Abstract
Objectives
Studies that demonstrate an association between rheumatoid arthritis (RA) and dysbiotic oral microbiomes are often confounded by the presence of extensive periodontitis in these individuals. Therefore, the present investigation sought to investigate the role of RA in modulating the periodontal microbiome by comparing periodontally healthy individuals with and without RA.
Methods
Subgingival plaque was collected from was collected periodontally healthy individuals (22 with and 19 without RA), and 16S gene sequenced on the Ilumina MiSeq platform. Bacterial biodiversity and co-occurrence patterns were examined using the QIIME and PhyloToAST pipelines.
Results
The subgingival microbiomes differed significantly based on both community membership and as well as the abundance of lineages, with 41.9% of the community differing in abundance and 19% in membership. In contrast to the sparse and predominantly congeneric co-occurrence networks seen in controls, RA subjects revealed a highly connected grid containing a large inter-generic hub anchored by known periodontal pathogens. Predictive metagenomic analysis (PICRUSt) demonstrated that arachidonic acid and ester lipid metabolism pathways might partly explain the robustness of this clustering. As expected from a periodontally healthy cohort, Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans were not significantly different between groups, however, Cryptobacterium curtum, another organism capable of producing large amounts of citrulline, emerged as a robust discriminant of the microbiome in individuals with RA.
Conclusions
Our data demonstrates that the oral microbiome in RA is enriched for inflammophilic and citrulline producing organisms, which may play a role in the production of autoantigenic citrullinated peptides in RA.
Keywords: Rheumatoid arthritis, periodontitis, DNA Sequence Analysis, oral microbiome
INTRODUCTION
Rheumatoid arthritis (RA) has been associated with periodontal disease (PD), a bacterially initiated chronic inflammation that leads to destruction of tooth-supporting tissues1. Although PD and RA share similar inflammatory pathways as well as genetic and environmental risk factors, these are insufficient to explain this connection1.
While the cause of RA remains unknown, it has been hypothesized that oral microbiota2,3 in particular the periodontal pathogens Porphyromonas gingivalis and Aggregatibacter actinomycemtemcomitans, may play a critical role in its pathogenesis4,5
Studies using next generation sequencing methods demonstrate the oral microbiome is altered in RA6,7. However, the majority of these studies included individuals with moderate to severe periodontitis7 or individuals whose periodontal health status was not established6. Periodontitis, by itself, is a significant modifier of the oral microbiome8, making it difficult to dissect the relative contributions of periodontitis and RA to the microbial dysbiosis.
Given the potential role oral bacteria may play in the etiopathogenesis of RA, we set out to characterize the periodontal microbiome in periodontally healthy individuals with and without RA, using next generation sequencing.
METHODS
The study sample included patients with RA and non-RA controls. All participants were periodontally healthy. Subgingival plaque samples were collected and analyzed using 16S rDNA sequencing. Detailed methods are described in supplementary information. The sequences are deposited in the Sequence Read Archive of NCBI (project number: PRJNA391575).
RESULTS
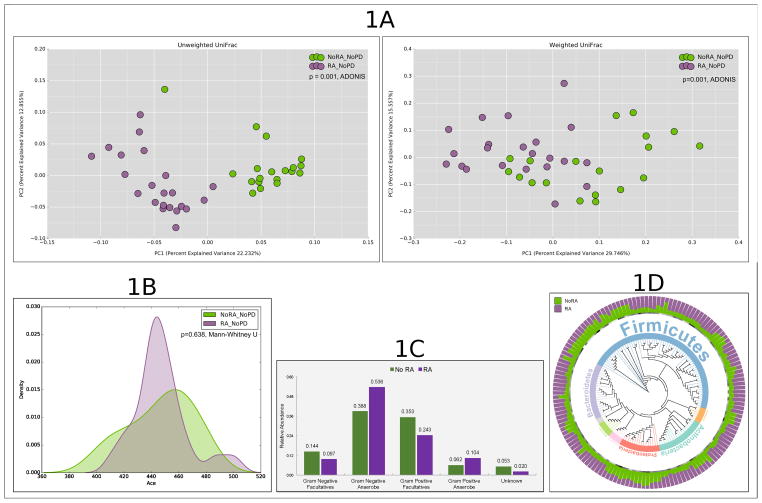
We examined 22 patients with RA and 19 non-RA controls. There was a statistically significant but clinically inconsequential difference between groups in periodontal measures, in particular PPD and CAL (Table 1). Principal Coordinate Analysis (PCoA) of both unweighted and weighted UniFrac distances demonstrated significant clustering of the microbiomes based on RA status (Figure 1, p=0.001, Adonis test), indicating that these groups differed both in presence or absence of lineages (community membership), as well as in the relative abundances of lineages within communities (community structure).
