Abstract
Aim
The aim of this article was to provide worldwide, population-based incidence rates for Merkel cell carcinoma (MCC).
Methods
We included 11,576 cases from 20 countries for time trend analyses (1990–2007) and 11,028 cases (2.5 billion person-years) from 21 countries for the period 2003–2007 extracted from Cancer Incidence in Five Continents. We computed age-standardised incidence rates (World Standard population) per million person years and sex ratios of these rates. We estimated annual percentage changes (EAPCs) of the incidence and studied the association between geographic latitude and MCC incidence. We examined the body site distribution of MCC.
Findings
In the majority of populations, the incidence has increased over time (EAPC, men 2.0–21.0%; women 1.6–27.2%). Rate differences between 1995 and 2007 were typically small (men: 0.8–2.2; women: 0.2–1.7). The incidence was relatively stable in some populations (men: U.S. blacks, Japan, Norway, Denmark; women: Denmark, Norway, Sweden). Incidences from 2003 to 2007 were highest in Australia, New Zealand, the United States and Israel among men and in New Zealand, Australia, Ireland and the Netherlands among women. The incidence of MCC and melanoma among white non-Hispanic males in North America was positively associated with living closer to the equator. The proportion of MCC on the head was higher with advanced age. The head was a less likely primary site among blacks as compared with any other ethnicity.
Interpretation
Several countries showed increases in MCC incidence among white non-Hispanics over time. Latitude closer to the equator was associated with the MCC incidence in North American men, but barely in women, possibly due to occupational sunlight exposure patterns.
Keywords: Epidemiology, Incidence, Carcinoma, Merkel cell, Carcinoma, Neuroendocrine, Registries
1. Introduction
Merkel cell carcinoma (MCC) was first described in 1972, when Toker presented the first five cases under the name ‘trabecular carcinoma of the skin’, assuming them to represent an eccrine, sweat gland–derived carcinoma. In electron microscopic studies, Tang and Toker later identified dense-core neuroendocrine granules within the tumour cells, thus assuming their origin from Merkel cells. However, this notion has been recently challenged by suggesting epidermal stem cells, fibroblasts or pro/pre-B cells as possible cells of origin with neuroendocrine differentiation as a consequence of the neoplastic transformation. To the pathologist, these lesions appear as sheets of undifferentiated tumour cells with little cytoplasm and dense nuclear chromatin. They are members of the group of ‘small round blue cell tumours’, which includes small cell carcinomas of the lung, lymphomas, and neuroblastomas. MCC is highly aggressive, and more than one-third of patients die of MCC, making it twice as lethal as malignant melanoma. Almost one-third of patients present at primary diagnosis with loco-regional metastases, e.g. in transit or lymph node metastases [1].
High-risk populations include the elderly and those with increased ultraviolet (UV) exposure. Several observations suggest that long-term exposure to UV sun light is a risk factor for MCC: the solar UVB index was positively associated with the incidence of MCC in the United States [2], MCC occurs most often on the head and neck [3], patients treated with psoralen and UVA (i.e. PUVA) have a higher risk of MCC [4], and many MCC patients have a history of other sun exposure–associated skin cancers [5]. Notably, skin pigmentation appears to protect against MCC as blacks, Asians and Hispanics have considerably lower risks of MCC than white non-Hispanic populations. Immunosuppression by HIV [6], after organ transplantation [7] or due to chronic lymphocytic leukaemia, small lymphocytic lymphoma, or Non-Hodgkin lymphoma (NHL) is considered to be another risk factor for MCC [8]. With the discovery of the Merkel cell polyomavirus (MCPyV) that is present in about 80% of all MCCs in the United States and Europe, a further aetiologic factor of MCC has been established [9]. Notably, MCPyV-negative MCC tumours are characterised by a UV mutational profile [10], whereas MCPyV-positive tumours have a very low UV mutational load.
Although several MCC incidence studies have been undertaken, the vast majority of them included only a single country (for example [2,11–21]). One of the few studies that compared MCC incidence time trends within several countries found that MCC incidence in the Nordic countries has been stable since 1995, whereas rates continued to increase up to 2005 in the Netherlands and the United States [16]. A comparison of MCC incidence across several countries is complicated because of differences in observation periods and age standards used for age standardisation. An international comparative incidence study that uses identical calendar periods, inclusion criteria for MCC and methods for age standardisation has not been reported. Consequently, the aim of our study was four-fold. First, we provide population-based incidence time trends of MCC. Second, we present the most recent population-based incidence rates of MCC. Third, we present analyses regarding geographic latitude and MCC incidence. Fourth, we present the body site distribution of incident MCC by ethnicity and age.
2. Material and methods
2.1. Definition of incident Merkel cell carcinoma cases
The morphology code for Merkel cell carcinoma (M8247/3) of the International Classification of Diseases for Oncology (ICD-O) was introduced in the second edition published in 1990 [22]. At best, the histology of MCCs is coded as 8247/3. However, depending on the source of report (pathologist, clinician, other healthcare provider), MCC may be reported as ‘cutaneous neuroendocrine carcinoma’ (abbreviated as NEC; ICD-O: M8246/3). In addition, the topography of MCC may not exactly be coded as skin (ICD-O topography codes C44.0-C44.9) but may include topographies that most likely reflect the skin as the origin including the lip (C00.0-C00.9), anus, not other specified (NOS) (C21.0), anal canal, anal sphincter (C21.1), connective, subcutaneous and other soft tissues: head, face or neck (C49.0), upper limb and shoulder (C49.1), lower limb and hip (C49.2), trunk, NOS (C49.6), vulva (C51.0-C51.9), female genital tract, NOS (C57.9), penis (C60.0-C60.9), scrotum (C63.2), overlapping lesion of the male genital organs (C63.8), male genital organs, NOS (C63.9), other and ill-defined sites at the head, face or neck, NOS (C76.0), pelvis, NOS (C76.3), upper limb, NOS (C76.4), lower limb, NOS (C76.5) and other ill-defined sites (back, flank, trunk, NOS; C76.7).
We distinguished three subgroups of MCC: first, those MCCs that were coded with the specific MCC morphology code (M8247/3) regardless of topography, second, MCCs coded as NEC (M8246/3) and a topography code of the skin in the narrow sense (C44.0-C44.9), and third, MCCs coded as NEC (M8246/3) and a topography code that most likely reflects dermal origin (see above). For the purpose of this study, the term MCC includes tumours of all three subgroups. However, 98% of MCC were coded with the most specific ICD-O code for Merkel cell carcinoma (ICD-O: 8247/3).
2.2. Included registries
2.2.1. Most recent time period (2003–2007)
Based on Cancer Incidence in Five Continents, Volume X [23], we first pooled the number of cases of regional cancer registries within countries. We included only countries that registered overall at least ten male and ten female cases during 2003 through 2007 to reach a minimum precision of the sex-specific rate estimates.
These countries included Australia (Capital Territory, New South Wales, Queensland, South Australia, Tasmania, Victoria); Canada (Alberta, British Columbia, Manitoba, New Brunswick, Newfoundland and Labrador, Nova Scotia, Ontario, Prince Edward Island, Saskatchewan); Costa Rica; Czech Republic; Denmark; Finland; France (Calvados, Doubs, Haut-Rhin, Isère, Hérault, Loire-Atlantique, Manche, Somme, Vendée); Germany (Brandenburg, Bremen, Hamburg, Mecklenburg-Western Pomerania, Munich, North Rhine-Westphalia, administrative district of Münster, Saarland; Schleswig-Holstein); Ireland; Israel; Italy (Biella, Brescia, Catania and Messina, Catanzaro, Como, Ferrara, Genoa, Latina, Lecco, Lombardy-South, Mantua, Naples, Palermo, Ragusa, Romagna, Sassari, Sondrio, South Tyrol, Syracuse, Turin, Varese); Japan (Aichi Prefecture, Hiroshima, Miyagi Prefecture, Nagasaki Prefecture, Osaka Prefecture); Netherlands; New Zealand; Norway; Slovakia; Spain (Albacete, Asturias, Basque Country, Canary Islands, Ciudad Real, Cuenca, Girona, Granada, Mallorca, Murcia, Navarra, Tarragona); Sweden; Switzerland (Basel, Geneva, Graubünden and Glarus, Neuchâtel, St Gall-Appenzell, Ticino, Valais, Vaud, Zurich); UK (East of England Region, Northern Ireland, Northern England and Yorkshire, North Western England, Oxford Region, South and Western Regions, Thames, Trent, West Midlands) and USA including five SEER-states (Connecticut, Hawaii, Iowa, New Mexico and Utah), the 42 cancer registries of NPCR and the cancer registry of Louisiana.
