Highlights
-
•
Pregnant Asian Indian women have bimodality in plasma glucose distribution.
-
•
Bimodality is evident even in very young pregnant women of age 18–23 years.
-
•
Glucose cut points may suggest thresholds for gestational diabetes diagnosis.
Keywords: Asian Indian women, Pregnancy, Bimodality, Gestational diabetes mellitus
Abstract
Aims
Presence of bimodality in plasma glucose distribution (BPG) and its relevance for gestational diabetes mellitus (GDM) diagnosis were studied in Asian Indian pregnant women.
Methods
Fasting (FPG) and two hour plasma glucose (2-h PG) values of oral glucose tolerance tests performed in 36,530 pregnant women for GDM screening (2006–16 period), were analyzed for BPG. A unimodal normal and a mixture of two normal distributions were fitted to log-transformed FPG and 2-h PG data. The mixture model was compared to unimodal model for BPG using likelihood ratio test (LRT) and the comparison was further verified by bootstrapping. The cut points of the two normal distribution curves in the mixture models of FPG and 2-h PG were noted.
Results
Fasting and 2-h PG distribution was bimodal in all pregnant women. The comparison of mixture and unimodal models using LRT revealed p value <0.001 in all age groups. The cut points for FPG and 2-h PG were 5.81 mmol/L (95% CI: 5.69–5.92) and 8.41 mmol/l (95% CI: 8.09–8.75) respectively.
Conclusion
BPG is noted for both FPG and 2-hPG in Asian Indian pregnant women. The cutpoints of normal distribution curves are close to threshold values for FPG and 2-h PG proposed in NICE (National Institute for health and Care Excellence) and IADPSG (International Association of Diabetes and Pregnancy Study Group) GDM diagnostic criteria respectively. Further research on BPG in pregnant women of racial groups with high GDM prevalence, is likely to be of value in GDM diagnosis.
Introduction
In ethnic groups with high prevalence of diabetes mellitus (DM), many community based studies have revealed bimodality in plasma glucose distribution (BPG) [1], [2], [3], [4]. Among Pima Indians in United States, BPG is clearly demonstrable marking a distinction between diabetic and non diabetic populations [1], which formed one of the parameters for setting up cut off Plasma Glucose values for diagnosis of diabetes mellitus in World Health Organization 1980 & 1985 criteria [5], [6]. Subsequently BPG was noted in several populations like Mexican Americans [7], Micronesians [8], certain Chinese ethnic groups [9], multi ethnic population in Malaysia [10] and Caucasians residing in USA [11]. Proneness for bimodality among Asian Indians was apparent in earlier studies conducted in migrant populations in South Africa [12] and Malaysia [10] as well as in native Indian participants of the ‘Evaluation of Screening and Early Detection Strategies for Type 2 Diabetes and Impaired Glucose Tolerance’ (DETECT-2) study [13]. Generally BPG is more apparent in elderly population especially when the sample size is large and the prevalence of diabetes is high.
Unlike the diagnosis of diabetes mellitus in non pregnant state, the screening and diagnosis of Gestational diabetes mellitus (GDM) remains without an international consensus [14], [15]. The International Association of Diabetes and Pregnancy Study Group (IADPSG) recommendations [16] for GDM diagnosis based on the maternal and fetal outcome data of multicenter Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study [17], are accepted by most preeminent organizations like World Health Organization (WHO), International Diabetes Federation (IDF), American Diabetes Association (ADA) and Federation of International Gynecologists and Obstetricians (FIGO). But it is not recommended by National Institute of health and care excellence (NICE) [18] and American College of Obstetrics and Gynecology (ACOG) [19]. Presently, the only consensus between these organizations is the acceptance of OGTT as the diagnostic test for GDM. But there is no agreement on the glucose load for the test, timing of blood sampling, plasma glucose (PG) cut off values and on the number of abnormal PG values required for diagnosis. The lack of a ‘gold standard’ criteria for GDM diagnosis continues to plague research as well as clinical management in GDM patients.
