Abstract
Objective:
It has been nearly 15 years since Kazdin and Nock published methodological and research recommendations for understanding mechanisms of change in child and adolescent therapy. Their arguments and enthusiasm for research on mechanisms of behavior change (MOBCs) resonated across disciplines and disorders, as it shined a light on the crucial importance of understanding how and for whom treatments instigate behavior change and how therapeutic mechanisms might be extended to “situations and settings of everyday life.” Initial efforts focused on how psychotherapy works and linear models, yet the use of theory to guide the study of mechanisms, and laboratory experiments to manipulate them, is broadly applicable.
Method:
This article considers dynamic physiological processes that support behavior change. Specifically, it examines the utility of psychophysiological methods to measure and promote behavior change. Moreover, it embeds the baroreflex mechanism, a well-defined heart–brain feedback loop, within the theories and strategies of MOBC research.
Results and Conclusion
Individuals’ subjective and expressive experience of change does not always align with their physiological reactivity. Thus, behavior change may be best understood when concurrently assessed across multiple biobehavioral levels. Further, behavior is initiated in the moment, often before conscious deliberation, suggesting that multilevel behavior change research may benefit from real-time methodological designs. Last, substance use trajectories vary widely, suggesting that different MOBCs are more or less active in individuals depending on their personal constituency and the functional need that their substance use serves; thus, methods that are amenable to personalized modeling approaches are important.
A central premise of most empirically supported behavioral treatments for alcohol use disorders (AUD) is that strengthening cognitive control will increase an individual’s ability to override the impulsive use of alcohol (McCrady & Epstein, 1999; Morgenstern et al., 2013). These behavioral treatments often are initially successful in helping persons to stop or greatly reduce drinking (Hettema et al., 2005; Martin & Rehm, 2012; Tripodi et al., 2010). After a short time, however, relapse becomes increasingly likely to occur (Witkiewitz & Marlatt, 2004; Zywiak et al., 2006). A key hurdle to understanding pathways to relapse stems from limited understanding of the active mechanisms that support drinking behavior change.
Years of research have identified treatments that work (albeit sometimes and for some individuals under some circumstances) (Hettema et al., 2005; Martin & Rehm, 2012; Tripodi et al., 2010), but not how or for whom they work. Mechanisms of change research seeks to increase and sustain the personal and public health benefits of behavioral interventions for AUD by moving away from static, one-sizefits-all perspectives of recovery. Instead, it considers recovery as a dynamic and ongoing process that is highly individual and dependent on many interrelated factors.
This article examines the value of psychophysiological methods in capturing multilevel, dynamic, and personalized change processes, including those associated with substance use initiation, maintenance, recovery, and relapse. Physiological research lends itself well to person-centered approaches tested across both short time scales (e.g., resting to challenge) and longer time scales (e.g., developmental trajectories, pre-, within-, post-treatment). Physiological methods are highly compatible with methods from other domains, including but not limited to psychological self-report assessments and cognitive batteries, neuroimaging modalities, facial affect characterization and social skills, genetic comparisons, and computational modeling. We present a sound scientific premise that physiological mechanisms are notable players in behavior change capacity, in addition to (not instead of) therapeutic, psychosocial, and cognitive mechanisms. Further, physiological mechanisms are malleable through easily adopted and widely available behavioral interventions that can increase brain–body communication “in-the-moment.” As such, psychophysiology offers novel and compelling intervention targets that are dynamic, linked to the operation of cognitive and emotional regulation systems, and suitable for personal implementation.
Psychophysiological measures in the multilevel determinants of behavior change
Body–brain communication occurs through numerous parallel and complementary feedback loops that have been well described in nearly all organ systems. Between the heart and brain is an anatomical feedback loop orchestrated through cranial and sympathetic nerves and baroreceptors located on vessel walls (Benarroch, 1997a; Goldstein, 2001). This feedback loop was named the baroreflex because of its reflexive pairing of malleable cardiac and vascular processes in the service of blood pressure control. The baroreflex mechanistically serves behavior by integrating visceral feedback from the heart and vasculature with cognitive-emotional information (body → brain; afferent stream) and relaying neural commands to the periphery to generate a coordinated, whole body response to stimuli (brain → body; efferent stream) (Benarroch, 1993, 1997a; Critchley & Harrison, 2013).
The baroreflex participates in the arousal and attentional processes needed to prepare for action (e.g., increasing heart rate) and initiate goal-directed behaviors (e.g., flee from danger, open a bottle of beer) (Kandel et al., 2000). As such, intact baroreflex function supports adaptive responding to extreme stressors as well as to everyday challenges. Importantly, the activity of the baroreflex can be gauged as variability in biosignals, such as heart rate (i.e., heart rate variability [HRV]), which can be noninvasively measured and manipulated using many inexpensive devices, most notably the ubiquitous smartphone.
The baroreflex has been extensively documented in terms of neurophysiology, functional anatomy, and molecular mechanisms in rodent, primate, and human models (Benarroch, 1997a; Goldstein, 2001). The neural circuit that controls the baroreflex, the central-autonomic network (Benarroch, 1997b), includes brain stem nuclei, multiple midbrain integration structures, and subcortical (e.g., amygdala) and cortical (e.g., anterior cingulate and medial prefrontal cortices) areas known to participate in cognitive-emotional processing. The central-autonomic network is responsible for relaying three different types of information to and from the body: steady state autonomic function, reflexive autonomic reaction, and adaptive autonomic reaction (Benarroch, 1997b). It follows, therefore, that the baroreflex and the neural systems that underlie it are instrumental in both acute and chronic stress reactions.
Prominent neuroscientific theories of addiction have not been elaborated yet to include signaling or mechanisms through which effector organs like the heart come to play in cognitive and affective regulation, although there is a substantial overlap between the well-characterized neurocardiac circuitry and the circuitry involved in prominent biobehavioral theories of addiction. The “three stages of addiction” model (Koob & Volkow, 2010; Volkow et al., 2016) directly implicates reciprocal innervations of the extended amygdala (central and bed nuclei) with subcortical relay structures (hypothalamus, brain stem) and effector organs in the negative reinforcement mechanisms involved in the transition to addiction. This theory also implicates the medial prefrontal, anterior cingulate, and insular cortices (and basolateral amygdala) in persistent craving mechanisms. The central-autonomic network comprises all of these structures.
Central-autonomic network structures including prefrontal, insular, and anterior cingulate cortices, amygdala, and hypothalamus also figure prominently in dual process models of information processing (Bechara, 2005; Noël et al., 2013). These dual process models propose a conflict between effortful processes that support conscious goals and intentions (i.e., “reflective,” “executive,” “cortical” processes) and automatic processes that derive from attention capture by salient cues in the environment and impulsive or habitual modes of affective regulation (i.e., “impulsive,” “automatic,” “limbic” processes). These circuitry parallels, along with other lines of evidence linking brain and cardiovascular physiology to immune system response, stress reactivity, and the behavioral consequences of allostatic load (McEwen, 2006; Tracey, 2009), point to the strong possibility that efficiency of the baroreflex mechanism is a novel transdiagnostic resilience/vulnerability factor that supports capacity for behavior change (Leventhal & Zvolensky, 2015). In other words, baroreflex functionality may serve as an overarching physiological force that affects mental and physical health, including both alcohol and drug use behaviors, because of its participation in a holistic stress response system.
