Abstract
The COPA syndrome is a monogenic, autoimmune lung and joint disorder first identified in 2015. This study sought to define the main pulmonary features of the COPA syndrome in an international cohort of patients, analyse patient responses to treatment and highlight when genetic testing should be considered.
We established a cohort of subjects (N=14) with COPA syndrome seen at multiple centres including the University of California, San Francisco, CA, USA. All subjects had one of the previously established mutations in the COPA gene, and had clinically apparent lung disease and arthritis. We analysed cohort characteristics using descriptive statistics.
All subjects manifested symptoms before the age of 12 years, had a family history of disease, and developed diffuse parenchymal lung disease and arthritis. 50% had diffuse alveolar haemorrhage. The most common pulmonary findings included cysts on chest computed tomography and evidence of follicular bronchiolitis on lung biopsy. All subjects were positive for anti-neutrophil cytoplasmic antibody, anti-nuclear antibody or both and 71% of subjects had rheumatoid factor positivity. All subjects received immunosuppressive therapy.
COPA syndrome is an autoimmune disorder defined by diffuse parenchymal lung disease and arthritis. We analysed an international cohort of subjects with genetically confirmed COPA syndrome and found that common pulmonary features included cysts, follicular bronchiolitis and diffuse alveolar haemorrhage. Common extrapulmonary features included early age of onset, family history of disease, autoantibody positivity and arthritis. Longitudinal data demonstrated improvement on chest radiology but an overall decline in pulmonary function despite chronic treatment.
Short abstract
When to consider COPA syndrome, a Mendelian disorder with lung disease and arthritis, plus a review of treatments used http://ow.ly/hWv130k21vT
Introduction
COPA syndrome is a monogenic autoimmune disorder manifested by lung and joint disease, identified in 2015 [1]. The COPA syndrome is inherited as an autosomal dominant, variably penetrant disease due to missense mutations in the COPA gene on chromosome 1. COPA encodes coatomer subunit α, a subunit of coat protein complex I (COPI). COPI is a carrier complex involved in retrograde transport of proteins from the Golgi to the endoplasmic reticulum. The COPA subunit binds proteins bearing a carboxyl-terminal dilysine motif [2]. All COPA mutations identified are missense single-nucleotide polymorphisms in exons encoding the WD40 domains of the COPA protein. Four mutations within the WD5 and WD6 repeats of the WD40 domain have been associated with the disease and include: Arg233His, Asp243Gly, Glu241Lys, and Lys230Asn. Functional analyses of the mutations demonstrated impaired binding of mutant COPA to dilysine-tagged proteins [1].
All patients reported to date with COPA syndrome have evidence of diffuse parenchymal lung disease (DPLD). However, the pulmonary features of patients have not been comprehensively described. Because the COPA syndrome can resemble a cystic lung disorder or pulmonary–renal syndrome, it may be difficult for the treating pulmonologist to recognise and establish the diagnosis.
The aim of our study was to provide a comprehensive descriptive analysis of the pulmonary features of COPA syndrome patients to aid clinicians in recognition, diagnosis and management. We describe the clinical, radiological and pathological features of the lung disease as well as treatments and lung function outcomes for 14 COPA syndrome subjects.
Materials and methods
Subjects
This protocol was approved by the institutional review boards of the University of California, San Francisco (UCSF) (USA) (approval number: 10-02467), Brigham and Women's Hospital (Boston, MA, USA), Istituto Giannina Gaslini (Genoa, Italy), the Hospital for Sick Children in Toronto (Canada) and the National Bioethics Committee in Iceland. Recruited subjects were diagnosed, treated and followed at their respective centres. 14 patients with a previously identified COPA mutation were identified by targeted sequencing and clinical symptoms. Written informed consent was obtained from all subjects.
Clinical information
Demographic and clinical data were abstracted from outpatient and inpatient encounters, pulmonary function tests (PFTs), chest computed tomography (CT) scans, and histopathology data. Data were collected for a minimum of 5 years, up to 25 years. The majority of PFTs were performed at UCSF according to American Thoracic Society (ATS) standards.