The diagnostic glucose threshold values, based on the onset of micro-vascular complications [20] are widely accepted for diagnosis of diabetes mellitus in non pregnant state. However a similar attempt to set glucose threshold values for GDM diagnosis by IADPSG, based on pregnancy outcome data of HAPO study, generated several controversies [21]. As bimodality in glucose distribution in high risk populations like Pima Indians was helpful in setting up DM diagnostic criteria in general population, the usefulness of a similar approach in diagnosing GDM is explored in the present study. There is an ongoing global diabetes epidemic and South Asia is projected as its epicenter [22]. Recent data from India [23] showed a high and rising prevalence of Type 2 diabetes in urban population with a concomitant rise in GDM prevalence [24], [25]. In the present hospital based study we tested the pattern of plasma glucose distribution among pregnant Asian Indian women; an ethnic group with high GDM prevalence as well as proneness for bimodal glucose distribution [10], [12], [13], [24].
Materials and methods
Participants
This retrospective study involved pregnant women of Asian Indian origin who attended routine antenatal clinics in St Stephen’s Hospital, a 600 bedded tertiary care hospital in New Delhi, during 2006 January to 2016 December period. The hospital delivers ∼3000 pregnant women annually and all of them are of Indian ethnic background, residing in New Delhi. 36,530 pregnant women who underwent 75 g OGTT as part of a universal one step GDM screening strategy were the candidates for this analysis. 454 women with known pre-gestational diabetes in whom OGTT was not done, were not included in the study. The FPG and 2-h PG values of the above mentioned 36,530 OGTTs formed the data for analysis for bimodality in glucose distribution. This study was approved by the institutional ethics committee.
Methods
The OGTTs were generally scheduled at 24–28 weeks of gestation, but done earlier if clinically indicated (previous GDM, family history of DM, bad obstetric history etc.) or later if women presented late for first booking. After 10 h overnight fast, standard protocol for the OGTT with ingestion of 75 g glucose [d-Glucose powder (Glaxo) 75 g dissolved in 200 ml distilled water consumed in 5 min] was followed in all women. Venous plasma glucose values were obtained at 0 h (FPG) and at 2 h after oral glucose (2-h PG) in all women. The OGTTs were supervised by a diabetic educator nurse who ensured proper pre test preparation, fasting state, full consumption of oral glucose and proper timing of blood sampling.
The plasma glucose was estimated by the glucose oxidase method on Beckman AU 680. The laboratory is certified by the National Accreditation Board for testing and calibration Laboratories and uses Biorad laboratories for proficiency testing. All the laboratory standards for glucose were met (i.e., imprecision < 2.9%, bias < 2.2% and total analytical error < 6.9% [26].
Statistical analysis
The data was analyzed by R-software 3.3.3. The distribution of FPG, 2-h PG values are generally skewed to the right. Log transformation was applied to remove the right skewness. A normal distribution and mixture of two normal distributions were fitted to log-transformed glucose data. The normal distribution was fitted using maximum likelihood method [27]. The mixture model of two normal components is where f(x) = density function for a normal distribution; α, 1-α are the mixture proportions; , are the means and are the standard deviations and it was fitted through the expectation–maximization (EM). The normal mix EM function from the Mixtools in R was applied [28]. To assess the presence of bimodality the mixture model was compared with unimodal distribution using likelihood ratio test in the total study group (Age 18–45 yrs) and in the age stratified groups (18–23, 24–30, 31–45 yrs) [29].
The variance of two normal distributions were quite different, thus for finding the p values for significance of bimodal as compared to unimodal, χ2 distribution with 6 degree of freedom was applied [27]. To overcome the regularity problems like identifiability of the mixture model, this comparison was further verified by bootstrapping method with 500 bootstraps as follows [30].
-
a)
A bootstrap sample was generated from the one-component normal distribution (H0-null hypothesis) with mean and variance as estimated from our data. The sample size of the generated data was also the same as that of each corresponding age group. The −2 log λ for the bootstrap sample was calculated ie where L0 = maximum likelihood estimates (MLE) under null hypothesis and L1 = MLE of alternative hypothesis i.e. bimodal distribution
-
b)
The above step was repeated 499 times to obtain 499 simulated −2 log λ.
-
c)
The −2 log λ for the observed data was calculated.