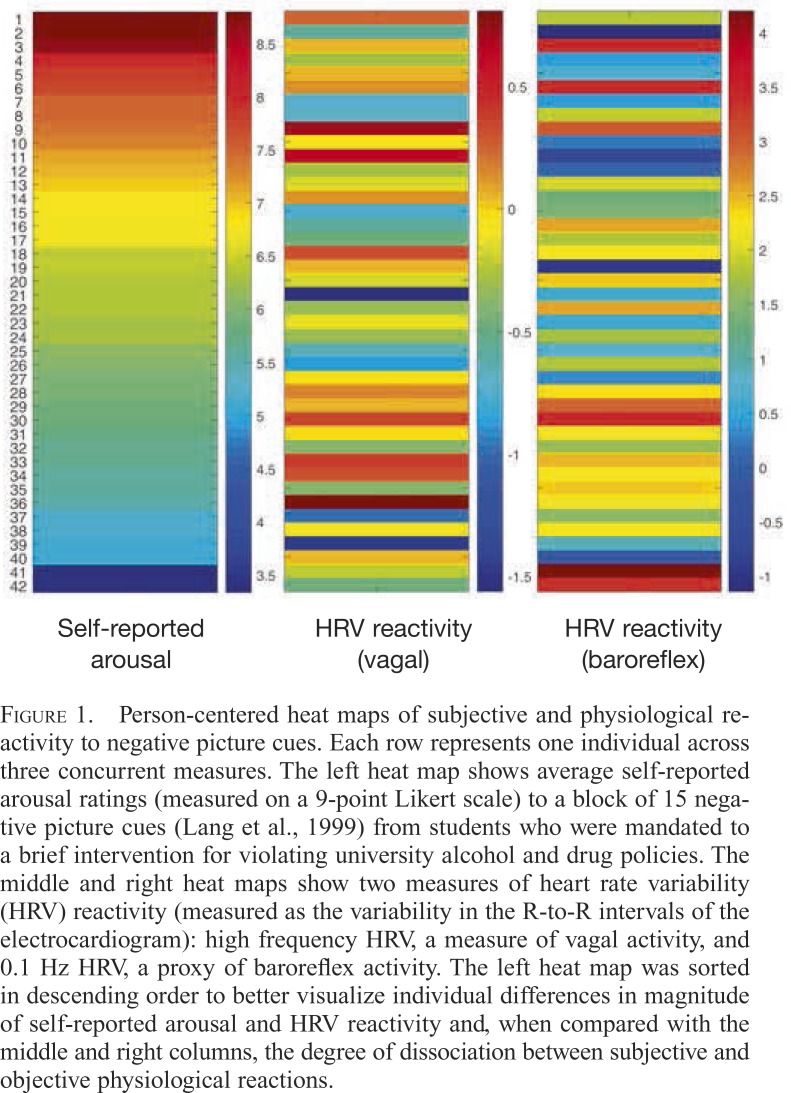
The ability of psychophysiological assessments to add nuance and depth to behavioral mechanism research is illustrated with two examples of individual-level, multisystem findings. First, Figure 1 shows self-reported arousal ratings and physiological reactivity to negative emotional visual cues from individuals who were mandated to a brief intervention after serious (e.g., emergency medical system involvement) or minor (e.g., in a dormitory room with alcohol) violations of university policies about on-campus alcohol use (Buckman et al., 2010). Each row of data represents an individual person across three variables.
Figure 1.
Person-centered heat maps of subjective and physiological reactivity to negative picture cues. Each row represents one individual across three concurrent measures. The left heat map shows average self-reported arousal ratings (measured on a 9-point Likert scale) to a block of 15 negative picture cues (Lang et al., 1999) from students who were mandated to a brief intervention for violating university alcohol and drug policies. The middle and right heat maps show two measures of heart rate variability (HRV) reactivity (measured as the variability in the R-to-R intervals of the electrocardiogram): high frequency HRV, a measure of vagal activity, and 0.1 Hz HRV, a proxy of baroreflex activity. The left heat map was sorted in descending order to better visualize individual differences in magnitude of self-reported arousal and HRV reactivity and, when compared with the middle and right columns, the degree of dissociation between subjective and objective physiological reactions.
In the left column, each individual’s arousal rating (Likert scale of 1 = not arousing to 9 = very arousing) to a block of 15 negative picture cues is shown; data are sorted in this heat map from high to low self-report arousal. In the middle and right heat maps are objective physiological reactivity data that were collected in parallel to these subjective data. These heat maps show reactivity to the block of negative pictures as measured by HRV. The middle heat map shows high frequency HRV, a measure of vagal (parasympathetic) activity, and the right map shows 0.1 Hz HRV, a proxy measure of baroreflex activity.
It is immediately evident that self-reported arousal often does not map onto cardiovascular arousal. In some cases (e.g., Rows 6, 39), there is considerable similarity across measurement domains. In other cases, there are considerable discrepancies. We previously reported that individuals who were mandated to the intervention after serious compared with minor violations of university policies about on-campus alcohol use exhibited a greater discrepancy between their self-reported and physiological arousal measures (Bates & Buckman, 2011); these individuals were not heavier drinkers but, rather, appeared to have substantially misjudged their drinking capacity on the violation occasion.
A second example comes from the comparison of emotional reactivity (as measured by general facial expressiveness) and vagal firing activity. The baroreflex shares neural circuitry with striated muscle–brain circuits via the vagal nerve. Thus, expressive facial action, which is crucial to social communication and engagement and thus an individual’s capacity to adapt and change (Porges, 2003, 2009), is intricately linked to autonomic nervous system activity from infancy (Field & Diego, 2008) through old age (Levenson et al., 1991).
Figure 2 shows facial expressiveness (as rated by facial movement) and cardiovascular reactivity (as estimated by computationally modeled vagal nerve firing rate) (Fonoberova et al., 2014) to negative emotional cues in young social-drinking adults. Each row shows data from one individual, and these data have been sorted from high to low facial expressiveness in the left heat map; the right heat map shows simultaneous physiological responding. This example also demonstrates that, in some individuals, facial expressiveness corresponded well with physiological response (e.g., Rows 18, 20). For others, however, facial expression and physiological reactivity were disparate (e.g., Rows 1, 21). This suggests that facial expression, like subjective self-report, can be dissociated from internal physiological processes.
Figure 2.
Person-centered heat maps of expressive and physiological reactivity to negative picture cues. Each row denotes data from one individual across measures. Social drinking college students participated in a cue reactivity study during which their facial expressiveness (left heat map) and physiological reactivity (right heat map) were simultaneously measured. Facial expressiveness was rated by trained observers of videotaped face recordings taken during negative emotional cues exposures. Scores ranged from 1 (low expressiveness) to 5 (high expressiveness) based on facial movement (not specific emotional expressions). Physiological reactivity was derived from a computational model parameter (Fonoberova et al., 2014) that captured vagal nerve firing rate. The left heat map was sorted in descending order to allow visualization of individual differences and comparison of the heat maps that showed substantial discrepancy between physiological and facial responsivity to negative emotional cues in many individuals.
Although the underpinnings of these observations are not clear, both examples demonstrate that psychophysiological assessments can provide a more holistic perspective of change by revealing synchrony or asynchrony across biobehavioral systems. There are multiple theoretical explanations for these dissociations, such as volitional differences (i.e., ability/desire to mask or exaggerate an emotional reaction), social desirability, or biologically mediated processes wherein physiological experiences simply are not concordant with expressive or subjective response (i.e., somatic dissociation). Characterization of neurocardiac processes that occur simultaneously with other dynamic system changes can allow new insights into how different people react to challenge in real time and the extent to which challenge ripples across receptive and expressive systems to be cognitively and emotionally processed. Conceivably, this type of multisystem assessment may tap into the individual’s interoceptive capacity and contribute to personalized treatment decisions (i.e., help determine who may benefit from biofeedback-type interventions).
Psychophysiological methods to capture the dynamic nature of behavior change
Everyday experiences of stress, negative affect, and salient alcohol and drug cues serve as potent triggers for substance use (Drummond, 2000; Marlatt, 1996) and undermine efforts for sustained recovery. The nature, salience, and power of these experiences are determined in the moment based on learning history and the interrelation of internal states (e.g., effortful and automatic cognitive processes, bodily sensations of arousal, moods) and input from the surrounding environment (e.g., visual, auditory, and olfactory cues, behavior of other persons). Thus, in addition to there being multifactorial determinants of behavior, these multiple information processing systems interact in real time during decisions to use drugs and alcohol, which are often momentary decisions, neither premeditated nor desired. Understanding how behavior change is accomplished on short, “moment-to-moment” time scales is thought to be fundamental to characterizing and intervening in alcohol and drug use behaviors (Shiffman et al., 2008; Witkiewitz & Marlatt, 2004).