Geographical distribution and sex ratios of age-standardised incidence rates of Merkel cell carcinoma among predominantly white non-Hispanic populations (2003–2007).
As skin pigmentation and latitude are associated (Hispanic and black populations living closer to the equator), we restricted this analysis to white non-Hispanic populations. For the United States, we used data from the North American Association of Central Cancer Registries and included overall 36 populations. We first identified all registries that provided data of white non-Hispanic populations (California, Los Angeles County; California, San Francisco Bay Area and New Mexico). Thereafter, we checked the proportion of white non-Hispanics among all whites for cancer registries that only provided data for whites overall by use of the U.S. census data from 2010 (https://www.census.gov/2010census/data/, accessed April 20, 2017). If this proportion was 80% or more, we considered the data as representing predominantly white non-Hispanic populations (Alabama; Arkansas; Connecticut; Delaware; Georgia; Illinois; Indiana; Louisiana; Massachusetts; Michigan; Missouri; Nebraska; North Carolina; Ohio; Oklahoma; Oregon; Pennsylvania; Rhode Island; South Carolina; Wisconsin; Virginia; Tennessee). Finally, for cancer registries that did not stratify their data by ethnicity at all, we checked the proportion of white non-Hispanic and included only those registries that had a proportion of 80% or more (Idaho; Iowa; Kentucky; Montana; New Hampshire; Maine; South Dakota; Utah; Vermont; West Virginia and Wyoming). Among countries other than the United States, we included Australia, Canada, Czech Republic, Denmark, Finland, France, Germany, Ireland, Israel (Jews), Italy, Netherlands, New Zealand (other populations than Pacific Islander or Maori), Norway, Slovakia, Sweden, Switzerland and UK. To study the association between geographic latitude of the populations and MCC incidence, we extracted the absolute value of the geographic latitude of each population by use of a web tool (http://www.latlong.net/, accessed June 23, 2017). Furthermore, to compare the geographic distribution of MCC with that of cutaneous melanoma (ICD-10: C43), we also extracted for these populations the corresponding age-standardised incidence rate of cutaneous melanoma. We separately studied the association between geographic latitude and incidence among North American white non-Hispanic populations (n = 37 populations) and among European populations excluding Spain (n = 13 populations).
2.2.2. Time trend analyses (1990–2007)
For the retrieval of coded Merkel cell carcinoma, it is essential that cancer registries used the second edition of ICD-O because the first edition contains only less specific codes like trabecular carcinoma (8190/3), small cell carcinoma of the skin (8002/3, 8041/3, 8043/3, 8044/3), undifferentiated carcinoma of the skin (8020/3), and anaplastic carcinoma of the skin (8021/3) [24]. Tumours with these codes do not necessarily represent MCC. We therefore restricted our time trend analyses to periods for which the second edition of ICD-O was used by the cancer registries. Time trend analyses covered a maximum period of 1990 through 2007 with the exception of SEER-9 for which publicly available data until 2012 were available. Based on volumes VII-X of Cancer Incidence in Five Continents [23,25–27], countries were eligible for time trend analyses, if the number of registered cases included at least 50 cases since 1990. These included Australia (New South Wales, Queensland, Tasmania, Victoria, South and Western Australia), Canada (all provinces but Quebec), France (Bas-Rhin, Calvados, Doubs, Haut-Rhin, Herault, Isere, Somme, Tarn), Czech Republic, Denmark, Finland, Germany (Saarland), Ireland, Israel, Italy (Lombardy - Varese province, Modena, Parma, Ragusa Province, Romagna, Torino), Japan (Miyagi Prefecture, Nagasaki Prefecture, Osaka Prefecture), Netherlands, New Zealand, Norway, Slovakia, Spain (Granada, Murcia, Navarra, Tarragona), Sweden, United Kingdom (Birmingham and West Midlands Region, East of England Region, England North Western, England South and Western Regions, Merseyside and Cheshire, Northern Ireland, Oxford, Scotland, Yorkshire) and the United States (SEER-9). Age-specific time trend analyses were only conducted when at least 100 male and 100 female cases were included the data sets.
3. Statistical methods
We computed crude, age-specific (40–59, 60–79, 80 and more years) and age-standardised incidence rates (cases per million person-years at risk) and the corresponding standard errors using the World Standard Population [28]. With a few exceptions, age groups for the age standardisation were 0–4, 5–9, …, 80–84 and 85+ years.
We computed annual percentage changes (EAPCs) of the age-standardised incidence rates by fitting regression lines to the natural logarithm of age-standardised annual rates for the period 1995 through 2007 if not specified otherwise. We used the calendar year as the predictor variable: Y = b x + c, where Y is the ln(rate), b is the regression coefficient, x is the calendar year and EAPC = 100 × (eb − 1). From the same models, we also estimated the absolute change of age-standardised incidence rates from 1995 through 2007.
To compare age-standardised rates (2003–2007) by sex, we estimated sex ratios with corresponding 95% confidence intervals [29]. For the association between geographic latitude of the capital city of each country and sex ratio, we used the inverse of the variance of the estimated sex ratios for weighting a non-parametric local regression smoothing (LOESS) [30,31] with cubic local polynomials and a smoothing parameter of 0.5. We used geographic latitude as a proxy for the annual ultraviolet dose in each country. We pooled ethnicity-specific cancer registry files for the study of the association between ethnicity and topographic (body site) distribution of MCC. We studied only ethnicities with at least 10 male and 10 female MCC cases.
We are calculating and reporting confidence intervals or standard errors throughout the manuscript to assess the precision of our estimates because our goal is estimation and not significance testing. We wish to avoid publication bias by preferential reporting of significant results. Instead, we judge the value of our estimates by their precision and validity.
4. Results
We included more than 11,000 newly diagnosed MCC cases for the incidence time trend analysis and for the estimation of the current incidence of MCC from 21 countries. The vast majority (98%) of MCC was coded with the most specific ICD-O code for Merkel cell carcinoma (ICD-O: 8247/3). With the exception of the US SEER program (data up to 2012), all cancer registries provided data up to 2007 (Table 1).
Table 1.
Included number of incident Merkel cell carcinoma for the incidence time trend analysis and study of current incidence rates.
| Characteristic | Time trend Analysis of analysis of rates | current incidence incidence rates |
|---|---|---|
| Number of countries | 20 | 21 |
| Time perioda | 1990–2007 | 2003–2007 |
| Number of cases (%) | 11,576 (100) | 11,028 (100) |
| Merkel cell carcinoma (8247/3) | 11,296 (97.6) | 10,676 (96.8) |
| Neuroendocrine carcinoma (8246/3), narrow definition of skin | 200 (1.7) | 142 (1.3) |
| Neuroendocrine carcinoma (8246/3), broader definition of skin | 80 (0.7) | 210 (1.9) |
Incidence trend analyses of the United States (SEER-9) cover the period 1990 through 2012.
Although the incidence of MCC was stable over time in some populations (men: U.S. blacks and others, Japan, Norway, Denmark; women: Denmark, Norway, Sweden), the majority of populations had increases over time (EAPC, men 2.0–21.0%; women 1.6–27.2%). However, the estimated rate differences between 1995 and 2007 were small (men: 0.8–2.2 per million person years; women: 0.2–1.7 per million person years; Fig. 1, Table 2). In countries where the overall incidence rate increased over time, the age-specific incidence rates for ages 40–59, 60–79, and 80 years and older showed comparable relative increases with the exception of Australia (women), Canada (women), France, (women), Israel (women), New Zealand (men and women), UK (men), where the incidence rate among people aged 40–59 years either remained stable or decreased (Supplementary Fig. S1 & Supplementary Table 1).
