Graphical abstract
Method name: Association of inorganic As and nano-TiO2 in algae
Keywords: Bioaccumulation, Engineered Nanomaterials, Dissociation, Algae, Trojan horse
Abstract
We developed the effect of titanium dioxide nanoparticles (nano-TiO2) on the bioaccumulation and biotransformation of arsenic (As), which remain largely unknown. We thus exposed two freshwater algae (Microcystis aeruginosa and Scenedesmus obliquus) to inorganic As with the aim of increasing our understanding on As bioaccumulation and methylation in the presence of nano-TiO2. Direct evidence of TEM and EDX image showed that nano-TiO2 (anatase) entered the exposed algae. Thus, nano-TiO2 as carriers boosted arsenic accumulation and methylation in these two algae species, which varied with both inorganic As speciation and algae species. Specifically, nano-TiO2 could enhance markedly arsenate accumulation in M. aerugginosa and arsenite accumulation in S. obliquus. Similarly, we found higher content of As methylation in M. aeruginosa of arsenite with 2 mg L−1 of nano-TiO2 treatment and in S. obliquus of arsenate treatment. Additionally, S. obliquus exhibited higher As methylation compared to M. aeruginosa, being more sensitive to As associated with nano-TiO2 than M. aeruginosa. Due to changes in pH levels inside these exposed algae, the As dissociation from nano-TiO2 inside algal cell enhanced As methylation. Accordingly, the potential influence of nanoparticles on the bioaccumulation and biotransformation of their co-contaminants deserves more attention.
-
•
Nano-TiO2 entry is assumed to promote As accumulation into exposed algae.
-
•
Nano-TiO2 had different carrying capacities for different forms of As and algae.
-
•
As dissociation from nano-TiO2 is assumed to enhance As methylation in algae.
| Subject area |
Select one of the following subject areas: Agricultural and Biological Sciences Engineering Environmental Science |
| More specific subject area | Combination pollution (Nanoparticles) |
| Method name | Association of inorganic As and nano-TiO2 in algae |
| Name and reference of original method | If applicable, include full bibliographic details of the main reference(s) describing the original method from which the new method was derived. |
| Resource availability | If applicable, include links to resources necessary to reproduce the method (e.g. data, software, hardware, reagent) |
Method
Nano-TiO2 and arsenic preparation
We used the anatase form of nano-TiO2 purchased from the Sigma-Aldrich Corporation with a particle size of less than 25 nm and a purity of > 99.7%. A nano-TiO2 stock suspension (1 g L-1) was prepared by first suspending nanoparticles in ultrapure water. We then sonicated the solution at 33 W for 30 min. The average hydrodynamic size of nano-TiO2 was 193 ± 10 nm, as measured by the dynamic light scattering technique (DLS, Malvern Instruments, UK) at automatic attenuator mode. Experimental nano-TiO2 concentrations of 100 μg L−1 and 2 mg L−1 were diluted from the stock suspension. The aggregate morphology of nano-TiO2 was observed by a scanning electron microscope (SEM, S-4800, Hitachi, Japan). We used Na3AsO4·12H2O and NaAsO2 to prepare As stock solutions at 1 mM, which were stored at 4 °C in the dark until further use. Additionally, we measured the average hydrodynamic diameter (dH) and zeta potential (ζ) using DLS, and pH levels of nano-TiO2 in BG-11 culture media with As(III) and As(V) of 10 μM at 0.1 mg L−1 and 2 mg L-1 TiO2 concentrations.
Exposed algae species
The two freshwater alga species (S. Obliquus and M. Aeruginosa) used in our experiments were inoculated under sterile conditions in BG-11 media in Erlenmeyer flasks at 25 °C. The light-dark cycle used was 16:8 with a light intensity of 115 μmol photons m2 s−1. For the following experiments, exposed algae were shaken at 100 rpm using a shaker to avoid settling.
