Abstract
Background
Women with a history of hypertensive disease of pregnancy have increased risks for early mortality from multiple causes. The effect of recurrent hypertensive disease of pregnancy on mortality risk and life expectancy is unknown.
Objective
To determine whether recurrent hypertensive disease of pregnancy is associated with increased mortality risks.
Study Design
In this retrospective cohort study, we used birth certificate data to determine the number of pregnancies affected by hypertensive disease of pregnancy for each woman delivering in Utah from 1939–2012. We assigned women to one of three groups based on number of affected pregnancies: 0, 1, or ≥2. Exposed women had ≥1 affected singleton pregnancy and lived in Utah for ≥1 year postpartum. Exposed women were matched 1:2 to unexposed women by age, year of childbirth, and parity. Underlying cause of death was determined from death certificates. Mortality risks by underlying cause of death were compared between exposed and unexposed women as a function of number of affected pregnancies. Cox regressions controlled for infant sex, gestational age, parental education, ethnicity, and marital status.
Results
We identified 57,384 women with ≥1 affected pregnancy (49,598 women with 1 affected pregnancy and 7,786 women with ≥2 affected pregnancies). These women were matched to 114,768 unexposed women. As of 2016, 11,894 women were deceased: 4,722 (8.2%) exposed and 7,172 (6.3%) unexposed. Women with ≥2 affected pregnancies had increased mortality from all causes [adjusted hazard ratio (aHR)=2.04, 95% CI 1.76–2.36], diabetes (aHR=4.33, 95% CI 2.21–8.47), ischemic heart disease (aHR=3.30, 95% CI 2.02–5.40), and stroke (aHR=5.10, 95% CI 2.62–9.92). For women whose index pregnancy delivered between 1939–1959 (n= 10,488), those with ≥2 affected pregnancies had shorter additional life expectancies than mothers who had only 1 or 0 hypertensive pregnancies (48.92 vs 51.91 vs 55.48 years, respectively).
Conclusion
Hypertensive diseases of pregnancy are associated with excess risks for early all-cause mortality and some cause-specific mortality, and these risks increase further with recurrent disease.
Keywords: Recurrent preeclampsia, pregnancy as a window to future health, survival analysis
Introduction
Certain medical complications of pregnancy are associated with the later development of chronic diseases (1–6). In particular, hypertensive disease of pregnancy is associated with subsequent chronic cardiovascular disease (7–9). Several studies have now demonstrated the association between a history of hypertensive disease of pregnancy and subsequent early mortality from cardiovascular and other causes (10–12).
Although previous studies have examined the effect of recurrent preeclampsia on subsequent cardiovascular morbidity and mortality (13, 14), there are currently no available data regarding the effect of recurrent pregnancies complicated by any hypertensive disease of pregnancy on subsequent all-cause mortality in an American cohort. In order to address this knowledge gap, we examined whether women with recurrent hypertensive disease of pregnancy have increased risk for early mortality and shorter life spans compared to women with either zero affected pregnancies or only one affected pregnancy.
Materials and Methods
Data Source
We used the Utah Population Database to identify a retrospective cohort of women who gave birth between 1939 and 2012. The Utah Population Database has been previously described in detail (14, 15). It consists of linked records pertaining to over 9 million people, and includes genealogy records from the Genealogical Society of Utah, official statewide birth and death records, and hospital discharge and ambulatory surgery records from the Utah State Department of Health. Its population is representative of a broad spectrum of the Caucasian United States population (16). Study approvals were obtained from the Resource for Genetic and Epidemiologic Research, a special review panel authorizing access to the Utah Population Database, as well as the University of Utah Institutional Review Board.
Inclusion Criteria
Women were included if they had at least one singleton pregnancy with birth certificate data during the study period and lived in Utah for at least one year following delivery. Using birth certificate data, we assigned a diagnosis of hypertensive disease of pregnancy to each affected pregnancy. Hypertensive disease diagnoses included gestational hypertension, preeclampsia, HELLP syndrome, and eclampsia, and were assigned as listed on the birth certificate for the affected pregnancy. When available, we used inpatient records from the time of delivery to confirm hypertensive diagnoses. We then determined the number of affected pregnancies for each woman, and assigned women to one of three categories: no affected pregnancies, one affected pregnancy, or two or more affected pregnancies.