Psychophysiological methods are capable of measuring moment-to-moment change and, as discussed below, physiological mechanisms are clearly acting on behavior on this momentary time scale. One theory of how the baroreflex mechanism participates in alcohol use behaviors is through its in-the-moment influence on attention capture by provocative cues, cue salience, and heightened visceral reactions to cues (Garland et al., 2012a, 2012b; Mun et al., 2008; Thayer & Lane, 2000). It appears likely that relatively diminished baroreflex function sets in motion a cascade of neurocardiac events that alter affect, emotional arousal, and stress response across the life span (El-Sheikh, 2001; El-Sheikh & Buckhalt, 2005; Hugdahl, 1996; Porges et al., 1994; Thayer & Lane, 2000), potentially increasing the likelihood that susceptible individuals will seek or use alcohol, even following extended periods of abstinence (Robinson & Berridge, 2008; Tiffany, 1995).
Growing evidence for the importance of neurocardiac mechanisms in behavior change capacity across development suggests the value of examining interventions that allow these otherwise automatic physiological mechanisms to be consciously activated in the moment, both as preventive interventions in high-risk children (Clark et al., 2016) and to protect recovering persons with substance use disorders from automatic capture by appetitive triggers. The most well-known intervention, HRV biofeedback (Lehrer & Vaschillo, 2004; Lehrer et al., 2000), is based on a comprehensive knowledge of cardiovascular mechanisms.
HRV biofeedback rhythmically stimulates the cardiovascular system with slow paced breathing (Song & Lehrer, 2003; Vaschillo et al., 2002). When breathing is slowed and paced to ∼6 respiratory cycles/minute, or 0.1 Hz, which is substantially slower and more rhythmic than normal breathing of ∼12–18 breaths per minute, the cardiac oscillations driven by respiratory sinus arrhythmia (i.e., the phenomena of heart acceleration with inhalation and deceleration with exhalation) are synchronized with the cardiac oscillations driven by the baroreflex (i.e., which links heart acceleration/deceleration to corresponding decreases/increases in blood pressure). Thus, two cardiovascular control mechanisms become synchronized and, crucially, this synchrony occurs at a natural resonance frequency (0.1 Hz). This resonance property results from the cardiovascular system being a closed-loop system with a built-in delay (i.e., it takes approximately 5 seconds for a change in blood pressure to initiate a change in heart rate and 5 additional seconds to feedback to blood pressure, a loop of 10 seconds or 0.1 Hz) (Vaschillo et al., 2002, 2006). The behavioral result is that heart rate oscillations are brought to their maximum amplitudes.
Studies of HRV biofeedback have focused primarily on positive therapeutic effects that may accrue over a series of weeks and sessions in various clinical populations (Eddie et al., 2014; Hassett et al., 2007; Karavidas et al., 2007; Reiner, 2008; Siepmann et al., 2008; Sutarto et al., 2012; Windhorst, 2007; Zucker et al., 2009). A pilot study of a brief (3-week) HRV biofeedback intervention in young male inpatients with substance use disorders found a nonsignificant, but nontrivial, effect size (Cohen’s d = 0.35) greater reduction in drug craving pre- to post-intervention compared with inpatient treatment as usual (Eddie et al., 2014). Similarly, Zucker et al. (2009) reported a nonsignificant, but medium, effect size reduction (Cohen’s d estimated at 0.58) in drug craving in a posttraumatic stress disorder sample with cooccurring substance use disorders. Penzlin et al. (2015) observed significantly greater decreases in craving and anxiety in an inpatient AUD treatment group receiving six sessions of HRV biofeedback compared with those receiving standard treatment. Thus, the baroreflex can be behaviorally manipulated over time (possibly in a dose-dependent manner) as a nonpharmacological, mechanism-based intervention to help regulate craving and negative affective states.
These changes, although clinically valuable, were labor and time intensive to achieve. Further, they may be limited in mitigating the intense momentary bouts of emotional dysregulation or craving that are crucial triggers for alcohol use and relapse outside the treatment context (Shiffman, 2009; Shiffman et al., 2008). Recent laboratory studies have focused on the “active ingredient” of HRV biofeedback—slow rhythmic breathing at 0.1 Hz, termed resonance breathing (Fonoberova et al., 2014; Vaschillo et al., 2006)—and emphasize the moment-to-moment impact of this technique on automatic-visceral reactivity. These studies demonstrated that a single brief exposure to resonance breathing in anticipation of psychosocial stress, or during induced stress, helped to control physiological arousal, reduced state anxiety, and improved cognitive performance (Prinsloo et al., 2011; Wells et al., 2012).
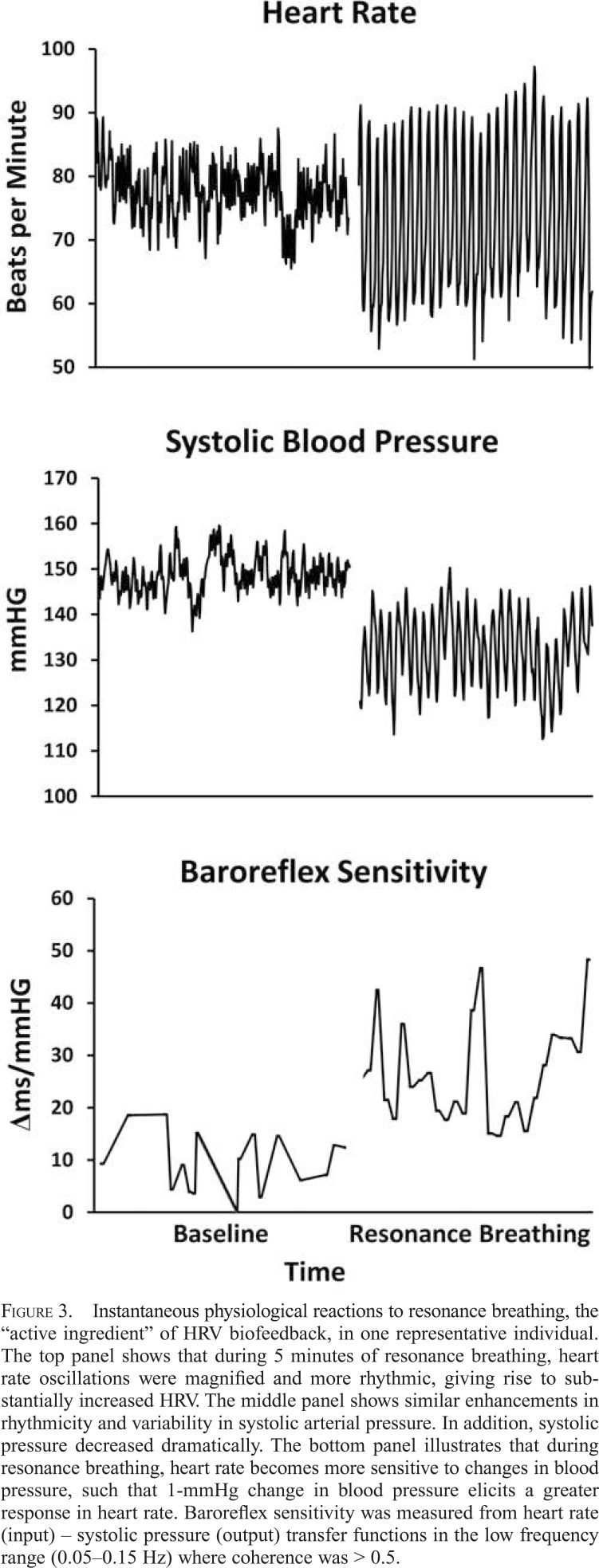
As shown in Figure 3, instantaneous changes in the cardiovascular system are induced during 5 minutes of resonance breathing performed in the laboratory, including substantially larger oscillations in heart rate (but negligible changes in average heart rate; top panel), reduced systolic blood pressure (middle panel), and increased baroreflex sensitivity, all of which are hypothesized to support chronic clinical benefits (Lehrer et al., 2003).
