Abstract
Objective:
The present study used youth’s in vivo reports of subjective responses to cannabis while smoking in their natural environments to identify real-world mechanisms of topiramate treatment for cannabis misuse.
Method:
Participants were 40 cannabis users (≥ twice weekly in past 30 days), ages 15–24 years (47.5% female), with at least one cannabis use episode during the final 3 weeks of a 6-week, randomized clinical trial. Youth reported subjective “high” while smoking, stimulation, sedation, stress, craving, and grams of marijuana used in the natural environment via wireless electronic devices. Bayesian multilevel structural equation modeling (MSEM) evaluated mediation via indirect effect tests.
Results:
Significant within (daily) and between (person) variability and distinctive within and between effects supported the MSEM approach. Subjective high while smoking was significantly reduced for youth in the topiramate condition, relative to placebo, and the indirect effect of reduced subjective high on total grams of cannabis smoked that day was significant. Indirect effects through other subjective responses were not significant.
Conclusions:
The results of this initial study suggest that altering subjective responses to smoking, specifically subjective high, may be a key target for developing adjunctive pharmacotherapies for cannabis misuse. More generally, this work provides an example for applying ecological momentary assessment and analytic techniques to evaluate mechanisms of behavior change in longitudinal data.
Adolescent cannabis misuse is increasingly recognized as potentially damaging to long-term health (Crean et al., 2011; Meier et al., 2012; Volkow et al., 2014). Yet, 1 in 17 high school seniors in the United States uses marijuana on a near daily basis (Johnston et al., 2015), and marijuana is the primary substance of abuse for the majority of adolescent substance use treatment admissions in the United States (Substance Abuse and Mental Health Services Administration, 2012). Unfortunately, the best treatment options yield only modest and temporary benefits, and many youth do not respond or have little sustained benefit (Bender et al., 2011; Davis et al., 2015). Thus, there is a pressing need to provide more effective treatments for adolescents seeking to reduce their cannabis use. Identifying the most beneficial treatment targets is an essential next step toward this goal.
We (Miranda & Treloar, 2016) and others (Belendiuk & Riggs, 2014; Gray et al., 2012) have suggested that adolescents may benefit from integrated treatments that include both pharmacological agents and psychotherapy. Three placebocontrolled randomized trials have evaluated the potential for pharmacotherapies to reduce cannabis misuse among youth. First, Cornelius and colleagues (2010) tested whether fluoxetine combined with cognitive behavioral therapy for major depressive disorder (MDD) and cannabis use disorder (CUD) and motivational enhancement therapy for CUD in a sample of 70 youth (ages 14–25) with concurrent MDD and CUD. Although depressive and CUD symptoms reduced over the 12-week trial, medication was not more effective than placebo, and there was no longer-term follow-up (Cornelius et al., 2010). Next, Gray and colleagues (2012) conducted an 8-week trial of N-acetylcysteine (NAC) combined with brief cessation counseling and contingency management among 116 treatment-seeking adolescents (ages 15–21) with cannabis dependence. Similar to standalone psychosocial interventions, NAC promoted short-term abstinence, relative to placebo, but these gains were not sustained (Gray et al., 2012). Last, our 6-week trial tested the efficacy of topiramate when combined with psychotherapy incorporating motivational enhancement and cognitive-behavioral techniques among 66 heavy cannabis users (ages 15–24). The frequency of cannabis use significantly decreased across the trial, but the percentage of use days did not differ between groups. Topiramate was more efficacious than placebo, however, at reducing the amount of cannabis youth smoked when they used (Miranda et al., 2016). On the whole, the results of clinical trials suggest the potential for pharmacotherapy combined with psychosocial interventions, but precisely how medications augment psychosocial treatment effects remains poorly understood.
Randomized controlled trials, such as those we review here, have been considered the gold standard for conducting psychotherapy outcome research for more than 30 years. Across psychiatric disorders and treatment types, these trials have generally found that many forms of psychotherapy, pharmacotherapy, or the combination are equally effective in promoting therapeutic change. Given that many modestly effective treatments have been developed, a crucial next step is to identify the treatment components that are most efficacious and the processes by which change occurs during successful treatment. By understanding how treatments work, researchers can leverage this information about mechanisms to optimize extant treatments or develop new iterations of more effective interventions.
Ecological momentary assessment (EMA) is a particularly useful method to capture mechanisms of behavior change in the context of addiction treatment, including adolescent cannabis use. EMA refers to a broad class of methods especially well suited to examine whether an intervention produces effective change in substance use, but also which treatment-induced changes accounted for the intervention effect and under what conditions (Shiffman, 2009; Trull & Ebner-Priemer, 2009). EMA studies collect data about participants’ subjective states and current behaviors at specified moments throughout the day as participants carry out their customary actions in their natural environments. The fine-grained, longitudinal assessment afforded by such assessments allows for temporally sequenced monitoring of mechanisms and substance use outcomes, thereby clarifying the putative causal chain from treatment to mediating variable to behavior change (Baraldi et al., 2015; Dallery et al., 2015). In the area of pharmaceutical development, EMA can elucidate mechanisms of a medication’s effects in an ecologically valid setting (Armeli et al., 2006; Kranzler et al., 2014a; Miranda et al., 2013, 2016; Tidey et al., 2008). This advantage is uniquely pertinent for capturing adolescent substance responses, for which the study of mechanisms and substance use behavior in the human laboratory is not legally or ethically plausible.