Histopathology
11 subjects underwent a lung biopsy, and pathology reports and/or slides for 10 (91%) subjects were reviewed. All available lung biopsy specimens were reviewed by a lung pathologist with expertise in DPLD (K.D. Jones) and analysed for prevalent histopathological findings including: cystic lung disease; bronchiolitis; hyperplasia of lymphoid tissue; germinal centre formation; lymphoid aggregates; airspace enlargement; peribronchiolar or luminal bronchiolar scarring; and acute or remote haemorrhage. Renal biopsies from three subjects were reviewed.
Imaging
11 (85%) out of 13 subjects had chest CT imaging available for review with a chest radiologist specialising interstitial lung disease (B.M. Elicker), who scored eight categories including 1) CT attributes, 2) ground glass opacity, 3) consolidation, 4) fibrosis, 5) airway disease, 6) nodules, 7) emphysema and 8) miscellaneous lung findings including cysts. Serial CT scans were assessed for progression, stability or resolution of disease.
Treatments and outcomes
Treatment regimens were extracted from outpatient and inpatient medical records. Data on outcomes were determined by reviewing progress notes, PFTs and CT scans. Data were collected to the present day.
Statistical analysis
The sample size for our study was 14 subjects. We used descriptive statistics to describe the clinical characteristics of this cohort including PFT data, findings on chest CT and histopathology of lung biopsies.
Results
Subject characteristics
Symptoms of COPA syndrome manifested in all patients before age 12 years. Initial presentation varied widely: three subjects had isolated joint pain, six subjects complained of pulmonary symptoms, and five subjects had a combination of joint pain and pulmonary symptoms. Four subjects had haemoptysis as their initial pulmonary symptom. Two subjects presented with fatigue and were found to have profound anaemia, thought to be due to diffuse alveolar haemorrhage (DAH). Those without haemoptysis reported shortness of breath, chronic cough and dyspnoea on exertion. Eventually, all subjects developed DPLD and arthritis. A subset acquired renal disease (three out of 14, 21%). Among those who developed arthritis as their first manifestation, all developed pulmonary symptoms 10–20 years later. Subjects were given several clinical diagnoses prior to discovery of COPA syndrome including juvenile idiopathic arthritis (JIA), rheumatoid arthritis, and idiopathic pulmonary haemosiderosis. The majority of subjects were female (table 1) and Caucasian (table S1).
TABLE 1.
Subject characteristics
| Characteristics | Patients |
| Subjects | 14 |
| Sex | |
| Male | 3 (21%) |
| Female | 11 (79%) |
| Age at presentation years | |
| <1 | 1 (7%) |
| 2–9 | 10 (71%) |
| 10–12 | 3 (21%) |
| Disease manifestations | |
| Lung disease | |
| Diffuse alveolar haemorrhage | 7 (50%) |
| DPLD | 14 (100%) |
| Arthritis | 14 (100%) |
| Renal disease | 3 (21%) |
DPLD: diffuse parenchymal lung disease.
Pulmonary manifestations
Diffuse alveolar haemorrhage
Half of the subjects developed DAH. In four subjects, haemoptysis was one of the first disease manifestations and two subjects presented with profound anaemia. Subjects with DAH were admitted to an intensive care unit for respiratory support and received immunosuppressive therapy. Notably, renal failure in combination with DAH was present in three (43%) out of seven subjects.
Pulmonary function tests
Spirometry was performed at least once on all subjects (table 2). Approximately half of the subjects received full PFTs including measurements of total lung capacity and diffusing capacity of the lung for carbon monoxide (DLCO). Two subjects, both younger than age 18 years, did not have spirometry that met ATS criteria and were excluded from the analysis. 17% (two out of 12) of subjects had a mixed obstructive/restrictive defect, 67% (eight out of 12) had a restrictive defect only and 8% (one out of 12) had an obstructive defect only. One subject had normal spirometry. Initial DLCO % predicted was abnormal for all subjects tested (eight out of eight, 100%).
TABLE 2.
