorska MM, et al. Increased frequency of dual-positive TH2/TH17 cells in bronchoalveolar lavage fluid characterizes a population of patients with severe asthma. J Allergy Clin Immunol. 2014;134:1175–86. e7. doi: 10.1016/j.jaci.2014.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu W, Liu S, Verma M, Zafar I, Good JT, Rollins D, et al. Mechanism of TH2/TH17-predominant and neutrophilic TH2/TH17-low subtypes of asthma. J Allergy Clin Immunol. 2017;139:1548–58. e4. doi: 10.1016/j.jaci.2016.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seys SF, Grabowski M, Adriaensen W, Decraene A, Dilissen E, Vanoirbeek JA, et al. Sputum cytokine mapping reveals an ‘IL-5, IL-17A, IL-25-high’ pattern associated with poorly controlled asthma. Clin Exp Allergy. 2013;43:1009–17. doi: 10.1111/cea.12125. [DOI] [PubMed] [Google Scholar]
- 13.Seys SF, Scheers H, Van den Brande P, Marijsse G, Dilissen E, Van Den Bergh A, et al. Cluster analysis of sputum cytokine-high profiles reveals diversity in T(h)2-high asthma patients. Respir Res. 2017;18:39. doi: 10.1186/s12931-017-0524-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chambers ES, Nanzer AM, Pfeffer PE, Richards DF, Timms PM, Martineau AR, et al. Distinct endotypes of steroid-resistant asthma characterized by IL-17A(high) and IFN-gamma(high) immunophenotypes: Potential benefits of calcitriol. J Allergy Clin Immunol. 2015;136:628–37. e4. doi: 10.1016/j.jaci.2015.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaminska M, Foley S, Maghni K, Storness-Bliss C, Coxson H, Ghezzo H, et al. Airway remodeling in subjects with severe asthma with or without chronic persistent airflow obstruction. J Allergy Clin Immunol. 2009;124:45–51. e1–4. doi: 10.1016/j.jaci.2009.03.049. [DOI] [PubMed] [Google Scholar]
- 16.Denlinger LC, Sorkness RL, Lee WM, Evans MD, Wolff MJ, Mathur SK, et al. Lower airway rhinovirus burden and the seasonal risk of asthma exacerbation. Am J Respir Crit Care Med. 2011;184:1007–14. doi: 10.1164/rccm.201103-0585OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esnault S, Kelly EA, Sorkness RL, Evans MD, Busse WW, Jarjour NN. Airway factor XIII associates with type 2 inflammation and airway obstruction in asthmatic patients. J Allergy Clin Immunol. 2016;137:767–73. e6. doi: 10.1016/j.jaci.2015.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Esnault S, Kelly EA, Nettenstrom LM, Cook EB, Seroogy CM, Jarjour NN. Human eosinophils release IL-1β and increase expression of IL-17A in activated CD4(+) T lymphocytes. Clin Exp Allergy. 2012;42:1756–64. doi: 10.1111/j.1365-2222.2012.04060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.R Core Team. R: A language and environment for statistical computing. Vienna, Austria: 2015. Available from https://www.R-project.org/ [Google Scholar]
- 20.Peng B, Yu RK, Dehoff KL, Amos CI. Normalizing a large number of quantitative traits using empirical normal quantile transformation. BMC Proc. 2007;1(Suppl 1):S156. doi: 10.1186/1753-6561-1-s1-s156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Josse J, Husson F. missMDA: A Package for Handling Missing Values in Multivariate Data Analysis. Journal of Statistical Software. 2016:70. <Go to ISI>://WOS:000373921300001. [Google Scholar]
- 22.Wei T. corrplot: Visualization of a correlation matrix. R package version 0.73. 2013 https://CRAN.R-project.org/package=corrplot.