Table 1.
Clinical and demographic characteristics in periodontally healthy subjects with rheumatoid arthritis (RA) and without RA (non-RA). Data represented as mean (25, 75 percentile) for ordinal data and percentage for categorical data. P values are calculated using Mann-Whitney test for ordinal data and Fisher’s test for categorical data and significant differences (p<0.05) indicated with an asterisk (*). Abbreviations: BMI, body mass index; PPD probing pocket depth; BoP, bleeding on probing; CAL, clinical attachment loss; ESR, erythrocyte sedimentation rate; VAS, visual analogue scale for patients global assessment of disease activity; DAS, disease activity score
| RAn=22 | Non-RAn=19 | |
|---|---|---|
| Age in years, mean (IQR) | 60 (54.1, 63.4)* | 36 (32.9, 41.6) |
| Gender (% Male) | 23 | 32 |
| Ethnicity (%) | ||
| White | 95 | 89 |
| Asian | 5 | 11 |
| Smoking history (%) | ||
| Never | 62 | 90 |
| Former | 29 | 5 |
| Current | 9 | 5 |
| Alcohol consumption (%) | ||
| Never | 11 | 14 |
| 1–4 times/month | 73 | 45 |
| 1–4 times/week | 16 | 41 |
| Clinical periodontal characteristics | ||
| PPD in mm, mean (IQR) | 2.3 (2.2, 2.4)* | 1.6 (1.5, 1.7) |
| Number of sites with PPD>4mm | 1.2 (0, 2) | 0.9 (0,3) |
| Number of sites with BoP, mean (IQR) | 6 (0, 19) | 4 (1, 16) |
| Gingival recession in mm, mean (IQR) | 0.28 (0.01, 0.26)* | 0.13 (0.04, 0.2) |
| Measures of RA severity | ||
| ESR | 8 (8.7, 21.7) | |
| VAS (global assessment of disease activity) | 41 (31.7, 58.5) | |
| DAS28 | 3.4 (2.7, 3.9) | |
p<0.001
Figure 1.
Differences in alpha and beta diversity metrics between periodontally healthy subjects with and without rheumatoid arthritis (RA). (A): Principal Coordinates Analysis (PCoA) plots of unweighted and weighted Unifrac distances (B): Kernel plots of alpha diversity (Abundance-based Coverage Estimator (ACE)). The peak indicates the median values for each group. The x-axis indicates the data range. (C): Distribution of species by gram staining and oxygen requirement characteristics. Groups that share the same symbol are significantly different from each other (p < 0.05, Kruskal Wallis test) (D): Phylogenetic tree representing normalized mean relative abundance (NMRA, stacked bar chart), core species (circles represent species present in ≥80% of samples in a group), significant frequency of detection (stars) and phylum-level taxonomic annotation (colored-strips and text) for significantly different and differentially abundant species-level OTUs (tree leaves). Data for figure 1D is presented in supplemental table 1.
Since patients with RA differed from controls in both community membership and structure, we identified species level operational taxonomic units (s-OTUs) that contributed to this difference using an increasingly granular top-down approach. Patients with RA present had greater abundances of obligate anaerobes (both gram-positive and gram-negative) while facultatives (especially gram-negative) were identified in greater abundance in non-RA controls (p<0.05 Wilcoxon signed rank test, Figure 1).
We then used DESeq29 to identify differentially abundant OTUs; with p-values <0.05 after adjusting for multiple testing, and Fisher’s exact test to examine the frequency of detection. We identified 558 OTUs from 3,963,291 classifiable sequences (mean of 107115 sequences per sample, range 69626–182993). Rarefaction curves demonstrated that all samples approached saturation or had plateaued. 229 OTUs (41.9% of the community) differed significantly in structure and 105 OTUs (19%) differed significantly in membership between groups (Figure 1 and supplementary table 1). Certain species were significantly more abundant in patients with RA, including those belonging to the genera Actinomyces (odds ratios (OR) varying from 4–9 for each species within the genus), Cryptobacterium (OR=36), Dialister, Desulfovibrio (ORs of 4 and 26), Fretibacterium (OR 9 to 12), Leptotrichia (OR 7 to 26) Prevotella (OR 0.04 to 6), Selenomonas (OR 0 to 7), Treponema (OR 0 to 7), and Veillonellaceae [G1] (OR 0 to 6).
