Abstract
Purpose
To report on a case of recurrence of paraproteinemic keratopathy (PPK) associated with monoclonal gammopathy after bilateral penetrating keratoplasty.
Observations
Penetrating keratoplasty was performed on both eyes of a 45-year-old man due to bilateral progressive corneal stromal clouding. Recurrence of the corneal stromal opacities accompanied by a decrease in visual acuity was observed on slit-lamp examination already two years after penetrating keratoplasty. Confocal laser scanning microscopy (CLSM) of the corneal grafts performed three years after penetrating keratoplasty showed bilateral morphological changes identical to that found in the patient's corneas prior to penetrating keratoplasty. A hematological work-up revealed monoclonal gammopathy of type IgG kappa. The histochemical examination of the explanted corneas confirmed the diagnosis of PPK.
Conclusions and importance
Paraproteinemic keratopathy is an underdiagnosed ophthalmological condition, which may be associated with potentially life-threatening hematologic disorders. A hematological workup should be performed in patients with corneal opacities of uncertain etiology. Penetrating keratoplasty should be performed with caution in patients with monoclonal gammopathy due to the possibility of a very fast recurrence of PPK in the corneal graft. This is the first presentation of the recurrence of flake-like PPK after penetrating keratoplasty assessed with CLSM.
Keywords: Paraproteinemic keratopathy, Monoclonal gammopathy of undetermined significance, Confocal microscopy, Corneal opacity
1. Introduction
Paraproteinemic keratopathy (PPK) or immunotactoid keratopathy is an umbrella term for a heterogenous group of corneal findings associated with monoclonal gammopathy. Monoclonal gammopathy is defined as a presence of abnormal protein (paraprotein), i.e. inoperable immunoglobulins or their parts (light or heavy chains), in the blood. Monoclonal gammopathies include disorders as: monoclonal gammopathy of undetermined significance (MGUS), smoldering multiple myeloma (SMM) or multiple myeloma (MM).
The incidence of MGUS is as high as 3.5% in people over 50 years of age and represents one of the most common premalignant disorders in Western countries.1,2 MGUS may precede not only MM, but also other potentially life-threatening diseases such as B-cell non-Hodgkin lymphoma or chronic myeloid leukaemia.3 According to hematological therapy guidelines, MGUS does not require any systemic therapy, but regular workup is needed in order to recognize its progression to MM or other immunoproliferative disorders.4
Early recognition of PPK by an ophthalmologist may help to diagnose some hematological disorders before a clinical progression occurs. The deposits of paraprotein in the cornea may reveal very distinct patterns and may involve all corneal layers. Crystalline and non-crystalline forms of PPK are known. Furthermore, PPK may imitate systemic and/or metabolic disorders; hereditary corneal dystrophies; inflammation or contact-lens related corneal damage. In addition to a detailed medical history, a general medicalexamination, check-up of family members and genetic analyses should be performed to exclude other reasons for corneal opacity. The new classification of PPK and detailed differential diagnoses have recently been reported by Lisch et al.5
In vivo confocal laser-scanning microscopy (CLSM) provides an insight into corneal morphology at the cellular level. Not only cells, but also extracellular deposits may be characterized.6 The literature regarding CLSM findings in PPK is poor. So far it consists of only a few case reports regarding crystalline keratopathy associated with MM and SMM as well as one case of crystalline keratopathy in a patient suffering from MGUS.7, 8, 9, 10 Micali et al. presented a comparison between confocal a histopathological changes in a patient with MM11
This report presents a diagnostic chain, which was conducted in a patient with bilateral corneal clouding. To the best of our knowledge, this is the first description of a recurrence of a stromal flake-like PPK after bilateral penetrating keratoplasty assessed with CLSM.