Exposure of Interest
Exposed women had at least one singleton pregnancy complicated by hypertensive disease of pregnancy. The index exposed pregnancy was defined as the most severely affected pregnancy in the Utah Population Database, with diagnoses ranked from least to most severe as follows: gestational hypertension, preeclampsia, HELLP syndrome, eclampsia. If two pregnancies were affected equally, then the earliest pregnancy was used. Exposed women were excluded if they had missing data on a key variable which would preclude matching or if they had documented medical comorbidities at the time of their pregnancy (chronic hypertension, antiphospholipid syndrome, pre-gestational diabetes, and chronic kidney disease). We also excluded exposed women who died within one year of delivery in order to assess the risk of long-term mortality as opposed to maternal mortality.
Unexposed women had pregnancies during the study period, but none were complicated by hypertensive disease of pregnancy. These unexposed women were excluded if they were missing data on a key variable which would preclude matching, if they died within one year of delivery, or if the birth or fetal death certificate listed a history of prior pregnancy complicated by hypertensive disease.
Matching
Exposed women were matched 1:2 to unexposed women by 5-year age groups, year of childbirth (within one year), and parity (1, 2, 3, 4, 5 or more) at the time of the index pregnancy.
Outcome of Interest
Our primary outcome of interest was mortality. The date of death was determined using the Utah Population Database genealogies, Utah death certificates, or the Social Security Death Index. Importantly, the Social Security Death Index is a national database, which allowed us to identify deaths which occurred in Utah or elsewhere. The underlying cause of death was determined from Utah death certificates based on the International Classification of Diseases (ICD) version used at the time of death and summarized into broad cause of death categories.
Adjustment for Confounders
In addition to the matching criteria, several confounders were included in the Cox models to generate hazard ratios for mortality among women with a history of hypertensive disease of pregnancy. These included infant sex, gestational age at delivery, parental education, maternal race/ethnicity, and maternal marital status. Infant sex was included as a potential confounder because of the known association between male fetal sex and maternal preeclampsia (17). Gestational age at delivery was included as a potential confounder because of the association between a history of preterm birth and adverse long-term maternal health outcomes (18, 19), but this did not distinguish between spontaneous and iatrogenic preterm births.
Statistical Methods
Cause-specific mortality risks were estimated based on comparisons between the number of affected pregnancies, using stratified Cox models to incorporate matching into the analysis. The time axis was years since index birth, and the number of affected pregnancies was defined at the subject level. Unadjusted and adjusted hazard ratios and 95% confidence intervals were calculated for all-cause and cause-specific mortality. The broad categories of causes of death were derived by the National Center for Health Statistics, which has implemented a standard broad classification of causes of death (14). These categories (and their ICD9 codes) include infectious diseases (001–139), neoplasms (140–239), endocrine/nutritional/metabolic diseases (240–279), diseases of blood and blood-forming organs (280–289), mental disorders (290–319), nervous system disorders (320–389), circulatory system disease (390–459), respiratory system disease (460–519), digestive system disease (520–579), genitourinary disease (580–629), musculoskeletal and connective tissue disease (710–739), ill-defined diseases (780–799), and external causes (E800–E999).
We then compared mortality risk by underlying cause of death among women with one affected pregnancy or two or more affected pregnancies versus matched women with no affected pregnancies for deaths occurring ≤50 years of age and deaths occurring >50 years of age. Adjusted hazard ratios (aHR) were calculated for each cause of mortality, as well as the p value for the difference in mortality risk between the two age groups.
Finally, we performed a companion analysis using life table methods to determine the difference in additional life expectancy in years for women with zero, one, or two or more affected pregnancies. Life table analysis accounts for the number of deaths occurring at each age among persons surviving to that age. This is the basis for determining the probabilities of survival to any age. Starting with maternal age at the time of the index pregnancy, remaining life expectancy at each subsequent age × (called ex) is also calculated. In our study, this life table analysis was performed only for women who delivered their index pregnancy between the years 1939 to 1959 in order to maximize the length of follow-up and the ability to observe adult deaths at mid-life or later.
We included all women identified with a diagnosis of hypertensive disease of pregnancy during the study period that met our inclusion criteria. Hypothesis tests were based on a Type I error of <0.05. Statistical analyses were performed using SAS software version 9.4 (Cary, NC).