-
d)
m, which is the total number of simulated values of −2 log λ greater than or equal to the observed value, was counted and the p value , was determined
These steps were done for FPG and 2-h PG values in the total study group and in each age stratified group. The 95% confidence intervals (CIs) of means of bimodal normal were estimated using bootstrapping with 1000 bootstraps [31]. Histograms of plasma glucose concentration (mmol/L) for both FPG and 2-h PG values, were plotted. On detection of bimodality in likelihood ratio test, the fitted bimodal distribution curves were superimposed on the histogram chart. The crossing point of two normal distribution curves of bimodal distribution was defined as the cut-off point. The approximate 95% CIs for the cut-off points were estimated by bootstrapping with 1000 bootstraps [31].
Results
The mean age of the 36,530 pregnant women of our study group was 27.02 ± 3.98 yrs. Gestational age at the time of OGTT was available in 33,242 (91%) women; 3590 (10.8%), 26,726 (80.4%) and 2926 (8.8%) women underwent the OGTT at <24, 24–28 and >28 weeks respectively. The FPG values were available for all, but due to vomiting during OGTT or blood sampling errors in 93 women (0.25%), reliable 2-h PG values could be obtained only in 36,437 women. The NICE criteria (either FPG > 5.6 mmol/L or 2-h PG > 7.8 mmol/L) was used for GDM diagnosis [18]. The GDM prevalence in the whole group (18–45 yrs) was 16.4% with rising trend as age advances; 9.38%, 15.4% and 26.65% respectively in 18–23 yrs, 24–30 yrs, 31–45 yrs age groups.
A normal distribution and mixture of two normal distributions were fitted to the log transformed PG values. The FPG and 2-h PG parameters for whole (18–45 yrs) and the age stratified 18–23, 24–30, 31–45 yrs groups are shown in Table 1, Table 2. Bimodal distribution was observed in all age groups for both FPG and 2-h PG values. The Log likelihood ratio statistics showed a significant difference between the unimodal and the normal bimodal distributions by chi square test with degree of freedom 6 (p < 0.001). Bootstrapping method for hypothesis testing with 499 bootstraps also produced similar results. None of the −2 log λ value of bootstrap exceeded the observed value of −2 log λ, which gave p value 0.002 (1/500) in all groups. The histograms for FPG and 2-h PG are shown in Fig. 1, Fig. 2. The fitted bimodal model revealed two distribution patterns for FPG and 2-h PG values. The cut points of two normal distribution curves of mixture model in the whole study group for FPG and 2-h PG values respectively were 5.81 mmol/L (95% CI: 5.69–5.92) and 8.41 mmol/l (95% CI: 8.09–8.75). In the whole group, the proportions of second mode in the normal bimodal distribution for the FPG and 2-h PG values were 0.08 and 0.18 respectively. The proportions for the age stratified groups are shown in Table 1, Table 2.
Table 1.
Statistical tests of unimodal and bimodal models of log transformed Fasting Plasma Glucose (FPG) concentrations by age.
| Age groups yrs | Number of women | Log mean mmol/I | Unimodal |
Biomodal |
Second mode proportion | Log likelihood value |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Log Fasting plasma Glucose mmol/I |
Mean mmol/I 95% Cl# |
|||||||||||||
| SD(s) | Mean* mmol/I 95% Cl‡ | m1 | S1 | m2 | s2 | Mean1 | Mean2 | Unimodal | Bimodal | P value† | ||||
| 18–45 | 36,530 | 1.519 | 0.122 | 4.60(4.59–4.61) | 1.512 | 0.103 | 1.592 | 0.234 | 4.56(4.55–4.57) | 5.05(4.98–5.13) | 0.084 | 24,975 | 26,335 | <0.001 |
| 18–23 | 6969 | 1.489 | 0.115 | 4.46(4.45–4.48) | 1.490 | 0.083 | 1.488 | 0.1521 | 4.45(4.43–4.47) | 4.48(4.45–4.52) | 0.387 | 5186 | 5294 | <0.001 |
| 24–30 | 23,106 | 1.519 | 0.119 | 4.60(4.59–4.61) | 1.513 | 0.101 | 1.584 | 0.229 | 4.56(4.55–4.57) | 5.02(4.93–5.11) | 0.082 | 16,441 | 17,230 | <0.001 |
| 31–45 | 6455 | 1.552 | 0.133 | 4.76(4.75–4.78) | 1.538 | 0.105 | 1.667 | 0.245 | 4.68(4.67–4.70) | 5.46(5.29–5.66) | 0.100 | 3876 | 4285 | <0.001 |
Meani = exp(mi + si2/2); i = 1, 2 where mi and si are log Fasting plasma glucose means and standard deviations of bimodal normal distribution respectively; exp = exponentiation.