2. Case report
2.1. Findings prior to penetrating keratoplasty
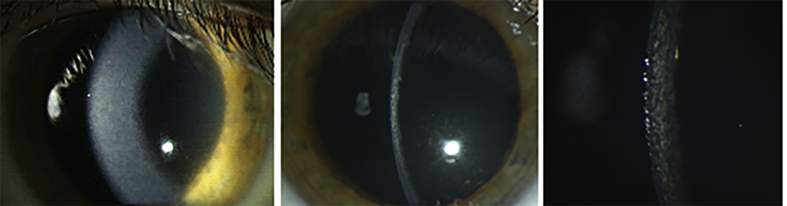
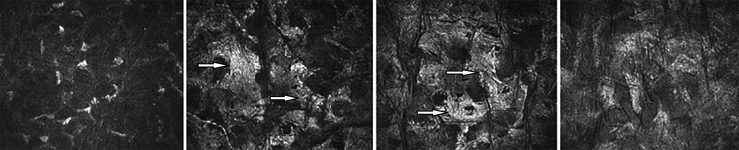
A 43-year-old man was examined for the first time at the Department of Ophthalmology of the University Medical Center in Mainz in 2009 due to bilateral worsening of visual acuity and photophobia. The patient's medical history was uneventful. He had never suffered from systemic or metabolic diseases. He had never used any systemic medication, eye drops or contact lenses. He had no history of any ocular or refractive surgery. The family history regarding ocular diseases was negative. At presentation, the BCVA was 0.63 in both eyes. Slit-lamp examination revealed bilateral diffuse yellowish corneal deposits localized in the corneal stroma (Fig. 1). Central corneal thickness was 543 μm in the right and 542 μm in the left eye. The intraocular pressure (IOP) was 15 mm Hg and the examination of the retina revealed normal findings. Bilateral progression of corneal clouding was accompanied by a decrease in visual acuity to 0.25 in the right and 0.16 in the left eye during the following three years. A genetic evaluation was performed as we suspected a congenital stromal corneal dystrophy (CSCD). However, it did not reveal any mutation in the transforming growth factor β-induced (TGFBI) and decorin (DCN) genes. The CLSM performed using the Heidelberg Retina Tomograph (HRT II) in conjunction with the Rostock Cornea Module (RCM) [Heidelberg Engineering GmbH, Heidelberg, Germany]12 revealed rarefied keratocytes and decreased transparency of the extracellular matrix in the anterior stroma (Fig. 2a); loss of keratocytes and “coral” – like hyperreflective structures in the mid stroma (Fig. 2b and c); more homogenous hyperreflection and obscured details in the posterior stroma (Fig. 2d). Increased reflectivity of the stroma prevented visualization of the corneal endothelium. The findings were similar in both eyes.
Fig. 1.
Corneal clouding in diffuse illumination (a) and in optical section (b–c). Lisch et al. Trans Am Ophthalmol Soc 2016; 114:T7 (1–21).
Fig. 2.
In vivo confocal laser-scanning microscopy of the clouded cornea of the right eye before penetrating keratopasty at the depth of a) 125 μm, b) 201 μm, c) 276 μm, d) 380 μm. Arrows indicate the “coral” – like hyperreflective structures in the middle stroma.
Non-HLA-matched penetrating keratoplasty was performed on the left eye in March 2012. HLA-matched penetrating keratoplasty was performed on the right eye in November 2012. The HLA-matching of the second PKP was due to participation in the FANCY study.13 Both keratoplasties were uneventful and fixed with double running sutures, which were removed one year after surgery. The postoperative course was also uneventful.
2.2. Findings after penetrating keratoplasty
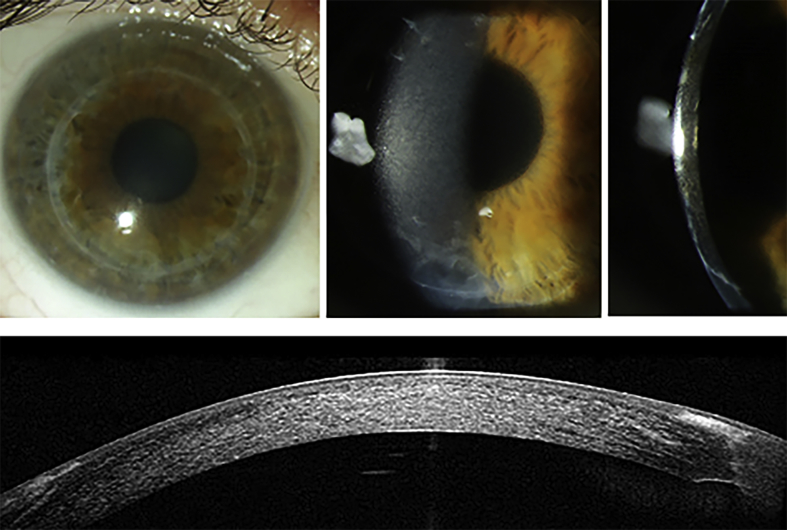
A follow-up examination conducted two years after penetrating keratoplasty revealed recurrence of the fine corneal stromal deposits. At this time point BCVA was 0.63 in the right and 0.8 in the left eye. The progressive corneal clouding caused decline of BCVA to 0.32 in the right and to 0.5 in the left eye three years postoperatively. At that time the slit lamp appearance of the corneal deposits was almost identical to the preoperative findings (Fig. 3). The anterior segment spectral domain - OCT revealed homogenous hyperreflectivity of the cornea consistent with the clinical features. IOP measurement and fundoscopy revealed normal findings. At the time of the manuscript submission BCVA declined further to 0.16 in the right and 0.32 in the left eye.