Fig. 1. Age-standardised (World Standard Population) annual incidence rates (per million person years) of Merkel cell carcinoma.
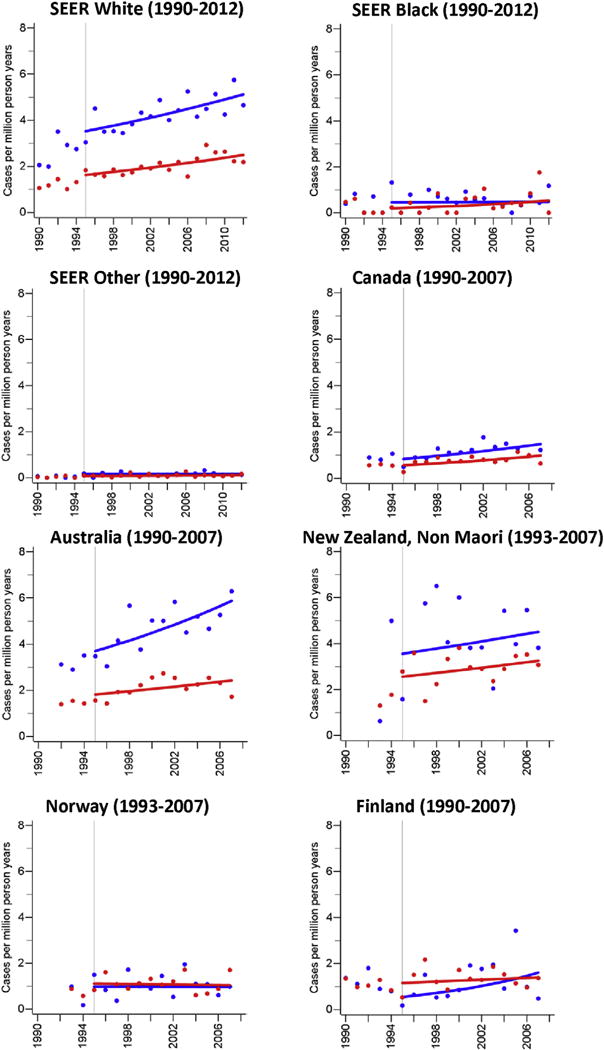
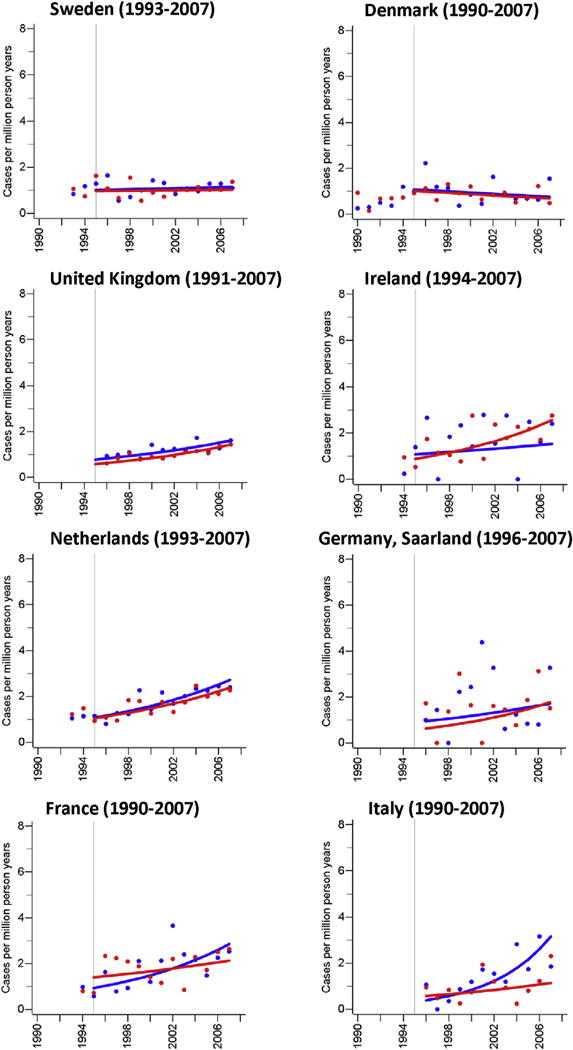
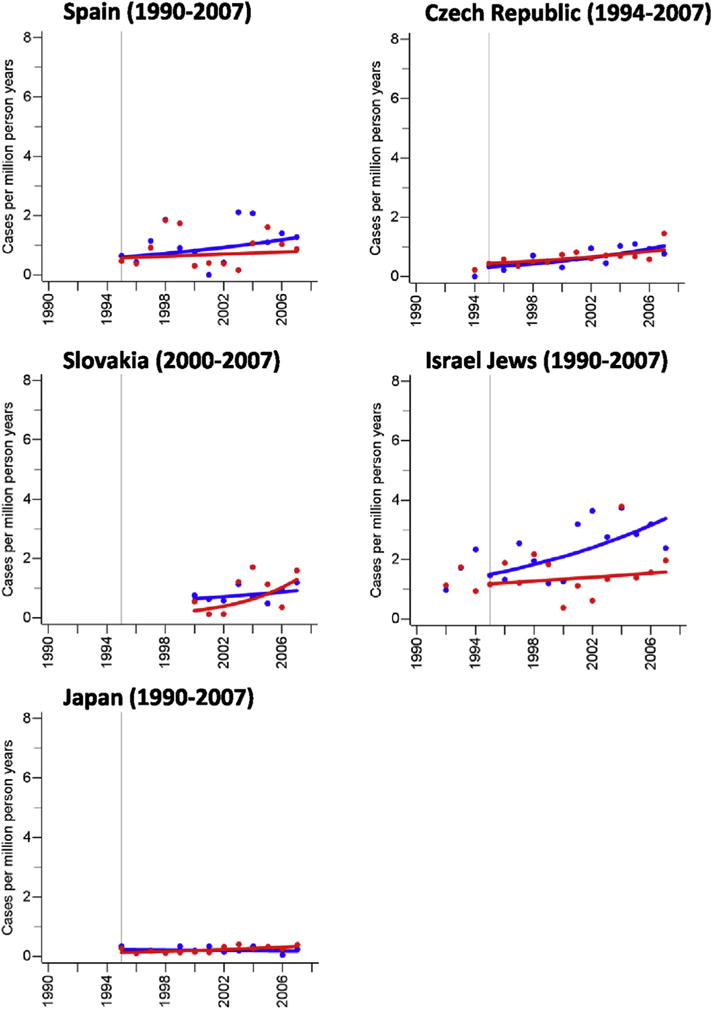
Blue dots and blue trend line: men; red dots and red trend line: women; cases include cancers coded as Merkel cell carcinoma (ICD-O: M8247/3) or neuroendocrine carcinoma (ICD-O: M8246/3) with either topography codes of the skin (ICD-O: C44.0-C44.9) or topography codes suggesting a skin cancer (see methods). (For interpretation of the references to color/colour in this figure legend, the reader is referred to the Web version of this article.)
Table 2.