Initial pulmonary function tests (PFTs)
| Subject | Age years | FEV1 % predicted | FVC % predicted | FEV1/FVC % | TLC % predicted | PFT pattern | DLCO % predicted |
| 1 | 31 | 55 | 61 | 72 | 68 | Restrictive | 37 |
| 3 | 23 | 35 | 48 | 62 | 56 | Mixed | 17 |
| 4 | 20 | 56 | 58 | 83 | 62 | Restrictive | 45 |
| 5 | 13 | 57 | 72 | 75 | 67 | Restrictive | 69 |
| 6 | 5 | 58 | 56 | 95 | NA | Restrictive | NA |
| 7 | 35 | 32 | 43 | 63 | 63 | Mixed | NA |
| 8 | 7 | 67 | 64 | 98 | 67 | Restrictive | NA |
| 10 | 8 | 82 | 76 | 91 | NA | Normal | NA |
| 11 | 28 | 67 | 69 | 83 | 76 | Restrictive | 68 |
| 12 | 35 | 69 | 69 | 80 | 69 | Restrictive | 41 |
| 13 | 15 | 89 | 91 | 79 | 101 | Obstructive | 58 |
| 14 | 9 | 59 | 61 | 89 | 72 | Restrictive | 48 |
FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; TLC: total lung capacity; DLCO: diffusing capacity of the lung for carbon monoxide; NA: not available.
Longitudinal spirometry data over multiple years were available for 10 subjects. There was a decline in % predicted forced expiratory volume in 1 s (FEV1) and % predicted forced vital capacity (FVC) over time for nearly all subjects (nine out of 10, 90%). The average decline in FEV1 % predicted was 2.6% per year and in FVC % predicted was 1.8% per year. One 9-year-old subject had stable FEV1 % predicted and FVC % predicted over only 1 year of follow-up.
Pulmonary imaging
Nearly all subjects underwent chest CT imaging (13 out of 14, 93%). The subject without a chest CT presented at age 2 years of age and DPLD was diagnosed via lung biopsy. The majority of scans were available for review by our radiologist (11 out of 13, 85%). All except two subjects had cysts (nine out of 11, 82%). One subject (subject 5) had a pneumothorax. Of the two subjects without cysts, one received a single scan at age 2 years that was difficult to interpret because of motion artefact and it remains unclear if cysts may develop over time. The other subject underwent a single scan at age 8 years that demonstrated multiple centrilobular nodules. Overall, cysts were thin-walled and scattered throughout the parenchyma in a variable distribution. Other chest CT findings included ground-glass opacities (six out of 11, 55%), nodules (five out of 11, 45%), and fibrosis (one out of 11, 1%).
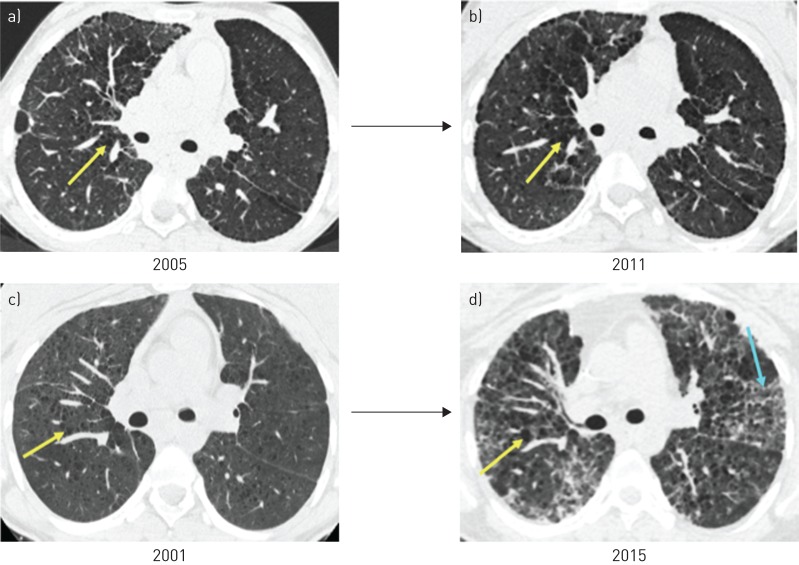
The majority of subjects had serial CT scans to review. In six (67%) out of nine subjects, CT scans stabilised or improved while on immunosuppression; improvement was indicated by fewer nodules and/or resolution of ground-glass opacities (figure 1a and b). Radiographic improvement did not correlate with increases in FEV1 % predicted and FVC % predicted, and these values remained stable or declined. In those with radiographic worsening of disease, there was an increase in cysts and new ground-glass opacities that correlated with decreases in both FEV1 % predicted and FVC % predicted (figure 1c and d).
FIGURE 1.