The rationale for developing pharmacotherapies for youth generally follows the same basic strategies as for adults: (a) make alcohol or drug use aversive, (b) replace the misused drug with a less harmful formulation for prolonged maintenance, (c) block reinforcing/addictive effects of the drug, (d) reduce craving, (e) facilitate detoxification and minimize withdrawal symptoms, and (f) treat psychiatric comorbidity (Miranda & Treloar, 2016). Although the U.S. Food and Drug Administration has approved several medications for these aforementioned targets among adults, no medication is approved for adolescent treatment. Extant reviews of the empirical literature on adolescent pharmacological treatment must qualify any demonstrated efficacy with the caveat that research in this area is at a nascent stage, and providers should proceed with caution (Belendiuk & Riggs, 2014; Bender et al., 2011; Clark, 2012; Courtney & Milin, 2015; Kim et al., 2011; Simkin & Grenoble, 2010; Upadhyaya & Deas, 2008; Winters et al., 2014). Narrowing this gap in medication development research for adolescent cannabis use treatment is key to optimizing and diversifying treatment options.
The present study used EMA to capture youth’s in vivo subjective responses to cannabis in their natural environments to identify real-world mechanisms of topiramate treatment for cannabis misuse. Topiramate is a sulfamatesubstituted fructopyranose derivative with multiple mechanisms of action, including potentiation of γ-aminobutyric acid (GABA), enhancement of GABAA receptor function, and antagonism of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)/kainate glutamate receptors (Kranzler et al., 2014; Shank et al., 2000; Simeone et al., 2006). Our prior work demonstrated initial evidence of topiramate’s potential to reduce the amount of cannabis that youth used when they smoked but not the overall frequency of use. This finding, combined with topiramate’s putative potentiation of GABA and antagonism of glutamate, suggests that topiramate may operate to reduce use through modulation of cannabinoid type 1 receptors—the primary target of cannabis—which are highly expressed on GABAergic and glutamatergic neurons and are believed to mediate most of the subjective effects of cannabis (Moreira et al., 2009).
We hypothesized that topiramate operates to reduce grams of cannabis that youth use when they smoke by blunting subjective effects of cannabis. This hypothesis is consistent with (a) conceptual models regarding how medications may reduce substance use; (b) the conjoint, putative mechanisms of action of cannabis and topiramate; and (c) our previous evaluations of topiramate for alcohol use in adults (Miranda et al., 2016). Furthermore, our primary outcome report showed that topiramate reduced grams of cannabis youth used when they smoked but not abstinence rates (Miranda et al., 2017). Thus, this medication may serve to reduce use levels through altering the acute subjective effects of cannabis.
Method
Participants and procedure
Sixty-six frequent cannabis users ages 15–24 were randomized to receive psychosocial treatment plus either adjunctive psychopharmacological treatment or placebo. Eligible youth who used cannabis at least twice weekly in the past 30 days were recruited from the community to participate in a study of whether a medication affects cannabis use. Excluded were those currently in treatment (past 30 days) for cannabis misuse; youth with current Axis I diagnoses other than cannabis, alcohol, nicotine, or disruptive behavior disorders; those actively suicidal or psychotic; and those with contraindicated medical conditions or medications. Written informed consent was obtained from the parents of minors and youth ages 18 or older; minors provided assent. Procedures were approved by Brown University’s institutional review board.
This is the first analysis of EMA data from the postrandomization period of Miranda and colleagues’ (2016) 6-week, double-blind trial of topiramate treatment for cannabis misuse. Youth received an individually tailored psychosocial intervention to support the reduction of cannabis use plus either treatment with topiramate or placebo. Topiramate was titrated slowly from 25 mg to 250 mg over 4 weeks to improve efficacy and tolerability (Albsoul-Younes et al., 2004). The manualbased psychosocial intervention was based on supported treatments for adolescent cannabis users and included both motivational and cognitive-behavioral components (Martin & Copeland, 2008; Walker et al., 2006). Master’s- or doctorallevel clinicians administered a series of three, individual, 50-minute sessions at study Weeks 1, 3, and 5.
The present analyses evaluated subjective responses to cannabis use as a mechanism by which topiramate reduced use. Thus, at least one cannabis-use episode at an effective dose of topiramate was required to be included. To maximize EMA data to test hypotheses of subjective-response mechanisms, analyses focused on Weeks 4–6, during which time topiramate dose ranged from 125 mg to 200 mg daily. Forty of the 66 randomized participants had EMA data from one or more use events in Weeks 4–6. Participants not included either withdrew before Week 4 (n = 20) or did not report use via EMA in Weeks 4–6 (n = 6).
Ecological momentary assessment protocol and assessments
EMA software was implemented on handheld Samsung Omnia electronic devices with procedures identical to those of Miranda et al. (2016). Participants self-initiated cannabis reports, which prompted a series of EMA questions, including, “How [tense, stressed, excited, energized, high, sedated, sluggish] do you feel right now?” and “How strong is your urge to smoke pot right now?” Participants recorded responses using a visual analog scale presented as a “slider” on the EMA screen, with endpoints labeled not at all and extremely for subjective states and endpoints labeled no urge and strongest ever for urge to smoke pot. Responses were scored on an 11-point scale from 0 to 10. Stress, stimulation, and sedation were averages of tense/stressed (α = .60), excited/energized (α = .77), and sedated/sluggish (α = .83) across study days. Participants also reported the total amount of marijuana used (grams) during each use episode, along with the number of people with whom they shared it. The total number of grams was divided by the number of those with whom they shared (+1 for the participant) to estimate grams for each episode.
Approach to mediation tests
Multilevel structural equation model (MSEM) analyses were implemented with Mplus 7.2 using Bayesian estimation. MSEM permitted a simultaneous test of the influence of pharmacological treatment on subjective responses to cannabis (a path) and the influence of these responses on subsequent cannabis use (b path). The MODEL CONSTRAINT command was used to calculate indirect effects (a × b). The advantages of MSEM over other approaches to test mediation and the specific procedures used for this analysis are described by Preacher and colleagues (2010, 2011).