P-value, log likelihood ratio test.
Bootstrap method using 1000 bootstrap and percentile (2.5%–97.5%) was used for 95% confidence interval.
Table 2.
Statistical tests of unimodal and bimodal models of log transformed post challenged 2 h plasma glucose concentrations by age.
| Age groups yrs | Number of women | Log mean mmol/I | Unimodal |
Biomodal |
Second mode proportion | Log likelihood value |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Log 2 h plasma glucose mmol/I |
Mean mmol/I 95% Cl# |
|||||||||||||
| SD(s) | Mean* mmol/I 95% Cl‡ | m1 | S1 | m2 | s2 | Mean1 | Mean2 | Unimodal | Bimodal | P value† | ||||
| 18–45 | 36,437 | 1.818 | 0.221 | 6.32(6.30–6.33) | 1.780 | 0.186 | 1.987 | 0.279 | 6.04(6.00–6.06) | 7.58(7.35–8.15) | 0.185 | 3250 | 3913 | <0.001 |
| 18–23 | 6930 | 1.757 | 0.203 | 5.92(5.89–5.95) | 1.719 | 0.172 | 1.896 | 0.242 | 5.66(5.58–5.74) | 6.86(6.46–8.12) | 0.217 | 1220 | 1313 | <0.001 |
| 24–30 | 23,063 | 1.815 | 0.217 | 6.29(6.27–6.31) | 1.782 | 0.184 | 1.971 | 0.280 | 6.04(6.00–6.08) | 7.47(7.23–8.15) | 0.177 | 2518 | 2893 | <0.001 |
| 31–45 | 6444 | 1.896 | 0.237 | 6.85(6.80–6.89) | 1.855 | 0.194 | 2.107 | 0.251 | 6.51(6.44–6.62) | 8.22(7.92–11.03) | 0.185 | 253 | 395 | <0.001 |
Meani = exp(mi + si2/2); i = 1, 2 where mi and si are log 2 h plasma glucose means and standard deviations distribution respectively; exp = exponentiation.
P-value, log likelihood ratio test.
Bootstrap method using 1000 bootstrap and percentile (2.5%–97.5%) was used for 95% confidence interval.
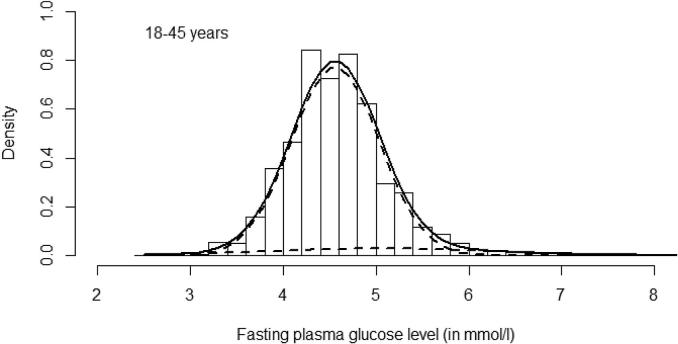
Fig. 1.
Histogram represents distribution data (intervals of 0.2 mmol/L) of Fasting Plasma Glucose in the whole study population (18–45 yrs). The superimposed solid curve is of fitted bimodal model and the dotted curves are of two underlying normal distributions.
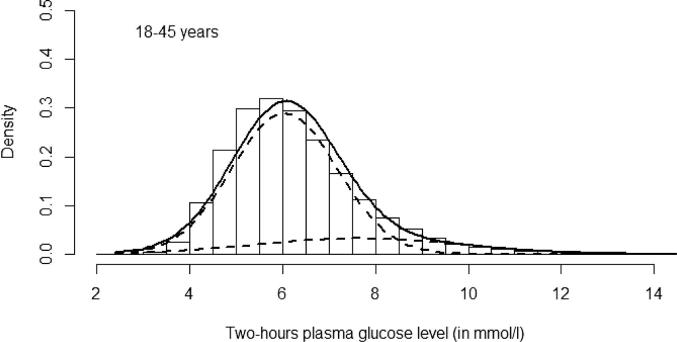
Fig. 2.
