Abstract
Anti-cancer effects of local anesthetics have been reported but the mode of action remains elusive. Here, we examined the bioenergetic and REDOX impact of levobupivacaine on human prostate cancer cells (DU145) and corresponding non-cancer primary human prostate cells (BHP). Levobupivacaine induced a combined inhibition of glycolysis and oxidative phosphorylation in cancer cells, resulting in a reduced cellular ATP production and consecutive bioenergetic crisis, along with reactive oxygen species generation. The dose-dependent inhibition of respiratory chain complex I activity by levobupivacaine explained the alteration of mitochondrial energy fluxes. Furthermore, the potency of levobupivacaine varied with glucose and oxygen availability as well as the cellular energy demand, in accordance with a bioenergetic anti-cancer mechanism. The levobupivacaine-induced bioenergetic crisis triggered cytostasis in prostate cancer cells as evidenced by a S-phase cell cycle arrest, without apoptosis induction. In DU145 cells, levobupivacaine also triggered the induction of autophagy and blockade of this process potentialized the anti-cancer effect of the local anesthetic. Therefore, our findings provide a better characterization of the REDOX mechanisms underpinning the anti-effect of levobupivacaine against human prostate cancer cells.
Keywords: Prostate cancer, Levobupivacaine, Glycolysis, Oxidative phosphorylation, Wortmannin
Highlights
-
•
Local anesthetics reduce cancer recurrence in prostate cancer.
-
•
Metabolic reprogramming in a hallmark of cancer.
-
•
Complex I inhibition is a potential anti-cancer bioenergetic therapeutic strategy.
-
•
Levobupivacaine inhibits complex I activity and mitochondrial respiration.
-
•
Autophagy blocker combined with levobupivacaine induces cytostasis in prostate cancer.
1. Introduction
Prostate cancer is the most common cancer in men and the second leading cause of death from cancer in men in the United States. Surgery remains the most common therapeutic option for the treatment of prostate cancer and the type of anesthesia used during prostatectomy impacts cancer recurrence [1] and patient survival [2], raising the need to better understand the interactions between anesthetic drugs and tumor biology. In particular, local anesthesia (LA) was shown to reduce cancer recurrence in prostate and ovarian tumors [1], and biochemical investigations in vitro revealed the anti-cancer potential of various local anesthetics. For instance, ropivacaine reduced the proliferation of colon cancer cells [3], bupivacaine altered the viability of melanoma cells [4], lidocaine reduced both the invasiveness of osteosarcoma cells [5] and the proliferation of tongue [6] and liver [7] cancer cells, and prilocaine, lidocaine and bupivacaine activated apoptosis in lymphoma cells [8]. In addition, we previously found that levobupivacaine induced a strong anti-proliferative effect on a panel of human cancer cells when compared to corresponding adult non-cancer primary cells [9]. Yet, the cytotoxic properties of levobupivacaine still remain elusive and the potential anti-cancer mode of action is unknown.
Levobupivacaine is a widely used long acting local anesthetic indicated for nerve block, infiltration, ophthalmic, epidural and intrathecal anesthesia. It is used for epidural anesthesia during prostatectomy [10] suggesting that levobupivacaine could theoretically have a local pharmacological anti-cancer effect on residual cancer cells. Levobupivacaine anesthetic mode of action requires the binding to sodium channels resulting in the blockade of sodium influx into nerve cells thereby preventing depolarization and the conduction of nerve impulses. Besides anesthesia, additional molecular effects of levobupivacaine were discovered on human cells as myoblasts [11]. By analogy with bupivacaine which targets the molecular pathways of cellular energy production as an analgesic side-effect (responsible for myotoxicity [11], [12], [13], [14], [15]), we hypothesized that levobupivacaine could induce a cancer cytotoxic or cytostatic effect by interfering with cancer cells REDOX biology at the interface between bioenergetics and autophagy [16]. Recently, cancer cells energy metabolism reprogramming was considered as a Hallmark of cancer and a potential site for therapeutic intervention [17]. Since the use of local anesthetics in clinics associates with a reduced recurrence of prostate cancer [1], [18], [19], the evaluation of levobupivacaine effect on prostate cancer cells is required. Moreover, targeting respiratory chain is a valid cytotoxic strategy on human prostate adenocarcinoma cells [20] and high-resolution respirometry studies further revealed that mitochondrial respiration is active in human prostate tumors [21].
In the present study, we observed a potent and specific antiproliferative effect of levobupivacaine on human prostate cancer cells as compared to non-cancer homologues. The mode of action of this local anesthetic included a multi-site inhibition of ATP production. We further observed that levobupivacaine activated autophagy in prostate cancer cells and combining levobupivacaine with a blocker of autophagy potentiated cytotoxicity. Altogether these observations delineate the mechanisms by which the local anesthetic levobupivacaine arrest proliferation of prostate cancer cells.
2. Material and methods
2.1. Chemicals
Levobupivacaine hydrochloride 0.5% (5 mg/ml) was purchased from ABBOTT (Rungis, France). All other reagents were purchased from Sigma, at the exception of the ATP monitoring kit (ATP Bioluminescence Assay Kit HS II from Roche Diagnostics GmbH, Mannheim, Germany), the ATP/ADP ratio kit (Abcam, Paris, France), the Caspase-Glo® 3/7 Assay (Promega, Madison, WI, USA) and the primary antibodies (Complex I NDUFB8 subunit antibody from MitoSciences, Eugene, OR, USA; LC3B and AKT-P from Abcam, Paris, France; and PARP from Santa Cruz Biotechnology, Santa Cruz, NM, USA).
2.2. Cell line and cell culture conditions
Human prostate cancer cells (DU145 – carcinoma from metastatic site) were obtained from ATCC (Bethesda, MD, USA). BHP primary epithelial prostate cells were obtained from Bergonié Cancer Center, Bordeaux (P. Pourquier). The cancer cells were grown in Dulbecco's Modified Eagle Media (DMEM) supplemented with 10% fetal bovine serum, 100 U/ml penicillin and 100U/ml of streptomycin. BHP cells were grown in optimal PrEGM Bullet Kit medium provided by Lonza, Walkersville, MD, USA. All of the cells were grown in 5% CO2 at 37 °C. For all experiments, the cells were harvested during the exponential phase of growth at 70% confluency. The solvents used for the dilution of the different drugs used in our study were as follow: rotenone used at 0.5–500 nM was dissolved in ethanol (1 mM) and diluted at 1/2000–1/20000, levobupivacaine used at 250–2500 µM was diluted in cell culture medium for cell treatments or in enzyme activity buffer for complex I assay, etoposide used at 50 µM was diluted in DMSO (50 mM stock solution) and diluted at 1/1000 in cell culture medium (DMEM) for cell treatments, iodoacetate used at 2.5 µM was diluted in cell culture medium (DMEM), resveratrol used at 20 µM was first dissolved in DMSO (100 mM stock solution) and diluted at 1/5000 in cell culture medium (DMEM) for cell treatments, N-acetylcysteine used at 1 mM was diluted in cell culture medium (DMEM), and alpha-tocopherol used at 400 µM was dissolved in DMSO (stock solution of 0.2 M) and diluted at 1/1000 for cell culture treatments. We administered DMSO and ethanol at dilution < 1/1000 to reduce toxicity of the solvents on cells.