- 23.Friendly M. Corrgrams: Exploratory displays for correlation matrices. American Statistician. 2002;56:316–24. doi: 10.1198/000313002533. [DOI] [Google Scholar]
- 24.Wold S, Sjostrom M, Eriksson L. PLS-regression: a basic tool of chemometrics. Chemometrics and Intelligent Laboratory Systems. 2001;58:109–30. doi: 10.1016/S0169-7439(01)00155-1. [DOI] [Google Scholar]
- 25.Abdi H, Williams LJ. Partial least squares methods: partial least squares correlation and partial least square regression. Methods Mol Biol. 2013;930:549–79. doi: 10.1007/978-1-62703-059-5_23. [DOI] [PubMed] [Google Scholar]
- 26.Mevik B-H, Wehrens R, Liland KH. pls: Partial Least Squares and Principal Component Regression. R package version 2. 5–0. 2015 https://CRAN.R-project.org/package=pls.
- 27.Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343–73. doi: 10.1183/09031936.00202013. [DOI] [PubMed] [Google Scholar]
- 28.Doe C, Bafadhel M, Siddiqui S, Desai D, Mistry V, Rugman P, et al. Expression of the T helper 17-associated cytokines IL-17A and IL-17F in asthma and COPD. Chest. 2010;138:1140–7. doi: 10.1378/chest.09-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, et al. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204:1849–61. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu H, Rohowsky-Kochan C. Regulation of IL-17 in human CCR6+ effector memory T cells. J Immunol. 2008;180:7948–57. doi: 10.4049/jimmunol.180.12.7948. [DOI] [PubMed] [Google Scholar]
- 31.Lee JH, Wang LC, Yu HH, Lin YT, Yang YH, Chiang BL. Type I IL-1 receptor (IL-1RI) as potential new therapeutic target for bronchial asthma. Mediat Inflamm. 2010;2010:567351. doi: 10.1155/2010/567351. Artn 567351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmitz N, Kurrer M, Kopf M. The IL-1 receptor 1 is critical for Th2 cell type airway immune responses in a mild but not in a more severe asthma model. Eur J Immunol. 2003;33:991–1000. doi: 10.1002/eji.200323801. [DOI] [PubMed] [Google Scholar]
- 33.Broide DH, Campbell K, Gifford T, Sriramarao P. Inhibition of eosinophilic inflammation in allergen-challenged, IL-1 receptor type 1-deficient mice is associated with reduced eosinophil rolling and adhesion on vascular endothelium. Blood. 2000;95:263–9. [PubMed] [Google Scholar]
- 34.Nakae S, Komiyama Y, Yokoyama H, Nambu A, Umeda M, Iwase M, et al. IL-1 is required for allergen-specific Th2 cell activation and the development of airway hypersensitivity response. Int Immunol. 2003;15:483–90. doi: 10.1093/intimm/dxg054. [DOI] [PubMed] [Google Scholar]
- 35.Thomas SS, Chhabra SK. A study on the serum levels of interleukin-1beta in bronchial asthma. J Indian Med Assoc. 2003;101:282. 4, 6 passim. [PubMed] [Google Scholar]
- 36.Konno S, Gonokami Y, Kurokawa M, Kawazu K, Asano K, Okamoto K, et al. Cytokine concentrations in sputum of asthmatic patients. International Archives of Allergy and Immunology. 1996;109:73–8. doi: 10.1159/000237234. [DOI] [PubMed] [Google Scholar]
- 37.Jarjour NN, Busse WW. Cytokines in bronchoalveolar lavage fluid of patients with nocturnal asthma. Am J Respir Crit Care Med. 1995;152:1474–7. doi: 10.1164/ajrccm.152.5.7582279. [DOI] [PubMed] [Google Scholar]
- 38.Baines KJ, Simpson JL, Wood LG, Scott RJ, Gibson PG. Transcriptional phenotypes of asthma defined by gene expression profiling of induced sputum samples. J Allergy Clin Immunol. 2011;127:153–60. 60. doi: 10.1016/j.jaci.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 39.Borish L, Mascali JJ, Dishuck J, Beam WR, Martin RJ, Rosenwasser LJ. Detection of alveolar macrophage-derived IL-1 beta in asthma. Inhibition with corticosteroids. J Immunol. 1992;149:3078–82. [PubMed] [Google Scholar]