Algae toxicity and stress
Toxicity of As(III) and As(V) was determined using 96 h growth rate bioassays. Algal cell at the exponential growth phase were added separately into a final concentration of As(III) (10 μM) and As(V) (10 μM) under increasing nano-TiO2 levels (from 0 to 200 mg L-1). The initial cell density of exposed algae was 106 cells mL-1. We used a hemocytometer and a microscope to measure algal cell density every 24 h until the end of the exposure experiment. The specific growth rate (μ) of cells was thus calculated according to the method reported by Zeng et al. [1]. Afterwards, the 96 h EC50 was calculated based on μ values of tested algal cells using a probability unit graphical method [2].
We examined alga stress from nano-TiO2 associated with 10 μM of As(III) or As(V). Final concentrations of nano-TiO2 were 0 mg L-1 (control), 0.1 mg L-1, and 2 mg L-1. Cells at the exponential growth phase were added individually to As(V) and As(III) under the three separate aforementioned nano-TiO2 concentrations. The initial cell density was 1 × 106 cells mL-1. At the same time, we conducted parallel experiments with final nano-TiO2 concentrations of 0 mg L-1, 0.1 mg L-1, and 2 mg L-1 (without the addition of As). We conducted Chl-a quantification after 96 h exposure. Additionally, we measured methane dicarboxylic aldehyde (MDA) to indicate the degree of lipid peroxidation (LPO) in this experiment, representing alga stress from As associated with nano-TiO2. We detected MDA content using the thiobarbituric acid reactive substances (TBARS) method by applying a reagent kit (the Nanjing Jiancheng Biotechnology Institute, China) according to the manufacturer’s instructions [3].
Furthermore, we characterized nano-TiO2 in algae and the culture media after both 0.1 and 2 mg/L of nano-TiO2 exposure associated with 10 μM of As(III) and As(V) using TEM as well as energy-dispersive X-ray spectroscopy (TEM, H-7650, Hitachi, Japan; EDX, Genesis XM2) [4]. In brief, we fixed exposed algal cells using 2.5% glutaraldehyde and then refrigerated them for 12 h. Afterwards, the treated algal cells were washed thrice in a 0.1 M phosphate buffer (pH 7.0), then postfixed in 1% osmium tetroxide for 1 h, dehydrated through a graded series of ethanol (30%, 50%, 70%, 90%, 95%, and 100%) and embedded for 12 h. The resultant algal cells were incised using a diamond blade in an ultramicrotome (Leica UC7, Germany) to obtain cell ultrathin sections (approximately 70 nm in thickness) for TEM and EDX observations without staining. Direct evidence of TEM image and EDX observations was provided in Fig. 1.
Fig. 1.
Morphology of titanium dioxide nanoparticles in algae and culture media. Note: Arrows point to nano-TiO2.  Nano-TiO2 in algae culture media;
Nano-TiO2 in algae culture media;  and
and  nano-TiO2 in Microcystis aeruginosa, indicating that nano-TiO2 can enter this algae cell;
nano-TiO2 in Microcystis aeruginosa, indicating that nano-TiO2 can enter this algae cell;  and
and  nano-TiO2 in Scenedesmus obliquus, indicating that nano-TiO2 can enter this algae cell;
nano-TiO2 in Scenedesmus obliquus, indicating that nano-TiO2 can enter this algae cell;  EDX analysis of areas pointed by arrows was confirmed to be nano-TiO2.
EDX analysis of areas pointed by arrows was confirmed to be nano-TiO2.