Fig. 3.
Corneal clouding in diffuse illumination (a–b) and in optical section (c); hyperreflectivity of the corneal stroma in the anterior segment Spectralis optical coherence tomography (d) of the right eye. Lisch et al. Trans Am Ophthalmol Soc 2016; 114:T7 (1–21).
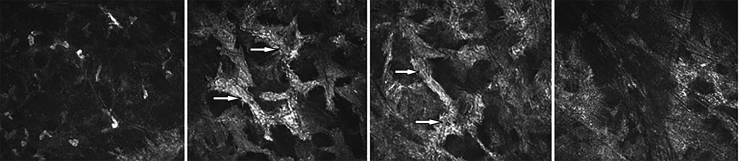
CLSM performed three years postoperatively revealed rarefied keratocytes and decreased transparency of the anterior stroma (Fig. 4 a); loss of keratocytes and “coral” – like hyperreflective structures in the middle stroma (Fig. 4b and c) with increasing homogenous hyperreflection in the posterior stroma (Fig. 4d) almost identical to the preoperative findings. The findings were again similar in both eyes.
Fig. 4.
In vivo confocal laser-scanning microscopy of the clouded cornea of the right eye three years after penetrating keratopasty at the depth of a) 137 μm, b) 227 μm, c) 368 μm, d) 457 μm. Arrows indicate the “coral” – like hyperreflective structures in the middle stroma.
2.3. Hematological workup
The patient had not presented B symptoms. The first hematological laboratory diagnostics performed as soon as stromal opacities recurred revealed IgG, IgM and IgA within the normal range; increased free kappa light chains by 19.7 mg/l (normal values 3.3–19.4 mg/l); increased kappa-lambda quotient 4.7 (normal values 1.3–2.6). No free light chains in the urine. The immunfixation of urine was normal. The plasma immunfixation confirmed the diagnosis of monoclonal gammopathy of type IgG kappa of intermediate-high risk.
Bone marrow biopsy presented no relevant proliferation of plasma cells. The whole-body low-dose computer tomography did not reveal any osteolysis.
The most recent laboratory evaluation revealed that IgG, IgM and IgA were still within the normal range; free kappa light chains increased to 22.9 mg/l (normal values 3.3–19.4 mg/l); the kappa-lambda quotient increased to 7.3 (normal values 1.3–2.6); M protein was at 7.8 g/l. No free light chains were detected in the urine. The immunfixation of urine was still normal.
2.4. Histopathology
Light microscopy of the corneal buttons revealed normal epithelium and degenerative stromal morphology. The Congo red staining did not reveal any amyloid deposits.
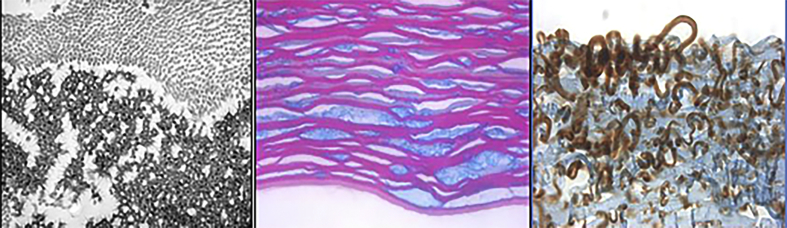
An expanded evaluation revealed: a) irregular corneal stromal deposits exhibiting placoid configuration (Masson's trichrome, × 136) (Fig. 5a); b) stromal extracellular, electron-dense amorphous deposits in between normal collagen fibrils (x 81,000) by transmission electron microscopy (x 36,000) (Fig. 5b); c) brownish staining of stromal deposits with antibodies against IgG (immunoperoxidase reaction, x100) (Fig. 5c). These findings confirmed the diagnosis of PPK.
Fig. 5.