Bayesian estimation used non-informative (diffuse) priors and posterior median point estimate. Specifically, prior distributions for mediation regression coefficients and indirect effect estimates were normal distributions with a prior mean of zero and an infinitely large prior variance. In practice, the Bayesian model with non-informative priors often provides similar estimates as maximum likelihood (ML) estimation (Muthén, 2010). A key difference lies in the assumptions about the posterior distribution of the indirect effect estimates. Whereas ML assumes that the distribution of these estimates will be normal based on asymptotic (large-sample) theory, a Bayes approach does not rely on large samples and provides 95% highest posterior density credibility intervals for the indirect effect estimates that can accommodate the asymmetric (skewed) sampling distribution of indirect effects (Muthén & Asparouhov, 2012).
An additional difference is the computational complexity of fitting an ML MSEM model with many latent variables. Whereas ML requires many dimensions of numerical integration, Bayes is less computationally demanding and can provide similar estimates while avoiding convergence issues. Convergence of the Bayesian MSEM was evaluated through inspecting the Proportional Scale Reduction (PSR) factor and trace plots. The first half of iterations constituted a “burn-in” phase, and thinning options were not used. After initial model estimation, the FBITERATIONS option (20,000 iterations) was added to evaluate whether parameter values remained stable and PSR values remained close to 1. Prior distributions for variance parameters were specified as inverse gamma (IG) distributions with a shape parameter of 0 and a scale parameter of -1. Sensitivity analyses specifying alternative variance priors (e.g., IG∼[.001, .001]) did not alter the substantive conclusions.
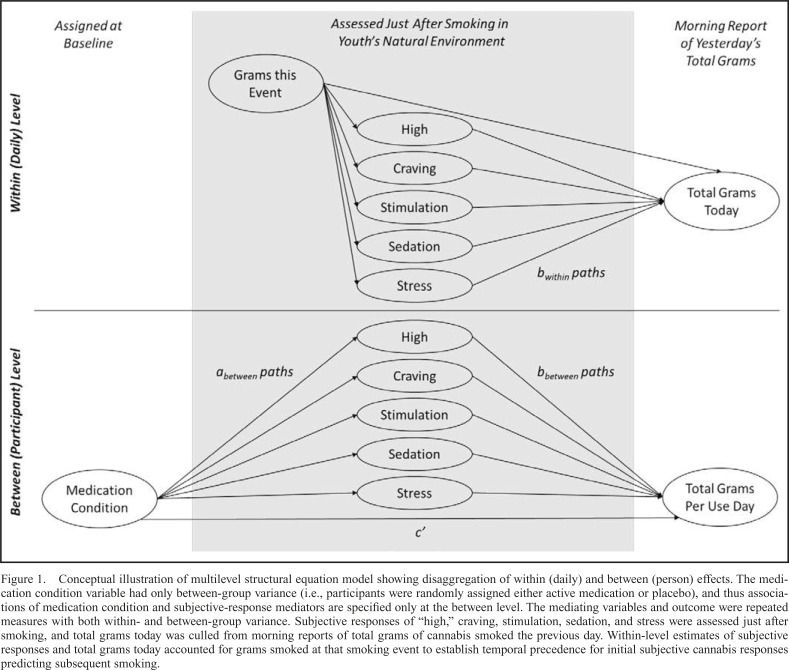
A conceptual 2-1-1 MSEM (Figure 1) illustrates hypothesized associations at each level of analysis. The “X” variable was the condition (0 = placebo, 1 = topiramate), with only between-group variance (i.e., participants were randomly assigned to one condition). The “M” and “Y” variables were repeated assessments with both within- and between-group variance. The “M” variables were subjective responses, and the “Y” was the total grams of cannabis smoked that day, culled from the next morning’s report of the total grams of cannabis smoked.
Figure 1.
Conceptual illustration of multilevel structural equation model showing disaggregation of within (daily) and between (person) effects. The medication condition variable had only between-group variance (i.e., participants were randomly assigned either active medication or placebo), and thus associations of medication condition and subjective-response mediators are specified only at the between level. The mediating variables and outcome were repeated measures with both within- and between-group variance. Subjective responses of “high,” craving, stimulation, sedation, and stress were assessed just after smoking, and total grams today was culled from morning reports of total grams of cannabis smoked the previous day. Within-level estimates of subjective responses and total grams today accounted for grams smoked at that smoking event to establish temporal precedence for initial subjective cannabis responses predicting subsequent smoking.
Hypotheses focused on how much additional cannabis was subsequently used that day following assessment of subjective responses. This temporally sequenced nature of the data is a major advantage of EMA. Inclusion of a covariate for grams of cannabis smoked at the initial event was key to establishing the temporal precedence of putative mediators on subsequent smoking. In addition, participants did not initiate an end-smoke report after a predetermined quantity of cannabis use. Therefore, the amount of cannabis used could vary considerably across events, influencing subjective responses and the additional amount of cannabis used that day. Subjective responses to cannabis may be dose dependent, and/or the use of cannabis on a given day may be dependent on prior use that day. Inasmuch as these are true, it was important to control for the amount of cannabis used at the first use event to capture the influence of subjective responses on subsequent use.
Results
Participants (n = 40; 47.5% female) were 19.4 years old, on average (SD = 2.2), and the majority (95%) met two or more criteria for current cannabis dependence (M = 4.6 symptoms, SD = 2.2). The average percentage of cannabis use days at baseline was 72.8% (SD = 25.9; range: 21.1– 100%), and the average grams of cannabis per use day was 0.72 g (SD = 0.56; range: 0.09–2.81 g). The most commonly reported race was White (57.5%) or Black (22.5%); 25.0% reported Hispanic or Latino ethnicity.