Dissociation of inorganic As from nano-TiO2 in algae cell homogenates
Inorganic arsenic dissociation in algal cell homogenates
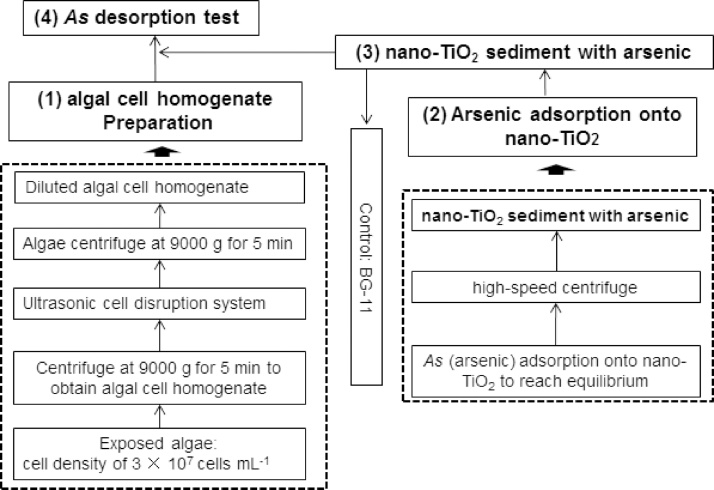
In order to identify whether As dissociated from nano-TiO2 inside algal cells, we conducted desorption experiments to detect the potential dissociation of inorganic As from nano-TiO2 in algal cell homogenates, which followed the flowchart provided in Fig. 2. This information could provide insight into oxidative stress of As entry into algal cells facilitated by the presence of nano-TiO2. In this experiment, the two algal species selected were cultured to a cell density of 3 × 107 cells mL-1. We collected an alga pellet in a 50 mL centrifuge tube using a high-speed centrifuge (9000g) for 5 min. BG-11 was then added into the tube containing the alga pellet, and the tube was shaken to re-suspend the species. After 3 min of disruption using an ultrasonic cell disruption system, the algal cell homogenate was separated at a centrifugal force of 9000g for 5 min to remove algae residue, such as cell wall material. Finally, the algal cell homogenate was diluted with BG-11 to 100 mL for further usage. At the same time, we measured algal cell homogenate pH levels and total organic carbon (TOC) to understand the potential mechanisms of the dissociation.
Fig. 2.
Flowchart of desorption of inorganic As from nano-TiO2 in algal cell homogenates.
Our preliminary results showed that As adsorption on nano-TiO2 rapidly reached equilibrium (within 1 h) in the BG-11 medium. In turn, the solution of As associated nano-TiO2 was prepared 1 h before use to make equilibrated adsorption of As(V) and As(III) onto nano-TiO2. The final concentration of nano-TiO2 and As was 2 mg L-1 and 10 μM, respectively, diluted from their stock solutions with BG-11 for As adsorption onto nano-TiO2 experiment. After mixing As and nano-TiO2 for 1 h, the solution was centrifuged for 10 min at 12 000g to obtain the sediment of nano-TiO2 associated with As by removing the supernatant. We then added 40 mL of the algal cell homogenate and BG-11 (used as the control) into the abovementioned sediment, respectively, to conduct the As desorption test in the algal cell homogenate. After shaking for 2 h at 180 rpm min1 in a shaker, we obtained the supernatant using a high-speed centrifuge (12 000g). We used 1 mL of the supernatant to measure As concentrations using inductively coupled plasma mass spectrometry (ICP-MS 7500a, Agilent). The difference in the final As concentration between BG-11 and the algal cell homogenate was the apparent As desorbed from nano-TiO2, caused by the algal cell homogenate. Three replicates were prepared for this test.
Inorganic arsenic depuration from algae
On the other hand, we examined the inorganic As depuration from the algae species to further identify whether the dissociation of As from nano-TiO2 inside algal cells occurred. The two selected alga species, with an initial cell density of 106 cells mL-1, were pre-exposed to As separately for 96 h in the presence of nano-TiO2, from which the final exposure concentrations were 1 μM As(V) and 0.8 μM As(III) in the presence of 20 mg L-1 nano-TiO2 for M. aeruginosa and 0.1 u M As(V) and 0.08 μM As(III) in the presence of 2 mg L-1 nano-TiO2 for S. obliquus. Under these exposure concentrations, algae did not exhibit any toxic effects. Moreover, As(III) and As(V) can absorb onto nano-TiO2 by 98.91% ± 0.91% and 98.23% ± 0.87%, respectively. Thus, the As accumulated in exposed algae was largely associated with nano-TiO2. The As solution was prepared by the same method described above, that is, it was prepared 1 h before use to equilibrate As(V) and As(III) adsorption on nano-TiO2. We then individually centrifuged each pre-exposed algae species into a pellet at 3800g for 10 min. Afterwards, we used sterile ultrapure water (18.2 mΩ cm−2) to twice wash each algal pellet before immersing each in an ice-cold phosphate buffer (1 mM K2HPO4, 5 mM MES, and 0.5 mM Ca (NO3)2) for 15 min to completely remove apoplastic As [5]. The collected pellets were then individually re-suspended in BG-11 media. After 12 h, 5 mL of supernatant was collected at 3800 for 10 min to measure total As and titanium (Ti) in media using ICP-MS [6,7]. At the same time, the desorption of As from nanoparticles in the absence of algae was comparably performed under the same treated conditions with the exception of using 12 000g centrifugation for 10 min, which further confirmed the depuration of As from algal cells. Moreover, we did not find any As desorbed from nano-TiO2. Thus, the amount of total As in the supernatant was the As depuration from the algae.