In contrast, several species belonging to the genera Aggregatibacter, Gemella, Granulicatella, Hemophilus, Neisseria and Streptoccoci not only demonstrated lower abundances but also were less frequently detected in RA. These significantly abundant species accounted for a median of 28% (range 12–82%) of each individual’s microbiome in patients with RA, indicating that these differences are not attributable to the rare biosphere.
Since the subgingival microbiome is known to be significantly heterogeneous among individuals10, we used the core microbiome (suite of species identified in ≥ 80% of subjects) to compare stable associations between groups. 326 OTUs were identified in the core microbiome of all study participants and 364 in patients with RA. 27.7% of the community (101 OTUs) differed significantly in structure and 10.9% (40 OTUs) in membership, with 38 species unique to the RA core microbiome (Figure 1). Importantly, 157 of the 229 species identified above belonged to the core microbiome.
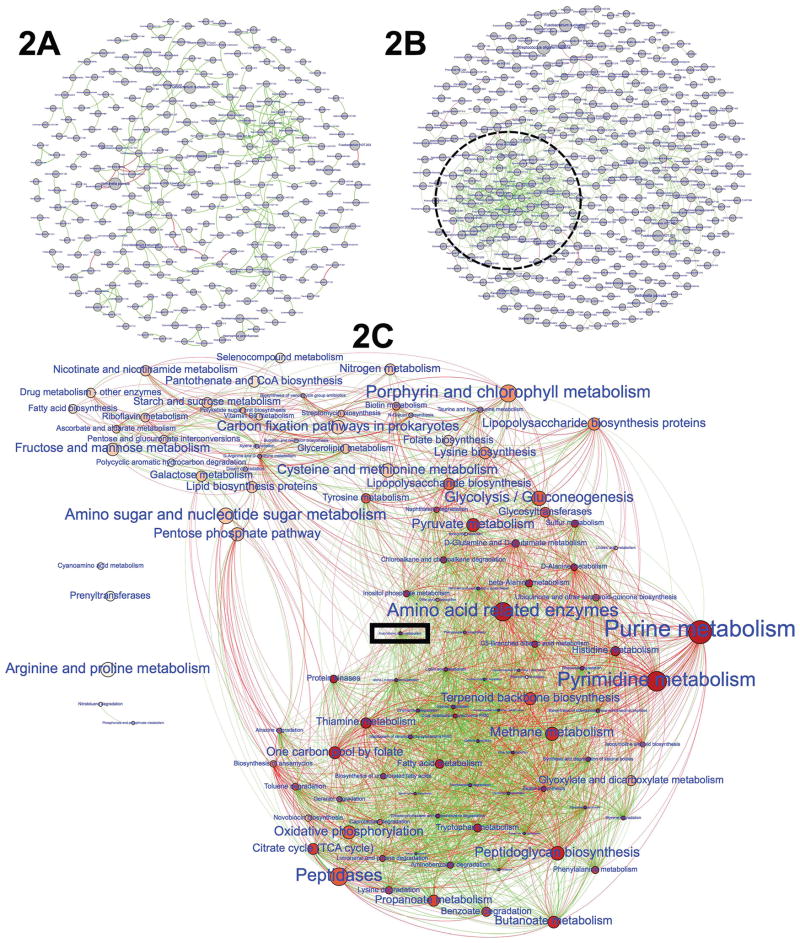
Sparse, congeneric networks were observed in non-RA controls (Figure 2). On the other hand, the network topology of individuals with RA revealed a highly connected grid with a robust intergeneric hub. 83 of the 157 core species were incorporated in this hub, further reinforcing our observation that in subjects with RA, the environment imposes a selection drive. Importantly, known pathogenic species belonging to Treponema, Selenomonas, Filifactor, Campylobacter and Fretibacterium were tightly interwoven into this hub, and 12 gram-negative species were identified as network anchors. Interestingly, species traditionally associated with RA, for example, P.gingivalis (Pg) and A.actinomycetemcomitans (Aa), were not part of the network cluster.
Figure 2.