- 40.Rosenkranz MA, Esnault S, Christian BT, Crisafi G, Gresham LK, Higgins AT, et al. Mind-body interactions in the regulation of airway inflammation in asthma: A PET study of acute and chronic stress. Brain Behav Immun. 2016 doi: 10.1016/j.bbi.2016.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strine TW, Mokdad AH, Balluz LS, Berry JT, Gonzalez O. Impact of depression and anxiety on quality of life, health behaviors, and asthma control among adults in the United States with asthma, 2006. J Asthma. 2008;45:123–33. doi: 10.1080/02770900701840238. [DOI] [PubMed] [Google Scholar]
- 42.Menzella F, Lusuardi M, Galeone C, Zucchi L. Tailored therapy for severe asthma. Multidiscip Respir Med. 2015;10:1. doi: 10.1186/2049-6958-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dhimolea E. Canakinumab. MAbs. 2010;2:3–13. doi: 10.4161/mabs.2.1.10328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pascoe S, Kanniess F, Bonner J, Lloyd P, Lowe P, Beier J, et al. A monoclonal antibody to IL-1β attenuates the late asthmatic response to antigen challenge in patients with mild asthma. Annu Congr Eur Resp Soc. 2006:115s. [Google Scholar]
- 45.Hernandez ML, Mills K, Almond M, Todoric K, Aleman MM, Zhang H, et al. IL-1 receptor antagonist reduces endotoxin-induced airway inflammation in healthy volunteers. J Allergy Clin Immunol. 2015;135:379–85. doi: 10.1016/j.jaci.2014.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–9. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 47.Lee WW, Kang SW, Choi J, Lee SH, Shah K, Eynon EE, et al. Regulating human Th17 cells via differential expression of IL-1 receptor. Blood. 2010;115:530–40. doi: 10.1182/blood-2009-08-236521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raffin C, Raimbaud I, Valmori D, Ayyoub M. Ex vivo IL-1 receptor type I expression in human CD4+ T cells identifies an early intermediate in the differentiation of Th17 from FOXP3+ naive regulatory T cells. J Immunol. 2011;187:5196–202. doi: 10.4049/jimmunol.1101742. [DOI] [PubMed] [Google Scholar]
- 49.Roussel L, Houle F, Chan C, Yao Y, Berube J, Olivenstein R, et al. IL-17 promotes p38 MAPK-dependent endothelial activation enhancing neutrophil recruitment to sites of inflammation. J Immunol. 2010;184:4531–7. doi: 10.4049/jimmunol.0903162. [DOI] [PubMed] [Google Scholar]
- 50.Prause O, Laan M, Lotvall J, Linden A. Pharmacological modulation of interleukin-17-induced GCP-2-, GRO-alpha- and interleukin-8 release in human bronchial epithelial cells. Eur J Pharmacol. 2003;462:193–8. doi: 10.1016/s0014-2999(03)01341-4. [DOI] [PubMed] [Google Scholar]
- 51.Hastie AT, Moore WC, Meyers DA, Vestal PL, Li H, Peters SP, et al. Analyses of asthma severity phenotypes and inflammatory proteins in subjects stratified by sputum granulocytes. Journal of Allergy and Clinical Immunology. 2010;125:1028–36. doi: 10.1016/j.jaci.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takii T, Akahoshi T, Kato K, Hayashi H, Marunouchi T, Onozaki K. Interleukin-1 up-regulates transcription of its own receptor in a human fibroblast cell line TIG-1: role of endogenous PGE2 and cAMP. Eur J Immunol. 1992;22:1221–7. doi: 10.1002/eji.1830220517. [DOI] [PubMed] [Google Scholar]
- 53.Bellehumeur C, Blanchet J, Fontaine JY, Bourcier N, Akoum A. Interleukin 1 regulates its own receptors in human endometrial cells via distinct mechanisms. Hum Reprod. 2009;24:2193–204. doi: 10.1093/humrep/dep192. [DOI] [PubMed] [Google Scholar]