EMA descriptive information
On average, youth reported smoking on 6.3 days (SD = 4.6; range: 1–20; Mdn = 5). Youth reported smoking 0.41 g for their first smoking event on a given smoking day (SD = 0.29) and 1.52 total grams per smoking day (SD = 1.48), on average. Independent samples t tests (unequal variances assumed) compared medication groups; EMA data replicated findings indicated by weekly self-reports of use in our prior, primary outcome analysis (Miranda et al., 2016). All youth reported reductions in the number of use days; youth in the placebo condition reported, on average, 7.7 use days in this 3-week period (SD = 6.3), whereas youth in the topiramate condition reported, on average, 5.9 use days (SD = 5.3). These group differences did not reach statistical significance, t(39) = 1.02, p = .314. Medication significantly influenced grams per use day, however, t(21) = 2.37, p = .027; youth in the topiramate group smoked, on average, 0.85 g per smoking day (SD = 0.45), whereas youth in the placebo group smoked, on average, 1.47 g per smoking day (SD = 1.45), Mdifference = 0.82 g per smoking day (SE = 0.35).
Within- and between-level variability
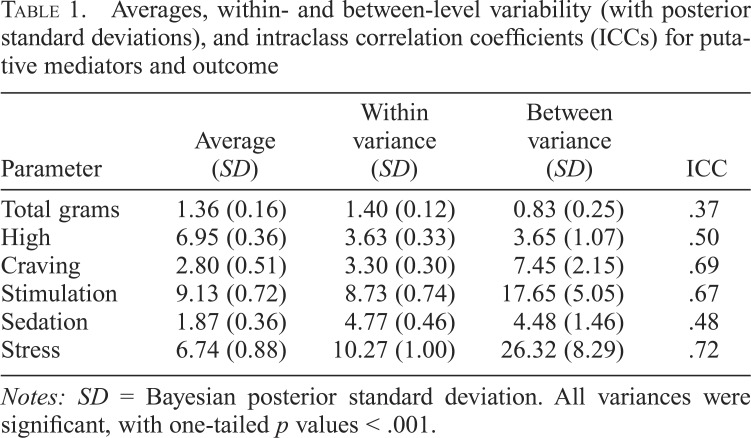
An initial MSEM estimated the overall average of the “Y” variable, the total grams per use day, and isolated within- and between-level variance to calculate an intraclass correlation coefficient (ICC). The ICC identifies the proportion of variability in grams per use day that was attributable to betweenperson effects (i.e., Varbetween/Varwithin + Varbetween = .37), indicating that more than one third (37%) of the variability in the total grams was attributable to participant effects. This finding supports the use of MSEM to identify mechanisms of the between-level effect of the medication condition on the total grams. All putative mediators also showed significant variability both within and between levels (Table 1). ICCs for subjective responses and craving ranged from .48 to .72, indicating, at minimum, that about half of the variability in putative mechanisms was attributable to between-level (participant) effects, again supporting mediation analysis.
Table 1.
Averages, within- and between-level variability (with posterior standard deviations), and intraclass correlation coefficients (ICCs) for putative mediators and outcome
| Parameter | Average (SD) | Within variance (SD) | Between variance (SD) | ICC |
| Total grams | 1.36 (0.16) | 1.40 (0.12) | 0.83 (0.25) | .37 |
| High | 6.95 (0.36) | 3.63 (0.33) | 3.65 (1.07) | .50 |
| Craving | 2.80 (0.51) | 3.30 (0.30) | 7.45 (2.15) | .69 |
| Stimulation | 9.13 (0.72) | 8.73 (0.74) | 17.65 (5.05) | .67 |
| Sedation | 1.87 (0.36) | 4.77 (0.46) | 4.48 (1.46) | .48 |
| Stress | 6.74 (0.88) | 10.27 (1.00) | 26.32 (8.29) | .72 |
Notes: SD = Bayesian posterior standard deviation. All variances were significant, with one-tailed p values < .001.
Direct effect
The first predictive MSEM model estimated the direct effect of topiramate versus placebo on the total grams used per use day. For positive effect estimates, the one-tailed p value reflects the proportion of the posterior distribution that is below zero, and for negative effects the p value reflects the proportion above zero. The intercept approximated the average grams per smoking day in the placebo group (estimate = 1.66), and the direct effect of topiramate on the total grams showed a significant reduction in the total grams per use day for the topiramate group (estimate = -0.65, 95% CI [-1.25, -0.06], one-tailed p = .020).
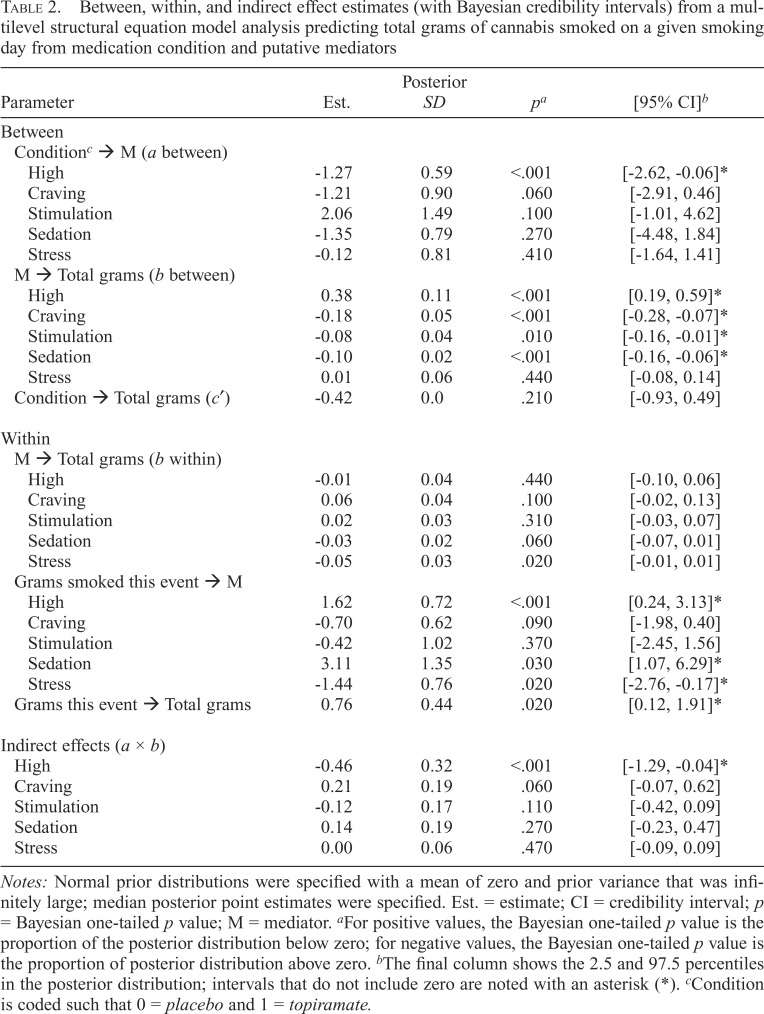
Mediation
Figure 1 and Table 2 show how between- and within effect estimates are disentangled using the MSEM approach. Bayesian MSEMs predicted the total grams of cannabis smoked on a given smoking day from the medication condition and putative mediators. Mediators were assessed at the first cannabis use event of a given smoking day, and the total grams outcome was culled from morning reports of the previous day’s total use. Bayesian credibility intervals that do not include zero imply a significant effect. The largest PSR value at 100 iterations was 1.075 for the effect of grams smoked at the event on the next morning’s report of the total grams smoked. Using the FBITERATIONS option to request 20,000 iterations showed the progression of PSR values with increasing iterations. PSR values stabilized from 1.001 to 1.003, and parameter values remained close to the original values shown in Table 2.