Serial imaging while on immunosuppressive therapy (two subjects). Axial computed tomography images from subject 5 demonstrate radiographic stability (a) baseline and b) 6 years later) in contrast to subject 4, who developed more cysts and ground-glass opacities over time (c) baseline and d) 14 years later). Yellow arrows indicate cysts and blue arrows indicate ground-glass opacity.
Lung pathology
The majority of subjects underwent lung biopsy (table 3). Detailed reports and/or slides were available for the majority of subjects. Eight out of 10 subjects underwent an open lung biopsy and two subjects underwent a transbronchial biopsy. The two most common histopathological features were follicular bronchiolitis and airspace enlargement/cystic changes. Radiological findings were compared to histological patterns. All seven subjects with follicular bronchiolitis had ground-glass opacities and/or nodules on CT scan (seven out of 10, 70%), consistent with published data [3]. Other findings included interstitial fibrosis in a non-usual interstitial pneumonitis pattern. Lung biopsy specimens of two female subjects with clinicoradiological cystic lung disease had negative immunohistochemical stains for HMB-45. Three subjects did not receive any lung biopsy.
TABLE 3.
Lung histopathological findings
| Features | Subjects (n with finding/n tested) |
| Number of biopsies available for review | 10 (10/11) |
| Type of biopsy | |
| Open lung biopsy | 8 (8/10) |
| Transbronchial biopsy | 2 (2/10) |
| Follicular bronchiolitis | 7 (7/10) |
| Alveolar haemorrhage | 4 (4/10) |
| Airspace enlargement/cystic changes | 2 (2/10) |
| Acute lung injury with capillaritis | 2 (2/10)# |
| Interstitial fibrosis | 2 (2/10)¶ |
| Nondiagnostic | 1 (1/10) |
#: both subjects with this finding were related (father and daughter); ¶: both biopsies demonstrated non-usual interstitial pneumonitis.
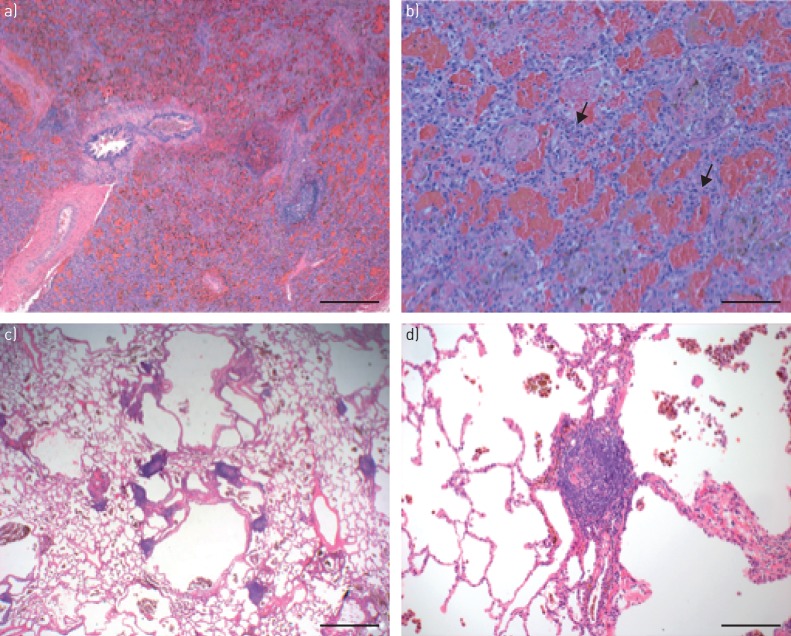
Three subjects were biopsied at the time they developed haemoptysis and diagnosed with DAH via bronchoscopy. Two of these subjects had evidence of acute lung injury with capillaritis. The other subject did not have a pathology report or slide available for review. Both subjects with capillaritis had increased interstitial and alveolar neutrophils on lung histopathology, and they were related (father and daughter, subjects 1 and 2) (figure 2a and b). Biopsy specimens of four subjects revealed alveolar haemorrhage. Among them was subject 4, who was treated with steroids and cyclophosphamide, and underwent a lung biopsy 2–3 weeks after resolution of DAH. Lung histopathology did not demonstrate capillaritis although alveolar haemorrhage was present (figure 2c and d).