Histological staining with Masson's trichrome (a), transmission electron microscopy (b) and immunohistochemistry (c) of the explanted cornea. Lisch et al. Trans Am Ophthalmol Soc 2016; 114:T7 (1–21).
3. Discussion
Paraproteinemic keratopathy comprises a number of corneal clouding patterns, which are symptoms of premalignant or malignant hematological disorders.5 These conditions are probably underdiagnosed in the ophthalmological daily routine because they are mistaken for corneal dystrophies or degenerations. On the other hand, the possible presence of PPK is not incorporated in any of the hematological diagnostic and therapy guidelines. Advanced corneal clouding leading to severe visual impairment and resulting in corneal transplantation is not defined as end-organ damage and the patient's monoclonal gammopathy is still regarded as one of undetermined significance.
It took us as long as six years to diagnose PPK and MGUS in our patient. The finding of bilateral diffuse stromal opacities suggested a hereditary dystrophy of the cornea, such as congenital stromal corneal dystrophy (CSCD), which was primarily taken into consideration. However, the clinical examination of the patient's mother and three sisters was negative for corneal disorders. In contrast to CSCD, corneal thickness was normal and the onset of the clinical manifestation was late.14 There were no mutations in the TGFBI and DCN genes. Furthermore, there was no indication for inflammatory, toxic or contact lens-induced damage. The CLSM performed three years after the first clinical manifestation was not diagnostically helpful as there were no published descriptions of the non-crystalline PPK assessed with CLSM. Hence, the images of “coral”-like hyperreflective structures in the middle and posterior stroma could not be properly interpreted.
The yellowish diffuse deposits recurred in the same morphological pattern in both corneal grafts as soon as two years after PKP. The quick recurrence led us once more evaluate possible systemic causes of corneal clouding. The hematological workup revealed MGUS of IgG kappa type. Under these circumstances and considering the new classification, the proper ophthalmological diagnosis “flake-like PPK” was made.5 The expanded histological, histochemical and electron microscopic evaluation of the explanted corneal buttons confirmed deposits of immunoglobulins in the corneal stroma.
Our patient has been undergoing a hematological follow-up every 3–6 months in order to exclude progression to MM since MGUS was diagnosed. The intervals of the hematological work-up depend on the individual risk profile and are defined by the International Myeloma Working Group.15 As we mentioned above, according to the hematological guidelines there is no indication for systemic chemotherapy at the moment. Buerk et al. found a regression of number and size of the corneal deposits assessed with CLSM in a patient with MM-associated crystalline keratopathy six months after onset of chemotherapy.7 However, there are no clinical trials or case reports regarding the outcome of MGUS-associated PPK under systemic therapy. Concerning PPK as end-organ damage, a systemic therapy with Bortezomib and dexamethasone has been taken into consideration, but has not yet been applied to our patient.
CLSM of corneal grafts showed the pattern of characteristic “coral”-like hyperreflective structures in the middle and posterior stroma identical to that seen at the first preoperative CLSM. There were no needle-shaped hyperreflective crystals or dendritic-shaped keratocytes, which were seen in CLSM in cases of MM- and SMM-associated crystalline PPK.7, 8, 9, 10, 11 To the best of our knowledge, this is the first presentation of the recurrence of flake-like stromal PPK associated with MGUS after penetrating keratoplasty assessed by CLSM.
4. Conclusions
PPK should be taken into consideration in every corneal disorder of undetermined entity. The hematological workup induced by an ophthalmologist may help to diagnose premalignant or even malignant disorders in a more timely fashion. In case of PPK, corneal transplantation should be indicated with caution as a swift recurrence in the corneal grafts is to be expected and frequent re-grafting results in increased risk of immunologic graft rejection.16 Deep anterior lamellar keratoplasty could be an alternative to PKP in case of assessable and intact endothelium. We believe that the non-invasive and quick examination using CLSM and, most of all, the correct interpretation of the images may lead to the proper ophthalmological and, consecutively, hematological diagnosis.
Source of funding
None.
Conflicts of interest
The following authors have no financial disclosures: JWP, AG, AD, USS, NP, WL.
Patient consent
The patient consented in writing to publication of medical record details and photographs.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Acknowledgements
Funding: No funding or grant support.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ajoc.2018.06.014.