Estimated changes of the age-standardised incidence rate of Merkel cell carcinoma.
| Country | Period for trend analysis | ICD-O 2 or 3 coding since | Population coverage (2005)
|
Men
|
Women
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| % | Cases | EAPC | 95% CI | Δ | Cases | EAPC | 95% CI | Δ | |||
| US, SEER9-White | 1995–2012 | 1990 | 8.7 | 1813 | 2.2 | 1.2; 3.3 | 1.6 | 1167 | 2.5 | 1.3; 3.7 | 0.9 |
| US, SEER9-Black | 1995–2012 | 1990 | 8.7 | 22 | 0.3 | −6.9; 8.1 | 0.0 | 29 | 6.4 | −1.8; 15.2 | 0.3 |
| US, SEER9-Other | 1995–2012 | 1990 | 18.1 | 81 | −0.1 | −3.0; 2.8 | 0.0 | 69 | 2.1 | −3.9; 8.5 | 0.0 |
| New Zealand | 1995–2007 | 1993 | 100.0 | 171 | 2.0 | −4.1; 8.5 | 0.9 | 172 | 2.0 | −1.6; 5.8 | 0.7 |
| United Kingdom | 1995–2007 | 1995 | 74.2 | 672 | 6.3 | 3.0; 9.7 | 0.8 | 872 | 7.9 | 4.9; 11.0 | 0.9 |
| Australia | 1995–2007 | 1992 | 98.8 | 1006 | 3.9 | 1.6; 6.3 | 2.2 | 609 | 2.5 | −0.3; 5.3 | 0.6 |
| Canada | 1995–2007 | 1992 | 76.3 | 298 | 4.9 | 1.0; 9.0 | 0.6 | 282 | 4.8 | 0.2; 9.6 | 0.4 |
| Czech Republic | 1995–2007 | 1994 | 100.0 | 62 | 10.4 | 4.8; 16.2 | 0.7 | 119 | 6.5 | 2.6; 10.6 | 0.5 |
| Denmark | 1995–2007 | 1990 | 100.0 | 70 | −2.8 | −10.0; 5.0 | −0.3 | 90 | −3.2 | −7.6; 1.5 | −0.3 |
| France | 1995–2007 | 1994 | 9.9 | 131 | 9.7 | 3.5; 16.3 | 1.9 | 192 | 3.5 | −2.6; 10.0 | 0.7 |
| Ireland | 1995–2007 | 1994 | 100.0 | 66 | 3.0 | −13.6; 22.7 | 0.4 | 100 | 9.3 | 2.9; 16.1 | 1.7 |
| Israel, Jews | 1995–2007 | 1992 | 79.5 | 110 | 7.1 | 2.0; 12.4 | 1.9 | 96 | 2.4 | −6.1; 11.8 | 0.4 |
| Italy | 1996–2007 | 1996 | 7.3 | 89 | 21.0 | 8.0; 35.5 | 2.8 | 88 | 6.4 | −5.0; 19.2 | 0.6 |
| Japan | 1995–2007 | 1995 | 10.1 | 34 | −1.9 | −10.1; 7.0 | 0.0 | 54 | 8.9 | 2.4; 15.9 | 0.2 |
| Netherlands | 1995–2007 | 1993 | 100.0 | 317 | 8.1 | 5.0; 11.3 | 1.6 | 416 | 7.1 | 4.1; 10.2 | 1.3 |
| Slovakia | 2000–2007 | 2000 | 100.0 | 22 | 5.2 | −4.6; 16.1 | 0.3 | 38 | 27.2 | −6.6; 73.2 | 1.0 |
| Spain | 1995–2007 | 1995 | 8.0 | 49 | 6.4 | −5.9; 20.2 | 0.7 | 48 | 2.6 | −8.6; 15.1 | 0.2 |
| Sweden | 1995–2007 | 1993 | 100.0 | 163 | 1.0 | −3.5; 5.8 | 0.1 | 207 | 0.5 | −4.2; 5.5 | 0.1 |
| Norway | 1995–2007 | 1993 | 100.0 | 60 | −0.1 | −7.2; 7.5 | 0.0 | 99 | −0.5 | −5.4; 4.6 | −0.1 |
| Finland | 1995–2007 | 1990 | 100.0 | 71 | 9.4 | −1.7; 21.6 | 1.1 | 149 | 1.6 | −0.4; 7.3 | 0.2 |
| Germany | 1996–2007 | 1996 | 1.3 | 20 | 5.5 | −11.2; 25.4 | 0.8 | 31 | 10.0 | −9.0; 33.0 | 1.2 |
The estimated percentage annual change (EAPC) and the estimated rate differences Δ per million person years are derived from log-binomial models; the time periods for these analyses are given in the second column.
Most recent age-standardised incidence rates among men and women are highest in Australia, New Zealand, the United States and Israel among men and in New Zealand, Australia, Ireland and Netherlands among women. Incidence rates of U.S. whites were 8.4-fold and 3.8-fold the rate of U.S. blacks among men and women, respectively. Japan, the only Asian population in our analysis, had a considerably lower incidence than other Non-Asian countries. Sex ratios of age-standardised incidence rates ranged from 0.7 (Slovakia) to 2.3 (Australia) with the vast majority of ratios above 1 (Table 3). Fig. 2 illustrates sex-specific age-standardised incidence rates of MCC in the world. For the United States, we only included white non-Hispanic populations. Our detailed analysis of geographic latitude and MCC incidence among white non-Hispanic men of North America shows that the MCC incidence becomes higher the closer the populations live to the equator. A similar, although weaker, association was observable for cutaneous melanoma. The MCC and cutaneous melanoma incidence of white non-Hispanic women of North America barely showed any association with geographic latitude (Fig. 3). Among the 13 European populations, we did not observe a clear pattern between MCC, cutaneous melanoma incidence and geographic latitude (Supplementary Fig. S2).
Table 3.
Number of person years, incident cases, age-standardised incidence rates (per million person-years) and sex ratio of age-standardised incidence rates of Merkel cell carcinoma in several countries, 2003–2007.
| Country | Population coverage (2005)
|
Men
|
Women
|
Sex ratiob
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | Person years | Casesa | Rates | SE | Person years | Casesa | Rates | SE | IRR | 95% CI | Precision | |
| Australia | 99.7 | 50,178,523 | 471 | 5.2 | 0.3 | 50,922,401 | 264 | 2.2 | 0.2 | 2.3 | 2.0; 2.7 | 0.72 |
| Canada | 100.0 | 61,025,391 | 149 | 1.3 | 0.1 | 62,078,324 | 134 | 0.9 | 0.1 | 1.5 | 1.1; 1.9 | 0.60 |
| Costa Rica | 100.0 | 10,822,379 | 11 | 1.1 | 0.3 | 10,492,838 | 12 | 1.2 | 0.4 | 0.9 | 0.4; 2.1 | 0.18 |
| Czech Rep | 100.0 | 24,992,499 | 34 | 0.8 | 0.2 | 26,239,502 | 60 | 0.9 | 0.1 | 1.0 | 0.6; 1.6 | 0.38 |
| Denmark | 100.0 | 13,411,122 | 26 | 1.0 | 0.2 | 13,689,087 | 41 | 0.9 | 0.2 | 1.1 | 0.6; 1.8 | 0.34 |
| Finland | 100.0 | 12,841,925 | 39 | 1.6 | 0.3 | 13,400,373 | 66 | 1.5 | 0.2 | 1.1 | 0.7; 1.7 | 0.41 |
| France | 11.3 | 16,864,758 | 25 | 0.8 | 0.2 | 17,736,506 | 26 | 0.5 | 0.1 | 1.5 | 0.8; 2.8 | 0.29 |
| Germany | 21.0 | 41,762,638 | 148 | 1.7 | 0.1 | 43,571,461 | 208 | 1.6 | 0.1 | 1.0 | 0.8; 1.3 | 0.63 |
| Ireland | 100.0 | 10,339,700 | 28 | 1.8 | 0.3 | 10,392,200 | 52 | 2.1 | 0.3 | 0.8 | 0.5; 1.3 | 0.38 |
| Israel | 100.0 | 16,429,900 | 57 | 2.8 | 0.4 | 16,754,200 | 54 | 1.9 | 0.3 | 1.4 | 1.0; 2.2 | 0.44 |
| Italy | 25.9 | 32,073,404 | 117 | 1.7 | 0.2 | 34,005,849 | 120 | 1.3 | 0.1 | 1.3 | 1.0; 1.8 | 0.55 |
| Japan | 12.1 | 37,269,211 | 21 | 0.2 | 0.0 | 39,363,569 | 33 | 0.3 | 0.1 | 0.7 | 0.4; 1.3 | 0.31 |
| Netherlands | 100.0 | 40,341,583 | 164 | 2.3 | 0.2 | 41,213,162 | 221 | 2.1 | 0.2 | 1.1 | 0.9; 1.3 | 0.65 |
| New Zealandc | 99.6 | 7,958,330 | 74 | 4.5 | 0.6 | 8,271,640 | 79 | 3.2 | 0.4 | 1.4 | 1.0; 2.0 | 0.49 |
| Norway | 100.0 | 11,488,658 | 27 | 1.1 | 0.2 | 11,660,966 | 45 | 1.1 | 0.2 | 1.0 | 0.6; 1.8 | 0.34 |
| Slovakia | 100.0 | 13,076,116 | 15 | 0.9 | 0.2 | 13,861,643 | 33 | 1.2 | 0.2 | 0.7 | 0.4; 1.3 | 0.29 |
| Spain | 24.7 | 25,795,259 | 65 | 1.1 | 0.1 | 26,254,868 | 56 | 0.8 | 0.1 | 1.4 | 0.9; 2.1 | 0.45 |
| Sweden | 100.0 | 22,418,578 | 69 | 1.1 | 0.2 | 22,791,398 | 91 | 1.1 | 0.1 | 1.0 | 0.7; 1.5 | 0.49 |
| Switzerland | 58.3 | 10,523,625 | 37 | 1.7 | 0.3 | 11,075,973 | 51 | 1.6 | 0.3 | 1.1 | 0.7; 1.7 | 0.39 |
| UK | 86.7 | 123,217,138 | 359 | 1.3 | 0.1 | 127,987,771 | 497 | 1.3 | 0.1 | 1.1 | 0.9; 1.2 | 0.74 |
| USA Whited | 94.4 | 546,817,586 | 4198 | 4.2 | 0.1 | 557,624,219 | 2552 | 1.9 | 0.0 | 2.2 | 2.1; 2.4 | 0.90 |
| USA Blackd | 94.4 | 87,406,801 | 46 | 0.5 | 0.1 | 95,996,619 | 66 | 0.5 | 0.1 | 1.0 | 0.7; 1.5 | 0.46 |
| USA Asian & Pacific Islanderd | 94.4 | 29,202,382 | 21 | 0.7 | 0.1 | 31,046,100 | 29 | 0.7 | 0.1 | 0.9 | 0.5; 1.6 | 0.32 |
| USA American Indiand | 94.4 | 7,192,643 | 11 | 1.7 | 0.5 | 7,193,208 | 11 | 1.4 | 0.4 | 1.2 | 0.5; 2.9 | 0.18 |
Narrow or broad topography definition.