Inorganic arsenic accumulation and methylation in the exposed algae
An initial cell density of 106 cells mL-1 was diluted from cell inoculum during the exponential growth phase. After being inoculated with M. aeruginosa or S. obliques, each culture was treated immediately and separately with As(V) and As(III) at 10 μM and the three different concentrations of nano-TiO2 (0 mg L-1, 0.1 mg L-1, and 2 mg L-1, respectively) throughout a 4 d uptake period to evaluate the effects of As bioaccumulation and methylation affected by the presence of nano-TiO2. Cultures devoid of nano-TiO2 (0 mg L-1) were used as controls. Each As(III) treatment was then monitored every 24 h by sampling 5 mL of the aliquot test solution until the end of experiment to detect whether As(III) oxidized into As(V). Finally, we individually collected two algal solutions of 25 mL each from separate flasks for all treatments, after which we centrifuged them into a pellet at 3800g for 10 min. Afterwards, two 5 mL supernatants were individually collected to measure total As and As speciation in the media, respectively, to calculate the bioconcentration factor (BCF) and to quantify As(III) oxidation in tested suspensions. Furthermore, before being immersed in the aforementioned ice-cold phosphate buffer for 15 min to completely remove apoplastic As, both algal pellets were washed twice using sterile ultrapure water (18.2 mΩ/cm2) [5]. Afterwards, the two algal pellets were respectively digested after one pellet was dried under warm conditions at 60 °C in an oven while the other was freeze-dried in a vacuum freeze dryer. At this point, total intracellular As was measured to determine As bioaccumulation using the oven-dried algae samples, while As speciation was analyzed to determine As methylation using the freeze-dried algae samples, including inorganic As, As(V), monomethylarsonic acid (MMA), and dimethylarsinic acid (DMA) [8]. The methylated amount of As inside algae was calculated as the sum of MMA and DMA in the exposed algae.
Arsenic determination in algae and medium
We determined total As and As speciation according to the previous methods used for algal sample preparation and As analysis [9]. In brief, approximately 0.02 g of oven-dried algal samples were treated overnight by means of microwave assisted digestion. Afterwards, samples were further diluted to measure As using ICP-MS. We had a good recovery rate (92.3% ± 5.6%) using a standard reference sample (GBW08521, the National Research Center for Standard Materials of China). Additionally, approximately 0.02 g of the freeze-dried algal samples was treated overnight. After microwave assisted digestion, samples were filtered using 0.45 μm filters. We then used HPLC-ICP-MS (Agilent LC1100 series coupled with the Agilent ICP-MS 7500a) to measure As speciation in algal extracts and media.
Calculation and statistics
The measured As bioaccumulation in algae included free As in algal cells and its association with nanoparticles on cell surface and internalized cells. We then used the dry weight of BCF to estimate As bioavailability affected by the presence of nano-TiO2, which was calculated using the following equation:
BCF = the As concentration in algae (μg/g dry weight)/the As concentration in media (which includes the As adsorbed on nanoparticles; μg L-1) × 1000 (1)
Moreover, SPSS 12.0 was used to perform statistical analysis on the data. The data are shown as means with standard deviations (SD). The differences within treated groups were evaluated by two-way analysis of variance (ANOVA) with a least significant difference (LSD) range test at P<0.05 significant levels.