Histogram represents distribution data (intervals of 0.5 mmol/L) of Post challenge 2 h plasma glucose in the whole study population (18–45 yrs). The superimposed solid curve is of fitted bimodal model and the dotted curves are of two underlying normal distributions.
Discussion
The present hospital based study showed that among Asian Indian pregnant women undergoing universal GDM screening, the plasma glucose distribution was bimodal rather than unimodal. The bimodality was evident in all age stratified groups (18–45, 18–23, 24–30 and 31–45 yrs) for both FPG and 2-h PG values (Table 1, Table 2). An interesting finding in our study is the presence of BPG in the pregnant women of 18–23 yrs age group. In the community based studies involving Asian Indians (men and non pregnant women), evidence for BPG was demonstrable only in persons above 30 yrs of age [10], [12]. South African Indians [12] with 18% DM prevalence, showed unequivocal evidence of bimodality for both FPG and 2-h PG in the 55–74 yrs age group only. For younger age group (25–34 yrs), it was limited to 2-h PG values in males. Among Malaysian Indians [10] with 16.3% (men) and 11.5% (women) DM prevalence, bimodality was apparent in 2-h PG values in both sexes above 30 yrs of age. BPG in subjects of <25 yrs of age (males and non pregnant females) was observed earlier in community based studies involving very high risk ethnic groups. A study among Pima Indians with DM prevalence from 18.2 to 49.5% (depending on age/sex), showed unimodal PG distribution in 5–14 yrs age group, early signs of bimodality in 15–24 yrs and unequivocal evidence of bimodality for FPG and 2-h PG values in those above 25 yrs of age [1]. The pattern of glucose distribution in the very young (18–23 yrs) pregnant Asian Indians is mimicking the BPG described in Pima Indians in 1971. Zimmet et al. postulated that when diabetes prevalence is above 10% in a community, bimodality in glucose distribution emerges [2]. Our study proved that concept true for pregnant women in a high risk population. With GDM prevalence of 16.4% (NICE criteria), BPG was apparent in all pregnant women for both FPG and 2-h PG values.
As BPG was evident among the pregnant women in this study, further analysis was done to identify its usefulness for GDM diagnosis. The fitted bimodal model revealed two distribution curves for both FPG and 2-h PG levels (Fig. 1, Fig. 2). The point of interception of the two curves of the bimodal distribution is generally regarded as the cut point of distinction between normal and abnormal populations i.e. normal and abnormal glucose tolerance in our study. The cut points for FPG and 2-h PG values in the present study, were 5.81 mmol/L and 8.41 mmol/L respectively. It is observed that the cut off value for 2-h PG is mostly in agreement with IADPSG criteria (8.5 mmol/L) [16] while that for FPG is closer to NICE criteria (5.6 mmol/L) [18]. If these findings are reliable, FPG of NICE criteria and 2-h PG of IADPSG criteria, can be proposed as likely glucose thresholds in OGTT for GDM diagnosis. But there are limitations in the interpretation of the two normal distribution curves observed in the fitted bimodal models (Fig. 1, Fig. 2) in our study. Earlier studies [10], [13] stress that the cut point of two curves in a bimodal model is regarded as biologically meaningful when it falls between the mean values of two modes of distribution. The crossing points of normal distribution curves for both FPG and 2-h PG in the present study were above the mean glucose values of the second mode, casting doubts on their relevance as threshold values for GDM diagnosis.
When the diabetes epidemic is well established as in Pima Indian population in Arizona, the two curves for the bimodal distribution are further apart, resulting in classic bimodal distribution curves [1] The cut points observed in the epidemiological studies in this population, clearly distinguished normal and abnormal glucose tolerance groups, hence were recommended for diagnosis of diabetes mellitus [5], [6]. But in earlier studies in Asian Indian and other populations where diabetes epidemic is evolving, the two distribution curves overlapped markedly and cut points were not always reliable [10], [13]. In the present study among Asian Indian pregnant woman, even in the setting of transient gestational glucose intolerance, statistically significant bimodality in glucose distribution was demonstrable. But the glucose distribution curves in the fitted mixture model were not widely separated to yield cutpoints of high biological relevance. But despite these limitations, the FPG and 2-h PG cut points of our study respectively were close to NICE and IADPSG glucose thresholds for GDM diagnosis. Further studies on plasma glucose distribution among pregnant women in ethnic groups like Pima Indians in whom strong BPG tendency and reliable cut points were evident in non pregnant state, are likely to yield diagnostic cut off values for GDM as well.