- 54.Guo H, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 2015;21:677–87. doi: 10.1038/nm.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krause K, Metz M, Makris M, Zuberbier T, Maurer M. The role of interleukin-1 in allergy-related disorders. Curr Opin Allergy Clin Immunol. 2012;12:477–84. doi: 10.1097/ACI.0b013e3283574d0c. [DOI] [PubMed] [Google Scholar]
- 56.Netea MG, van de Veerdonk FL, van der Meer JW, Dinarello CA, Joosten LA. Inflammasome-independent regulation of IL-1-family cytokines. Annu Rev Immunol. 2015;33:49–77. doi: 10.1146/annurev-immunol-032414-112306. [DOI] [PubMed] [Google Scholar]
- 57.Denlinger LC, Shi L, Guadarrama A, Schell K, Green D, Morrin A, et al. Attenuated P2X7 pore function as a risk factor for virus-induced loss of asthma control. Am J Respir Crit Care Med. 2009;179:265–70. doi: 10.1164/rccm.200802-293OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Denlinger LC, Manthei DM, Seibold MA, Ahn K, Bleecker E, Boushey HA, et al. P2X7-regulated protection from exacerbations and loss of control is independent of asthma maintenance therapy. Am J Respir Crit Care Med. 2013;187:28–33. doi: 10.1164/rccm.201204-0750OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Simpson JL, Phipps S, Baines KJ, Oreo KM, Gunawardhana L, Gibson PG. Elevated expression of the NLRP3 inflammasome in neutrophilic asthma. Eur Respir J. 2014;43:1067–76. doi: 10.1183/09031936.00105013. [DOI] [PubMed] [Google Scholar]
- 60.Kuo CS, Pavlidis S, Loza M, Baribaud F, Rowe A, Pandis I, et al. T-helper cell type 2 (Th2) and non-Th2 molecular phenotypes of asthma using sputum transcriptomics in U-BIOPRED. Eur Respir J. 2017:49. doi: 10.1183/13993003.02135-2016. [DOI] [PubMed] [Google Scholar]
- 61.Sorkness RL, Bleecker ER, Busse WW, Calhoun WJ, Castro M, Chung KF, et al. Lung function in adults with stable but severe asthma: air trapping and incomplete reversal of obstruction with bronchodilation. J Appl Physiol. 2008;104:394–403. doi: 10.1152/japplphysiol.00329.2007. [DOI] [PubMed] [Google Scholar]
- 62.Taylor-Robinson AW, Phillips RS. Expression of the IL-1 receptor discriminates Th2 from Th1 cloned CD4+ T cells specific for Plasmodium chabaudi. Immunology. 1994;81:216–21. [PMC free article] [PubMed] [Google Scholar]
- 63.Suwara MI, Green NJ, Borthwick LA, Mann J, Mayer-Barber KD, Barron L, et al. IL-1alpha released from damaged epithelial cells is sufficient and essential to trigger inflammatory responses in human lung fibroblasts. Mucosal Immunol. 2014;7:684–93. doi: 10.1038/mi.2013.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Whelan R, Kim C, Chen M, Leiter J, Grunstein MM, Hakonarson H. Role and regulation of interleukin-1 molecules in pro-asthmatic sensitised airway smooth muscle. Eur Respir J. 2004;24:559–67. doi: 10.1183/09031936.04.00133803. [DOI] [PubMed] [Google Scholar]
- 65.Fujisawa T, Velichko S, Thai P, Hung LY, Huang F, Wu R. Regulation of airway MUC5AC expression by IL-1beta and IL-17A; the NF-kappaB paradigm. Journal of Immunology. 2009;183:6236–43. doi: 10.4049/jimmunol.0900614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dragon S, Rahman MS, Yang J, Unruh H, Halayko AJ, Gounni AS. IL-17 enhances IL-1beta-mediated CXCL-8 release from human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1023–L9. doi: 10.1152/ajplung.00306.2006. [DOI] [PubMed] [Google Scholar]
- 67.Unutmaz D. RORC2: the master of human Th17 cell programming. Eur J Immunol. 2009;39:1452–5. doi: 10.1002/eji.200939540. [DOI] [PubMed] [Google Scholar]