FIGURE 2.
Lung histopathology from two subjects demonstrating either capillaritis or prior alveolar haemorrhage. Subject 2: a) low-power view shows diffuse alveolar haemorrhage with filling of alveolar spaces by red blood cells. Focal lymphoid hyperplasia is present. Scale bar=500 µm. b) High-power view shows alveolar haemorrhage and prominent septal neutrophils (arrows) consistent with alveolar capillaritis. Scale bar=100 µm. Subject 4: c) low-power view shows bronchiolocentric airspace enlargement/cystic change, prominent lymphoid hyperplasia and increased haemosiderin-filled intra-alveolar macrophages. Scale bar=1 mm. d) High-power view shows lymphoid hyperplasia and haemosiderin-filled macrophages, indicative of prior alveolar haemorrhage. Scale bar=200 µm.
Autoantibodies
All patients were positive for ANA, ANCA or both (12 out of 14, 86%; nine out of 12, 64%; and nine out of 12, 64%, respectively) with titres ranging from 1:40 to 1:320. Of those with ANCA positivity, three (33%) out of nine subjects were positive for PR3 and two (22%) out of nine subjects were positive for MPO. 10 (71%) out of 14 subjects were positive for rheumatoid factor (RF). Cyclic citrullinated peptide antibodies were positive in three (21%) out of 14 subjects; two of these three were siblings. 10 patients were tested for anti-Ro/SSA, anti-La/SSB, anti-Smith and anti-RNP, and were negative for all four. Complement (C3 and C4) was tested in 10 subjects and seven (70%) had low levels.
Extrapulmonary disease
All subjects developed arthritis. Five (35%) out of 14 subjects were diagnosed with juvenile idiopathic arthritis (JIA) and two (14%) out of 14 subjects with rheumatoid arthritis (RA) prior to the diagnosis of COPA syndrome. Five out of 10 subjects with who were positive for RF had previously been diagnosed with JIA or RA. Other subjects developed polyarticular arthritis but were not classified as having JIA or RA. Affected joints included large joints, such as shoulders and knees, and small joints, such as metacarpophalangeal, proximal interphalangeal and distal interphalangeal joints. Two out of five subjects developed cervical spine disease based on clinical and radiological examinations. One subject developed rheumatoid nodules and another had bilateral swan neck deformities. No subject developed uveitis. All arthritis symptoms responded to immunosuppression.
Three (21%) out of 14 patients developed biopsy confirmed immune-mediated kidney disease and concurrent DAH. Two were ANCA positive (one for PR3, the other was negative for both PR3 and MPO) and one was only ANA positive. Two subjects had evidence of IgM, IgG, IgA, C3 and C1q positivity on immunofluorescence. The subject with IgA nephropathy had strongly positive IgA immunofluorescence staining, and perimesangial and mesangial deposits by electron microscopy. Subjects with renal disease did not require haemodialysis.
Treatment
Acute treatment for DAH
All seven subjects with DAH received high-dose corticosteroids. Doses ranged from methylprednisolone at 30 mg·kg−1 intravenously for 3 days (children) and 500–1000 mg i.v. for 3 days (adults). All seven subjects with DAH also received i.v. cyclophosphamide at doses ranging from six doses of 500 mg·m−2 (children) to six doses of 750 mg·m−2 (adults), which resulted in prompt resolution of DAH for six subjects. Subject 2 had persistent recurrent DAH despite treatment with both i.v. methylprednisolone and cyclophosphamide, and was subsequently treated with four doses of rituximab at 375 mg·m−2 i.v., resulting in resolution of DAH. Plasmapheresis was not used for any subject.
Current treatment
Data on current treatment regimens were available for 12 out of 14 subjects (table 4). The majority of subjects remain on oral low-dose prednisone at 1–2.5 mg (children) or 5–10 mg daily (adults). The majority were on two more agents. The most common second agents were mycophenolic acid at 720 mg daily (children), mycophenolate mofetil 1000–2000 mg daily (adult), and hydroxychloroquine 70–75 mg daily (child) or 200 mg daily (adult). Other steroid-sparing agents included azathioprine at 40 mg orally daily (child) and 100–125 mg orally daily (adult), rituximab at 375 mg·m−2 i.v. once weekly for 4 weeks (child), etanercept at 25 mg subcutaneously (s.c.) weekly (child) and 50 mg s.c. weekly (adult), and methotrexate at 12.5 mg s.c. weekly (child). Two subjects received bilateral lung transplants.