Figure 3.
Instantaneous physiological reactions to resonance breathing, the “active ingredient” of HRV biofeedback, in one representative individual. The top panel shows that during 5 minutes of resonance breathing, heart rate oscillations were magnified and more rhythmic, giving rise to substantially increased HRV. The middle panel shows similar enhancements in rhythmicity and variability in systolic arterial pressure. In addition, systolic pressure decreased dramatically. The bottom panel illustrates that during resonance breathing, heart rate becomes more sensitive to changes in blood pressure, such that 1-mmHg change in blood pressure elicits a greater response in heart rate. Baroreflex sensitivity was measured from heart rate (input) – systolic pressure (output) transfer functions in the low frequency range (0.05–0.15 Hz) where coherence was > 0.5.
No published study to our knowledge has examined the effects of resonance breathing in real time and ecologically valid settings, although such trials are in progress (ClinicalTrials.gov ID: NCT02579317). Data from such studies will allow baroreflex modulation to be linked to relapse triggers and drinking behaviors as they unfold in real time in everyday life. Such studies are possible because of the advent of smartphone application technology to teach people resonance breathing by acquiring cardiac rhythms through the phone’s camera LED light and/or providing a breathing pacer on the screen. Thus, individuals can use resonance breathing interventions strategically following treatment in their natural environment, to help interrupt the cascade of emotional arousal that often characterizes high-risk situations. A compelling research question for the future is whether routine, app-assisted engagement in resonance frequency breathing over time will eventually instigate an automatic resonance breathing response through consistent mapping to drinking or drug use triggers, potentially dampening visceral arousal long enough for cognitive behavioral coping skills to be engaged when needed.
Personalized modeling of behavior change with psychophysiological data
Hypothetically, a prime benefit of isolating active MOBCs should be improved prediction of “for whom” an intervention will be most effective. A persisting challenge in improving the outcomes of addiction treatment has been the inability to match specific individuals to specific interventions to achieve the highest likelihood of successful outcomes. In this article, we argue for the value of measuring behavior change as it propagates across biological and behavioral systems and dynamically unfolds in real time. To this, we add the utility of psychophysiological measurements in computational modeling approaches that seek to capture multilevel, dynamic change processes at the level of the person.
On average, baroreflex functioning has a robust relationship to physical and mental health (Abboud, 2010; Tracey, 2009) and alcohol and drug use (Bär et al., 2006; Wray et al., 2008). On average, there appear to be immediate as well as chronic clinical benefits of resonance breathing and HRV biofeedback in multiple populations (Eisenberg et al., 2004; Hassett et al., 2007; Karavidas et al., 2007; Lehrer & Vaschillo, 2004; Reiner, 2008; Sherlin & Wyckoff, 2008; Siepmann et al., 2008; Sutarto et al., 2012; Zucker et al., 2009). Yet, momentary baroreflex reactivity appears to be affected by the individual’s cumulative developmental history of autonomic regulation patterns that are compromised by early childhood experiences of psychosocial stress (Clark et al., 2016).
Reduced health, emotion regulation, resiliency, and social competence in childhood (Clark et al., 2016; Doussard-Roosevelt et al., 1997; El-Sheikh & Erath, 2011; El-Sheikh et al., 2001, 2009; Katz & Gottman, 1995, 1997) have been related to distinctive patterns of cardiac response (often lower resting HRV, vagal withdrawal, increased sympathetic response), suggesting that well-known psychosocial risk factors for AUD parallel those associated with inefficient cardiac physiology mediated by the baroreflex. Genetic factors also are known to influence baroreflex activity (Parmer et al., 1992; Xing-Sheng et al., 2010) and may account for about 40% of individual variability in its sensitivity at rest (Tank et al., 2001). It follows that individuals will likely differ quantitatively in the extent to which baroreflex activation results in positive physiological and psychological changes.
A computational modeling approach is personalized and dynamic. It mathematically models unobservable physiological processes in a behavioral testing situation (Hubenko et al., 2011; Mezić, 2004, 2005; Mezić & Banaszuk, 2004; Mezić & Runolfsson, 2008; Rowley et al., 2009; Vainchtein & Mezić, 2004) and provides a noninvasive paradigm to capture how a system as a whole adapts to change, which system elements are most sensitive to a given type of change, and, most important for patient-treatment matching, whether system dynamics vary between persons. Such strategies for understanding mechanisms that support behavior change provide an approach for person-mechanism matching and conceivably could set the stage for future person-treatment matching strategy development that is transformative.
We developed a computational model of the baroreflex mechanism (Fonoberova et al., 2014) based on validated animal models (Magosso & Ursino, 2002; Magosso et al., 2001; Ursino, 1998; Ursino & Magosso, 2000, 2003) that represents the baroreflex mechanism as the output of a complex human brain–body system that includes dynamics of heart and vascular musculature, ascending and descending neural tracts, and an array of chemo- and mechanoreceptors. This approach captured changes in observable and unobservable physiological processes that are active when the baroreflex loop is in its basal (regular breathing, about 12–18 breaths per minute) and activated (resonance breathing, about 6 breaths per minute) state (Fonoberova et al., 2014).
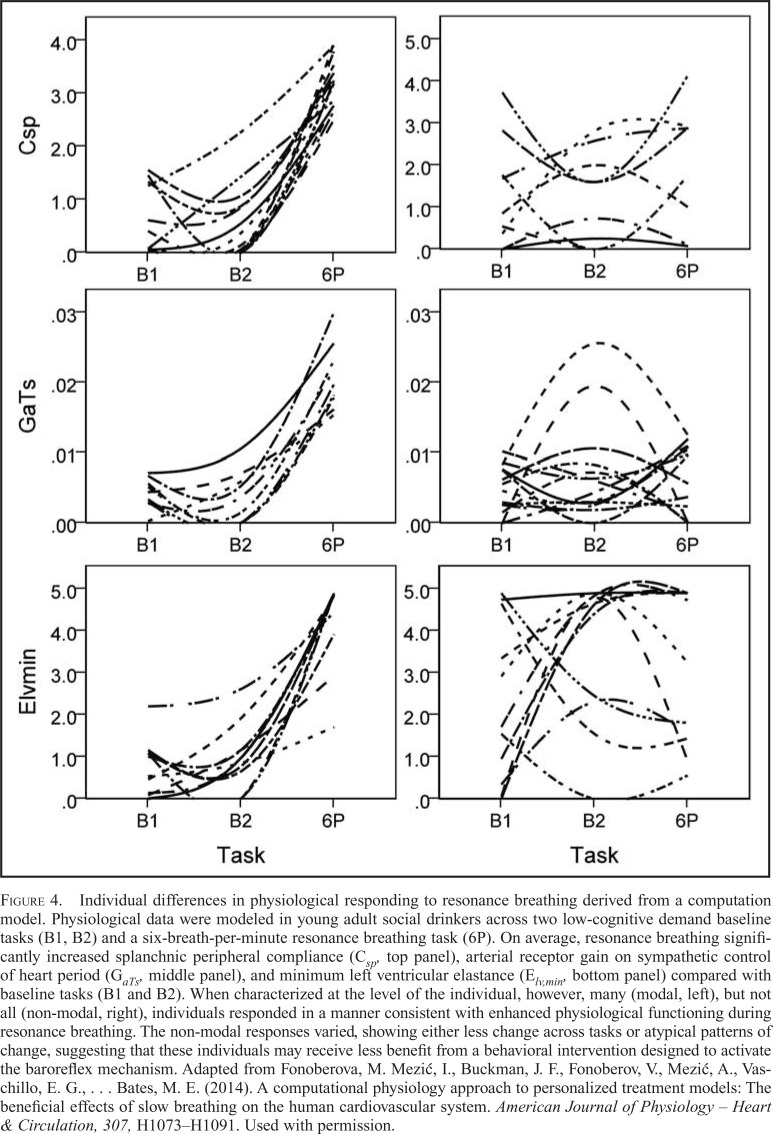
In addition, the approach revealed that health-promoting system plasticity existed only in a subset of the sample. Although many participants experienced positive functional increases in physiological processes in response to 5 minutes of resonance breathing (Figure 4, left panels), others showed a less dynamic pattern of cardiovascular responding or an atypical, unexpected pattern of responding (Figure 4, right panels). This implies that a dynamic computational approach may be used to determine whether a brief baroreflex intervention reliably induces positive physiological changes and, possibly, whether the presence of these changes can be used to predict the likelihood that an individual will benefit from the inclusion of a baroreflex intervention (i.e., resonance breathing or HRV biofeedback) in the broader addiction treatment context.