2.3. Cell viability and enumeration
Cell proliferation rate was evaluated by counting the cells during the exponential phase of growth in the growing medium using a Malassez hemocytometer. The cells were seeded in 6-well plates (100,000 cells per well), treated or not with 1 mM levobupivacaine, 50 μM etoposide, rotenone (0.5, 5, 50 or 500 nM) or 2.5 µM iodoacetate as precised in the text. We selected a dose of 1 mM based on our previous findings on levobupivacaine cytotoxicity on cancer and non-cancer cells. At this dose, cancer cells viability was strongly reduced while non-cancer cells remained unaltered [9]. Moreover, in a previous study performed in rat, we evaluated the dose of levobupivacaine accumulated in the muscle, at the vicinity of the injection point, to be close to 1 mM and we discussed this point thoroughlly elsewhere [13], [15]. Therefore, the clinically-relevant dose of levobupivacaine used for local analgesia is in the range of the dose used to provide an antiproliferative effect on cancer cells. Cells were counted manually on a Mallassez counting chamber (n ≥ 3 for each condition). To investigate the role of reactive oxygen species in the cytotoxic effect of levobupivacaine 1 mM, we repeated the enumeration procedure in the presence of the following antioxidants: 20 µM resveratrol, 1 mM N-acetyl-L-cysteine or 0.4 mM α-tocopherol (n ≥ 3 for each condition). We verified that in these concentrations the different antioxidants had no impact on cell viability (data not shown). To determine the role of autophagy on levobupivacaine induced-cytotoxicity, we repeated the enumeration procedure in the presence of 100 nM wortmannin. After 6, 48 or 72 h of local anesthetic exposure, the DU145 cells were washed with PBS and trypsinized, and 25 µL sample of this cell suspension was used for staining with Trypan Blue (1:1). A live/dead cell count was performed. Caspase activity was assessed to evaluate cell death induction with the Caspase-Glo® 3/7 Assay (Promega, Madison, WI, USA) according to the manufacturer recommandation after 24 h of treatment with or without 1 mM levobupivacaine or 50 μM etoposide. DU145 cell line proliferation was also evaluated after 24 h of treatment with 1 mM levobupivacaine and subsequent wash-out of the cells with DMEM medium for 24–48 h.
2.4. Cell respiration and adenosine triphosphate measurements
Endogenous respiratory rate was assayed in intact cells using high-resolution respirometry (Oroboros Instruments, Innsbruck, Austria). Respiration was measured at 37 °C with 2 × 106 cells/ml in DMEM.15 Levobupivacaine (0.1 µM to 3 mM) or rotenone (0.5–3.5 nM) was titrated at steady state, and the respiratory rates were expressed as ng atom O/min/1 × 106 cells. The intracellular ATP content was measured using the bioluminescent ATP kit HS II (Roche Applied Science). 5uL of a cell suspension of 2 × 106 cells/ml were plated in a white 96-well plate. When attached to the plate, they were treated or not with 1 mM levobupivacaine for 2 h. When precised in the text, cells were pre-adapted for 2 h in galactose medium before levobupivacaine treatment. For each condition, four wells were used to measure the total ATP content, and four wells were treated with 2.5 µM oligomycin A for 10 min to block mitochondrial ATP synthesis and allow ATP turnover via ATP consuming processes. In a third subset of four wells, glycolysis was blocked with 2.5 µM iodoacetate over a 10-min period to evaluate the participation of glycolysis to cellular ATP synthesis. Then, cells were immediately lysed to release the intracellular ATP using the lysis buffer provided with the kit (volume ratio 1:1) for 5 min at room temperature. ATP concentration is determined with the light-emitting, luciferase-catalyzed oxidation of luciferin with ATP. A total of 100 µL of luciferase reagent provided by the kit was injected into the wells and bioluminescence was measured (10 s integration time) on a luminometer (Luminoskan, Labsystems, Finland). Standardization was performed with known quantities of standard ATP provided with the kit measured under the same conditions. To evaluate the ATP/ADP ratio, we used the Abcam ATP/ADP Ratio Assay Kit (Bioluminescent) and followed the protocol of the manufacturer. The ratio of ATP to ADP in the cell is a measure of the available metabolic energy.
2.5. Western blotting
Total cell lysis was performed by sonication (total time of 5 min with a cycle of 30 s sonication, 30 s rest and 45 °C amplitude at 4 °C) on a Epishear multi-sample sonicator (Active Motif, La Hulpe, Belgium). Samples were diluted in an SDS-PAGE tricine sample buffer (Bio-Rad Laboratories) containing 2% β-mercaptoethanol by incubation for 30 min at 37 °C. The samples were then separated on a 4–20% SDS polyacrylamide gradient mini-gel (Bio-Rad Laboratories) at 150 V. Proteins (30 µg of proteins per well) were transferred electrophoretically to 0.45 µm polyvinylidine difluoride membranes for 2 h at 100 mA in CAPS buffer (3.3 g CAPS, 1.5 L 10% methanol, pH 11) on ice. The membranes were blocked overnight in 5% milk-PBS + 0.02% azide and incubated for 4 h with the primary antibodies. After six washes with PBS with 0.05% Tween 20, the membranes were incubated for 1 h with horseradish peroxidase-conjugated goat anti-rabbit or goat anti-mouse (Bio-Rad Laboratories) diluted in PBS with 5% milk. This secondary antibody was detected in a Chemidoc (Bio-Rad Laboratories) using the chemiluminescent ECL PlusTM reagent (Amersham Biosciences, GE Healthcare, Uppsala, Sweden). The signal was quantified by densitometric analysis using Image J (National Institutes of Health, Bethesda, MD, USA) software.
2.6. Flow cytometric cell cycle assay
The cells were treated with 1 mM levobupivacaine and allowed to incorporate BrdU for 3 h at 37 °C before the end of the 24 h treatment. The cells were trypsinized and counted, and 2 × 106 cells/ml were fixed with the solution provided in the manufacturer's kit (BrdU Flow Kit, BD Biosciences, France) for 25 min at 0 °C. Then, the cells were incubated with DNase 30% overnight at 0 °C and stained with 7-AAD. Subsequent detection of BrdU was accomplished using antibodies for BrdU (1:100) according to the manufacturer's instructions and visualized by flow cytometry (CANTO).
2.7. Fluorometric measurement of cellular ROS production
Cells were grown in 5 mM glucose DMEM and treated for 24 H with 1 mM levobupivacaine, in presence or not of 20 µM resveratrol as precised in the text. Then, the cells were trypsinized, counted, and the changes in cytosolic reactive oxygen species levels were monitored using the CM-H2DCFDA probe. Please note that this probe is not specific to H2O2 and other reactive oxygen species so that changes in H2-DCFDA fluorescence are more indicative of alteration of the global redox state of the cell [22]. Therefore, one has to consider this limitation for proper interpretation of the data. The probe was added to the cell suspension in the presence of levobupivacaine and incubated for 30 min at 37 °C, according to the manufacturer's protocol. The cells were then washed twice in PBS, and fluorescence was measured in a quartz cuvette on a Xenius spectrofluorometer (SAFAS, Monaco, France). A second reading was performed with the addition of 100 µM H2O2 in the cuvette to verify the response and absence of saturation of the CM-H2DCFDA probe. The signal increased immediately after the addition of H2O2 in a dose-dependent manner (data not shown).
2.8. Mitochondrial complex I and catalase activity enzymatic assays
The enzymatic activity of complex I was assessed by monitoring the oxidation of nicotinamide-adenine-dinucleotide disodium-salt (NADH). First, a cell homogenate was prepared as follow: 10 million cells were detached from the plate using trypsin-EDTA 0.25% solution and a cell pellet was obtained by centrifugation 10 min at 300 g. The pellet was frozen and − 80 °C and thawed at room temperature. Then, mechanical cell lysis was performed using a glass/glass potter at 4 °C (10 strokes in complex I enzyme assay buffer (35 mM KH2PO4, 5 mM MgCl2, 2 mM KCN, pH7.2)). A cell homogenate was obtained and protein concentration was determined using the BCA assay (Thermofisher). Then, the rate of NADH oxidation by complex I was recorded using the ubiquinone analogue decylubiquinone (Sigma D-7911) as electron acceptor. Enzyme activity was measured in the complex I enzyme assay buffer supplemented with 2.5 mg/ml BSA, 5 µg/ml antimycinA, 65 µM UQ1decylubiquinone and 0.13 mM NADH in a final volume of 1 ml. The reaction was initiated with 80 μg of total cell lysate. The decrease in absorbance due to NADH oxidation was measured at 340 nm. The extinction coefficient used for NADH was 6.22 Mm/cm. Then rotenone 5 µg/ml was added and the rotenone-sensitive activity (complex I activity) was calculated. The enzymatic activity of catalase was monitored as described previously.17 The decrease in NADH absorbance due to H2O2 deoxydation was measured at 340 nm. The extinction coefficient used was 40 mM/cm.