Co-occurrence networks in periodontally healthy subjects with or without rheumatoid arthritis (RA): Each network graph contains nodes (circles) and edges (connections representing Spearman’s ρ). Edges are colored green for positive correlation and red for negative correlation. Nodes represent species-level OTUs in 2A and 2B and genes encoding for metabolic functions in 2C; and are sized by relative abundance. Edges represent significant and robust Spearman’s correlation (p<0.05, ρ≥0.75). Data for figure 2C is presented in supplemental table 2.
Since there is little literature-based information to provide insights into the biological basis for this tight clustering, we combined predictive metagenomic analysis (PICRUSt11) with network graph theory and core microbiome analysis to explore if shared functionality could explain co-occurrence (Figure 2). Bacterial arachidonic acid and ether lipid metabolism genes exhibited the greatest betweenness centrality (reflecting the amount of control that these node exerts over the interactions of other nodes in the network12), and the highest degree centrality (an indication that they are the central focal point of the structure).
DISCUSSION
Gram-negative anaerobes are known to play important roles in initiating periodontitis, and emerging evidence also implicates them in the etiopathogenesis of RA6,13. Our results show that even in periodontally healthy RA patients, gram-negative anaerobes are significantly more abundant in RA, consistent with a dysbiotic state. Such a status might indicate a pre-clinical phase of periodontitis. As expected from a periodontally healthy adult cohort, Pg and Aa were neither dominant members of the microbiome nor significantly different between groups. Taken together with previous studies13, our data implies that gram-negative bacteria other than Pg and Aa may play a role in initiation of RA, while the evidence from literature suggests that these two species may be critical to disease perpetuation.
Recent investigations demonstrate that while substantial microbial heterogeneity exists among healthy individuals, a robust core microbiome is identifiable in individuals who smoke or are pregnant14–16. The findings of the present study parallel these previous observations and support the ecological plaque hypothesis17, suggesting that RA imposes a habitat filtering on the subgingival environment, preferentially promoting the growth of certain organisms.
Traditional statistical methods assume bacterial presence and abundance to be independent variables, but in reality bacterial presence in a biofilm is driven by inter-dependent nutritional and metabolic interactions. Therefore, we combined network graph theory with DESeq and core microbiome analysis to examine co-occurrence patterns and identify important community members (network anchors). No network anchors were identifiable in controls (since betweenness centrality was homogeneous between species), indicating that this is an ecological niche in equilibrium. However, the tightly woven hub of anaerobes suggest that a small group of anaerobic bacteria play an important role controlling the flow of resources in the RA-influenced microbiome, implying that even small changes in these anchors could impact upon community assembly in people with RA. These species may be potential targets for microbial disruption.
Arachidonic acid (AA) is essential for cell membrane integrity. It is metabolized to prostaglandin E2 (PGE2) and other pro-inflammatory eicosanoids, which are implicated in the development of RA. The ability to metabolize AA into pro-inflammatory eicosanoids is an emergent property of opportunistic pathogens18. AA is also known to inhibit the growth and epithelial adhesion of beneficial species in the gut19. Taken together, the data indicate that the subgingival microbiome is both influenced by, and influences, the inflammatory burden of RA.
One of the most intriguing findings was the identification of Cryptobacterium curtum as a predominant member of the RA-influenced periodontal microbiome. This gram-positive, assacharolytic, anaerobic rod (which was previously misclassified as Eubacterium saburreum) degrades arginine through the arginine deiminase pathway and produces substantial amounts of citrulline, ornithine and ammonia20. We have previously identified this as a periodontal pathogen21, and translocation from oral sources has been implicated in the etiology of distant infections such as pelvic abscesses, gynecologic infections, and wounds22. More importantly, C. curtum is enriched in the oral and gut microbiomes of early RA cases6,23. In line with previous studies, we observed that this species was a member of the core microbiome in RA patients. Compared to non-RA controls, this species demonstrated a 100-fold greater abundance in RA with 39-fold greater odds of detection. While this unusually high association does not necessarily suggest an etiopathogenic role for C.curtum, this organism is certainly a candidate for further studies. In light of evidence that antibodies against citrullinated protein and peptides (ACPA) precede the clinical onset of RA by several years, have high specificity for RA at over 95%24,25 and that we previously observed antibodies characteristic of RA, including citrullinated and uncitrullinated peptides of the RA autoantigens in individuals with periodontitis3, the ability of C.curtum to degrade arginine via the arginine deiminase pathway and to produce substantial amounts of citrulline is of particular interest. Presence of C.curtum in the plaque may therefore be a contributing factor in the development of RA autoantigens and warrants further investigation.