Conclusion
Nano-TiO2 promoted the accumulation and methylation of inorganic As in the two selected freshwater algae species investigated in this study. Evidence showed that nano-TiO2 promotes As accumulation into exposed algae species. The dissociation of inorganic As from nano-TiO2 contributes to its increased toxic effects on exposed algae, which be demonstrated by Chl-a and MDA. Subsequently, stress levels increased as a result of this As and nano-TiO2 dissociation, thus potentially leading to greater As methylation in algae species. It is clear that As metabolism is variable between As forms and algae species as well as nanoparticles and environmental factors. Furthermore, As contamination is globally recognized as being high risk.
Acknowledgements
We would like to thank the anonymous reviewers for their helpful comments. This study was jointly supported by the National Nature Science Foundation of China (41271484, 41401552, and 41629101), USDA-NIFA Hatch program (MAS 00475) and the Nature Science Foundation of Fujian Province (2016J01691 and 2017Y0081). Z. Luo. thanks the China Scholarship Council (201304910008) for supporting him to study at University of Massachusetts for one year.
Contributor Information
Zhuanxi Luo, Email: zxluoire@163.com.
Baoshan Xing, Email: bx@umass.edu.
References
- 1.Zeng J., Yang L., Wang W.X. Cadmium and zinc uptake and toxicity in two strains of Microcystis aeruginosa predicted by metal free ion activity and intracellular concentration. Aquat. Toxicol. 2009;91(3):212–220. doi: 10.1016/j.aquatox.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Li M. Arsenate accumulation, distribution, and toxicity associated with titanium dioxide nanoparticles in Daphnia magna. Environ. Sci. Technol. 2016;50(17):9636–9643. doi: 10.1021/acs.est.6b01215. [DOI] [PubMed] [Google Scholar]
- 3.Rocchetta I. Effect of chromium on the fatty acid composition of two strains of Euglena gracilis. Environ. Pollut. 2006;141(2):353–358. doi: 10.1016/j.envpol.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 4.Lin D.H. The influence of dissolved and surface-bound humic acid on the toxicity of TiO2 nanoparticles to Chlorella sp. Water Res. 2012;46(14):4477–4487. doi: 10.1016/j.watres.2012.05.035. [DOI] [PubMed] [Google Scholar]
- 5.Wang Z.H. Arsenic uptake and depuration kinetics in Microcystis aeruginosa under different phosphate regimes. J. Hazard. Mater. 2014;276:393–399. doi: 10.1016/j.jhazmat.2014.05.049. [DOI] [PubMed] [Google Scholar]
- 6.Luo Z.X. Spatial distribution, electron microscopy analysis of titanium and its correlation to heavy metals: occurrence and sources of titanium nanomaterials in surface sediments from Xiamen Bay. China. J. Environ. Monitor. 2011;13(4):1046–1052. doi: 10.1039/c0em00199f. [DOI] [PubMed] [Google Scholar]
- 7.Wang Z.H. Impacts of environmental factors on arsenate biotransformation and release in Microcystis aeruginosa using the Taguchi experimental design approach. Water Res. 2017;118:167–176. doi: 10.1016/j.watres.2017.04.036. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Z.X. CE-MS analysis of heroin and its basic impurities using a charged polymer-protected gold nanoparticle-coated capillary. Electrophoresis. 2009;30(2):379–387. doi: 10.1002/elps.200800069. [DOI] [PubMed] [Google Scholar]
- 9.Wang Z.H., Luo Z.X., Yan C.Z. Accumulation, transformation, and release of inorganic arsenic by the freshwater cyanobacterium Microcystis aeruginosa. Environ. Sci. Pollut. Res. 2013;20(10):7286–7295. doi: 10.1007/s11356-013-1741-7. [DOI] [PubMed] [Google Scholar]