2.9. Statistical analysis
All of the data presented in this study correspond to the mean value of n experiments ± SD, with a minimum of n ≥ 3. Comparison of the data sets (control versus treated cells) was performed with Student's t-test in normally distribution data, and the Mann-Withney U-test for non-normally distributed data. SigmaStat 3.1 (Systat Software Inc., San Jose, CA, USA) was used for the statistical analyses. Two sets of data were considered statistically different when P < 0.05.
3. Results
3.1. Levobupivacaine inhibits human prostate cancer cell proliferation
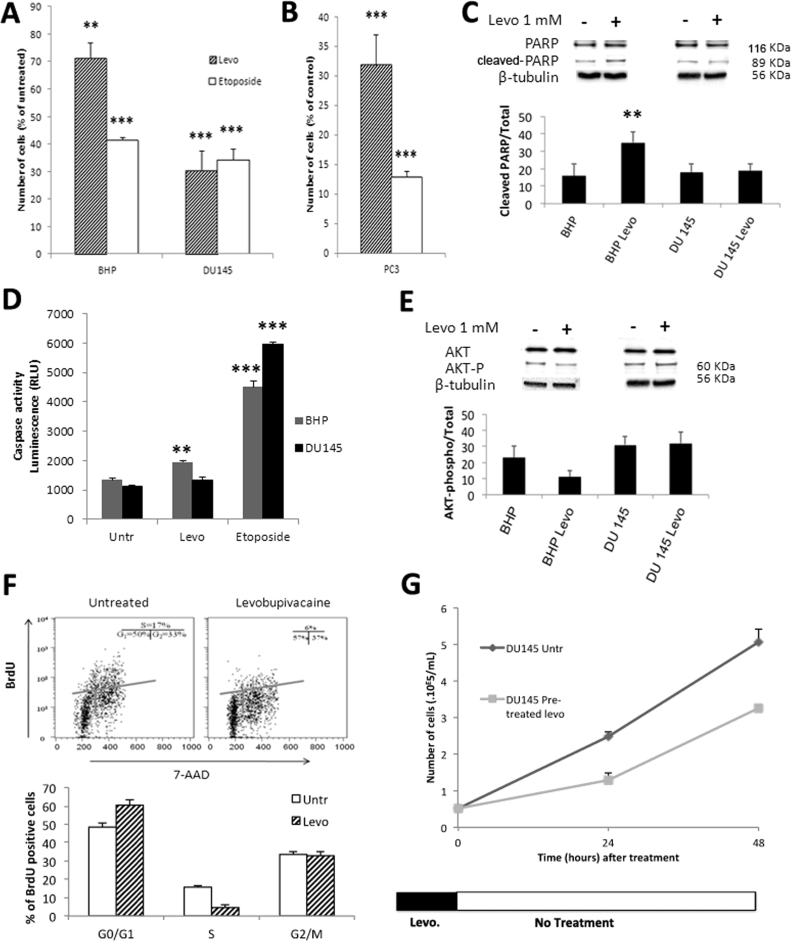
We performed cell enumeration analyses on BHP and on DU145, in the presence of either 50 µM etoposide, a validated chemotherapeutic agent or 1 mM of the local anesthetic levobupivacaine for 24 h (Fig. 1A). Although DU145 and PC3 prostate cancer cell lines are androgen-independent, previous in vitro analyses showed that etoposide alters the viability of those cell lines at doses ranging between 50 and 100 µM [23]. In our study, levobupivacaine or etoposide delivered during 24 h induced a significant (P < 0.005) reduction in cell number in the cancer cell line (Fig. 1A) and levobupivacaine even showed a higher cancer-specificity. For instance, both levobupivacaine and etoposide reduced DU145 cell number by 65% while levobupivacaine reduced cell number by 60% in BHP cells (and 28% by etoposide). Similar data (Fig. 1B) were obtained with another prostate cancer cell line (PC3 adenocarcinoma). To determine whether the reduction in cancer cell growth mediated by 1 mM levobupivacaine was caused by cell death or by cell growth arrest, we evaluated the former by measuring the cellular content of active (ADP)-Ribose Polymerase (PARP) by western blot (normalized to tubulin) (Fig. 1C). No significant increase in PARP was observed in DU145 cells treated with 1 mM levobupivacaine over the 24-h period compared to the untreated control (P = 0.1975). Likewise, in treated DU145 cells, no significant increase of the caspase 3/7 activity was observed (Fig. 1D) while etoposide induced apoptosis (Fig. 1D). When the incubation period with 1 mM levobupivacaine was raised to 72 h, no further loss of cell viability was measured with the trypan blue exclusion method (4.95% ± 2.4 of dead cells compared to 0.01% ± 0.002 in the untreated control (P = 0.2137), suggesting that 1 mM levobupivacaine decreased the rate of cancer cell proliferation rather than induced cell death. In contrast, we observed a significant (32.7 ± 3.9%) increase in active PARP content in the primary cell line BHP treated with 1 mM levobupivacaine during 24 h (Fig. 1C) in good correspondance with the 31.3 ± 3.4% higher caspase 3/7 activity in these cells (Fig. 1D). Such increase in apoptosis markers observed in BHP cells was also in good correspondence with the decrease in cell number (−28.7 ± 5.3%) induced by 1 mM levobupivacaine (Fig. 1A). Previous work reported that levobupivacaine was able to reduce Akt activity and subsequently to activate apoptosis in C2C12 non-cancer cells [24]. Accordingly, we found a significant reduction of the active form of Akt kinase in primary BHP cells for which apoptosis was observed (Fig. 1E). This effect was not observed in DU145 cancer cells for which levobupivacaine failed to activate apoptosis.
Fig. 1.
Impact of levobupivacaine and etoposide on cell proliferation. (A) Cell number of BHP and DU145 cell lines after 24 h treatments with 1 mM of levobupivacaine or 50 µM etoposide. (B) Cell number of PC3 cell line after 24 h treatments with 1 mM of levobupivacaine or 50 µM etoposide. (C) Cell death induction by levobupivacaine was assessed by following the expression level of PARP or (D) Caspase activity on cells treated with levobupivacaine (Levo) or untreated (Untr). The effect of 50 µM etoposide on Caspase activity has also been assessed. (E) Expression level of Akt and P-AKT. (F) Cell cycle arrest induced by levobupivacaine was determined in DU145 using flow cytometry analysis (BrDU and 7-AAD staining). (G) Effect of levobupivacaine pre-treatment on cell proliferation rate. Cell enumeration was performed over 48 h of growth after 24 h of treatment and subsequent wash-off. All the data shown correspond to the mean value ± SD of N ≥ 3 different experiments. Significantly different from the untreated cells at: * P ≤ 0.05, ** P ≤ 0.01, *** P ≤ 0.001.
3.2. Levobupivacaine arrests cancer cell cycle progression prior to the S-phase
The impact of 1 mM levobupivacaine on DU145 cell cycle progression was investigated by flow cytometry and western blot. First, we analyzed the proportion of cancer cells entering the different stages of the cell cycle, using BrdU and 7-AAD as fluorescent indicators of DNA active synthesis and DNA content, respectively. We found a significant difference in the distribution of the cell population between the cell cycle phases (P = 0.0441 for the G0-G1 phase and P = 0.0054 for the S phase) (Fig. 1F). Indeed, 1 mM levobupivacaine led to the accumulation of the prostate cancer cells in the non-proliferating G0/G1 phase indicative of a potential failure to enter the high energy demanding S phase (Fig. 1E). This observation illustrates the anti-proliferative potency of 1 mM levobupivacaine on DU145 prostate cancer cells. This effect occurred after removal of the drug as DU145 cells required a longer time to perform cell doubling over 48 h (Fig. 1G).