In summary, our data suggest that RA plays a major role in shaping the oral microbiome. The microbiome in RA is enriched for pro-inflammatory organisms and those capable of producing substantial amounts of citrulline (pro-antigenic). An ability to metabolize arachidonic acid and ether lipids appears to be a shared function among the species observed in individuals with RA. Our findings lend further credence to a link between the oral microbiome and RA; however, longitudinal studies are needed to understand directionality and causality, and also to characterize potentially “driver species” that could serve as biomarkers for RA.
Supplementary Material
Supplemental Table 1: Species level OTU matrix highlighting results of abundance analysis (mean ± standard deviation), differential abundance (DESEq2), differential detection frequency, and presence in core (observed in ≥80% of samples in a group) for periodontally healthy subjects with and without rheumatoid arthritis (RA).
Supplemental Table 2: Correlation matrix of significant (p<0.05) Spearman’s correlation among metabolism related KEGG level 3 gene functions of periodontally healthy subjects with rheumatoid arthritis (RA).
Supplemental File 1: Methods
Acknowledgments
Sources of Funding: This paper presents independent research partially funded by the National Institute for Health Research (NIHR) under its Research for Patient Benefit (RfPB) Programme (Grant Reference Number PB-PG-0609-19100), by the GSK Research award 2016 (Oral & Dental Research Trust) and the Philips oral healthcare young investigator research grant. Praveen Sharma is funded by an NIHR Doctoral Research Fellowship (Grant Reference Number DRF-2014-07-109). Paola de Pablo is supported by an NIHR fellowship (Grant Code: NIHR PDF-2014-07-055). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health. KR And AF are supported by the National Institute for Health Research (NIHR) Birmingham Biomedical Research Centre. The sequencing effort was supported by a NIDCR grant (R01-DE022579) to Purnima Kumar. Akshay Paropkari is supported by a grant from the NCI (U01 CA188250).
References
- 1.Kaur S, White S, Bartold M. Periodontal Disease as a Risk Factor for Rheumatoid Arthritis: A Systematic Review. JBI library of systematic reviews. 2012;10(42 Suppl):1–12. doi: 10.11124/jbisrir-2012-288. published Online First: 2012/01/01. [DOI] [PubMed] [Google Scholar]
- 2.de Pablo P, Chapple IL, Buckley CD, et al. Periodontitis in systemic rheumatic diseases. Nat Rev Rheumatol. 2009;5(4):218–24. doi: 10.1038/nrrheum.2009.28. published Online First: 2009/04/02. [DOI] [PubMed] [Google Scholar]
- 3.de Pablo P, Dietrich T, Chapple IL, et al. The autoantibody repertoire in periodontitis: a role in the induction of autoimmunity to citrullinated proteins in rheumatoid arthritis? Ann Rheum Dis. 2014;73(3):580–6. doi: 10.1136/annrheumdis-2012-202701. [DOI] [PubMed] [Google Scholar]
- 4.Wegner N, Wait R, Sroka A, et al. Peptidylarginine deiminase from Porphyromonas gingivalis citrullinates human fibrinogen and α-enolase: Implications for autoimmunity in rheumatoid arthritis. Arthritis & Rheumatism. 2010;62(9):2662–72. doi: 10.1002/art.27552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Konig MF, Abusleme L, Reinholdt J, et al. <em>Aggregatibacter actinomycetemcomitans</em>–induced hypercitrullination links periodontal infection to autoimmunity in rheumatoid arthritis. Science translational medicine. 2016;8(369):369ra176–369ra176. doi: 10.1126/scitranslmed.aaj1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang X, Zhang D, Jia H, et al. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nature medicine. 2015;21(8):895–905. doi: 10.1038/nm.3914. published Online First: 2015/07/28. [DOI] [PubMed] [Google Scholar]
- 7.Scher JU, Ubeda C, Equinda M, et al. Periodontal disease and the oral microbiota in new-onset rheumatoid arthritis. Arthritis and rheumatism. 2012;64(10):3083–94. doi: 10.1002/art.34539. published Online First: 2012/05/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Griffen AL, Beall CJ, Campbell JH, et al. Distinct and complex bacterial profiles in human periodontitis and health revealed by 16S pyrosequencing. The ISME journal. 2012;6(6):1176–85. doi: 10.1038/ismej.2011.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paster BJ, Boches SK, Galvin JL, et al. Bacterial diversity in human subgingival plaque. J Bacteriol. 2001;183(12):3770–83. doi: 10.1128/JB.183.12.3770-3783.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Langille MG, Zaneveld J, Caporaso JG, et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nature biotechnology. 2013;31(9):814–21. doi: 10.1038/nbt.2676. published Online First: 2013/08/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoon J, Blumer A, Lee K. An algorithm for modularity analysis of directed and weighted biological networks based on edge-betweenness centrality. Bioinformatics. 2006;22(24):3106–8. doi: 10.1093/bioinformatics/btl533. published Online First: 2006/10/25. [DOI] [PubMed] [Google Scholar]