Results
We identified 932,788 women who had one or more singleton births between 1939 and 2012. Within this group, 62,489 women had at least one pregnancy complicated by hypertensive disease. After excluding 5,103 women, we included 57,384 exposed women in our analysis (49,598 women with one affected pregnancy and 7,786 women with two or more affected pregnancies). These affected pregnancies included 27,546 cases of gestational hypertension, 27,818 cases of preeclampsia, 884 cases of HELLP syndrome, and 1,136 cases of eclampsia. These exposed women were matched to 114,768 unexposed women with no history of hypertensive disease in any pregnancies in the Utah Population Database. Baseline characteristics of exposed and unexposed women are reported in Table 1. Exposed women were significantly more likely to deliver at an earlier gestational age, to deliver a neonate with lower birth weight, to have lower maternal and paternal Nam-Powers socioeconomic scores [a census-derived score relating to occupation (20)], and to have fewer years of maternal education.
Table 1.
Baseline characteristics of exposed (one or more pregnancies affected by hypertensive disease of pregnancy) and unexposed women (no affected pregnancies) at the time of the index pregnancy.
| Variable | No affected pregnancies (n=114,768) |
One affected pregnancy (n=49,598) |
Two or more affected pregnancies (n=7,786) |
P |
|---|---|---|---|---|
|
| ||||
| Year of childbirth | 1994.4 ± 16.0 | 1994.1 ± 16.3 | 1996.2 ± 13.4 | <0.01 |
|
| ||||
| Maternal age (years) | 26.2 ± 6.0 | 26.2 ± 6.0 | 26.4 ± 5.5 | <0.01 |
|
| ||||
| Maternal birth year | 1967.9 ± 16.6 | 1967.7 ± 17.0 | 1969.4 ± 13.8 | <0.01 |
|
| ||||
| Maternal ethnicity [n (%)] | ||||
| African | 726 (6.3%) | 365 (7.4%) | 51 (6.6%) | 0.06 |
| Hispanic | 13,712 (11.9%) | 5,915 (11.9%) | 814 (10.5%) | <0.01 |
| Caucasian | 109,379 (95.3%) | 47,227 (95.2%) | 7,420 (95.3%) | 0.76 |
|
| ||||
| Paternal age (years) | 28.8 ± 6.5 | 28.7 ± 6.6 | 28.8 ± 6.0 | 0.16 |
|
| ||||
| Paternal birth year | 1965.1 ± 17.2 | 1964.9 ± 17.7 | 1967.0 ± 14.3 | <0.01 |
|
| ||||
| Gestational age at delivery (weeks) | 38.9 ± 1.9 | 38.0 ± 2.5 | 37.0 ± 2.9 | <0.01 |
|
| ||||
| Birth weight (g) | 3319.3 ± 513.1 | 3100.4 ± 694.5 | 2946.6 ± 756.6 | <0.01 |
|
| ||||
| Birth order [median (IQR)] | 1.0 (1.0 – 2.0) | 1.0 (1.0 – 2.0) | 2.0 (1.0 – 3.0) | <0.01 |
|
| ||||
| Total number of children [median (IQR)] | 3.0 (2.0 – 4.0) | 3.0 (2.0 – 4.0) | 3.0 (3.0 – 4.0) | <0.01 |
|
| ||||
| Maternal Nam-Powers score | 50.7 ± 24.3 | 49.7 ±24.1 | 50.6 ±23.8 | 0.02 |
|
| ||||
| Paternal Nam-Powers score | 55.1 ± 24.8 | 52.4 ± 24.2 | 53.7 ± 24.6 | <0.01 |
|
| ||||
| Maternal education years | 13.5 ± 4.5 | 13.2 ±4.1 | 13.4 ± 4.0 | <0.01 |
Data presented as mean ± standard deviation unless otherwise specified.