TABLE 4.
Current immunosuppression maintenance regimens
| Type of immunomodulation | Subjects# |
| Low-dose corticosteroids | 9 (75%) |
| Mycophenolate mofetil | 5 (42%) |
| Hydroxychloroquine | 4 (33%) |
| Azathioprine | 3 (25%) |
| Rituximab | 2 (16%) |
| Etanercept | 2 (16%) |
| Methotrexate | 2 (16%) |
| IVIG | 2 (16%) |
| Combination therapies | |
| One agents | 4 (33%) |
| Two agents | 3 (25%) |
| More than two agents | 5 (42%) |
IVIG: intravenous immunoglobulin. #: N=12.
Overall effect of continued immunosuppression on pulmonary disease
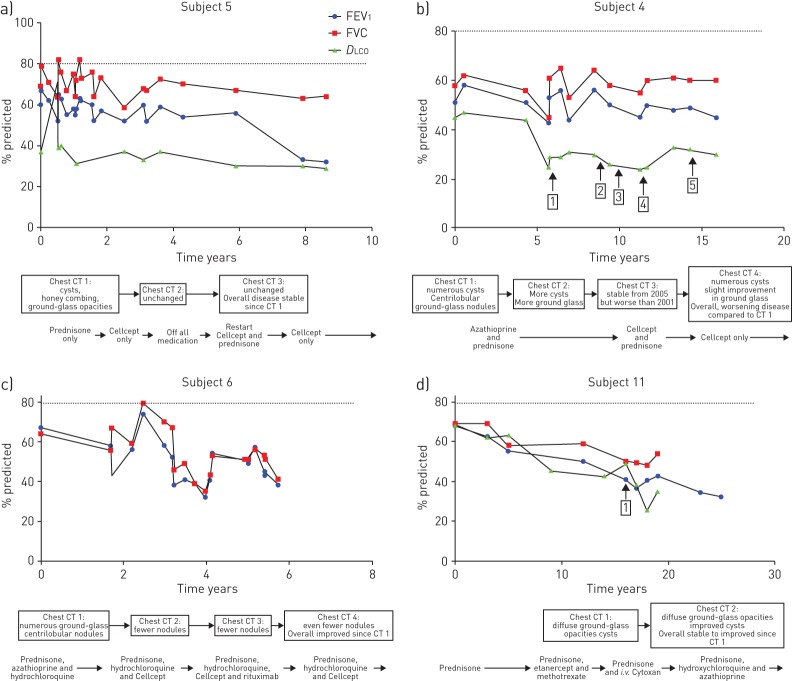
Detailed longitudinal data for five subjects were analysed including serial PFTs spanning 5–20 years, chest CT scans, episodes of DAH and treatment regimens; data from four subjects are shown (figure 3). Overall, immunosuppression did not improve % predicted FEV1, FVC or DLCO values, and in general, these measures declined over time. With respect to episodes of DAH, two subjects experienced no recurrence on immunosuppression (subjects 8 and 11). Most subjects had radiological stability or improvement as evidenced by resolution of ground-glass opacities and fewer nodules (subjects 5, 6 and 11). Subject 4 experienced multiple recurrences of DAH despite treatment and an increase in cysts on chest CT. She had stopped immunosuppression multiple times for various reasons, including noncompliance.
FIGURE 3.
Impact of immunosuppressive therapy on pulmonary function, recurrence of diffuse alveolar haemorrhage (DAH) and chest computed tomography (CT) findings over time (four subjects). a) Subject 5 demonstrated decline in pulmonary function as indicated by % predicted forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC) and diffusing capacity of the lung for carbon monoxide (DLCO) but had radiographic stability while on treatment, while in contrast, her sibling, b) subject 4, struggled with medication noncompliance and developed multiple episodes of DAH as well as radiographic decline based on serial CT scans. Both c) subject 6 and d) subject 11 experienced physiological decline but radiographic stability and/or improvement with immunosuppression. Time axis is years from initial study. Vertical arrows within the plots indicate episodes of DAH; numbers in boxes indicate the episode number. Major chest CT scan findings and treatment regimen are indicated below. Dotted line represents 80% predicted FEV1, FVC and DLCO, which are the cut-offs for normal.