Figure 4.
Individual differences in physiological responding to resonance breathing derived from a computation model. Physiological data were modeled in young adult social drinkers across two low-cognitive demand baseline tasks (B1, B2) and a six-breath-per-minute resonance breathing task (6P). On average, resonance breathing significantly increased splanchnic peripheral compliance (Csp, top panel), arterial receptor gain on sympathetic control of heart period (GaTs, middle panel), and minimum left ventricular elastance (Elv,min, bottom panel) compared with baseline tasks (B1 and B2). When characterized at the level of the individual, however, many (modal, left), but not all (non-modal, right), individuals responded in a manner consistent with enhanced physiological functioning during resonance breathing. The non-modal responses varied, showing either less change across tasks or atypical patterns of change, suggesting that these individuals may receive less benefit from a behavioral intervention designed to activate the baroreflex mechanism. Adapted from Fonoberova, M. Mezić, I., Buckman, J. F., Fonoberov, V., Mezić, A., Vaschillo, E. G., … Bates, M. E. (2014). A computational physiology approach to personalized treatment models: The beneficial effects of slow breathing on the human cardiovascular system. American Journal of Physiology – Heart & Circulation, 307, H1073–H1091. Used with permission.
A great deal of work remains to be done in developing dynamic personalized models of behavior change, but the results to date recommend this approach for matching clients to interventions and potentially identifying addiction subtypes based on dynamic and multifactorial information. Computational physiology modeling makes it possible to translate “down” to characterize biological change within largely unobservable body and brain systems, as well as translate “up” to characterize how an automatic-visceral process can support or impede drinking change behavior. Future computational physiology models can be elaborated to include genetic variation, neural computations, and drinking/behavioral information to ultimately build a holistic perspective on how change occurs within interacting systems (Mezić, 2005; Mezić & Banaszuk, 2004).
Conclusions
This article aimed to shed light on the advantages of pursuing multilevel and multimethod characterizations of MOBCs that include physiological as well as affective, cognitive, and other psychological and behavioral regulation processes in order to promote progress in understanding behavioral flexibility toward alcohol and drugs. Converging evidence suggests that these processes share neural substrates and that their operation is dynamically interwoven. Often a narrow question has been asked, “What is the correlation between self-report and physiological response to a challenge?” Yet, what often appears most notable in these multilevel data is the heterogeneity between individuals, for example, in the relation of self-reported levels of arousal to emotional/substance use-related cues compared with physiological and behavioral (e.g., facial expressiveness) levels of arousal. This observation raises new clinically relevant questions about whether the degree of integration and/or dissociation between different interrelated systems’ response to triggers for alcohol and drug use is a source of resilience or risk—or perhaps a source of information for personalized intervention targets. We suggest that these may be heuristic transdiagnostic research questions.
Better understanding of neurocardiac and other physiological mechanisms in relation to intentional cognitive mechanisms instilled by most behavioral AUD treatments will strengthen the foundation from which to bridge basic and clinical MOBC science. Willenbring (2006) suggested more than a decade ago that the addiction field’s limited progress in client-treatment matching, based on our understanding of MOBCs, strongly argues for translational research to provide innovation. Using both laboratory and clinical research to understand the intersections of intentional and automatic control systems and their mechanisms of action appears necessary for continued progress in AUD prevention and intervention development, given the importance of both top-down and bottom-up paths to self-regulation (Bridgett et al., 2015).
Progress thus far has not been rapid, and translational research conducted to transform scientific discoveries from laboratory and clinical studies into clinical applications has presented many challenges across the public health problems targeted by the National Institutes of Health (Butler, 2008). Recently, however, progress has been made in definition and classification metrics (Surkis et al., 2016) that can be used to organize and track the stage, chart progress, and understand the impact of translational research in MOBCs.
In the context of the current problem of how to accelerate progress on MOBCs, the time is ripe for translational research on the baroreflex and other neurocardiac mechanisms that support behavioral flexibility. Technical advances in the tools available to assess and intervene in physiological functions in everyday life offer new research opportunities that could be implemented efficiently within the context of clinical trials that are designed to collect rich data on, for example, psychosocial or neurocognitive mechanisms. Especially when used together with ecological momentary assessments of self-reported states and behavior, the potential to understand and eventually intervene in momentary processes at the individual level is great. Development of behavioral interventions that help mitigate stress reactivity and negative emotional states has been suggested to help normalize balance in brain circuitry affected by drugs (Volkow et al., 2016). HRV biofeedback, resonance breathing, and other biobehavioral techniques that manipulate physiological mechanisms to restore autonomic balance and improve brain–body communication hold promise in positively affecting overlapping addiction and central autonomic network circuitry.
Footnotes
This study was supported by National Institute on Alcohol Abuse and Alcoholism Grants R01 AA015248, R01 AA015248-05S1, K02AA000325, K01 AA017473, K24AA021778, and Contract HHSN275201000003C, and by National Institute on Drug Abuse Grant P20 DA017552.