- 13.Mikuls TR, Payne JB, Yu F, et al. Periodontitis and Porphyromonas gingivalis in Patients with Rheumatoid Arthritis. Arthritis and rheumatism. 2014 doi: 10.1002/art.38348. published Online First: 2014/01/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mason MR, Preshaw PM, Nagaraja HN, et al. The subgingival microbiome of clinically healthy current and never smokers. Isme Journal. 2015;9(1):268–72. doi: 10.1038/ismej.2014.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paropkari AD, Leblebicioglu B, Christian LM, et al. Smoking, pregnancy and the subgingival microbiome. Scientific reports. 2016;6:30388. doi: 10.1038/srep30388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jetté ME, Dill-McFarland KA, Hanshew AS, et al. The human laryngeal microbiome: effects of cigarette smoke and reflux. Scientific reports. 2016;6:35882. doi: 10.1038/srep35882. http://www.nature.com/articles/srep35882 - supplementary-information. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marsh PD. Microbial ecology of dental plaque and its significance in health and disease. Advances in dental research. 1994;8(2):263–71. doi: 10.1177/08959374940080022001. [DOI] [PubMed] [Google Scholar]
- 18.Fourie R, Ells R, Swart CW, et al. Candida albicans and Pseudomonas aeruginosa Interaction, with Focus on the Role of Eicosanoids. Frontiers in physiology. 2016;7:64. doi: 10.3389/fphys.2016.00064. published Online First: 2016/03/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kankaanpaa P, Yang B, Kallio H, et al. Effects of polyunsaturated fatty acids in growth medium on lipid composition and on physicochemical surface properties of lactobacilli. Appl Environ Microbiol. 2004;70(1):129–36. doi: 10.1128/AEM.70.1.129-136.2004. published Online First: 2004/01/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uematsu H, Sato N, Djais A, et al. Degradation of arginine by Slackia exigua ATCC 700122 and Cryptobacterium curtum ATCC 700683. Oral Microbiology and Immunology. 2006;21(6):381–84. doi: 10.1111/j.1399-302X.2006.00307.x. [DOI] [PubMed] [Google Scholar]
- 21.Kumar PS, Griffen AL, Barton JA, et al. New bacterial species associated with chronic periodontitis. J Dent Res. 2003;5(82):338–44. doi: 10.1177/154405910308200503. [DOI] [PubMed] [Google Scholar]
- 22.Brook I, Frazier EH. Significant recovery of nonsporulating anaerobic rods from clinical specimens. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 1993;16(4):476–80. doi: 10.1093/clind/16.4.476. published Online First: 1993/04/01. [DOI] [PubMed] [Google Scholar]
- 23.Vaahtovuo J, Munukka E, Korkeamaki M, et al. Fecal microbiota in early rheumatoid arthritis. The Journal of rheumatology. 2008;35(8):1500–5. published Online First: 2008/06/06. [PubMed] [Google Scholar]
- 24.Schellekens GA, de Jong BA, van den Hoogen FH, et al. Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis-specific autoantibodies. J Clin Invest. 1998;101(1):273–81. doi: 10.1172/JCI1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schellekens GA, Visser H, de Jong BA, et al. The diagnostic properties of rheumatoid arthritis antibodies recognizing a cyclic citrullinated peptide. Arthritis and rheumatism. 2000;43(1):155–63. doi: 10.1002/1529-0131(200001)43:1<155::AID-ANR20>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1: Species level OTU matrix highlighting results of abundance analysis (mean ± standard deviation), differential abundance (DESEq2), differential detection frequency, and presence in core (observed in ≥80% of samples in a group) for periodontally healthy subjects with and without rheumatoid arthritis (RA).
Supplemental Table 2: Correlation matrix of significant (p<0.05) Spearman’s correlation among metabolism related KEGG level 3 gene functions of periodontally healthy subjects with rheumatoid arthritis (RA).
Supplemental File 1: Methods