3.3. Levobupivacaine alters complex I activity and generates ROS
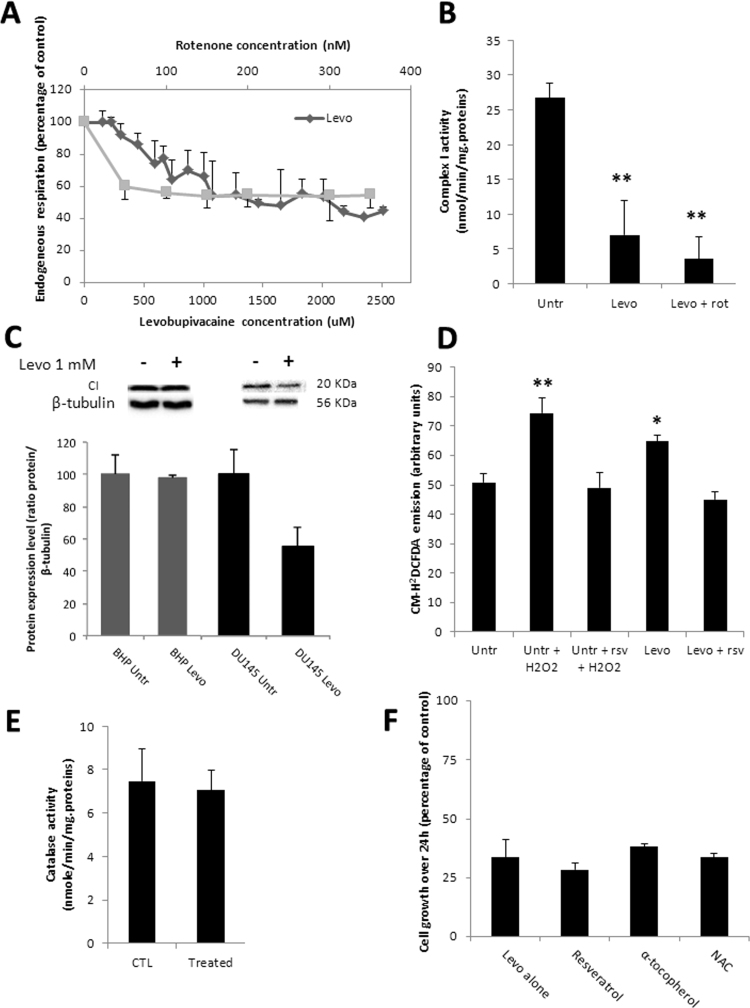
We assayed the direct effect of levobupivacaine on mitochondrial function by performing a titration of cellular oxygen consumption with levobupivacaine concentrations ranging from 10 µM to 3 mM on DU145 intact cells (Fig. 2A). Levobupivacaine inhibited cell respiration in a dose-dependent manner with an apparent Ki of 600 µM while considering the complex I-dependent respiration. Titration of cell respiration with rotenone revealed a significant part of oxygen consumption independent of complex I (potentially supported by complex II). The impact of levobupivacaine on cell respiration was explained by the direct inhibition of respiratory chain complex I (P = 0.0068) as shown by the measurement of complex I specific activity by spectrophotometry (Fig. 2B). In addition to this direct effect of levobupivacaine on complex I enzyme activity, we analyzed the impact of this drug on mitochondrial biogenesis, based on previous analyses which revealed an additional reduction of mitochondrial content in the muscle of rats exposed to bupivacaine [12]. Accordingly, the western blot analysis of DU145 cells treated with 1 mM levobupivacaine showed a significant decrease in complex I NDUFB8 subunit content normalized to tubulin (P < 0.05) (Fig. 2C). Several studies have indicated that the inhibition of CI enzyme activity can trigger the excessive production of ROS [25]. Using the intracellular fluorescent indicator CM-H2DCFDA, we estimated the potential increase in cellular H2O2 concentration induced by exposure of the DU145 cells to 1 mM levobupivacaine over a 24-h period (Fig. 2D). We observed a significant increase of approximately 22% of CM-H2DCFDA fluorescence in the treated cells as compared the untreated control (P = 0.0285). To identify the eventual activation of the antioxidant systems in cells treated with 1 mM levobupivacaine, we measured catalase activity following 24 h of treatment (Fig. 2E). No difference was observed in this enzyme activity suggesting that oxidative stress caused by levobupivacaine was not sufficient to activate the antioxydant defense program. To further evaluate the possible impact of the ROS increase on cancer cell proliferation, we performed a co-treatment of DU145 cells with 1 mM levobupivacaine and the antioxidant resveratrol used at 20 µM. As expected, this co-treatment prevented the increase in ROS production (Fig. 2F) but no diminution of the anti-proliferative effect of levobupivacaine was noticed. Similar results were obtained with the antioxydants α-tocopherol and N-Acetyl-L-cystein (NAC) (Fig. 2F). These findings suggest that levobupivacaine-mediated ROS overproduction does not contribute to its anti-proliferative property.
Fig. 2.
Impact of 1 mM levobupivacaine on mitochondrial function and composition. (A) High resolution respirometry analysis (Oroboros oxygraph 2-k) of DU145 challenged with a titration of levobupivacaine (diamond) or rotenone (square). (B) Complex I specific activity determined by spectrophotometry on DU145 after 24 h of treatment with 1 mM levobupivacaine, before and after the addition of 100 nM rotenone. (C) Complex I protein expression level (20KDa subunit) in BHP and DU145 cells after 24 h of treatment with 1 mM levobupivacaine. (D) CM-H2DCFDA fluorescence on DU145 cells treated or untreated with 1 mM levobupivacaine for 24 h, with or without co-treatment with resveratrol (rsv) and/or H2O2. (E) Catalase activity measured in DU145 cells treated or untreated with 1 mM Levobupivacaine. (F) Cell proliferative capacity measured on DU145 cells after 24 h of treatment with 1 mM levobupivacaine, with or without addition of anti-oxidants (NAC, Resveratrol and α-tocopherol). All the data shown correspond to the mean value ± SD of N ≥ 3 different experiments. Significantly different from the untreated cells at: * P ≤ 0.05, ** P ≤ 0.01.