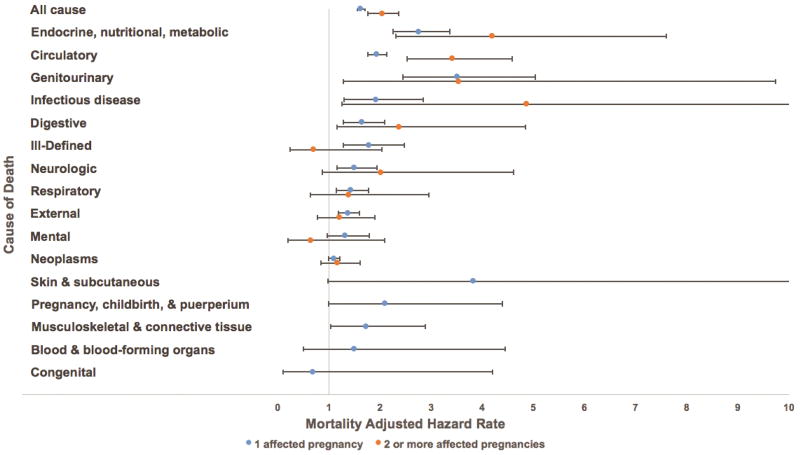
As of 2015, 11,894 women were deceased: 4,722 (8.2%) exposed and 7,172 (6.3%) unexposed. Figure 1 is a forest plot illustrating the aHRs and 95% confidence intervals for mortality from several causes for women with 1 affected pregnancy (represented by blue dots) and 2 or more affected pregnancies (represented by orange dots) compared to matched women with no affected pregnancies. In relation to matched women with no affected pregnancies, the aHRs for mortality for women with two or more affected pregnancies were significantly higher than those for women with one affected pregnancy for deaths from all causes (aHR 2.04 vs 1.62, p<0.01), infectious disease (aHR 4.86 vs 1.92, p<0.01), endocrine, nutritional, and metabolic disease (aHR 4.19 vs 2.76, p<0.01), circulatory disease (aHR 3.41 vs 1.93, p<0.01), digestive disease (aHR 2.37 vs 1.64, p<0.01), and genitourinary disease (aHR 3.54 vs 3.51, p<0.01).
Figure 1.
Adjusted hazard rate ratios and 95% confidence intervals for the association between number of pregnancies complicated by hypertensive disease of pregnancy and general causes of mortality.
In addition to these broader causes of mortality, we assessed whether women with recurrent hypertensive disease of pregnancy had increased risks for several important subsets of cause-specific mortality, which were chosen based on previously published findings (10). Compared to matched women with no affected pregnancies, we found that women with 2 or more affected pregnancies had increased risks of mortality from diabetes (aHR 4.33, 95% CI 2.21–8.47), ischemic heart disease (aHR 3.30, 95% CI 2.02–5.40), and stroke (aHR 5.10, 95% CI 2.62–9.92). Again, in relation to matched women with no affected pregnancies, the aHRs for mortality for women with two or more affected pregnancies were significantly higher than those for women with one affected pregnancy for deaths from diabetes (aHR 4.33 vs 3.04, p<0.01), ischemic heart disease (aHR 3.30 vs 2.08, p<0.05), and stroke (aHR 5.10 vs 1.86, p<0.01).
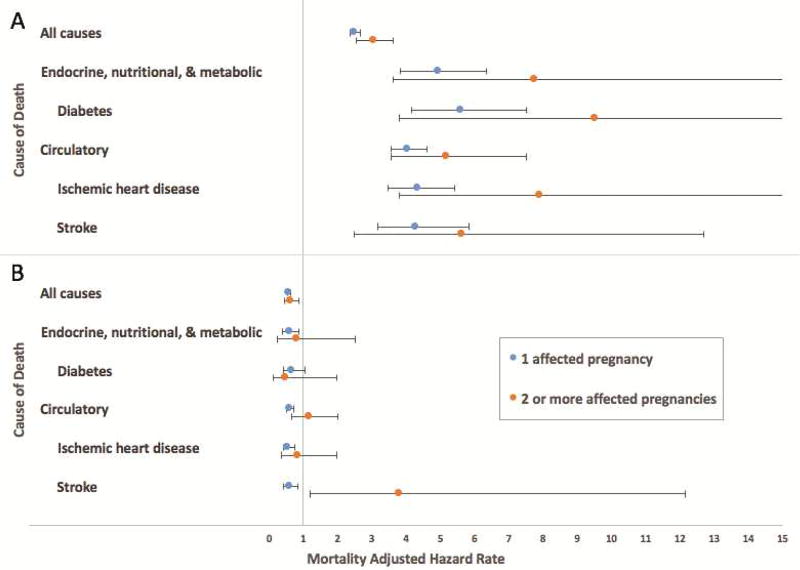
When we compared mortality risk by primary cause of death for deaths occurring at age ≤50 years versus age >50 years, we found that the association between hypertensive disease of pregnancy and mortality from each cause of death was significant only for deaths occurring ≤50 years of age (Figure 2), with the exception of deaths from stroke among women with two or more pregnancies complicated by hypertensive disease of pregnancy.