Table 2.
Between, within, and indirect effect estimates (with Bayesian credibility intervals) from a multilevel structural equation model analysis predicting total grams of cannabis smoked on a given smoking day from medication condition and putative mediators
| Parameter | Est. | Posterior SD | pa | [95% CI]b |
| Between | ||||
| Conditionc → M (a between) | ||||
| High | -1.27 | 0.59 | <.001 | [-2.62, -0.06]* |
| Craving | -1.21 | 0.90 | .060 | [-2.91, 0.46] |
| Stimulation | 2.06 | 1.49 | .100 | [-1.01, 4.62] |
| Sedation | -1.35 | 0.79 | .270 | [-4.48, 1.84] |
| Stress | -0.12 | 0.81 | .410 | [-1.64, 1.41] |
| M → Total grams (b between) | ||||
| High | 0.38 | 0.11 | <.001 | [0.19, 0.59]* |
| Craving | -0.18 | 0.05 | <.001 | [-0.28, -0.07]* |
| Stimulation | -0.08 | 0.04 | .010 | [-0.16, -0.01]* |
| Sedation | -0.10 | 0.02 | <.001 | [-0.16, -0.06]* |
| Stress | 0.01 | 0.06 | .440 | [-0.08, 0.14] |
| Condition → Total grams (c′) | -0.42 | 0.0 | .210 | [-0.93, 0.49] |
| Within | ||||
| M → Total grams (b within) | ||||
| High | -0.01 | 0.04 | .440 | [-0.10, 0.06] |
| Craving | 0.06 | 0.04 | .100 | [-0.02, 0.13] |
| Stimulation | 0.02 | 0.03 | .310 | [-0.03, 0.07] |
| Sedation | -0.03 | 0.02 | .060 | [-0.07, 0.01] |
| Stress | -0.05 | 0.03 | .020 | [-0.01, 0.01] |
| Grams smoked this event → M | ||||
| High | 1.62 | 0.72 | <.001 | [0.24, 3.13]* |
| Craving | -0.70 | 0.62 | .090 | [-1.98, 0.40] |
| Stimulation | -0.42 | 1.02 | .370 | [-2.45, 1.56] |
| Sedation | 3.11 | 1.35 | .030 | [1.07, 6.29]* |
| Stress | -1.44 | 0.76 | .020 | [-2.76, -0.17]* |
| Grams this event → Total grams | 0.76 | 0.44 | .020 | [0.12, 1.91]* |
| Indirect effects (a × b) | ||||
| High | -0.46 | 0.32 | <.001 | [-1.29, -0.04]* |
| Craving | 0.21 | 0.19 | .060 | [-0.07, 0.62] |
| Stimulation | -0.12 | 0.17 | .110 | [-0.42, 0.09] |
| Sedation | 0.14 | 0.19 | .270 | [-0.23, 0.47] |
| Stress | 0.00 | 0.06 | .470 | [-0.09, 0.09] |
Notes: Normal prior distributions were specified with a mean of zero and prior variance that was infinitely large; median posterior point estimates were specified. Est. = estimate; CI = credibility interval; p = Bayesian one-tailed p value; M = mediator. aFor positive values, the Bayesian one-tailed p value is the proportion of the posterior distribution below zero; for negative values, the Bayesian one-tailed p value is the proportion of posterior distribution above zero. bThe final column shows the 2.5 and 97.5 percentiles in the posterior distribution; intervals that do not include zero are noted with an asterisk (*). cCondition is coded such that 0 = placebo and 1 = topiramate.
The top panel of Table 2 shows between-level effects. After including subjective responses and craving as mediators, the effect of the medication condition on the total grams (c′) was no longer significant, one-tailed p = .210. The effects of the medication condition on putative mediators (a path between) suggested reductions in subjective high for the topiramate group (estimate = -1.27, 95% CI [-2.62, -0.06]). Also at the between level, greater craving, stimulation, and sedation during initial smoking events were each associated with a reduced total grams of cannabis smoked per use day (b paths between; see Table 2). In contrast, a greater high was associated with an increased total grams per use day (estimate = 0.38, 95% CI [0.19, 0.59], one-tailed p < .001).