Ratio of the unrounded age-standardised incidence rates (male/female), precision: the inverse of the confidence limit ratio, that is, the ratio of the upper over the lower confidence limit; higher values indicate higher precision.
Only data for other populations than Maori and Pacific Islanders are presented as Maori and Pacific Islanders provided only 3 male and 2 female cases respectively.
Includes 42 cancer registries of NPCR, Connecticut, New Mexico, Hawaii, Iowa, Utah and Louisiana.
Fig. 2.
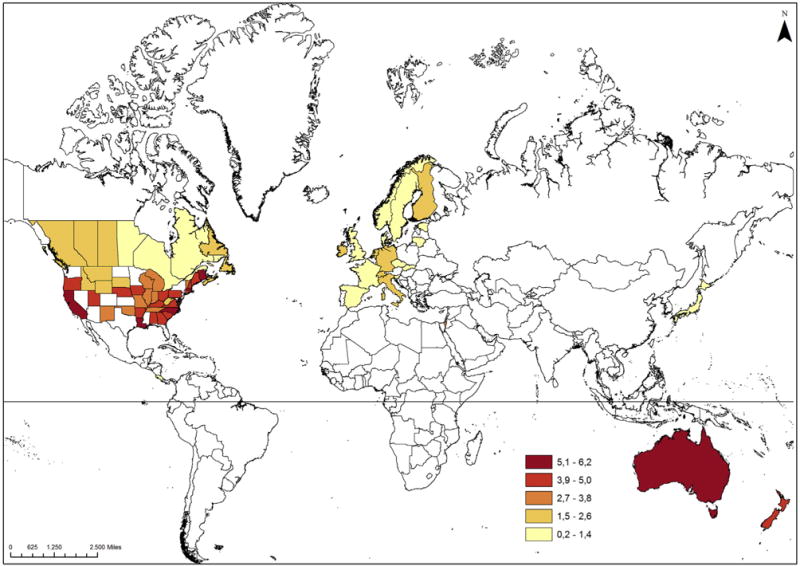
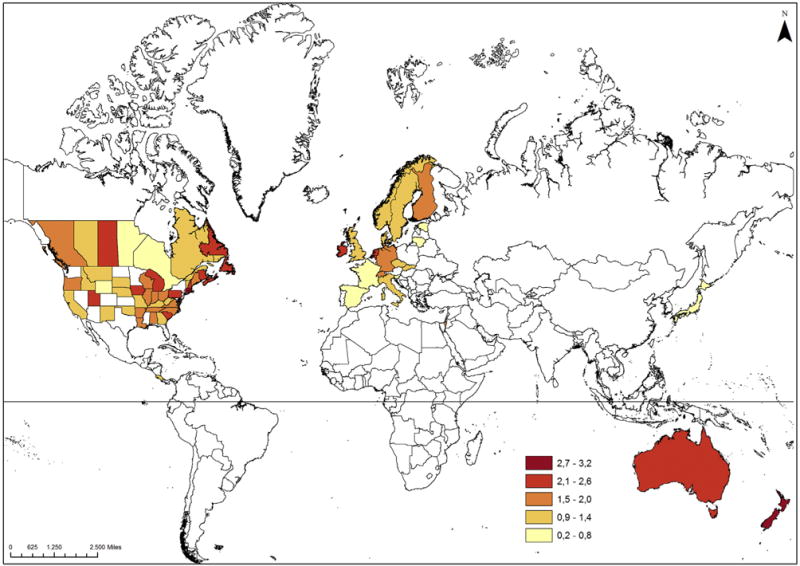
Age-standardised incidence rates (cases per million person-years) of Merkel cell carcinoma in the world, 2003–2007 (upper figure: men, lower figure: women). Esri, DeLorme Publishing Company, Inc., U.S. Central Intelligence Agency; Projection type: WGS 1984, Web Mercator (auxiliary sphere).
Fig. 3. Degree of geographical latitude (absolute value), age-standardised incidence rate and sex ratios of Merkel cell carcinoma among 37 white non-Hispanic populations in Northern America, 2003–2007.
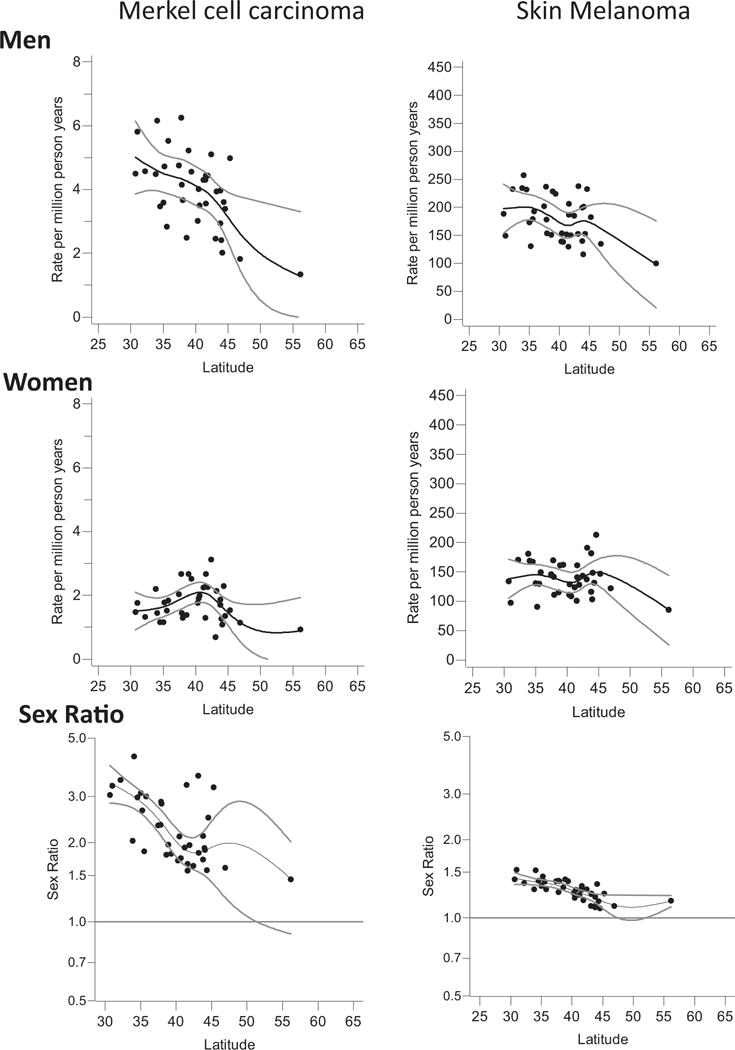
Gray lines indicate 95% confidence limits of the flexibly modelled precision-weighted regression line; populations include 36 U.S. populations and Canada.