Outcomes
At the time of writing, the oldest subject was 53 years old and the youngest was 9 years old. Four subjects underwent lung transplant or were being considered for lung transplantation (29%). Based on our data, we were unable to predict which patients would require lung transplant. Three out of four subjects were in their third or fourth decade of life, suggesting that the lung disease may progress with age despite chronic immunosuppression. Subject 4 had a long history of medication noncompliance, which may have contributed to declining lung function. Subject 3, who was 25 years old and listed for transplant, presented with respiratory symptoms at 3 months, whereas most subjects presented after age 2 years. The early disease onset in subject 3 may have influenced the need for earlier lung transplant compared to other patients in the cohort.
Discussion
The COPA syndrome is a recently described monogenic autoimmune disorder that may be difficult for clinicians to recognise because it is rare. Because the lung disease tends to dominate in COPA syndrome, we analysed a cohort of subjects to provide a comprehensive and detailed evaluation of pulmonary findings. From our analysis, we identified several pulmonary characteristics common to subjects with genetically confirmed disease: 1) cysts, 2) follicular bronchiolitis and 3) DAH (50% of subjects). Common extrapulmonary features included 1) early age of onset, 2) family history of disease, 3) ANA, ANCA or RF positivity, and 4) arthritis.
At present, four missense mutations in exons 8 and 9 of the COPA gene have been associated with the COPA syndrome. Targeted sequencing should be performed in all subjects suspected of having the disease to establish the diagnosis and distinguish the COPA syndrome from other disorders in which DPLD may occur such as lymphangioleiomyomatosis, Sjögren's syndrome and JIA (table 5). Bioinformatic tools may help predict the likelihood of a novel mutation to cause disease. However, further studies may be required to confirm whether novel mutations result in an impairment of the protein consistent with a defect in COPA function. This may be important for amino acid changes outside of the WD5 and WD6 repeats of the WD40 domain.
TABLE 5.
When to consider genetic testing for COPA syndrome
| Pulmonary features |
| Evidence of follicular bronchiolitis or cysts |
| Diffuse alveolar haemorrhage |
| Extrapulmonary features |
| Early age of onset (<12 years) |
| Family history of disease |
| Positive for ANA or ANCA, ±RF |
| Arthritis |
Consider targeted sequencing for exons 8–9 of COPA in patients with one pulmonary feature and two extrapulmonary features, and strongly consider sequencing in patients with one pulmonary and three extrapulmonary features. ANA: anti-nuclear antibody; ANCA: anti-neutrophil cytoplasmic antibody; RF: rheumatoid factor.
The presence of ANA and/or ANCA in the COPA syndrome suggests a connection to systemic lupus erythematous (SLE) or ANCA-associated vasculitides such as granulomatosis with polyangiitis (GPA) or microscopic polyangiitis (MPA). The presence of immune complex deposition observed in the renal biopsies of patients with COPA syndrome is suggestive of SLE, and alveolar haemorrhage is a recognised, but rare, manifestation of SLE. However, the lack of ANA subserologies such as anti-double-stranded DNA is less consistent with SLE, as is the presence of DPLD. While ANCA positivity, DAH and renal involvement can be consistent with ANCA-associated vasculitis, the lack of upper airway involvement and immune complex deposition on renal biopsy distinguish COPA syndrome from GPA and MPA [4]. In addition, GPA and MPA are primarily associated with PR3-ANCA and MPO-ANCA, while in contrast, only half of COPA subjects with ANCA were positive for either PR3 or MPO. Future studies will be needed to determine whether the COPA syndrome represents a form of SLE or ANCA-associated vasculitis, or a disorder with distinct disease pathogenesis.
Because those with COPA syndrome demonstrate evidence of systemic autoimmunity, in our experience, immunosuppression is central to treatment. In our study, the pulmonary disease was severe and progressive, and frequently the clinical parameter that dictated management. Subjects with DAH were given combination immunosuppression consisting of methylprednisolone and cyclophosphamide followed by steroid-sparing (“maintenance”) immunosuppressive agents such as mycophenolate mofetil or azathioprine. Overall, this regimen was effective at terminating further bleeding. Importantly, maintenance immunosuppression appeared to decrease recurrence of DAH and lead to radiographic improvement on chest CT imaging. Given the limited size of our cohort, we were unable to conclude whether treatment slowed the decline of lung function on PFTs. Four subjects on treatment ultimately required lung transplantation or were listed for lung transplant, suggesting that current immunosuppressive therapies are limited in their ability to prevent long-term progression of lung disease.