References
- Abboud F. M. In search of autonomic balance: The good, the bad, and the ugly. American Journal of Physiology—Regulatory, Integrative and Comparative Physiology. 2010;298:R1449–R1467. doi: 10.1152/ajpregu.00130.2010. doi:10.1152/ajpregu.00130.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bär K.-J., Boettger M. K., Boettger S., Grotelüschen M., Neubauer R., Jochum T., Voss A. Reduced baroreflex sensitivity in acute alcohol withdrawal syndrome and in abstained alcoholics. Drug and Alcohol Dependence. 2006;85:66–74. doi: 10.1016/j.drugalcdep.2006.03.014. doi:10.1016/j.drugalcdep.2006.03.01. [DOI] [PubMed] [Google Scholar]
- Bates M. E., Buckman J. F. Emotional dysregulation in the moment: Why some college students may not mature out of hazardous alcohol and drug use. In: White H. R., Rabiner D. L., editors. College drinking and drug use. New York, NY: Guilford Press; 2011. pp. 83–101. [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: A neurocognitive perspective. Nature Neuroscience. 2005;8:1458–1463. doi: 10.1038/nn1584. doi:10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Benarroch E. E. The central autonomic network: Functional organization, dysfunction, and perspective. Mayo Clinic Proceedings. 1993;68:988–1001. doi: 10.1016/s0025-6196(12)62272-1. doi:10.1016/S0025-6196(12)62272-1. [DOI] [PubMed] [Google Scholar]
- Benarroch E. E. The central autonomic network. In: Low P. A., editor. Clinical autonomic disorders. 2nd ed. Philadelphia, PA: Lippincott-Raven; 1997a. pp. 17–23. [Google Scholar]
- Benarroch E. E. Armonk, NY: Futura Publishing Co; 1997b. Central autonomic network: Functional organization and clinical correlations. [Google Scholar]
- Bridgett D. J., Burt N. M., Edwards E. S., Deater-Deckard K. Intergenerational transmission of self-regulation: A multidisciplinary review and integrative conceptual framework. Psychological Bulletin. 2015;141:602–654. doi: 10.1037/a0038662. doi:10.1037/a003866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckman J. F., White H. R., Bates M. E. Psychophysiological reactivity to emotional picture cues two years after college students were mandated for alcohol interventions. Addictive Behaviors. 2010;35:786–790. doi: 10.1016/j.addbeh.2010.03.017. doi:10.1016/j.addbeh.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler D. Translational research: Crossing the valley of death. Nature. 2008;453:840–842. doi: 10.1038/453840a. doi:10.1038/453840a. [DOI] [PubMed] [Google Scholar]
- Clark C. A., Skowron E. A., Giuliano R. J., Fisher P. A. Intersections between cardiac physiology, emotion regulation and interpersonal warmth in preschoolers: Implications for drug abuse prevention from translational neuroscience. Drug and Alcohol Dependence. 2016;163(Supplement 1):S60–S69. doi: 10.1016/j.drugalcdep.2016.01.033. doi:10.1016/j.drugalcdep.2016.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley H. D., Harrison N. A. Visceral influences on brain and behavior. Neuron. 2013;77:624–638. doi: 10.1016/j.neuron.2013.02.008. doi:10.1016/j.neuron.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Doussard-Roosevelt J. A., Porges S. W., Scanlon J. W., Alemi B., Scanlon K. B. Vagal regulation of heart rate in the prediction of developmental outcome for very low birth weight preterm infants. Child Development. 1997;68:173–186. doi:10.2307/1131844. [PubMed] [Google Scholar]
- Drummond D. C. What does cue-reactivity have to offer clinical research? Addiction. 2000;95(Supplement 2):S129–S144. doi: 10.1080/09652140050111708. doi:10.1046/j.1360-0443.95.8s2.2.x. [DOI] [PubMed] [Google Scholar]
- Eddie D., Kim C., Lehrer P., Deneke E., Bates M. E. A pilot study of brief heart rate variability biofeedback to reduce craving in young adult men receiving inpatient treatment for substance use disorders. Applied Psychophysiology and Biofeedback. 2014;39:181–192. doi: 10.1007/s10484-014-9251-z. doi:10.1007/s10484-014-9251-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg J., Ben-Daniel N., Mei-Tal G., Wertman E. An autonomic nervous system biofeedback modality for the treatment of attention deficit hyperactivity disorder—an open pilot study. Israel Journal of Psychiatry and Related Sciences. 2004;41:45–53. [PubMed] [Google Scholar]
- El-Sheikh M. Parental drinking problems and children’s adjustment: Vagal regulation and emotional reactivity as pathways and moderators of risk. Journal of Abnormal Psychology. 2001;110:499–515. doi: 10.1037//0021-843x.110.4.499. doi:10.1037/0021-843X.110.4.499. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M., Buckhalt J. A. Vagal regulation and emotional intensity predict children’s sleep problems. Developmental Psychobiology. 2005;46:307–317. doi: 10.1002/dev.20066. doi:10.1002/dev.20066. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M., Erath S. A. Family conflict, autonomic nervous system functioning, and child adaptation: State of the science and future directions. Development and Psychopathology. 2011;23:703–721. doi: 10.1017/S0954579411000034. doi:10.1017/S0954579411000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sheikh M., Harger J., Whitson S. M. Exposure to interparental conflict and children’s adjustment and physical health: The moderating role of vagal tone. Child Development. 2001;72:1617–1636. doi: 10.1111/1467-8624.00369. doi:10.1111/1467-8624.00369. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M., Kouros C. D., Erath S., Cummings E. M., Keller P., Staton L. Marital conflict and children’s externalizing behavior: Interactions between parasympathetic and sympathetic nervous system activity. Monographs of the Society for Research in Child Development. 2009;74(vii):1–79. doi: 10.1111/j.1540-5834.2009.00501.x. doi:10.1111/j.1540-5834.2009.00501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field T., Diego M. Vagal activity, early growth and emotional development. Infant Behavior and Development. 2008;31:361–373. doi: 10.1016/j.infbeh.2007.12.008. doi:10.1016/j.infbeh.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonoberova M., Mezić I., Buckman J. F., Fonoberov V. A., Mezić A., Vaschillo E. G., Bates M. E. A computational physiology approach to personalized treatment models: The beneficial effects of slow breathing on the human cardiovascular system. American Journal of Physiology — Heart and Circulatory Physiology. 2014;307:H1073–H1091. doi: 10.1152/ajpheart.01011.2013. doi:10.1152/ajpheart.01011.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland E. L., Carter K., Ropes K., Howard M. O. Thought suppression, impaired regulation of urges, and Addiction-Stroop predict affect-modulated cue-reactivity among alcohol dependent adults. Biological Psychology. 2012a;89:87–93. doi: 10.1016/j.biopsycho.2011.09.010. doi:10.1016/j.biopsycho.2011. 09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland E. L., Franken I. H., Sheetz J. J., Howard M. O. Alcohol attentional bias is associated with autonomic indices of stressprimed alcohol cue-reactivity in alcohol-dependent patients. Experimental and Clinical Psychopharmacology. 2012b;20:225–235. doi: 10.1037/a0027199. doi:10.1037/a0027199. [DOI] [PubMed] [Google Scholar]
- Goldstein D. S. New York, NY: Marcel Dekker; 2001. The autonomic nervous system in health and disease. [Google Scholar]
- Hassett A. L., Radvanski D. C., Vaschillo E. G., Vaschillo B., Sigal L. H., Karavidas M. K., Lehrer P. M. A pilot study of the efficacy of heart rate variability (HRV) biofeedback in patients with fibromyalgia. Applied Psychophysiology and Biofeedback. 2007;32:1–10. doi: 10.1007/s10484-006-9028-0. doi:10.1007/s10484-006-9028-0. [DOI] [PubMed] [Google Scholar]
- Hettema J., Steele J., Miller W. R. Motivational interviewing. Annual Review of Clinical Psychology. 2005;1:91–111. doi: 10.1146/annurev.clinpsy.1.102803.143833. doi:10.1146/annurev.clinpsy.1.102803.143833. [DOI] [PubMed] [Google Scholar]