Regardless of geographic latitude, the incidence of MCC was positively associated with the incidence of cutaneous melanoma (Supplementary Fig. S3).
A detailed analysis of the body site (topographical) distribution of 8068 MCC revealed that 49% (men) and 46% (women) of MCC occurred on the head followed by the arms (men 23%, women 22%). The topographical distribution differed by sex. The proportion of MCC on the legs was considerably higher among women than men (22% versus 13% respectively). Notably, although the proportion of MCC on the legs declined by age among men, this proportion increased by age among women (Fig. 4).
Fig. 4. Sex-specific site distribution of Merkel cell carcinoma among 7.737 white non-Hispanic populations, 2003–2007.
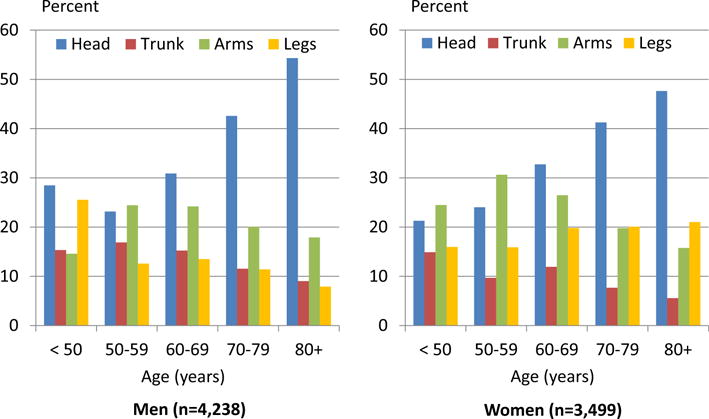
Unspecified sites (M: 13%, F: 9%), overlapping sites (M: 0.4%, F: 0.6%) and anogenital sites (M: 0.9%, F: 1.7%) were excluded.
Ethnicity was also associated with the topographical distribution: the proportion of MCC on the head was considerably lower among blacks than any other ethnicity. Furthermore, blacks had a higher percentage of anogenital MCC compared with other ethnicities (Table 4). Albeit the total number of MCC in blacks is low, 11% and 18% of MCC among males and females respectively were localised in the anogenital area as compared with 1% and 2%, respectively in white non-Hispanic. The topographical distribution among white non-Hispanic populations was associated with age: the proportion of MCC at the head increased monotonically by age.
Table 4.
Body site distribution of 8068 Merkel cell carcinomas among selected ethnic groups, 2003–2007.
| Populationa | Cases | Body site
|
Distribution of body sites among determined subsite localisations (%)
|
|||||
|---|---|---|---|---|---|---|---|---|
| Indeterminedb | Determined | Head | Trunk | Arms | Legs | Ano-genitalc | ||
| Men | ||||||||
| Total | 4390 | 583 | 3807 | 49 | 14 | 23 | 13 | 1 |
| Black | 42 | 5 | 37 | 24 | 16 | 16 | 32 | 11 |
| Japanese | 25 | 3 | 22 | 41 | 14 | 23 | 23 | 0 |
| White, Hispanic | 85 | 8 | 77 | 51 | 10 | 18 | 19 | 1 |
| White, non-Hispanic | 4238 | 567 | 3671 | 49 | 14 | 23 | 13 | 1 |
| Women | ||||||||
| Total | 3678 | 353 | 3325 | 46 | 8 | 22 | 22 | 2 |
| Black | 62 | 5 | 57 | 28 | 11 | 19 | 25 | 18 |
| Japanese | 38 | 4 | 34 | 76 | 6 | 9 | 9 | 0 |
| White, Hispanic | 79 | 8 | 71 | 54 | 3 | 20 | 21 | 3 |
| White, non-Hispanic | 3499 | 336 | 3163 | 46 | 8 | 22 | 22 | 2 |
Black populations included exclusively populations from the US; Japanese populations included Japanese populations from California, Los Angeles County, Hawaii and Japan; Hispanic populations included Hispanic populations from California, San Francisco Bay, Los Angeles County, New Mexico and Spain; White non-Hispanic populations: Australia, Canada, Czech Rep, Denmark, Finland, France, Germany, Ireland, Israel, Italy, Netherlands, New Zealand, Norway, Slovakia, Sweden, Switzerland, UK, and US.
Indetermined subsites included: unspecified subsites, overlapping sites, lymph node or inner organ codes for topography of 8247/3 coded MCCs.
Black, males: four anal MCC; white Hispanic, males: one anal canal MCC; white non-Hispanic, males: 15 anal MCC, 12 anal canal MCC, five penis MCC, three scrotal MCC, two MCC at male genital organ, not other specified; Black, females: four anal MCC, two anal canal MCC, three vulva MCC, one female genital organ, not other specified; white Hispanic, females: one vulva MCC; white non-Hispanic, females: 19 anal MCC, 19 anal canal MCC, 15 vulva MCC, one vaginal MCC, six MCC at female genital organs, not other specified.
5. Discussion
To the best of our knowledge, this is the largest and most comprehensive international overview of the incidence of Merkel cell carcinoma. Our analysis included more than 11,000 newly diagnosed cases between 2003 and 2007 from high-quality population-based cancer registries of 21 countries. Several countries showed incidence increases over time although the absolute rate increases were small. Currently, the highest incidence rates have been observed in Australia, New Zealand and the United States, i.e. the same countries with the strongest incidence increases. Besides sex, age and ethnicity, geographic latitude is associated with MCC incidence among white non-Hispanic men. The MCC incidence is higher among North American white non-Hispanic men living closer to the equator. Furthermore, the topographic distribution of MCC was associated with sex, age and ethnicity, and among white non-Hispanics, it was comparable to that of cutaneous squamous cell carcinomas [32]. Interestingly, blacks had a lower proportion of MCC on the head and a substantially higher proportion of anogenital MCC.
Agelli and Clegg [2] observed a plateau or slight decline in incidence rates during 1996–1999 in the United States (SEER-9). We provided trend data from 1990 through 2012 for SEER-9 and found a continuous upward trend of the age-standardised incidence rate of MCC of the white population. It has been speculated that the increase in the incidence of MCC over time may reflect improvements in cancer registration, histopathologic diagnosis, immunohistochemistry (most importantly cytokeratin 20), increased awareness and familiarity with this cancer by physicians [2,11,13,16,18,33–35]. These factors would be expected to have their effects globally, and thus do not explain why the incidence in the Nordic countries remained fairly stable incidence over time in our analyses.
Australia had the highest incidence rate of MCC in 2003–2007 in our analyses. A study using Australian tissue samples from MCC patients reported that only 24% of the tumours contained the Merkel cell polyomavirus (MCPyV), whereas in northern Europe, up to 80% of the tumours are associated with MCPyV [36]. This observation suggests that high sun exposure in Australia may make a greater contribution to the incidence of MCC than the Merkel polyomavirus [37]. It is tempting to speculate that the MCPyV-associated MCC incidence and incidence trend is comparable in white non-Hispanics in Australia and Europe; however, the UV-associated MCC incidence and incidence trend is considerably higher or stronger respectively in Australia than Europe.
The vast majority of sex ratios of the age-standardised incidence were above one indicating a higher incidence among men than women. Sex ratios of MCC incidence are remarkably influenced by the geographic latitude of populations. We found that Non-Hispanic white men in North America living closer to the equator (and therefore being exposed to more sun light) tend to have higher a MCC and cutaneous melanoma incidence than Non-Hispanic white men living farther from the equator. We did not observe these associations among North American women and among European populations. The findings of North America may be explained by a higher prevalence of outdoor jobs (and therefore more UV radiation exposure) among men than women. The distribution of lighter skin types in Europe may be a reason why the latitude-incidence association for MCC and cutaneous melanoma among predominantly white men is not apparent: men in Southern Europe more frequently have darker skin types than men in Northern Europe; however, outdoor UV radiation is considerably stronger in Southern Europe than Northern Europe. In contrast, in North America, there is not a strong relation of skin type and latitude among white non-Hispanics.