The immunopathogenesis of the COPA syndrome remains to be elucidated and it is unclear which immune modulating drugs are most beneficial. T helper type 17 (Th17) cells were shown previously to be elevated in a subset of subjects, although it may be premature to recommend therapies targeting Th17 cells until this finding is replicated. Moreover, it is unclear whether Th17 cell-directed therapies would be effective since it is unknown whether they are central to driving disease or arise in response to systemic inflammation or other factors. Interestingly, limited experience with rituximab suggests the drug may be effective for the lung disease or arthritis and that B-cells may have a role in disease pathogenesis. Finally, a recent study showed activation of the type I interferon pathway in peripheral blood mononuclear cells of patients with COPA syndrome, which suggests a close relationship with SLE given similar activation of type I interferon signalling. Given activation of this pathway, Janus kinase inhibitors or anti-interferon antibodies might serve as another treatment approach [5].
The COPA syndrome is a rare disease and because it has only recently been described is not widely recognised. Although it is possible that the clinical presentation may vary from the features described here, based on our analysis, we recommend that physicians or genetic counsellors consider the disorder when patients present with evidence of follicular bronchiolitis or DAH and extrapulmonary features of the disease such as arthritis, early age of disease onset, family history and typical autoantibodies (table 5). In this setting, targeted sequencing for a COPA mutation may establish the diagnosis, although given the small size of this study, future research with larger groups of patients will likely be needed to conclusively establish diagnostic criteria for the disease.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
TABLE S1 Subject characteristics (detailed) 00017-2018_tableS1 (184KB, pdf)
Acknowledgements
We thank the patients and their families for their involvement in these studies.
Footnotes
This article has supplementary material available from openres.ersjournals.com
Author contributions: J.L. Tsui analysed the data and wrote the manuscript. O.A. Estrada recruited subjects and gathered clinical data. Z. Deng, K.M. Wang and C.S. Law analysed the data and wrote the manuscript. B.M. Elicker provided radiology services including analysis. K.D. Jones provided pathology services including analysis. S.D. Dell, G. Gudmundsson, S. Hansdottir, S.M. Helfgott, S. Volpi, M. Gattorino, A.Y. Chan and M.R. Waterfield provided clinical care and analysed clinical data. B. Ley and S.A. Chung analysed the data and wrote the manuscript. A.K. Shum supervised the study and wrote the manuscript.
Conflict of interest: None declared
Support statement: A.K. Shum is supported by NIH grants R01HL122533 and R01AI137249, J.L. Tsui is supported by NIH grant F32HL131234, G. Gudmundsson is supported by a project grant from the Icelandic Research Fund (141513-051) and from the Landspitali Scientific Fund (A-2015-030 and A-2016-023), M.R. Waterfield is supported by NIH K08 AI121513-03, S. Volpi is supported by grant Telethon GGP15241A, and A.Y. Chan is supported by the Rheumatology Research Foundation Investigator Award. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Levi BW, Jessen B, Wiszniewski W, et al. COPA mutations impair ER–Golgi transport and cause hereditary autoimmune-mediated lung disease and arthritis. Nat Genet 2015; 47: 654–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kirchhausen T. Three ways to make a vesicle. Nat Rev Mol Cell Biol 2001; 2: 216. [DOI] [PubMed] [Google Scholar]
- 3.Tashtoush B, Okafor NC, Ramirez JF, et al. Follicular bronchiolitis: a literature review. J Clin Diagn Res 2015; 9: OE01–OE05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pagnoux C. Updates in ANCA-associated vasculitis. Eur J Rheumatol 2016; 3: 122–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Volpi S, Tsui J, Mariani M, et al. Type I interferon pathway activation in COPA syndrome. Clin Immunol 2018; 187: 33–36. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
TABLE S1 Subject characteristics (detailed) 00017-2018_tableS1 (184KB, pdf)