Figure 2.
Adjusted hazard rate ratios and 95% confidence intervals for the association between number of pregnancies complicated by hypertensive disease of pregnancy and (A) deaths occurring ≤50 years of age and (B) deaths occurring >50 years of age.
In our analyses using life table methods, for women in this sample whose index pregnancy delivered between 1939–1959 (n=10,488), we found a trend toward shorter life expectancy with increasing number of affected pregnancies. For example, within the first decade following the index pregnancy, the life expectancy for women with two or more pregnancies complicated by hypertensive disease of pregnancy was 48.92 years, while mothers with only one affected pregnancy had a life expectancy of 51.91 years and mothers with no affected pregnancies had a life expectancy of 55.48 years.
Comment
We found that recurrent hypertensive disease of pregnancy is associated with increased risks for early all-cause and some cause-specific mortality. This association is significant for deaths occurring at age ≤50 years. Our findings are consistent with those published regarding the association between recurrent preeclampsia and subsequent early-onset cardiovascular morbidity (21). This is important because many women with recurrent hypertensive disease of pregnancy may not begin screening for the chronic diseases underlying many of these causes of death (hypertension, diabetes, and dyslipidemia) until age 45–50, thereby missing an opportunity to intervene and potentially improve outcomes. The American College of Obstetricians and Gynecologists and the American Heart Association both acknowledge hypertensive disease of pregnancy as a significant risk factor for early-onset cardiovascular disease in women, but there are insufficient data available at this time to determine when and how screening and intervention should be undertaken (22, 23).
Strengths of our study include our large population-based sample and retrospective design, which allowed us to examine the association between the exposure of interest and mortality in many women over many years. Our retrospective design is also a limitation, as there is the potential for selection bias and residual confounding. Obesity, substance use, and the development of chronic hypertension following pregnancy are important potential confounders for which we could not adjust due to lack of available data during the decades of follow-up within the Utah Population Database. The association between hypertensive disease of pregnancy and mortality from some causes which are not intuitively related (such as gastrointestinal disease) may be representative of unmeasured confounding to some degree; however, hypertensive disease of pregnancy is a systemic process with well-known end-organ effects on the gastrointestinal tract (specifically, the liver), so we would not readily dismiss this association. Additionally, because our study period encompassed a long period of time, historical risks may not reflect current risks in the setting of contemporary medical practices.
Our use of birth certificate data constitutes another limitation of our study. In order to confirm birth certificate diagnoses and to limit inconsistencies between providers over time in assigning maternal diagnoses of hypertensive disease of pregnancy, we used inpatient records when available; however, we would expect that any residual misclassification of hypertensive diagnoses should trend our results toward the null. As a result, we may have underestimated the magnitude of our positive findings, rather than erroneously rejecting the null hypothesis. Similarly, it is possible that women may have had pregnancies outside the state of Utah, and these pregnancies may have been complicated by hypertensive disease. We attempted to minimize this limitation by excluding women with prior hypertensive disease listed on the birth certificate, but we cannot exclude the possibility that women may have had subsequent affected pregnancies after leaving Utah. Such an error would make our exposure groups more similar, increasing the potential for type II (but not type I) error.
Additionally, our study population was limited to women with at least one viable pregnancy while residing in Utah, a population that remains predominantly white and non-obese (24, 25). This limits the generalizability of our findings.
Despite these limitations, our study constitutes a meaningful contribution to the literature on long-term health outcomes among American women following hypertensive disease of pregnancy. While obstetricians are making great strides in the prevention of immediate maternal morbidity and mortality related to hypertensive disease of pregnancy with a focus on safety bundles and treatment algorithms, there may be additional opportunities to improve women’s long-term health in the months and years following pregnancy. We still do not know whether the association between hypertensive disease of pregnancy and subsequent mortality reflects a causal relationship or an underlying predisposition to chronic disease which is unmasked during pregnancy; regardless, we have identified an at-risk population of women who may benefit from early screening and intervention for chronic diseases in order to prevent early mortality (7). Prospective studies are needed to determine whether adjusted health screening schedules may be beneficial in this population.
Implications and Contributions.
To determine whether women with recurrent hypertensive disease of pregnancy have increased mortality risks.