A factor which may have altered the distribution curve of the second mode of the fitted bimodal model is the exclusion of patients with pre-gestational DM from the study. Undiagnosed DM patients (which form 30% of DM in urban Indian population) [23] are included in our analysis. Addition of 454 diagnosed pre-gestational DM women would have shifted the second mode of bimodal model to right, leading to a cut point of better discriminative value. But as these women were not candidates for GDM screening, they were excluded from our study. Vistisen et al., on analysis of the DETECT-2 study data for bimodality of glucose distribution, observed that inclusion or exclusion of subjects with known diabetes produced great variation in FPG and 2-h PG cut off points [13]. They commented that the mean of the second component in bimodal distribution is likely to be lower when participants with known diabetes are excluded. The exclusion makes the two components less distinct and thereby decreases the probability of detecting a meaningful cut point. Ideal study design for assessment of bimodality in glucose distribution is to have a population that includes ‘treatment naive’ diabetic patients. But this design raises certain therapeutic concerns in pregnancy. Performing OGTT on diabetic pregnant women or withholding anti-hyperglycemic treatment in them, can put the fetus at risk.
Interestingly, in the pregnant women of this study, segregation to normal and abnormal glucose tolerance groups occurred at a lower plasma glucose than in non pregnant state. The cut points were lower than those observed in earlier studies in non-obstetric populations. On DETECT-2 study data analysis, among ethnic groups with clinically meaningful bimodality, FPG and 2-h PG cut points were 6.7mmol/l and 10.9 mmol/l respectively [13]. In the Malaysian study, 2-h PG cut point was ∼12mmol/l in all ethnic groups (including Asian Indians) [10]. We do not have any follow up data on the glucose distribution pattern of our study group after delivery. Further studies evaluating BPG in pregnant women, with further post partum reassessment of their glucose distribution will be interesting in two aspects; (a) To look for persistence or disappearance of BPG in post partum state (b) If bimodality is evident after delivery, any shifting of cut point to a higher glucose value.
In populations where glucose distribution is bimodal, DM prevalence corresponds to the proportion of individuals identified in the second mode of bimodal distribution [11], [12]. The 2-h PG analysis in our study group, the proportion of women in second mode was 18.4% in the whole group which was higher than the16.4% GDM prevalence noted by NICE guideline. The age stratified group analysis revealed proportions ranging from 17.7 to 21.7%, which were not in agreement with the GDM prevalence in each group. The FPG value analysis was confusing and did not yield any reliable conclusions (Table 1).
To the best of our knowledge, there are no earlier studies evaluating the pattern of plasma glucose distribution in an obstetric population in any ethnic group. In this retrospective analysis of the OGTT data of a large number of pregnant women from a major hospital in urban India. the bimodality of glucose distribution was evident. In the absence of a clear international or national guideline, hospitals in developing countries follow different GDM screening strategies and OGTT protocols. Hence in a retrospective study, in the present scenario, there are practical difficulties in obtaining identical OGTT data for bimodality assessment, from different hospitals in Delhi. We propose more prospective studies in this unexplored field, which may reveal more relevant data to settle some of the controversies in GDM diagnosis.
Conclusions
In conclusion, present study involving pregnant Asian Indian women revealed statistically significant bimodality of glucose distribution for both fasting and 2-h PG values. Compared to non obstetric population, the segregation to normal and abnormal glucose groups occurs at a lower level of plasma glucose in pregnancy. Despite the limitations in the pattern of normal distribution curves in the plotted graph of this study, the identified FPG and 2-h PG cut points are close to the FPG and 2-h PG threshold glucose values suggested in NICE and IADPSG criteria respectively. Further studies to assess BPG in pregnant women of ethnic groups with very high GDM prevalence with inclusion of women with pregestational diabetes, may yield more reliable cut off glucose values for GDM diagnosis.
Acknowledgments
Acknowledgement
We gratefully acknowledge the immense help received from M/s Sheeba Samuel, Sapna Robinson (diabetes nurses) and Mr. Aashish Kumar (diabetes educator) of Dept of Endocrinology, St. Stephen’s Hospital.