The strengths of our study include the large number of incident MCC cases from many high qualities, representative population-based cancer registries, the use of identical selection criteria of MCC in the cancer registry files, and the use of an identical statistical approach to calculate incidence rates across registries. This study also has limitations. First, despite our quality-based selection criteria of cancer registries, detailed quality controls of the cancer registry data, and exclusion of the early 1990s for time trend analyses, improvement in MCC detection by dermatologists and pathologists, ICD-O coding quality, and completeness of cancer registration could still be an alternative to an aetiologic explanation of the incidence increase of MCC. Second, we could neither review the pathology reports nor reassess the histological slides or tumour tissues. However, Girschik et al. [13] reviewed the pathology reports of 226 MCC cases acquired by the cancer registry of Western Australia between 1993 and 2007. Where doubt existed as to the diagnosis of MCC, they attempted to obtain stored histopathological slides and tissue blocks for further review. They found that 95% of the registered MCC cases could be confirmed as MCC. The remaining cases contained insufficient histological or immunohistochemical information, and the original slides or tissue blocks could not be obtained. Similarly, a tissue sample review of 193 MCC cases reported to the Finnish Cancer Registry between 1983 and 2004 revealed that 181 cases (94%) could be confirmed as MCC at re-evaluation [16].
6. Conclusions
Since the mid 1990s, several countries have experienced an increase in the incidence of MCC which can only be partially explained by improved detection, classification and coding of MCC. The incidence of MCC is particularly strongly associated with latitude and therefore UV sun exposure among white non-Hispanic men in North America.
Supplementary Material
Acknowledgments
Andreas Stang was supported by a grant, German Federal Ministry of Education and Science (BMBF), grant number 01ER1704. The authors thank the following population-based cancer registries that provided us their data for this project. Andreas Stang contributed to the conception of the project, data access applications, statistical analyses, data interpretation, writing. Jürgen Becker contributed to the conception of the project, data interpretation, writing. Paul Nghiem contributed to the conception of the project, data interpretation, writing. Jacques Ferlay contributed to the conception of the project, data provision, data interpretation, writing. Furthermore, we thank Salman Ahmed, Center for Urban Epidemiology, Institute of Medical Informatics, Biometry and Epidemiology, University Duisburg-Essen for preparing the geographic maps. In addition, we thank an anonymous reviewer of the European Journal of Cancer for helpful comments.
Most recent period
Colombia: Bucaramanga, Pasto; Costa Rica; Canada: Alberta, British Columbia, Manitoba, New Brunswick, Newfoundland and Labrador, Northwest Territories, Nova Scotia, Ontario, Prince Edward Island, Saskatchewan; USA: California, Los Angeles County, Louisiana, NPCR (42 states), SEER-9; Israel; Japan; Czech Republic; Denmark; Estonia; Finland; France: Calvados, Doubs, Haut-Rhin, Isère, Hérault, Loire-Atlantique, Manche, Somme, Vendée; Germany: Brandenburg, Bremen, Hamburg, Mecklenburg-Western Pomerania, Munich, North Rhine-Westphalia, Saarland, Schleswig-Holstein; Ireland; Italy: Biella, Brescia, Catanzaro, Catania and Messina, Como, Ferrara, Friuli-Venezia Giulia, Genoa, Latina, Lecco, Lombardy South, Mantua, Naples, Palermo, Ragusa, Romagna, Sassari, Sondrio, South Tyrol, Syracuse, Trento, Turin, Varese; Lithuania; The Netherlands; Norway; Slovakia; Slovenia; Spain: Albacete, Asturias, Basque Country, Canary Islands, Ciudad Real, Cuenca, Girona, Granada, Mallorca, Murcia, Navarra, Tarragona; Sweden; Switzerland: Basel, Geneva, Graubünden and Glarus, Neuchâtel, St Gall-Appenzell, Ticino, Valais, Vaud, Zurich; UK: East of England Region, England, Northern and Yorkshire, Northern Ireland, North Western, Oxford Region, South and Western Regions, Thames, Trent, West Midlands; Australia: Australian Capital Territory, New South Wales, Queensland, South Australia, Tasmania, Victoria, Western Australia; New Zealand.
Time trend analyses
Cancer registries from Australia; Austria: Tyrol; Bulgaria; Canada; China: Shanghai; Colombia: Cali; Costa Rica; Czech Republic; Denmark; Ecuador; Estonia; Finland; France; Germany: Saarland; India; Ireland; Israel; Italy; Japan; Latvia; Lithuania; Netherlands; New Zealand; Norway; Philippines: Manila; Poland; Slovakia; Slovenia; Spain; Sweden; Switzerland; Thailand, Chiang Mai; UK; USA SEER-9 including Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco-Oakland, Seattle-Puget Sound, and Utah.
Role of the funding source
This work was supported by the German Federal Ministry of Education and Science (BMBF) [grant number 01ER1704]. The funding source had no role in the study design, in the collection, analysis and interpretation of data, in the writing of the report, and in the decision to submit the paper for publication.
Appendix A. Supplementary data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ejca.2018.02.003.
Footnotes
Conflict of interest statement
Andreas Stang received a speaker honorarium from MerckSerono. Jürgen C. Becker has received speaker honoraria from Amgen, MerckSerono and Pfizer and has received advisory board honoraria from Amgen, CureVac, eTheRNA, Lytix, MerckSerono, Novartis, Rigontec and Takeda; and he has received research funding from Boehringer Ingelheim, BMS and Merck-Serono. Paul Nghiem has received honoraria from EMD Serono, Pfizer and Merck for consulting work. His institution has received research funding from Bristol Myers Squibb. The other authors have no conflicts to declare.