Indirect effect tests for putative mediators are shown in the bottom panel of Table 2. A significant indirect effect estimate for high suggested that topiramate reduced the total grams smoked by reducing the initial subjective high from cannabis use (estimate = -0.46, 95% CI [-1.29, -0.04], onetailed p < .001). Although the general pattern of betweenlevel effects was also suggestive of mediation for craving, the indirect effect was not significant, one-tailed p = .060.
Discussion
Cannabis misuse among youth is potentially damaging to long-term health (Crean et al., 2011; Meier et al., 2012; Volkow et al., 2014); yet, the majority of youth who receive treatment will not show lasting reductions in use (Bender et al., 2011; Davis et al., 2015). Identifying the most beneficial treatment targets is an essential step toward improving extant treatment options and requires keen understanding of how treatments work. The present study built on our prior work (Miranda et al., 2016) by identifying putative mechanisms by which topiramate treatment for cannabis misuse reduced the quantity of cannabis use in youth. The intended purpose was to highlight the importance of ecological research in the natural environment for identifying mechanistic aspects of successful treatment for adolescent substance use. The results of this initial study suggest that altering subjective responses to cannabis, specifically subjective high, may be a key target for developing adjunctive pharmacotherapies for cannabis misuse in youth.
In addition to the substantive findings for topiramate and the subjective states on the quantity of cannabis use, this application from a pharmacotherapy trial with adolescent cannabis users illustrated the importance of (a) capturing the temporal order of putative mediators and outcomes, and (b) disaggregating the distinctive within- (daily) and between- (participant) level effects when evaluating mechanisms of treatment effects. Each of these points is reviewed in turn.
To be a true mechanism, the mediator (M) must change as a result of treatment (X) and, in turn, bring about change in the outcome (Y). Thus, true mediation requires a specific temporal order for focal variables such that X precedes M and M precedes Y (Cole & Maxwell, 2003). The necessity of establishing a timeline to infer a causal relation or a mediation of change is described as the “Achilles heel of treatment studies” (Kazdin, 2007, p. 5). Thus, one of the key strengths of this study, and of EMA generally, is the rich temporal structure of repeated-measures data collected in real time as behavior unfolds naturally in participants’ usual settings. In our study, reports of subjective states, grams of cannabis smoked, and the number of persons shared with in real time allowed for estimating the specific number of grams of cannabis smoked at the time initial subjective responses were reported. Further, the inherent lag of this report to the next morning’s report of total use that day allowed for establishing a clear timeline between the mediator and outcome.
In repeated-measures designs, variables assessed at Level 1 (repeated assessments) typically have both between (person) and within (state) components. Although a multilevel model disaggregates within and between variance, a traditional multilevel model approach will conflate between and within effects when testing mediation, obscuring tests of indirect effects (Preacher et al., 2011). The importance of distinguishing within and between effects is highlighted in our study through distinctive effects of high on outcome at the within (daily) and between (participant) levels. At the level of the person, a greater high is associated with increased total grams smoked, on average. This effect is intuitive and consistent with conceptual models of addiction—that is, those with more positive subjective experiences of the pharmacological effects of drugs are more likely to be repeat users and to use at higher levels (Volkow et al., 2016). At the daily level, however, the direction of this effect is reversed such that a greater high is associated with reduced total grams of cannabis smoked that day. Although purely speculative, this effect suggests that a greater experience of a subjective high initially during a smoking episode may lead the user to titrate use. Although the distinctive within-level effect of a high on the outcome was not statistically significant, it illustrates how evaluation of the between-groups mediation effect may be masked by opposing within (state) and between (person) influences of the same mediating variables on outcomes.
Despite the advantage of EMA combined with MSEM to establish temporal order and disaggregate between and within effects, respectively, evidence for mediation in any between-groups randomized trial is inherently established at the between (participant) level. This is because persons are assigned to only one treatment group—in this case, either topiramate or placebo. Because there is no variation in the medication condition within persons, the medication condition can exert only between-level effects, regardless of when and how mediators and outcomes are assessed (Preacher et al., 2011). Nonetheless, through extracting within-level variance from the repeated EMA assessments, we were able to establish a purer test of the between-groups mechanisms underlying topiramate’s pharmacotherapeutic benefit to reduce the quantity of cannabis use.
Limitations of the parent proof-of-concept study and the present analysis should be considered. The present study used 3 weeks of data from a small sample of youth at a potentially efficacious topiramate dose. Findings support the need for additional trials implemented in larger samples and with longer stabilization and evaluation of dose-response relationships. The youth in the present trial may be representative of likely candidates for adjunctive pharmacotherapy— that is, those who use cannabis regularly, who use it heavily, and who have multiple symptoms of dependence. But generalizability of mechanisms to adults and across levels of use and problems remains to be considered. Further evaluations of sample size for MSEM, particularly for Bayesian applications and those in the context of mediation, are also needed (McNeish, 2017; Preacher et al., 2011, 2010). Evaluations of other putative mechanisms or moderation of indirect pathways are also exciting avenues for future work.
This study contributes to the literature on mechanisms of behavior change not only by addressing a substantive research question but also by providing an example for applying EMA and a Bayesian MSEM analytic approach to evaluate indirect treatment effects in longitudinal data. Topiramate’s beneficial effects on cannabis use appeared to be attributable to its ability to reduce subjective high from use, showing specificity over other putative mediators—that is, craving, stimulation, sedation, and stress. A treatment implication of this finding is that topiramate may be especially beneficial for youth who use cannabis for positive subjective effects or whose primary treatment goal is to reduce their cannabis use to less harmful levels. Consistency of our findings across studies, samples, and conditions is imperative to draw firm inferences about the associations implicated by these conclusions.
Footnotes
This work was supported by National Institute on Drug Abuse Grant R01 DA026778 and National Institute on AlcoholAbuse and Alcoholism Grants K23 AA024808 and R01 AA007850.