Author contributions
JP conceptualized the idea and prepared the manuscript, RM carried out statistical analysis and contributed to manuscript KS & AM contributed to discussion and made constructive criticism to manuscript AS & NC helped in collecting data and contributed to manuscript.
Disclosures
Nil
Funding sources
Nil
References
- 1.Rushforth N.B., Bennett P.H., Steinberg A.G., Burch T.A., Miller M. Diabetes in the Pima Indians: evidence of bimodality in glucose tolerance distributions. Diabetes. 1971;20(11):756–765. doi: 10.2337/diab.20.11.756. [DOI] [PubMed] [Google Scholar]
- 2.Zimmet P., Whitehouse S. Bimodality of fasting and two-hour glucose tolerance distributions in a Micronesian population. Diabetes. 1978;27(8):793–800. doi: 10.2337/diab.27.8.793. [DOI] [PubMed] [Google Scholar]
- 3.Raper L.R., Taylor R., Zimmet P., Milne B., Balkau B. Bimodality in glucose tolerance distributions in the urban Polynesian population of Western Samoa. Diabetes Res. 1984;1(1):19–26. [PubMed] [Google Scholar]
- 4.Dowse G.K., Spark R.A., Mavo B., Hodge A.M., Erasmus R.T., Gwalimu M. Extraordinary prevalence of non insulin dependent diabetes mellitus and bimodal plasma glucose distribution in the Wanigela people of Papua New Guinea. Med J Aust. 1994;160(12):767–774. doi: 10.5694/j.1326-5377.1994.tb125945.x. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization WHO expert committee on diabetes mellitus: second report. Geneva, World Health Org. World Health Organ Tech Rep Ser 646. 1980;646:1–80. [PubMed] [Google Scholar]
- 6.World Health Organization Diabetes mellitus: report of a WHO study group. Geneva, World Health Org. World Health Organ Tech Rep Ser 727. 1985;727:1–113. [PubMed] [Google Scholar]
- 7.Rosenthal M., McMahan C.A., Stern M.P., Eifler C.W., Haffner S.M., Hazuda H.P. Evidence of bimodality of two hour plasma glucose concentrations in Mexican Americans: results from the San Antonio Heart study. J Chronic Dis. 1985;38(1):5–16. doi: 10.1016/0021-9681(85)90003-7. [DOI] [PubMed] [Google Scholar]
- 8.Loo S.G., Dowse G.K., Finch C., Zimmet P. Bimodality analysis of frequency distributions of 2-hour plasma glucose concentrations in the urban Micronesian population of Kiribati. J Diabetes Complications. 1993;7(2):73–80. doi: 10.1016/1056-8727(93)90029-x. [DOI] [PubMed] [Google Scholar]
- 9.Gong Haiying, Pa Lize, Wang Ke, Hebuli Mu, Dong Fen, Ya Shengjiang. Bimodal distribution of fasting plasma glucose in the Uyghur and Han populations of Xinjiang, China. Asia Pac J Clin Nutr. 2017;26(4):708–712. doi: 10.6133/apjcn.052016.07. [DOI] [PubMed] [Google Scholar]
- 10.Lim T.O., Bakri R., Morad Z., Hamid M.A. Bimodality in blood glucose distribution: is it universal? Diabetes Care. 2002;25(12):2212–2217. doi: 10.2337/diacare.25.12.2212. [DOI] [PubMed] [Google Scholar]
- 11.Fan J., May S.J., Zhou Y., Barrett-Connor E. Bimodality of 2-h plasma glucose distributions in whites: the Rancho Bernardo study. Diabetes Care. 2005;28(6):1451–1456. doi: 10.2337/diacare.28.6.1451. [DOI] [PubMed] [Google Scholar]
- 12.Omar M.A., Seedat M.A., Dyer R.B., Motala A.A., Knight L.T., Becker P.J. South African Indians show a high prevalence of NIDDM and bimodality in plasma glucose distribution patterns. Diabetes Care. 1994;17(1):70–73. doi: 10.2337/diacare.17.1.70. [DOI] [PubMed] [Google Scholar]
- 13.Vistisen D., Colagiuri S., Borch Johnsen K., Collaboration D. Bimodal distribution of glucose is not universally useful for diagnosing diabetes. Diabetes Care. 2009;32(3):397–403. doi: 10.2337/dc08-0867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sacks D.B. Diagnosis of gestational diabetes mellitus: it is time for international consensus. Clin Chem. 2014;60(1):141–143. doi: 10.1373/clinchem.2013.206920. [DOI] [PubMed] [Google Scholar]