- Hubenko A., Fonoberov V. A., Mathew G., Mezić I. Multiscale adaptive search. IEEE Transactions on Systems, Man, and Cybernetics. Part B, Cybernetics. 2011;41:1076–1087. doi: 10.1109/TSMCB.2011.2106207. doi:10.1109/TSMCB.2011.2106207. [DOI] [PubMed] [Google Scholar]
- Hugdahl K. Cognitive influences on human autonomic nervous system function. Current Opinion in Neurobiology. 1996;6:252–258. doi: 10.1016/s0959-4388(96)80080-8. doi:10.1016/S0959-4388(96)80080- [DOI] [PubMed] [Google Scholar]
- Kandel E. R., Schwartz J. H., Jessell T. M. New York, NY: McGraw-Hill; 2000. Principles of neural science. [Google Scholar]
- Karavidas M. K., Lehrer P. M., Vaschillo E., Vaschillo B., Marin H., Buyske S., Hassett A. Preliminary results of an open label study of heart rate variability biofeedback for the treatment of major depression. Applied Psychophysiology and Biofeedback. 2007;32:19–30. doi: 10.1007/s10484-006-9029-z. doi:10.1007/s10484-006-9029-z. [DOI] [PubMed] [Google Scholar]
- Katz L. F., Gottman J. M. Vagal tone protects children from marital conflict. Development and Psychopathology. 1995;7:83–92. doi:10.1017/S0954579400006350. [Google Scholar]
- Katz L. F., Gottman J. M. Buffering children from marital conflict and dissolution. Journal of Clinical Child Psychology. 1997;26:157–171. doi: 10.1207/s15374424jccp2602_4. doi:10.1207/s15374424jccp2602_4. [DOI] [PubMed] [Google Scholar]
- Koob G. F., Volkow N. D. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. doi:10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang P. J., Bradley M. M., Cuthbert B. N. Gainesville, FL: The Center for Research in Psychophysiology, University of Florida; 1999. International Affective Picture System (IAPS): Instruction manual and affective ratings. [Google Scholar]
- Lehrer P., Vaschillo E. Heart rate variability biofeedback: A new tool for improving autonomic homeostasis and treating emotional and psychosomatic diseases. Japanese Journal of Biofeedback. 2004;30:7–16. [Google Scholar]
- Lehrer P. M., Vaschillo E., Vaschillo B. Resonant frequency biofeedback training to increase cardiac variability: Rationale and manual for training. Applied Psychophysiology and Biofeedback. 2000;25:177–191. doi: 10.1023/a:1009554825745. doi:10.1023/A:1009554825745. [DOI] [PubMed] [Google Scholar]
- Lehrer P. M., Vaschillo E., Vaschillo B., Lu S.-E., Eckberg D. L., Edelberg R., Hamer R. M. Heart rate variability biofeedback increases baroreflex gain and peak expiratory flow. Psychosomatic Medicine. 2003;65:796–805. doi: 10.1097/01.psy.0000089200.81962.19. doi:10.1097/01.PSY.0000089200.81962.1. [DOI] [PubMed] [Google Scholar]
- Levenson R. W., Carstensen L. L., Friesen W. V., Ekman P. Emotion, physiology, and expression in old age. Psychology and Aging. 1991;6:28–35. doi: 10.1037//0882-7974.6.1.28. doi:10.1037/0882-7974.6.1.28. [DOI] [PubMed] [Google Scholar]
- Leventhal A. M., Zvolensky M. J. Anxiety, depression, and cigarette smoking: A transdiagnostic vulnerability framework to understanding emotion-smoking comorbidity. Psychological Bulletin. 2015;141:176–212. doi: 10.1037/bul0000003. doi:10.1037/bul000000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magosso E., Biavati V., Ursino N. Paper presented at the 23rd Annual International Conference of the IEEE Engineering in Medicine and Biology Society; Istanbul, Turkey: 2001. Analysis of cardiovascular instability by mathematical model of baroreflex control. [Google Scholar]
- Magosso E., Ursino M. Cardiovascular response to dynamic aerobic exercise: A mathematical model. Medical & Biological Engineering & Computing. 2002;40:660–674. doi: 10.1007/BF02345305. doi:10.1007/BF02345305. [DOI] [PubMed] [Google Scholar]
- Marlatt G. A. Taxonomy of high-risk situations for alcohol relapse: Evolution and development of a cognitive-behavioral model. Addiction. 1996;91(Supplement):S37–S49. doi:10.1111/j.1360-0443.1996.tb02326.x. [PubMed] [Google Scholar]
- Martin G. W., Rehm J. The effectiveness of psychosocial modalities in the treatment of alcohol problems in adults: A review of the evidence. Canadian Journal of Psychiatry. 2012;57:350–358. doi: 10.1177/070674371205700604. doi:10.1177/070674371205700604. [DOI] [PubMed] [Google Scholar]
- McCrady B. S., Epstein E. New York, NY: Oxford University Press; 1999. Addictions: A comprehensive guidebook. [Google Scholar]
- McEwen B. S. Protective and damaging effects of stress mediators: Central role of the brain. Dialogues in Clinical Neuroscience. 2006;8:367–381. doi: 10.31887/DCNS.2006.8.4/bmcewen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezić I. Presented at the 43rd IEEE Conference on Decision and Control; Nassau, Bahamas: 2004. Coupled nonlinear dynamical systems: Asymptotic behavior and uncertainty propagation. [Google Scholar]
- Mezić I. Spectral properties of dynamical systems, model reduction and decompositions. Nonlinear Dynamics. 2005;41:309–325. doi:10.1007/s11071-005-2824-x. [Google Scholar]
- Mezić I., Banaszuk A. Comparison of systems with complex behavior. Physica D: Nonlinear Phenomena. 2004;197:101–133. doi:10.1016/j.physd.2004.06.015. [Google Scholar]
- Mezić I., Runolfsson T. Uncertainty propagation in dynamical systems. Automatica. 2008;44:3003–3013. doi:10.1016/j.automatica.2008.04.020. [Google Scholar]
- Morgenstern J., Naqvi N. H., Debellis R., Breiter H. C. The contributions of cognitive neuroscience and neuroimaging to understanding mechanisms of behavior change in addiction. Psychology of Addictive Behaviors. 2013;27:336–350. doi: 10.1037/a0032435. doi:10.1037/a0032435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mun E. Y., von Eye A., Bates M. E., Vaschillo E. G. Finding groups using model-based cluster analysis: Heterogeneous emotional self-regulatory processes and heavy alcohol use risk. Developmental Psychology. 2008;44:481–495. doi: 10.1037/0012-1649.44.2.481. doi:10.1037/0012-1649.44.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noël X., Brevers D., Bechara A. A neurocognitive approach to understanding the neurobiology of addiction. Current Opinions in Neurobiology. 2013;23:632–638. doi: 10.1016/j.conb.2013.01.018. doi:10.1016/j.conb.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmer R. J., Cervenka J. H., Stone R. A. Baroreflex sensitivity and heredity in essential hypertension. Circulation. 1992;85:497–503. doi: 10.1161/01.cir.85.2.497. doi:10.1161/01.CIR.85.2.497. [DOI] [PubMed] [Google Scholar]
- Penzlin A. I., Siepmann T., Illigens B. M., Weidner K., Siepmann M. Heart rate variability biofeedback in patients with alcohol dependence: A randomized controlled study. Neuropsychiatric Disease and Treatment. 2015;11:2619–2627. doi: 10.2147/NDT.S84798. doi:10.2147/NDT.S84798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges S. W. Social engagement and attachment: A phylogenetic perspective. Annals of the New York Academy of Sciences. 2003;1008:31–47. doi: 10.1196/annals.1301.004. doi:10.1196/annals.1301.004. [DOI] [PubMed] [Google Scholar]
- Porges S. W. The polyvagal theory: New insights into adaptive reactions of the autonomic nervous system. Cleveland Clinic Journal of Medicine. 2009;76(Supplement 2):S86–S90. doi: 10.3949/ccjm.76.s2.17. doi:10.3949/ccjm.76.s2.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges S. W., Doussard-Roosevelt J. A., Maiti A. K. Vagal tone and the physiological regulation of emotion. Monographs of the Society for Research in Child Development. 1994;59(167–186):250–283. doi:10.1111/j.1540-5834.1994.tb01283.x. [PubMed] [Google Scholar]
- Prinsloo G. E., Rauch H. G. L., Lambert M. I., Muench F., Noakes T. D., Derman W. E. The effect of short duration heart rate variability (HRV) biofeedback on cognitive performance during laboratory induced cognitive stress. Applied Cognitive Psychology. 2011;25:792–801. doi:10.1002/acp.1750. [Google Scholar]
- Reiner R. Integrating a portable biofeedback device into clinical practice for patients with anxiety disorders: Results of a pilot study. Applied Psychophysiology and Biofeedback. 2008;33:55–61. doi: 10.1007/s10484-007-9046-6. doi:10.1007/s10484-007-9046-6. [DOI] [PubMed] [Google Scholar]