References
- Albsoul-Younes A. M., Salem H. A., Ajlouni S. F., Al-Safi S. A. Topiramate slow dose titration: Improved efficacy and tolerability. Pediatric Neurology. 2004;31:349–352. doi: 10.1016/j.pediatrneurol.2004.04.012. doi:10.1016/j.pediatrneurol.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Armeli S., Feinn R., Tennen H., Kranzler H. R. The effects of naltrexone on alcohol consumption and affect reactivity to daily interpersonal events among heavy drinkers. Experimental and Clinical Psychopharmacology. 2006;14:199–208. doi: 10.1037/1064-1297.14.2.199. doi:10.1037/1064-1297.14.2.199. [DOI] [PubMed] [Google Scholar]
- Baraldi A. N., Wurpts I. C., MacKinnon D. P., Lockhart G. Evaluating mechanisms of behavior change to inform and evaluate technology-based interventions. In: Marsch L. A., Lord S. E., Dallery J., editors. Behavioral healthcare and technology. New York, NY: Oxford University Press; 2015. pp. 187–199. [Google Scholar]
- Belendiuk K. A., Riggs P. Treatment of adolescent substance use disorders. Current Treatment Options in Psychiatry. 2014;1:175–188. doi: 10.1007/s40501-014-0016-3. doi:10.1007/s40501-014-0016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender K., Tripodi S. J., Sarteschi C., Vaughn M. G. A metaanalysis of interventions to reduce adolescent cannabis use. Research on Social Work Practice. 2011;21:153–164. doi:10.1177/1049731510380226. [Google Scholar]
- Clark D. B. Pharmacotherapy for adolescent alcohol use disorder. CNS Drugs. 2012;26:559–569. doi: 10.2165/11634330-000000000-00000. doi:10.2165/11634330-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Cole D. A., Maxwell S. E. Testing mediational models with longitudinal data: Questions and tips in the use of structural equation modeling. Journal of Abnormal Psychology. 2003;112:558–577. doi: 10.1037/0021-843X.112.4.558. doi:10.1037/0021-843X.112.4.558. [DOI] [PubMed] [Google Scholar]
- Cornelius J. R., Bukstein O. G., Douaihy A. B., Clark D. B., Chung T. A., Daley D. C., Brown S. J. Double-blind fluoxetine trial in comorbid MDD–CUD youth and young adults. Drug and Alcohol Dependence. 2010;112:39–45. doi: 10.1016/j.drugalcdep.2010.05.010. doi:10.1016/j.drugalcdep.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney D. B., Milin R. Pharmacotherapy for adolescents with substance use disorders. Current Treatment Options in Psychiatry. 2015;2:312–325. doi:10.1007/s40501-015-0053-6. [Google Scholar]
- Crean R. D., Crane N. A., Mason B. J. An evidence based review of acute and long-term effects of cannabis use on executive cognitive functions. Journal of Addiction Medicine. 2011;5:1–8. doi: 10.1097/ADM.0b013e31820c23fa. doi:10.1097/ADM.0b013e31820c23fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallery J., Jarvis B., Marsch L., Xie H. Mechanisms of change associated with technology-based interventions for substance use. Drug and Alcohol Dependence. 2015;150:14–23. doi: 10.1016/j.drugalcdep.2015.02.036. doi:10.1016/j.drugalcdep.2015.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. L., Powers M. B., Handelsman P., Medina J. L., Zvolensky M., Smits J. A. Behavioral therapies for treatment-seeking cannabis users: A meta-analysis of randomized controlled trials. Evaluation & the Health Professions. 2015;38:94–114. doi: 10.1177/0163278714529970. doi:10.1177/0163278714529970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray K. M., Carpenter M. J., Baker N. L., DeSantis S. M., Kryway E., Hartwell K. J., Brady K. T. A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. American Journal of Psychiatry. 2012;169:805–812. doi: 10.1176/appi.ajp.2012.12010055. doi:10.1176/appi.ajp.2012.12010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston L. D., O’Malley P. M., Miech R. A., Bachman J. G., Schulenberg J. E. Ann Arbor, MI: Institute for Social Research, The University of Michigan; 2015. Monitoring the Future national survey results on drug use, 1975-2014: Overview, key findings on adolescent drug use. [Google Scholar]
- Kazdin A. E. Mediators and mechanisms of change in psychotherapy research. Annual Review of Clinical Psychology. 2007;3:1–27. doi: 10.1146/annurev.clinpsy.3.022806.091432. doi:10.1146/annurev.clinpsy.3.022806.091432. [DOI] [PubMed] [Google Scholar]
- Kim Y., Myung S. K., Jeon Y. J., Lee E. H., Park C. H., Seo H. G., Huh B. Y. Effectiveness of pharmacologic therapy for smoking cessation in adolescent smokers: Meta-analysis of randomized controlled trials. American Journal of Health-System Pharmacy. 2011;68:219–226. doi: 10.2146/ajhp100296. doi:10.2146/ajhp100296. [DOI] [PubMed] [Google Scholar]
- Kranzler H. R., Armeli S., Feinn R., Tennen H., Gelernter J., Covault J. GRIK1 genotype moderates topiramate’s effects on daily drinking level, expectations of alcohol’s positive effects and desire to drink. International Journal of Neuropsychopharmacology. 2014a;17:1549–1556. doi: 10.1017/S1461145714000510. doi:10.1017/S1461145714000510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler H. R., Covault J., Feinn R., Armeli S., Tennen H., Arias A. J., Kampman K. M. Topiramate treatment for heavy drinkers: Moderation by a GRIK1 polymorphism. American Journal of Psychiatry. 2014b;171:445–52. doi: 10.1176/appi.ajp.2013.13081014. doi:10.1176/appi.ajp.2013.13081014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G., Copeland J. The adolescent cannabis check-up: Randomized trial of a brief intervention for young cannabis users. Journal of Substance Abuse Treatment. 2008;34:407–414. doi: 10.1016/j.jsat.2007.07.004. doi:10.1016/j.jsat.2007.07.004. [DOI] [PubMed] [Google Scholar]