3.4. The anti-proliferative potency of levobupivacaine on DU145 cancer cells is mediated by multi-site inhibition of energy production
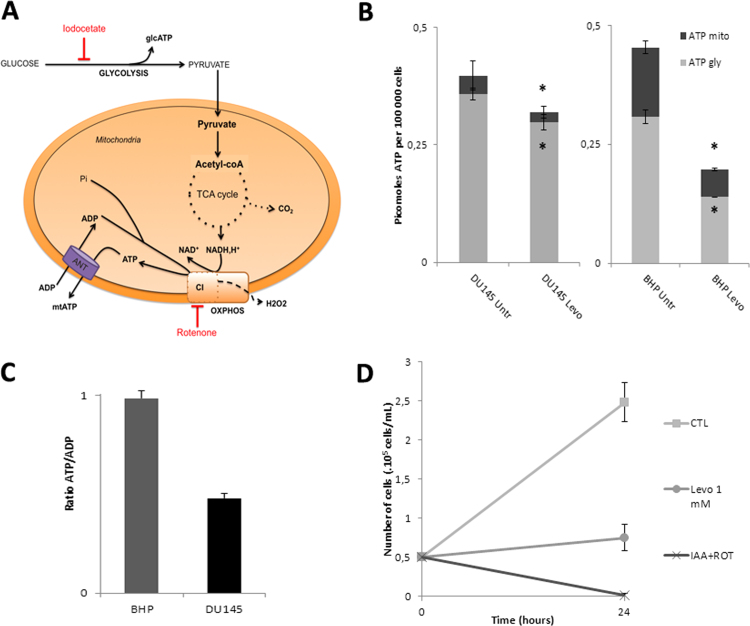
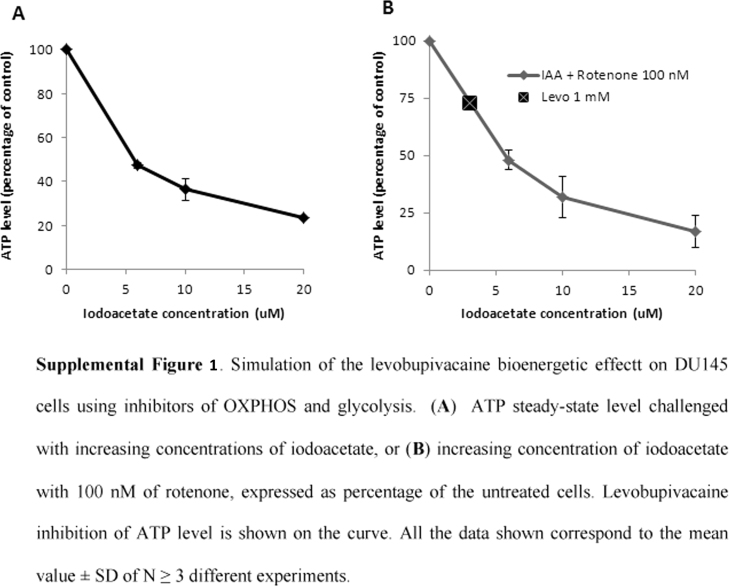
To decipher the mechanisms by which levobupivacaine altered the growth of DU145 cancer cells we measured the impact of this drug on overall energy metabolism. We assessed the change in the respective contribution of glycolysis or oxidative phosphorylation to cellular ATP synthesis as illustrated in Fig. 3A. The changes in the steady-state level of cellular ATP and mitochondrial ATP in DU145 and BHP cells exposed for 2 h to 1 mM levobupivacaine are shown in Fig. 4B. The total ATP content was reduced by 20% in DU145 cells and 57% in BHP cell line (P = 0.0264 and P = 0.0059, respectively). In DU145 cancer cells, this reduction of ATP content had two components: mitochondrial ATP production was diminished by approximately 50% and glycolytic ATP production by 17%. In BHP cells mitochondrial ATP production was diminished by 60% and glycolytic ATP production by 55% (Fig. 4B). To verify whether the observed reduction of intracellular total ATP content induced by levobupivacaine was the primary cause of cell cycle arrest, we mimicked the dual inhibition of the two energy production systems, namely glycolysis and OXPHOS, by using specific inhibitors of those two pathways (see Fig. 3A). Rotenone was used as a specific inhibitor of respiratory chain complex I, and iodoacetate as an inhibitor of glycolysis. In order to accurately reproduce the bioenergetic impact of levobupivacaine we had first to determine the doses of rotenone and of iodoacetate, which could reduce mitochondrial and glycolytic ATP production to the same levels as levobupivacaine did. We chose to use rotenone at 100 nM as this dose mimicked the inhibitory effect of levobupivacaine on complex I-dependent respiration. Iodoacetate decreased the total ATP levels in a dose-dependent manner in DU145 cells (supplementary data Fig. S1A) and the combination of 100 nM rotenone with 2.5 µM iodoacetate mimicked the inhibition of 20% of the total ATP (as obtained with levobupivacaine in DU145 cells; supplementary data Fig. S1B). This combination of inhibitors triggered a strong decrease in DU145 cell growth after 24 h of treatment, as also obtained with levobupivacaine (Fig. 4C). To understand the basis of the cancer specific effect of levobupivacaine we considered that the repercussion of a given reduction of ATP production could have different consequences on cell proliferation, in accordance with the metabolic state of the cells and the metabolic control analysis [26]. To evaluate the energy state of DU145 and BHP cells we measured the ATP/ADP ratio at steady-state which gives a measure of the balance between ATP synthesis and ATP consumption. We found a twice lower ATP/ADP ratio in DU145 cells as compared to BHP cells indicative of a higher rate of ATP consumption in the cancer cells (Fig. 4D) which also showed a higher rate of proliferation (doubling time of 10.4 h as compared to BHP (19.8 h)). Although those two cell types showed similar steady-state ATP content values (Fig. 4B) the higher ATP demand of DU145 cancer cells was indicated by the twice higher steady-state ADP content.
Fig. 3.
Impact of levobupivacaine on the bioenergetic state of BHP and DU145 cells. (A) Metabolic chart showing the two main sources of ATP synthesis in mammalian cells: glcolysis and oxidative phosphorylation. (B) Total ATP steady-state levels of BHP and DU145 cells treated with 1 mM levobupivacaine. (C) Contribution of mitochondria and glycolysis in cellular ATP production, as assessed using inhibitors of those pathways (iodoacetate and oligomycin, respectively). (D) Bioenergetic status of BHP and DU145 cell lines as determined by the ATP/ADP ratio measured at steady state. All the data shown correspond to the mean value ± SD of N ≥ 3 different experiments. Significantly different from the untreated cells at: * P ≤ 0.05.
Fig. 4.
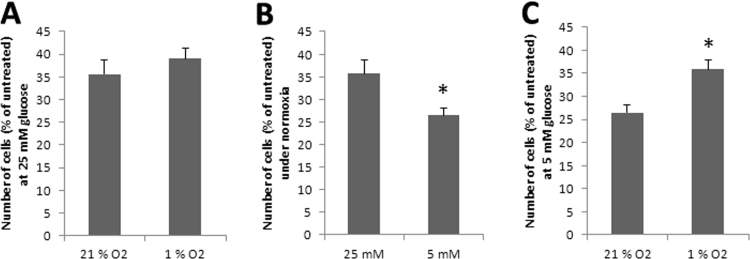
Impact of levobupivacaine (1 mM; 24 h) in relevant microenvironmental tumor bioenergetic conditions. The effect of 1 mM levobupivacaine on DU145 cell proliferation was assessed in (A) 25 mM ‘high’ glucose medium under normoxia and hypoxia, (B) 5 mM ‘low’ glucose medium under normoxia and (C), 5 mM ‘low’ glucose medium under hypoxia. All the data shown correspond to the mean value ± SD of N ≥ 3 different experiments. Significantly different from the untreated cells at: * P ≤ 0.05.
3.5. Influence of tumor-like microenvironmental conditions on levobupivacaine antiproliferative effect
To investigate the impact of levobupivacaine on energy production in challenging bioenergetic conditions we placed the DU145 cells in situations of reduced glucose concentration and oxygen tension, as occurs in solid tumors. Cell culture is typically performed under 21% oxygen while in vivo oxygen tension varies between 1% and 10% according to the tumor type. So, we placed DU145 cells under 1% O2 and treated with levobupivacaine for 24 h. We found no difference in the proliferation of cells grown in 25 mM glucose in normoxia or hypoxia (Fig. 4A). As most cancer cells are typically grown in high glucose media (25 mM glucose) as performed above, we also analyzed the effect of levobupivacaine in DU145 cells grown in a 5 mM glucose medium (Fig. 4B). The results indicate a higher anti-proliferative effect of levobupivacaine in those conditions more representative of cancer physiology (P = 0.0303). Conversely, at low oxygen tension (1%) this effect was no longer observed (Fig. 4C), indicating the influence of the microenvironment on cancer cell sensitivity to levobupivacaine.