References
- 1.Becker JC, Stang A, DeCaprio JA, Cerroni L, Lebbe C, Veness A, et al. Merkel cell carcinoma. Nat Rev Dis Primers. 2017;3:17077. doi: 10.1038/nrdp.2017.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agelli M, Clegg LX. Epidemiology of primary Merkel cell carcinoma in the United States. J Am Acad Dermatol. 2003;49:832–41. doi: 10.1016/s0190-9622(03)02108-x. [DOI] [PubMed] [Google Scholar]
- 3.Pellitteri PK, Takes RP, Lewis JS, Jr, Devaney KO, Harlor EJ, Strojan P, et al. Merkel cell carcinoma of the head and neck. Head Neck. 2012;34:1346–54. doi: 10.1002/hed.21787. [DOI] [PubMed] [Google Scholar]
- 4.Lunder EJ, Stern RS. Merkel-cell carcinomas in patients treated with methoxsalen and ultraviolet A radiation. N Engl J Med. 1998;339:1247–8. doi: 10.1056/NEJM199810223391715. [DOI] [PubMed] [Google Scholar]
- 5.Howard RA, Dores GM, Curtis RE, Anderson WF, Travis LB. Merkel cell carcinoma and multiple primary cancers. Cancer Epidemiol Biomark Prev. 2006;15:1545–9. doi: 10.1158/1055-9965.EPI-05-0895. [DOI] [PubMed] [Google Scholar]
- 6.Lanoy E, Dores GM, Madeleine MM, Toro JR, Fraumeni JF, Jr, Engels EA. Epidemiology of nonkeratinocytic skin cancers among persons with AIDS in the United States. AIDS. 2009;23:385–93. doi: 10.1097/QAD.0b013e3283213046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clarke CA, Robbins HA, Tatalovich Z, Lynch CF, Pawlish KS, Finch JL, et al. Risk of merkel cell carcinoma after solid organ transplantation. J Natl Cancer Inst. 2015;107 doi: 10.1093/jnci/dju382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brewer JD, Shanafelt TD, Otley CC, Roenigk RK, Cerhan JR, Kay NE, et al. Chronic lymphocytic leukemia is associated with decreased survival of patients with malignant melanoma and Merkel cell carcinoma in a SEER population-based study. J Clin Oncol. 2012;30:843–9. doi: 10.1200/JCO.2011.34.9605. [DOI] [PubMed] [Google Scholar]
- 9.Santos-Juanes J, Fernandez-Vega I, Fuentes N, Galache C, Coto-Segura P, Vivanco B, et al. Merkel cell carcinoma and Merkel cell polyomavirus: a systematic review and meta-analysis. Br J Dermatol. 2015;173:42–9. doi: 10.1111/bjd.13870. [DOI] [PubMed] [Google Scholar]
- 10.Harms PW, Vats P, Verhaegen ME, Robinson DR, Wu YM, Dhanasekaran SM, et al. The distinctive mutational spectra of polyomavirus-negative merkel cell carcinoma. Cancer Res. 2015;75:3720–7. doi: 10.1158/0008-5472.CAN-15-0702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaae J, Hansen AV, Biggar RJ, Boyd HA, Moore PS, Wohlfahrt J, et al. Merkel cell carcinoma: incidence, mortality, and risk of other cancers. J Natl Cancer Inst. 2010;102:793–801. doi: 10.1093/jnci/djq120. [DOI] [PubMed] [Google Scholar]
- 12.Hussain SK, Sundquist J, Hemminki K. Incidence trends of squamous cell and rare skin cancers in the Swedish national cancer registry point to calendar year and age-dependent increases. J Investig Dermatol. 2010;130:1323–8. doi: 10.1038/jid.2009.426. [DOI] [PubMed] [Google Scholar]
- 13.Girschik J, Thorn K, Beer TW, Heenan PJ, Fritschi L. Merkel cell carcinoma in Western Australia: a population-based study of incidence and survival. Br J Dermatol. 2011;165:1051–7. doi: 10.1111/j.1365-2133.2011.10493.x. [DOI] [PubMed] [Google Scholar]
- 14.Reichgelt BA, Visser O. Epidemiology and survival of Merkel cell carcinoma in the Netherlands. A population-based study of 808 cases in 1993-2007. Eur J Cancer. 2011;47:579–85. doi: 10.1016/j.ejca.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Lyhne D, Lock-Andersen J, Dahlstrom K, Drzewiecki KT, Balslev E, Muhic A, et al. Rising incidence of Merkel cell carcinoma. J Plast Surg Hand Surg. 2011;45:274–80. doi: 10.3109/2000656X.2011.613233. [DOI] [PubMed] [Google Scholar]
- 16.Kukko H, Bohling T, Koljonen V, Tukiainen E, Haglund C, Pokhrel A, et al. Merkel cell carcinoma - a population-based epidemiological study in Finland with a clinical series of 181 cases. Eur J Cancer. 2012;48:737–42. doi: 10.1016/j.ejca.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 17.Youlden DR, Soyer HP, Youl PH, Fritschi L, Baade PD. Incidence and survival for Merkel cell carcinoma in Queensland, Australia, 1993-2010. JAMA Dermatol. 2014;150:864–72. doi: 10.1001/jamadermatol.2014.124. [DOI] [PubMed] [Google Scholar]
- 18.Zaar O, Gillstedt M, Lindelof B, Wennberg-Larko AM, Paoli J. Merkel cell carcinoma incidence is increasing in Sweden. J Eur Acad Dermatol Venereol. 2016;30:1708–13. doi: 10.1111/jdv.13698. [DOI] [PubMed] [Google Scholar]
- 19.Goon PK, Greenberg DC, Igali L, Levell NJ. Merkel cell carcinoma: rising incidence in the East of England. J Eur Acad Dermatol Venereol. 2016;30:2052–5. doi: 10.1111/jdv.13828. [DOI] [PubMed] [Google Scholar]
- 20.Rubio-Casadevall J, Hernandez-Pujol AM, Ferreira-Santos MC, Morey-Esteve G, Vilardell L, Osca-Gelis G, et al. Trends in incidence and survival analysis in non-melanoma skin cancer from 1994 to 2012 in Girona, Spain: a population-based study. Cancer Epidemiol. 2016;45:6–10. doi: 10.1016/j.canep.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Fondain M, Dereure O, Uhry Z, Guizard AV, Woronoff AS, Colonna M, et al. Merkel cell carcinoma in France: a registries-based, comprehensive epidemiological survey. J Eur Acad Dermatol Venereol. 2018 doi: 10.1111/jdv.14798. https://doi.org/10.1111/jdv.14798 [Epub ahead of print] [DOI] [PubMed]
- 22.Percy CVHV, Muir C. International classification of diseases for oncology (ICD-O) 2nd. Geneva: World Health Organization; 1990. [Google Scholar]
- 23.Forman D, Bray F, Brewster DH, Gombe Mbalawa C, Kohler B, Steliarova-Foucher E, et al. Cancer incidence in five Continents. X. Lyon: International Agency for Research on Cancer; 2014. [DOI] [PubMed] [Google Scholar]
- 24.World Health O. International classification of diseases for oncology. 1st. Geneva: WHO; 1976. [Google Scholar]
- 25.Parkin DM, Whelan SL, Ferlay J, Raymond L, Young J. Cancer incidence in five Continents. VII. Lyon: International Agency for Research on Cancer; 1997. [Google Scholar]
- 26.Parkin DM, Whelan SL, Ferlay J, Teppo L, Thomas DB. Cancer incidence in five continents. VIII. Lyon: International Agency for Research on Cancer; 2002. [Google Scholar]
- 27.Curado MP, Edwards B, Shin HR, Strom H, Ferlay J, Heanue M, et al. Cancer incidence in five continents. IX. Lyon: IARC; 2007. [Google Scholar]
- 28.Doll R, Cook P. Summarizing indices for comparison of cancer incidence data. Int J Cancer. 1967;2:269–79. doi: 10.1002/ijc.2910020310. [DOI] [PubMed] [Google Scholar]
- 29.Boyle P, Parkin DM. Statistical methods for registries. In: Jensen OM, Parkin DM, MacLennan R, editors. Cancer registration: principles and methods. Lyon; 1991. pp. 136–9. [PubMed] [Google Scholar]
- 30.Cleveland WS, Devlin S, Grosse E. Regression by local fitting. J Econometrics. 1988;37:87–114. [Google Scholar]
- 31.Cleveland WS, Grosse E. Computational methods for local regression. Stat Comput. 1991;1:47–62. [Google Scholar]
- 32.Dal H, Boldemann C, Lindelof B. Trends during a half century in relative squamous cell carcinoma distribution by body site in the Swedish population: support for accumulated sun exposure as the main risk factor. J Dermatol. 2008;35:55–62. doi: 10.1111/j.1346-8138.2008.00416.x. [DOI] [PubMed] [Google Scholar]
- 33.Hodgson NC. Merkel cell carcinoma: changing incidence trends. J Surg Oncol. 2005;89:1–4. doi: 10.1002/jso.20167. [DOI] [PubMed] [Google Scholar]
- 34.Albores-Saavedra J, Batich K, Chable-Montero F, Sagy N, Schwartz AM, Henson DE. Merkel cell carcinoma demographics, morphology, and survival based on 3870 cases: a population based study. J Cutan Pathol. 2010;37:20–7. doi: 10.1111/j.1600-0560.2009.01370.x. [DOI] [PubMed] [Google Scholar]
- 35.Pulitzer MP, Amin BD, Busam KJ. Merkel cell carcinoma: review. Adv Anat Pathol. 2009;16:135–44. doi: 10.1097/PAP.0b013e3181a12f5a. [DOI] [PubMed] [Google Scholar]
- 36.Schrama D, Peitsch WK, Zapatka M, Kneitz H, Houben R, Eib S, et al. Merkel cell polyomavirus status is not associated with clinical course of Merkel cell carcinoma. J Investig Dermatol. 2011;131:1631–8. doi: 10.1038/jid.2011.115. [DOI] [PubMed] [Google Scholar]
- 37.Allen PJ, Zhang ZF, Coit DG. Surgical management of Merkel cell carcinoma. Ann Surg. 1999;229:97–105. doi: 10.1097/00000658-199901000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