- 68.Zhang Y, Collier F, Naselli G, Saffery R, Tang ML, Allen KJ, et al. Cord blood monocyte-derived inflammatory cytokines suppress IL-2 and induce nonclassic “T(H)2-type” immunity associated with development of food allergy. Sci Transl Med. 2016;8:321ra8. doi: 10.1126/scitranslmed.aad4322. [DOI] [PubMed] [Google Scholar]
- 69.Zhu J, Cote-Sierra J, Guo L, Paul WE. Stat5 activation plays a critical role in Th2 differentiation. Immunity. 2003;19:739–48. doi: 10.1016/s1074-7613(03)00292-9. [DOI] [PubMed] [Google Scholar]
- 70.Dokter WH, Borger P, Hendriks D, van der Horst I, Halie MR, Vellenga E. Interleukin-4 (IL-4) receptor expression on human T cells is affected by different intracellular signaling pathways and by IL-4 at transcriptional and posttranscriptional level. Blood. 1992;80:2721–8. [PubMed] [Google Scholar]
- 71.Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE. The IL-4 receptor: signaling mechanisms and biologic functions. Annu Rev Immunol. 1999;17:701–38. doi: 10.1146/annurev.immunol.17.1.701. [DOI] [PubMed] [Google Scholar]
- 72.Hoang LT, Tolfvenstam T, Ooi EE, Khor CC, Naim AN, Ho EX, et al. Patient-based transcriptome-wide analysis identify interferon and ubiquination pathways as potential predictors of influenza A disease severity. PLoS One. 2014;9:e111640. doi: 10.1371/journal.pone.0111640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Edwards RH, Marquitz AR, Raab-Traub N. Changes in expression induced by Epstein-Barr Virus LMP1-CTAR1: potential role of bcl3. MBio. 2015:6. doi: 10.1128/mBio.00441-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Patel OP, Giorno RC, Dibbern DA, Andrews KY, Durairaj S, Dreskin SC. Gene expression profiles in chronic idiopathic (spontaneous) urticaria. Allergy Rhinol (Providence) 2015;6:101–10. doi: 10.2500/ar.2015.6.0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Raedler D, Ballenberger N, Klucker E, Bock A, Otto R, Prazeres da Costa O, et al. Identification of novel immune phenotypes for allergic and nonallergic childhood asthma. J Allergy Clin Immunol. 2015;135:81–91. doi: 10.1016/j.jaci.2014.07.046. [DOI] [PubMed] [Google Scholar]
- 76.Woodruff PG, Modrek B, Choy DF, Jia G, Abbas AR, Ellwanger A, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009;180:388–95. doi: 10.1164/rccm.200903-0392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure E1. Cross-validation (black solid line) and bias-adjusted (red, dashed line) cross-validation root mean squared error of prediction (RMSEP) estimators used to select the number of components.
Figure E2. Actual versus predicted cell differentials (centered and scaled) from the three-component PLS model.
Figure E3. Regression coefficients with 95% confidence intervals for ordinary multiple linear regression models of sputum cells.
Figure E4. Loadings plots for the first component (horizontal axis) and second component (vertical axis) of each pulmonary function PLS model.
Figure E5. Regression coefficients with 95% confidence intervals for PLS model of gene expression and sputum cells including additional predictors for age, steroid dose level, and asthma severity.
Figure E6. Regression coefficients with 95% confidence intervals for PLS model of gene expression and sputum cells in 31 non-severe asthma subjects.
Figure E7. Regression coefficients with 95% confidence intervals for PLS model of gene expression and sputum cells in 17 severe asthma subjects.
Table E1. Primer sequences used for real-time PCR
Table E2. Variation explained (%) by cell differential model
Table E3. Variation explained (%) by pulmonary function models