3.6. Levobupivacaine anti-cancer cytotoxic effect is potentialized by blockade of autophagy with Wortmannin
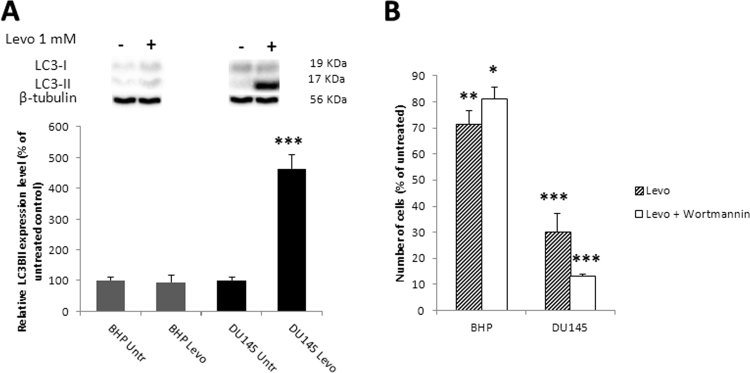
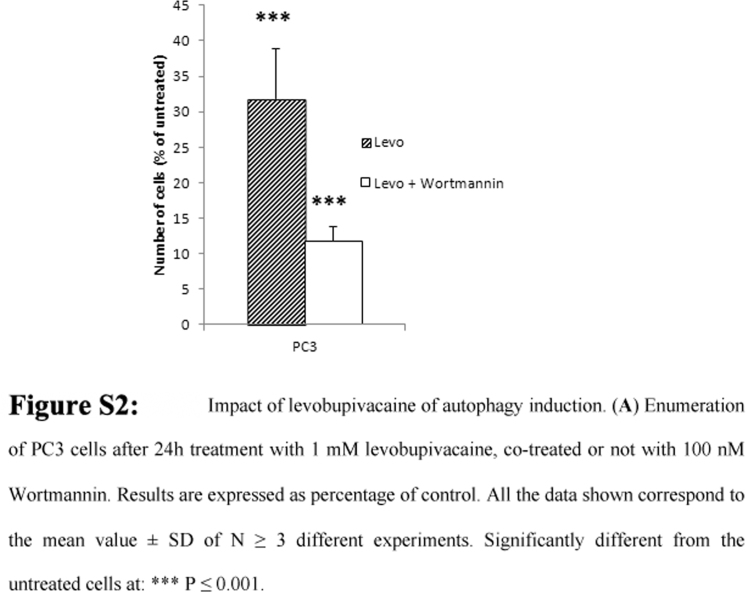
Autophagy is a survival pathway activated in response to nutrient deprivation, metabolic stress and exposure to anticancer drugs. The reduction in respiratory chain protein content in DU145 cells exposed to levobupivacaine (see Fig. 2C) led us to investigate the possible activation of autophagy by this drug. Levobupivacaine strongly induced the activation of LC3B, a marker of autophagy in the DU145 cancer cells, while no significant change was detected in the primary prostate cell line BHP (Fig. 5A). To further evaluate whether such autophagy was potentially used as a death or a survival pathway in these cells, we blocked autophagy with 100 nM Wortmannin and reassessed the impact of 1 mM levobupivacaine on cell enumeration after 24 h. In cancer cells, the amino acids released by autophagy are essential for cell survival and in these conditions, autophagy can be considered as a pro-survival mechanism. In our study, Wortmannin alone had no effect the viability of both cell lines grown in complete medium (data not shown) while it specifically synergized the cytotoxic effect of levobupivacaine on the prostate cancer cells (Fig. 5B). Similar findings were obtained with the PC3 cell line (Supplementary Fig. S2)
Fig. 5.
Impact of levobupivacaine of autophagy induction. (A) Autophagy induction was assessed by following the activation of LC3, as assessed by the expression ratio between LC3B I and LC3B II, on BHP and DU145 cell lines after 24 h of treatment with 1 mM levobupivacaine. (B) Enumeration of BHP and DU145 cell lines after 24 h of treatment with 1 mM levobupivacaine co-treated or not with 100 nM Wortmannin. Results are expressed as percentage of control. All the data shown correspond to the mean value ± SD of N ≥ 3 different experiments. Significantly different from the untreated cells at: * P ≤ 0.05, ** P ≤ 0.01, *** P ≤ 0.001.
4. Discussion
The objective of this work was to study the bioenergetic and related-cytotoxic effect of the local anesthetic levobupivacaine on human prostate cancer cell. Our results indicate that levobupivacaine could be considered as a cytostatic compound on DU145 cells since this drug induces cancer-specific cell growth arrest with a minor impact on non-cancer cells. Moreover, the mechanism of action (MOA) of levobupivacaine differs between DU145 cancer cells and BHP non-cancer cells. In cancer cells, the MOA involves multi-site alteration of energy homeostasis and further depends on cellular microenvironmental conditions. On the non-cancer cells, levobupivacaine triggered moderate apoptosis as revealed by PARP and caspases 3/7 activation. Such induction of apoptosis was associated with the reduction of Akt activation, as also observed on a different primary cell line [24]. Therefore, levobupivacaine-induced activation of apoptosis was sufficient to explain to reduction of cell number over 24 h in the non-cancer cells. However, we pursued the investigation of the cytotoxic mechanism of levobupivacaine in cancer cells since no apoptosis was observed with this drug. Yet, apoptosis could be activated by etoposide in DU145, which further suggests the existence of a different mode of action of levobupivacaine on non-cancer cells. Indeed, the investigation of DU145 cell cycle progression by flow cytometry revealed that 1 mM levobupivacaine arrested cell growth before the S-phase. Our findings that the cell cycle is stuck in a phase of high energy expenditure - is not surprising with this substance which inhibits energy supplies. Given the reported deleterious impact of local anesthetics on energy metabolism [15], we postulated that cell growth arrest could have occurred in response to cellular energy deprivation. Therefore, we investigated the bioenergetic changes, which occur after the exposure of DU145 cancer cells to 1 mM levobupivacaine over a 24h period. We observed first a direct inhibition of mitochondrial respiratory chain complex I activity with a subsequent decline in both cell respiration and mitochondrially produced ATP. A similar inhibition of oxidative phosphorylation has already been shown for bupivacaine in different experimental models [11], [12], [14], [27], but little is known for what concerns cancer cells. Given the impact of levobupivacaine on complex I, we followed possible changes at the level of ROS production. Accordingly, we found a moderate (15%) increase in the oxidative power of the cytosol but all the antioxydants tested (resveratrol, alpha-tocopherol and NAC) failed to block the anti-proliferative effect of levobupivacaine on cancer cells. Moreover, H2-DCFDA was used to monitor the intracellular ROS levels, but this probe has strong limitations since it is not specific to H2O2 and other reactive oxygen species. Yet, H2-DCFDA can be considered as a REDOX state indicator so that changes in H2-DCFDA fluorescence are more indicative of alterations in the global redox state of the cell [22]. Therefore, one has to consider this limitation for proper interpretation of the data. We conclude that the observed increase in cytosolic oxidative power cannot explain the alteration of DU145 cell proliferation. We then focused deeper on the study of energy metabolism and found a multi-site inhibition of levobupivacaine on cellular ATP synthesis. Alike lidocaine and bupivacaine [4], levobupivacaine reduced the activity of glycolysis and of OXPHOS. Lidocaine and bupivacaine have been shown to induce a marked dose-dependent detachment of the glycolytic enzymes, phosphofructokinase (PFK) and aldolase, from the cytoskeleton of B16 melanoma cells [28] in correlation with a loss of cell viability. Likewise, PFK and lactate dehydrogenase activity were reduced by 50% in non-cancer muscle cells treated with bupivacaine [29]. By attempting to mimick the respective impact of levobupivacaine on complex I activity (70% inhibition) and on glycolytic ATP production (17% inhibition), we were able to resume the anti-growth effect of levobupivacaine. When selected doses of rotenone and iodoacetate were given as co-treatment, the impact on cell viability was stronger than that of levobupivacaine, and this may be explained by a synergistic effect between those two compounds. Paradoxically, in BHP cells, levobupivacaine triggered a stronger reduction of ATP levels produced both by glycolysis and OXPHOS but the cells only showed a minute impairment of proliferation. Thus, the higher sensitivity of DU145 cells to a bioenergetic impairment could reflect a stronger utilization of ATP for cell viability possibly linked with the faster proliferation rate of those cells as compared to BHP. Accordingly, the ATP/ADP ratio measured in DU145 cells in absence of treatment was half of that obtained in BHP non-cancer cells. The energy-state of the targeted cells is not the sole determinant of levobupivacaine potency as modification of glucose concentration in the medium and of oxygen delivery to the cells also influenced the anti-proliferative effect of this drug.