Recurrent hypertensive disease is strongly associated with increased mortality from diabetes, heart disease, and stroke, and women with recurrent disease had shorter life expectancy compared to women with zero or one affected pregnancy.
This study shows that women with recurrent hypertensive disease have higher risk for adverse long-term health outcomes than women without hypertensive disease of pregnancy or with only one affected pregnancy.
Acknowledgments
Funding: We thank the Pedigree and Population Resource of the Huntsman Cancer Institute, University of Utah (funded in part by the Huntsman Cancer Foundation) for its role in the ongoing collection, maintenance, and support of the Utah Population Database. We also acknowledge partial support for the Utah Population Database through grant P30 CA2014 from the Huntsman Cancer Foundation, University of Utah and from the University of Utah’s Program in Personalized Health and Center for Clinical and Translational Science. Huong Meeks, Alison Fraser, and Ken Smith were supported by R01AG022095 (Early Life Conditions, Survival, and Health: A Pedigree-Based Population Study) (PI Smith). Michael Varner is supported by NIH/NCATS 1UL1TR001067 and by the HA and Edna Benning Presidential Endowment.
The authors thank Jennifer West, who is employed as a Utah Population Database navigator through the Huntsman Cancer Institute, for creating the figures for this manuscript. The individual named in this section has consented to such acknowledgment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest/disclosure: The authors report no conflicts of interest.
Presentation: Data from this study were presented as a Fellows Plenary oral presentation at the 2017 SMFM Annual Meeting in Las Vegas, NV.
References
- 1.Sandvik MK, Hallan S, Svarstad E, Vikse BE. Preeclampsia and prevalence of microalbuminuria 10 years later. Clin J Am Soc Nephrol. 2013;8:1126–34. doi: 10.2215/CJN.10641012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghossein-Doha C, Peeters L, van Heijster S, van Kuijk S, Spaan J, Delhaas T, et al. Hypertension after preeclampsia is preceded by changes in cardiac structure and function. Hypertension. 2013;62:382–90. doi: 10.1161/HYPERTENSIONAHA.113.01319. [DOI] [PubMed] [Google Scholar]
- 3.Breetveld NM, Ghossein-Doha C, van Kuijk S, van Dijk AP, van der Vlugt MJ, Heidema WM, et al. Cardiovascular disease risk is only elevated in hypertensive, formerly preeclamptic women. BJOG. 2015;122:1092–100. doi: 10.1111/1471-0528.13057. [DOI] [PubMed] [Google Scholar]
- 4.McDonald SD, Malinowski A, Zhou Q, Yusuf S, Devereaux PJ. Cardiovascular sequelae of preeclampsia/eclampsia: a systematic review and meta-analyses. Am Heart J. 2008;156:918–30. doi: 10.1016/j.ahj.2008.06.042. [DOI] [PubMed] [Google Scholar]
- 5.Gaugler-Senden IP, Berends AL, de Groot CJ, Steegers EA. Severe, very early onset preeclampsia: subsequent pregnancies and future parental cardiovascular health. Eur J Obstet Gynecol Reprod Biol. 2008;140:171–7. doi: 10.1016/j.ejogrb.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Chesley LC. Recognition of the long-term sequelae of eclampsia. Am J Obstet Gynecol. 2000;182:249–50. doi: 10.1016/s0002-9378(00)70521-7. [DOI] [PubMed] [Google Scholar]
- 7.Rich-Edwards JW, Fraser A, Lawlor DA, Catov JM. Pregnancy characteristics and women’s future cardiovascular health: an underused opportunity to improve women’s health? Epidemiol Rev. 2014;36:57–70. doi: 10.1093/epirev/mxt006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cain MA, Salemi JL, Tanner JP, Kirby RS, Salihu HM, Louis JM. Pregnancy as a window to future health: maternal placental syndromes and short-term cardiovascular outcomes. Am J Obstet Gynecol. 2016;215:484.e1–484.e14. doi: 10.1016/j.ajog.2016.05.047. [DOI] [PubMed] [Google Scholar]