- 15.Agarwal M.M., Dhatt G.S., Punnose J., Koster G. Gestational diabetes: dilemma caused by multiple international diagnostic criteria. Diabetes Med. 2005;22(12):1731–1736. doi: 10.1111/j.1464-5491.2005.01706.x. [DOI] [PubMed] [Google Scholar]
- 16.International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33(3):676–682. doi: 10.2337/dc09-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study: associations with neonatal anthropometrics. Diabetes. 2009;58(2):453–459. doi: 10.2337/db08-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Institute for Health & Care Excellence (NICE). Diabetes in pregnancy: management Diabetes in pregnancy: management from preconception to the postnatal period. NICE guideline [NG 3;1-] 201, 567 nice.org.uk/guidance/ng. [PubMed]
- 19.Practice bulletin No. 180: gestational diabetes mellitus. Obstet Gynecol. 2017;130:e17–e37. doi: 10.1097/AOG.0000000000002159. [DOI] [PubMed] [Google Scholar]
- 20.Engelgau M.M., Thompson T.J., Herman W.H., Boyle J.P., Aubert R.E., Kenny S.J. Comparison of fasting and 2-hour glucose and HbA1c levels for diagnosing diabetes: diagnostic criteria and performance revisited. Diabetes Care. 1997;20(5):785–791. doi: 10.2337/diacare.20.5.785. [DOI] [PubMed] [Google Scholar]
- 21.Ryan E.A. Diagnosing gestational diabetes. Diabetologia. 2011;54:480–486. doi: 10.1007/s00125-010-2005-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zimmet P.Z. Diabetes and its drivers: the largest epidemic in human history? Clin Diabetes Endocrinol. 2017;3(1):1. doi: 10.1186/s40842-016-0039-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anjana R.M., Deepa M., Pradeepa R., Mahanta J., Narain K., Das H.K. Prevalence of diabetes and prediabetes in 15 states of India: results from the ICMR–INDIAB population-based cross-sectional study. Lancet Diabetes Endocrinol. 2017;5(8):585–596. doi: 10.1016/S2213-8587(17)30174-2. [DOI] [PubMed] [Google Scholar]
- 24.Arora G.P., Thaman R.G., Prasad R.B., Almgren P., Brøns C., Groop L.C. Prevalence and risk factors of gestational diabetes in Punjab, North India: results from a population screening program. Eur J Endocrinol. 2015;173(2):257–267. doi: 10.1530/EJE-14-0428. [DOI] [PubMed] [Google Scholar]
- 25.Mithal A., Bansal B., Kalra S. Gestational diabetes in India: science and society. Indian J Endocrinol Metab. 2015;19(6):701–704. doi: 10.4103/2230-8210.164031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sacks D.B., Arnold M., Bakris G.L., Bruns D.E., Horvath A.R., Kirkman M.S. Executive summary: guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem. 2011;57(6):793–798. doi: 10.1373/clinchem.2011.163634. [DOI] [PubMed] [Google Scholar]
- 27.MacLean C.J., Morton N.E., Elston R.C., Yee S. Skewness in commingled distributions. Biometrics. 1976;32(695–69):9. [PubMed] [Google Scholar]
- 28.Benalgia T., Chauveau D., Hunter D.R., Young D.S. Mixtools: an R package for analysing mixture models. J Stat Softw. 2009;32:1–29. [Google Scholar]
- 29.Thode H.C., Finch S.J., Mendell N.R. Simulated percentage points for the null distribution of the likelihood ratio test for a mixture of two normals. Biometrics. 1988;44:1195–1201. [PubMed] [Google Scholar]
- 30.McLachlan G.J. On bootstrapping the likelihood ratio test statistic for the number of components in a normal mixture. Appl Stat. 1987;36:318–324. [Google Scholar]
- 31.Efron B., Tibshirani R.J. Chapman & Hall; New York: 1993. An introduction to bootstrap. [Google Scholar]