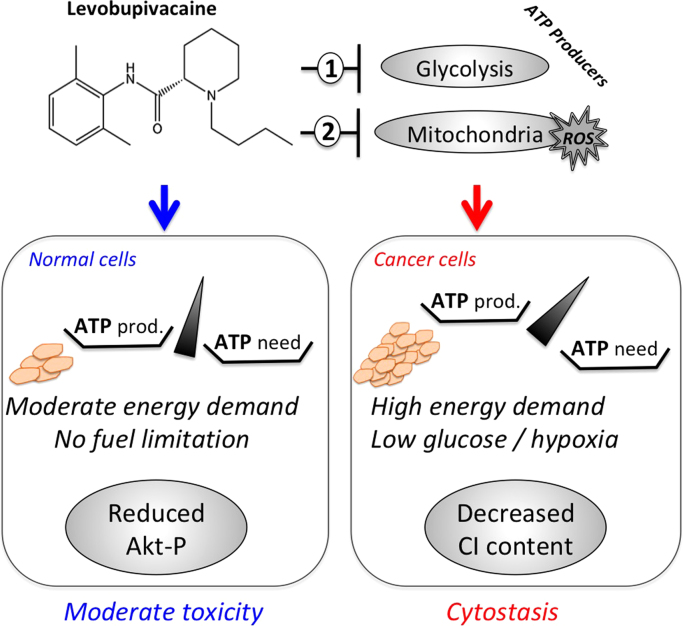
Our study indicates that levobupivacaine has a different impact on cell proliferation between cancer and non-cancer prostate epithelial cells. Highly proliferative cells such as DU145 cells with a doubling time of 10.4 h have a high energy demand as compared to normal cells like BHP that proliferate more slowly (DT=19.8 h). To face the challenge caused by levobupivacaine, cancer cells could activate compensatory mechanisms to stimulate ATP synthesis and this might occur via an increased energy substrate supply to the bioenergetic machineries. A typical way to extract metabolites and recycle molecules for biosynthetic or metabolic reactions involves autophagy in cancer cells and promising therapeutic approachs aim to target these processes. Autophagy can be activated as a survival pathway in conditions of challenging energy transduction [30] and we found that levobupivacaine strongly induced autophagy in DU145 cancer cells, while no induction occurred in the normal cells. This could suggest that the normal cells are less affected by an energetic perturbation than the cancer cells. Glycolysis and mitochondria although being energy producers are also important in producing building blocks for the daughter-cells. By inhibiting both metabolic pathways, levobupivacaine blocks the possibility to reproduce the cell's content necessary to construct a new cell. We found that autophagy was induced as a pro-survival mechanism as its blockade with Wortmannin, a specific inhibitor of PI3K, enhances levobupivacaine anti-tumor efficiency. Moreover, activation of autophagy could be responsible for the decreased complex I content observed in the treated cancer cells. The MOA of levobupivacaine on cancer cells is schematized in Fig. 6. Still, further analyses will be necessary on a larger number of cancer cell lines and corresponding non-cancer cells to fully decipher the mechanism of action of levobupivacaine as well as the specificity of the observed cytostatic effect between cancer and non cancer cells.
Fig. 6.
Bioenergetic and REDOX mechanism of levobupivacaine anti-cancer effect in prostate carcinoma (model). Levobupivacaine inhibits mitochondrial and glycolytic ATP level to specific extent, leading to a reduction of ATP level in the cell. Normal BHP cells respond by decreasing AKT survival pathway thereby triggering a moderate activation of apoptosis. Cancer cells with higher energy demand arrest proliferation and activate autophagy. Wortmannin blocks autophagy and potentializes levobupivacaine toxicity in cancer cells.
5. Conclusion
Levobupivacaine is widely used molecule for regional anesthesia and this drug alters the proliferation of human prostate cancer cells. Our findings identify a bioenergetic cytostatic mode of action of levobupivacaine on human prostate cancer cells, as well as potentiation by autophagy blockers. Our results have implication for the design of bioenergetic therapies of prostate cancer and point out the importance of compensatory mechanisms as autophagy. We also found that the metabolic microenvironment (oxygen tension and glucose levels) modifies the cytotoxic potency of levobupivacaine, fostering the need to evaluate its anti-cancer effect in vivo. The moderate dual inhibition of mitochondrial and glycolytic ATP synthesis might explain the strongest toxicity of this compound on cells with high-energy demand, as cancer cells. Therefore, levobupivacaine may be tested in the next future in combined prostate cancer therapies in the context of the "reuse of old/already approved drugs" with a well known toxicity profile. Also, the localized delivery of the drug directly on the tumor tissue might reduce the risk of systemic toxicity on the heart or the brain, and drug-delivery methods will have to be optimized to foster the use of levobupivacaine in prostate cancer therapy.
Acknowledgments
Authors contributions
CJ., EHC., NA., ER. and EO. performed the experiments of bioenergetics and cell biology. DL., HR., and PP. participated to data analysis and scientific discussions. KNG. and RR. designed the study, analyzed the data and wrote the manuscript.
Funding
The authors acknowledge financial support from INSERM (U1211), INCA (Grant 2017-040), CAPES-COFECUB and Fondation Arc (N.A), H2020-MSCA-ITN-2016/722605 –TRANSMIT (Translating the Role of Mitochondria in Tumorigenesis) and SIRIC Brio.
Disclosure of potential conflicts of interest
The authors declare no conflict of interest.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2018.05.014.
Contributor Information
Karine Nouette-Gaulain, Email: karine.nouette-gaulain@u-bordeaux.fr.
Rodrigue Rossignol, Email: rodrigue.rossignol@u-bordeaux.fr.
Appendix A. Supplementary material
Fig S1.
.
Fig S2.
.
References
- 1.Biki B., Mascha E., Moriarty D.C., Fitzpatrick J.M., Sessler D.I., Buggy D.J. Anesthetic technique for radical prostatectomy surgery affects cancer recurrence: a retrospective analysis. Anesthesiology. 2008;109:180–187. doi: 10.1097/ALN.0b013e31817f5b73. (pii) [DOI] [PubMed] [Google Scholar]
- 2.Lee E.K., Ahn H.J., Zo J.I., Kim K., Jung D.M., Park J.H. Paravertebral block does not reduce cancer recurrence, but is related to higher overall survival in lung cancer surgery. Anesth. Analg. 2017;125:1322–1328. doi: 10.1213/ANE.0000000000002342. [DOI] [PubMed] [Google Scholar]
- 3.Martinsson T. Ropivacaine inhibits serum-induced proliferation of colon adenocarcinoma cells in vitro. J. Pharmacol. Exp. Ther. 1999;288:660–664. 〈http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9918572〉 [PubMed] [Google Scholar]
- 4.Karniel M., Beitner R. Local anesthetics induce a decrease in the levels of glucose 1, 6-bisphosphate, fructose 1,6-bisphosphate, and ATP, and in the viability of melanoma cells. Mol. Genet Metab. 2000;69:40–45. doi: 10.1006/mgme.1999.2954. (pii) [DOI] [PubMed] [Google Scholar]
- 5.Mammoto T., Higashiyama S., Mukai M., Mammoto A., Ayaki M., Mashimo T., Hayashi Y., Kishi Y., Nakamura H., Akedo H. Infiltration anesthetic lidocaine inhibits cancer cell invasion by modulating ectodomain shedding of heparin-binding epidermal growth factor-like growth factor (HB-EGF) J. Cell Physiol. 2002;192:351–358. doi: 10.1002/jcp.10145. [DOI] [PubMed] [Google Scholar]
- 6.Sakaguchi M., Kuroda Y., Hirose M. The antiproliferative effect of lidocaine on human tongue cancer cells with inhibition of the activity of epidermal growth factor receptor. Anesth. Analg. 2006;102:1103–1107. doi: 10.1213/01.ane.0000198330.84341.35. (doi:102/4/1103 )(pii)(10.1213/01.ane.0000198330.84341.35) [DOI] [PubMed] [Google Scholar]
- 7.Le Gac G., Angenard G., Clément B., Laviolle B., Coulouarn C., Beloeil H. Local anesthetics inhibit the growth of human hepatocellular carcinoma cells. Anesth. Analg. 2017:1. doi: 10.1213/ANE.0000000000002429. [DOI] [PubMed] [Google Scholar]
- 8.Arai Y., Kondo T., Tanabe K., Zhao Q.L., Li F.J., Ogawa R., Li M., Kasuya M. Enhancement of hyperthermia-induced apoptosis by local anesthetics on human histiocytic lymphoma U937 cells. J. Biol. Chem. 2002;277:18986–18993. doi: 10.1074/jbc.M108084200. (pii) [DOI] [PubMed] [Google Scholar]