Appendix ASupplementary data
The following is the supplementary data related to this article:
References
- 1.Kyle R.A., Durie B.G.M., Rajkumar S.V. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma. IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia. 2010;24:1121–1127. doi: 10.1038/leu.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kyle R.A. “Benign” monoclonal gammopathy: a misnomer? J Am Med Assoc. 1984;251(4):1849–1854. [PubMed] [Google Scholar]
- 3.Wasielica-Poslednik J., Gericke A., Munder M., Pfeiffer N., Lisch W. Hematological diagnosis in the corneal consultation. Ophthalmologe. 2017 Nov 17 doi: 10.1007/s00347-017-0615-7. [Epub ahead of print] German. [DOI] [PubMed] [Google Scholar]
- 4.International Myeloma Working Group Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol. 2003;121(5):749–757. [PubMed] [Google Scholar]
- 5.Lisch W., Wasielica-Poslednik J., Kivelä T. The hematologic definition of monoclonal gammopathy of undetermined significance in relation to paraproteinemic keratopathy (an American ophthalmological society thesis) Trans Am Ophthalmol Soc. 2016;114:T7. [PMC free article] [PubMed] [Google Scholar]
- 6.Wasielica-Poslednik J., Pfeiffer N., Reinke J., Pitz S. Confocal laser-scanning microscopy allows differentiation between Fabry disease and amiodarone-induced keratopathy. Graefes Arch Clin Exp Ophthalmol. 2011 Nov;249(11):1689–1696. doi: 10.1007/s00417-011-1726-5. [DOI] [PubMed] [Google Scholar]
- 7.Buerk B.M., Tu E. Confocal microscopy in multiple myeloma crystalline keratopathy. Cornea. 2002 Aug;21(6):619–620. doi: 10.1097/00003226-200208000-00020. [DOI] [PubMed] [Google Scholar]
- 8.Houben N., Foets B. Confocal microscopy in multiple myeloma associated crystalline keratopathy: case report. Bull Soc Belge Ophtalmol. 2006;300:13–17. [PubMed] [Google Scholar]
- 9.Mazzotta C., Caragiuli S., Caporossi A. Confocal microscopy in a case of crystalline keratopathy in a patient with smouldering multiple myeloma. Int Ophthalmol. 2014 Jun;34(3):651–654. doi: 10.1007/s10792-013-9838-z. Epub 2013 Aug 9. [DOI] [PubMed] [Google Scholar]
- 10.Kocabeyoglu S., Mocan M.C., Haznedaroglu I.C., Uner A., Uzunosmanoglu E., Irkec M. In vivo confocal microscopic characteristics of crystalline keratopathy in patients with monoclonal gammopathy: report of two cases. Indian J Ophthalmol. 2014 Sep;62(9):938–940. doi: 10.4103/0301-4738.143933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Micali A., Roszkowska A.M., Postorino E.I. Comparative confocal and histopathological study of corneal changes in multiple myeloma. Cornea. 2017 Jan;36(1):123–126. doi: 10.1097/ICO.0000000000001038. [DOI] [PubMed] [Google Scholar]
- 12.Stave J., Zinser G., Grümmer G., Guthoff R. Der modifizierte Heidelberg-Retina-Tomograph HRT. Erste Ergebnisse einer Invivo-Darstellung von kornealen Strukturen. Ophthalmol Times. 2002;99:276–280. doi: 10.1007/s003470100535. [DOI] [PubMed] [Google Scholar]
- 13.Böhringer D., Ihorst G., Grotejohann B., Maurer J., Spierings E., Reinhard T., FANCY study group Functional antigen matching in corneal transplantation: matching for the HLA-A, -B and -DRB1 antigens (FANCY) - study protocol. BMC Ophthalmol. 2014 Dec 13;14:156. doi: 10.1186/1471-2415-14-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiss J.S., Møller H.U., Aldave A.J. IC3D classification of corneal dystrophies–edition 2. Cornea. 2015 Feb;34(2):117–159. doi: 10.1097/ICO.0000000000000307. [DOI] [PubMed] [Google Scholar]
- 15.http://imwg.myeloma.org/monoclonal-gammopathy-of-undetermined-significance-mgus-and-smoldering-asymptomatic-multiple-myeloma-imwg-consensus-perspectives-risk-factors-for-progression-and-guidelines-for-monitoring-and-man/(June 2018).
- 16.Qazi Yureeda, Hamrah Pedram. Corneal allograft rejection: immunopathogenesis to therapeutics. J Clin Cell Immunol. 2013 Nov 20;2013(Suppl 9):006. doi: 10.4172/2155-9899.S9-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.