- Robinson T. E., Berridge K. C. The incentive sensitization theory of addiction: Some current issues. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2008;363:3137–3146. doi: 10.1098/rstb.2008.0093. doi:10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley C. W., Mezić I., Bagheri S., Schlatter P., Henningson D. S. Spectral analysis of nonlinear flows. Journal of Fluid Mechanics. 2009;641:115–127. doi:10.1017/S0022112009992059. [Google Scholar]
- Sherlin L., Wyckoff S. Respiratory sinus arrhythmia feedback impact upon limbic current source density in an anxiety population demonstrated by standardized low resolution electromagnetic tomography (sLORETA) Applied Psychophysiology and Biofeedback. 2008;33:173–174. [Google Scholar]
- Shiffman S. Ecological momentary assessment (EMA) in studies of substance use. Psychological Assessment. 2009;21:486–497. doi: 10.1037/a0017074. doi:10.1037/a0017074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S., Stone A. A., Hufford M. R. Ecological momentary assessment. Annual Review of Clinical Psychology. 2008;4:1–32. doi: 10.1146/annurev.clinpsy.3.022806.091415. doi:10.1146/annurev.clinpsy.3.022806.091415. [DOI] [PubMed] [Google Scholar]
- Siepmann M., Aykac V., Unterdörfer J., Petrowski K., Mueck-Weymann M. A pilot study on the effects of heart rate variability biofeedback in patients with depression and in healthy subjects. Applied Psychophysiology and Biofeedback. 2008;33:195–201. doi: 10.1007/s10484-008-9064-z. doi:10.1007/s10484-008-9064-z. [DOI] [PubMed] [Google Scholar]
- Song H. S., Lehrer P. M. The effects of specific respiratory rates on heart rate and heart rate variability. Applied Psychophysiology and Biofeedback. 2003;28:13–23. doi: 10.1023/a:1022312815649. doi:10.1023/A:1022312815649. [DOI] [PubMed] [Google Scholar]
- Surkis A., Hogle J. A., DiazGranados D., Hunt J. D., Mazmanian P. E., Connors E., Aphinyanaphongs Y. Classifying publications from the clinical and translational science award program along the translational research spectrum: A machine learning approach. Journal of Translational Medicine. 2016;14:235. doi: 10.1186/s12967-016-0992-8. doi:10.1186/s12967-016-0992-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutarto A. P., Wahab M. N., Zin N. M. Resonant breathing biofeedback training for stress reduction among manufacturing operators. International Journal of Occupational Safety and Ergonomics. 2012;18:549–561. doi: 10.1080/10803548.2012.11076959. doi:10.1080/10803548.2012.11076959. [DOI] [PubMed] [Google Scholar]
- Tank J., Jordan J., Diedrich A., Stoffels M., Franke G., Faulhaber H.-D., Busjahn A. Genetic influences on baroreflex function in normal twins. Hypertension. 2001;37:907–910. doi: 10.1161/01.hyp.37.3.907. doi:10.1161/01.HYP.37.3.907. [DOI] [PubMed] [Google Scholar]
- Thayer J. F., Lane R. D. A model of neurovisceral integration in emotion regulation and dysregulation. Journal of Affective Disorders. 2000;61:201–216. doi: 10.1016/s0165-0327(00)00338-4. doi:10.1016/S0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]
- Tiffany S. T. The role of cognitive factors in reactivity to drug cues. In: Drummond D. C., Tiffany S. T., editors. Addictive behaviour: Cue exposure theory and practice. New York, NY: Wiley; 1995. [Google Scholar]
- Tracey K. J. Reflex control of immunity. Nature Reviews Immunology. 2009;9:418–428. doi: 10.1038/nri2566. doi:10.1038/nri2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripodi S. J., Bender K., Litschge C., Vaughn M. G. Interventions for reducing adolescent alcohol abuse: A meta-analytic review. Archives of Pediatrics & Adolescent Medicine. 2010;164:85–91. doi: 10.1001/archpediatrics.2009.235. doi:10.1001/archpediatrics.2009.235. [DOI] [PubMed] [Google Scholar]
- Ursino M. Interaction between carotid baroregulation and the pulsating heart: A mathematical model. American Journal of Physiology. 1998;275:H1733–H1747. doi: 10.1152/ajpheart.1998.275.5.H1733. [DOI] [PubMed] [Google Scholar]
- Ursino M., Magosso E. Acute cardiovascular response to isocapnic hypoxia. I. A mathematical model. American Journal of Physiology: Heart and Circulatory Physiology. 2000;279:H149–H165. doi: 10.1152/ajpheart.2000.279.1.H149. [DOI] [PubMed] [Google Scholar]
- Ursino M., Magosso E. Role of short-term cardiovascular regulation in heart period variability: A modeling study. American Journal of Physiology: Heart and Circulatory Physiology. 2003;284:H1479–H1493. doi: 10.1152/ajpheart.00850.2002. doi:10.1152/ajpheart.00850.2002. [DOI] [PubMed] [Google Scholar]
- Vainchtein D., Mezić I. Capture into resonance: A method for efficient control. Physical Review Letters. 2004;93:084301. doi: 10.1103/PhysRevLett.93.084301. doi:10.1103/PhysRevLett.93.084301. [DOI] [PubMed] [Google Scholar]
- Vaschillo E., Lehrer P., Rishe N., Konstantinov M. Heart rate variability biofeedback as a method for assessing baroreflex function: A preliminary study of resonance in the cardiovascular system. Applied Psychophysiology and Biofeedback. 2002;27:1–27. doi: 10.1023/a:1014587304314. doi:10.1023/A:1014587304314. [DOI] [PubMed] [Google Scholar]
- Vaschillo E. G., Vaschillo B., Lehrer P. M. Characteristics of resonance in heart rate variability stimulated by biofeedback. Applied Psychophysiology and Biofeedback. 2006;31:129–142. doi: 10.1007/s10484-006-9009-3. doi:10.1007/s10484-006-9009-3. [DOI] [PubMed] [Google Scholar]
- Volkow N. D., Koob G. F., McLellan A. T. Neurobiologic advances from the brain disease model of addiction. The New England Journal of Medicine. 2016;374:363–371. doi: 10.1056/NEJMra1511480. doi:10.1056/NEJMra1511480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells R., Outhred T., Heathers J. A., Quintana D. S., Kemp A. H. Matter over mind: A randomised-controlled trial of singlesession biofeedback training on performance anxiety and heart rate variability in musicians. PLoS ONE. 2012;7(10):e46597. doi: 10.1371/journal.pone.0046597. doi:10.1371/journal.pone.0046597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willenbring M. Paper presented at the MOBC Satellite Symposium; RSA Baltimore, MD: 2006. The NIAAA MOBC Research Initiative. [Google Scholar]
- Windhorst U. Muscle proprioceptive feedback and spinal networks. Brain Research Bulletin. 2007;73:155–202. doi: 10.1016/j.brainresbull.2007.03.010. doi:10.1016/j.brainresbull.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Witkiewitz K., Marlatt G. A. Relapse prevention for alcohol and drug problems: That was Zen, this is Tao. American Psychologist. 2004;59:224–235. doi: 10.1037/0003-066X.59.4.224. doi:10.1037/0003-066X.59.4.224. [DOI] [PubMed] [Google Scholar]
- Wray D. W., Raven P. B., Sander M. Diminished baroreflexinduced vasoconstriction following alpha-2 adrenergic receptor blockade in humans. Autonomic Neuroscience. 2008;138:114–117. doi: 10.1016/j.autneu.2007.11.002. doi:10.1016/j.autneu.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing-Sheng Y., Yong-Zhi L., Jie-Xin L., Yu-Qing G., Zhang-Huang C., Chong-Fa Z., Shu-Zheng L. Genetic influence on baroreflex sensitivity in normotensive young men. American Journal of Hypertension. 2010;23:655–659. doi: 10.1038/ajh.2010.30. doi:10.1038/ajh.2010.30. [DOI] [PubMed] [Google Scholar]
- Zucker T. L., Samuelson K. W., Muench F., Greenberg M. A., Gevirtz R. N. The effects of respiratory sinus arrhythmia biofeedback on heart rate variability and posttraumatic stress disorder symptoms: A pilot study. Applied Psychophysiology and Biofeedback. 2009;34:135–143. doi: 10.1007/s10484-009-9085-2. doi:10.1007/s10484-009-9085-2. [DOI] [PubMed] [Google Scholar]
- Zywiak W. H., Stout R. L., Longabaugh R., Dyck I., Connors G. J., Maisto S. A. Relapse-onset factors in Project MATCH: The relapse questionnaire. Journal of Substance Abuse Treatment. 2006;31:341–345. doi: 10.1016/j.jsat.2006.05.007. doi:10.1016/j.jsat.2006.05.007. [DOI] [PubMed] [Google Scholar]