- 9.Jose C., Bellance N., Chatelain E.H., Benard G., Nouette-Gaulain K., Rossignol R. Antiproliferative activity of levobupivacaine and aminoimidazole carboxamide ribonucleotide on human cancer cells of variable bioenergetic profile. Mitochondrion. 2012;12:100–109. doi: 10.1016/j.mito.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 10.Y. Baydilek, B.S. Yurtlu, V. Hanci, H. Ayoğlu, R.D. Okyay, G.E. Kayhan, H. Tokgöz, G. Mungan, I. Özkoçak, The comparison of levobupivacaine in continuous or single dose spinal anesthesia for transurethral resection of prostate surgery, Brazilian J. Anesthesiol. (EnglishEd. 64 89–97. doi: 10.1016/j.bjane.2013.03.007, 2014. [DOI] [PubMed]
- 11.Nouette-Gaulain K., Sirvent P., Canal-Raffin M., Morau D., Malgat M., Molimard M., Mercier J., Lacampagne A., Sztark F., Capdevila X. Effects of intermittent femoral nerve injections of bupivacaine, levobupivacaine, and ropivacaine on mitochondrial energy metabolism and intracellular calcium homeostasis in rat psoas muscle. Anesthesiology. 2007;106:1026–1034. doi: 10.1097/01.anes.0000265164.29630.b4. (pii) [DOI] [PubMed] [Google Scholar]
- 12.Nouette-Gaulain K., Bellance N., Prevost B., Passerieux E., Pertuiset C., Galbes O., Smolkova K., Masson F., Miraux S., Delage J.P., Letellier T., Rossignol R., Capdevila X., Sztark F. Erythropoietin protects against local anesthetic myotoxicity during continuous regional analgesia. Anesthesiology. 2009;110:648–659. doi: 10.1097/ALN.0b013e3181974f7a. [DOI] [PubMed] [Google Scholar]
- 13.Nouette-Gaulain K., Bringuier S., Canal-Raffin M., Bernard N., Lopez S., Dadure C., Masson F., Mercier J., Sztark F., Rossignol R., Capdevila X. Time course of mitochondrial metabolism alterations to repeated injections of bupivacaine in rat muscle. Can. J. Anaesth. 2010;57:836–842. doi: 10.1007/s12630-010-9347-8. [DOI] [PubMed] [Google Scholar]
- 14.Nouette-Gaulain K., Dadure C., Morau D., Pertuiset C., Galbes O., Hayot M., Mercier J., Sztark F., Rossignol R., Capdevila X. Age-dependent bupivacaine-induced muscle toxicity during continuous peripheral nerve block in rats. Anesthesiology. 2009;111:1120–1127. doi: 10.1097/ALN.0b013e3181bbc949. [DOI] [PubMed] [Google Scholar]
- 15.Nouette-Gaulain K., Jose C., Capdevila X., Rossignol R. From analgesia to myopathy: when local anesthetics impair the mitochondrion. Int J. Biochem Cell Biol. 2010;43:14–19. doi: 10.1016/j.biocel.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Wende A.R., Young M.E., Chatham J., Zhang J., Rajasekaran N.S., Darley-Usmar V.M. Redox biology and the interface between bioenergetics, autophagy and circadian control of metabolism. Free Radic. Biol. Med. 2016;100:94–107. doi: 10.1016/j.freeradbiomed.2016.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amoedo N.D., Obre E., Rossignol R. Drug discovery strategies in the field of tumor energy metabolism: limitations by metabolic flexibility and metabolic resistance to chemotherapy. Biochim. Biophys. Acta - Bioenerg. 2017;1858:674–685. doi: 10.1016/j.bbabio.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 18.Wuethrich P.Y., Hsu Schmitz S.F., Kessler T.M., Thalmann G.N., Studer U.E., Stueber F., Burkhard F.C. Potential influence of the anesthetic technique used during open radical prostatectomy on prostate cancer-related outcome: a retrospective study. Anesthesiology. 2010;113:570–576. doi: 10.1097/ALN.0b013e3181e4f6ec. [DOI] [PubMed] [Google Scholar]
- 19.Tsui B.C., Rashiq S., Schopflocher D., Murtha A., Broemling S., Pillay J., Finucane B.T. Epidural anesthesia and cancer recurrence rates after radical prostatectomy. Can. J. Anaesth. 2010;57:107–112. doi: 10.1007/s12630-009-9214-7. [DOI] [PubMed] [Google Scholar]
- 20.Loubiere C., Clavel S., Gilleron J., Harisseh R., Fauconnier J., Ben-Sahra I., Kaminski L., Laurent K., Herkenne S., Lacas-Gervais S., Ambrosetti D., Alcor D., Rocchi S., Cormont M., Michiels J.-F., Mari B., Mazure N.M., Scorrano L., Lacampagne A., Gharib A., Tanti J.-F., Bost F. The energy disruptor metformin targets mitochondrial integrity via modification of calcium flux in cancer cells. Sci. Rep. 2017;7:5040. doi: 10.1038/s41598-017-05052-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schöpf B., Schäfer G., Weber A., Talasz H., Eder I.E., Klocker H., Gnaiger E. Oxidative phosphorylation and mitochondrial function differ between human prostate tissue and cultured cells. FEBS J. 2016;283:2181–2196. doi: 10.1111/febs.13733. [DOI] [PubMed] [Google Scholar]
- 22.Kalyanaraman B., Darley-Usmar V., Davies K.J.A., Dennery P.A., Forman H.J., Grisham M.B., Mann G.E., Moore K., Roberts L.J., Ischiropoulos H. Measuring reactive oxygen and nitrogen species with fluorescent probes: challenges and limitations. Free Radic. Biol. Med. 2012;52:1–6. doi: 10.1016/j.freeradbiomed.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma R., Kline R. Chemosensitivity assay in mice prostate tumor: preliminary report of flow cytometry, DNA fragmentation, ion ratiometric methods of anti-neoplastic drug monitoring. Cancer Cell Int. 2004;4:3. doi: 10.1186/1475-2867-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maurice J.M., Gan Y., MA F., Chang Y., Hibner M., Huang Y. Bupivacaine causes cytotoxicity in mouse C2C12 myoblast cells: involvement of ERK and Akt signaling pathways. Acta Pharmacol. Sin. 2010;31:493–500. doi: 10.1038/aps.2010.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cela O., Piccoli C., Scrima R., Quarato G., Marolla A., Cinnella G., Dambrosio M., Capitanio N. Bupivacaine uncouples the mitochondrial oxidative phosphorylation, inhibits respiratory chain complexes I and III and enhances ROS production: results of a study on cell cultures. Mitochondrion. 2010;10:487–496. doi: 10.1016/j.mito.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 26.Fell D.A., Fell D.A. Metabolic control analysis: a survey of its theoretical and experimental development. Biochem. J. 1992;286:313–330. doi: 10.1042/bj2860313. 〈http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=1530563〉 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Irwin W., Fontaine E., Agnolucci L., Penzo D., Betto R., Bortolotto S., Reggiani C., Salviati G., Bernardi P. Bupivacaine myotoxicity is mediated by mitochondria. J. Biol. Chem. 2002;277:12221–12227. doi: 10.1074/jbc.M108938200. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz D., Beitner R. Detachment of the glycolytic enzymes, phosphofructokinase and aldolase, from cytoskeleton of melanoma cells, induced by local anesthetics. Mol. Genet. Metab. 2000;69:159–164. doi: 10.1006/mgme.2000.2960. [DOI] [PubMed] [Google Scholar]
- 29.Duguez S., Féasson L., Denis C., Freyssenet D. Mitochondrial biogenesis during skeletal muscle regeneration. Am. J. Physiol. Endocrinol. Metab. 2002;282:E802–E809. doi: 10.1152/ajpendo.00343.2001. [DOI] [PubMed] [Google Scholar]
- 30.Ogier-Denis E., Codogno P. Autophagy: a barrier or an adaptive response to cancer. Biochim. Biophys. Acta. 2003;1603:113–128. doi: 10.1016/s0304-419x(03)00004-0. 〈http://www.ncbi.nlm.nih.gov/pubmed/12618311〉 (Accessed 7 October 2017) [DOI] [PubMed] [Google Scholar]