- 9.White WM, Mielke MM, Araoz PA, Lahr BD, Bailey KR, Jayachandran M, Miller VM, Garovic VD. A history of preeclampsia is associated with a risk for coronary artery calcification 3 decades later. Am J Obstet Gynecol. 2016;214:519.e1–519.e8. doi: 10.1016/j.ajog.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cirillo PM, Cohn BA. Pregnancy complications and cardiovascular disease death: 50-year follow-up of the Child Health and Development Studies pregnancy cohort. Circulation. 2015;132:1234–42. doi: 10.1161/CIRCULATIONAHA.113.003901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mongraw-Chaffin ML, Cirillo PM, Cohn BA. Preeclampsia and cardiovascular disease death: prospective evidence from the Child Health and Development Studies Cohort. Hypertension. 2010;56:166–71. doi: 10.1161/HYPERTENSIONAHA.110.150078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Theilen LH, Fraser A, Hollingshaus MS, Schliep KC, Varner MW, Smith KR, Esplin MS. All-cause and cause-specific mortality after hypertensive disease of pregnancy. Obstet Gynecol. 2016;128:238–44. doi: 10.1097/AOG.0000000000001534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Funai EF, Friedlander Y, Paltiel O, Tiram E, Xue X, Deutsch L, Harlap S. Long-term mortality after preeclampsia. Epidemiology. 2005;16:206–15. doi: 10.1097/01.ede.0000152912.02042.cd. [DOI] [PubMed] [Google Scholar]
- 14.Skjaerven R, Wilcox AJ, Klungsoyr K, Irgens LM, Vikse BE, Vatten LJ, Lie RT. Cardiovascular mortality after pre-eclampsia in one child mothers: prospective, population based cohort study. BMJ. 2012;345:e7677. doi: 10.1136/bmj.e7677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Esplin MS, Fausett MB, Fraser A, Kerber R, Mineau G, Carrillo J, et al. Paternal and maternal components of the predisposition to preeclampsia. N Engl J Med. 2001;344:867–72. doi: 10.1056/NEJM200103223441201. [DOI] [PubMed] [Google Scholar]
- 16.Smith KR, Mineau GP, Bean LL. Fertility and post-reproductive longevity. Soc Biol. 2002;49:185–205. [PubMed] [Google Scholar]
- 17.Jorde LB. The genetic structure of the Utah Mormons: migration analysis. Hum Biol. 1982;54:583–97. [PubMed] [Google Scholar]
- 18.Jaskolka D, Retnakaran R, Zinman B, Kramer CK. Fetal sex and maternal risk of pre-eclampsia/eclampsia: a systematic review and meta-analysis. BJOG. 2016 doi: 10.1111/1471-0528.14163. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 19.Pariente G, Kessous R, Sergienko R, Sheiner E. Is preterm delivery an independent risk factor for long-term maternal kidney disease? J Matern Fetal Neonatal Med. 2016 doi: 10.1080/14767058.2016.1205022. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 20.Kessous R, Shoham-Vardi I, Pariente G, Holcberg G, Sheiner E. An association between preterm delivery and long-term maternal cardiovascular morbidity. Am J Obstet Gynecol. 2013;209:368.e1–8. doi: 10.1016/j.ajog.2013.05.041. [DOI] [PubMed] [Google Scholar]
- 21.Berkman L, Macintyre S. The measurement of social class in health studies: old measures and new formulations. In: Kovenias, et al., editors. Social Inequalities and Cancer. Lyon, France: International Agency for Research on Cancer; 1997. pp. 51–64. IARC Publication 138. [PubMed] [Google Scholar]
- 22.Auger N, Fraser WD, Schnitzer M, Leduc L, Healy-Profitos J, Paradis G. Recurrent pre-eclampsia and subsequent cardiovascular risk. Heart. 2017;103:235–43. doi: 10.1136/heartjnl-2016-309671. [DOI] [PubMed] [Google Scholar]
- 23.The American College of Obstetricians and Gynecologists: Task Force on Hypertension in Pregnancy. Hypertension in Pregnancy: report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122:1122–31. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 24.Goff DC, Lloyd-Jones DM, Bennett G, Coady S, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S49–73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 25.U.S. Census Bureau. QuickFacts. Washington, D.C: U.S. Census Bureau; 2016. http://www.census.gov/quickfacts/UT. [Google Scholar]
- 26.Utah Department of Health. Utah Health Status Update: Obesity and associated chronic conditions. Utah Department of Health, Center for Health Data and Informatics, Indicator-Based Information System for Public Health; 2010. http://ibis.health.utah.gov/pdf/opha/publication/hsu/2010/10May_Obesity.pdf. [Google Scholar]