- McNeish D. Multilevel mediation with small samples: A cautionary note on the multilevel structural equation modeling framework. Structural Equation Modeling: A Multidisciplinary Journal. 2017;24:1–17. doi:10.1080/10705511.2017.1280797. [Google Scholar]
- Meier M. H., Caspi A., Ambler A., Harrington H., Houts R., Keefe R. S. E., Moffitt T. E. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E2657–E2664. doi: 10.1073/pnas.1206820109. doi:10.1073/pnas.1206820109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda R. J., MacKillop J., Treloar H., Blanchard A., Tidey J. W., Swift R. M., Monti P. M. Biobehavioral mechanisms of topiramate’s effects on alcohol use: An investigation pairing laboratory and ecological momentary assessments. Addiction Biology. 2016;21:171–182. doi: 10.1111/adb.12192. doi:10.1111/adb.12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda R., Ray L., Blanchard A., Reynolds E. K., Monti P. M., Chun T., Ramirez J. Effects of naltrexone on adolescent alcohol cue reactivity and sensitivity: An initial randomized trial. Addiction Biology. 2013;19:941–954. doi: 10.1111/adb.12050. doi:10.1111/adb.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda R., Treloar H. Emerging pharmacologic treatments for adolescent substance use: Challenges and new directions. Current Addiction Reports. 2016;3:145–156. doi: 10.1007/s40429-016-0098-7. doi:10.1007/s40429-016-0098-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda R., Treloar H., Blanchard A., Justus A., Monti P. M., Chun T., Gwaltney C. J. Topiramate and motivational enhancement therapy for cannabis use among youth: A randomized placebo-controlled pilot study. Addiction Biology. 2017;22:779–790. doi: 10.1111/adb.12350. doi:10.1111/adb.12350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira F. A., Grieb M., Lutz B. Central side-effects of therapies based on CB1 cannabinoid receptor agonists and antagonists: Focus on anxiety and depression. Best Practice & Research Clinical Endocrinology & Metabolism. 2009;23:133–144. doi: 10.1016/j.beem.2008.09.003. doi:10.1016/j.beem.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Muthén B. Bayesian analysis in Mplus: A brief introduction. 2010 Retrieved from http://www.statmodel.com/download/IntroBayesVersion%203.pdf.
- Muthén B., Asparouhov T. Bayesian structural equation modeling: A more flexible representation of substantive theory. Psychological Methods. 2012;17:313–335. doi: 10.1037/a0026802. doi:10.1037/a0026802. [DOI] [PubMed] [Google Scholar]
- Preacher K. J., Zhang Z., Zyphur M. J. Alternative methods for assessing mediation in multilevel data: The advantages of multilevel SEM. Structural Equation Modeling: A Multidisciplinary Journal. 2011;18:161–182. doi:10.1080/10705511.2011.557329. [Google Scholar]
- Preacher K. J., Zyphur M. J., Zhang Z. A general multilevel SEM framework for assessing multilevel mediation. Psychological Methods. 2010;15:209–233. doi: 10.1037/a0020141. doi:10.1037/a0020141. [DOI] [PubMed] [Google Scholar]
- Shank R. P., Gardocki J. F., Streeter A. J., Maryanoff B. E. An overview of the preclinical aspects of topiramate: Pharmacology, pharmacokinetics, and mechanism of action. Epilepsia. 2000;41(Supplement s1):3–9. doi:10.1111/j.1528-1157.2000.tb02163.x. [PubMed] [Google Scholar]
- Shiffman S. Ecological momentary assessment (EMA) in studies of substance use. Psychological Assessment. 2009;21:486–497. doi: 10.1037/a0017074. doi:10.1037/a0017074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeone T. A., Wilcox K. S., White H. S. Subunit selectivity of topiramate modulation of heteromeric GABA(A) receptors. Neuropharmacology. 2006;50:845–857. doi: 10.1016/j.neuropharm.2005.12.006. doi:10.1016/j.neuropharm.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Simkin D. R., Grenoble S. Pharmacotherapies for adolescent substance use disorders. Child and Adolescent Psychiatric Clinics of North America. 2010;19:591–608. doi: 10.1016/j.chc.2010.03.010. doi:10.1016/j.chc.2010.03.010. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. DASIS Series S-61, HHS Publication No. (SMA) 12–4701; Rockville, MD: 2012. Treatment Episode Data Set (TEDS) 2000–2010. Author. Retrieved from https://wwwdasis.samhsa.gov/dasis2/teds_pubs/2010_teds_rpt_natl.pdf. [Google Scholar]
- Tidey J. W., Monti P. M., Rohsenow D. J., Gwaltney C. J., Miranda R., McGeary J. E., Paty J. A. Moderators of naltrexone’s effects on drinking, urge, and alcohol effects in non-treatment-seeking heavy drinkers in the natural environment. Alcoholism: Clinical and Experimental Research. 2008;32:58–66. doi: 10.1111/j.1530-0277.2007.00545.x. doi:10.1111/j.1530-0277.2007.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trull T. J., Ebner-Priemer U. W. Using experience sampling methods/ecological momentary assessment (ESM/EMA) in clinical assessment and clinical research: Introduction to the special section. Psychological Assessment. 2009;21:457–462. doi: 10.1037/a0017653. doi:10.1037/a0017653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyaya H. P., Deas D. Pharmacological interventions for adolescent substance use disorders. In: Kaminer Y., Bukstein O. G., editors. Adolescent substance abuse: Psychiatric comorbidity and highrisk behaviors. New York, NY: Taylor & Francis Group; 2008. pp. 145–161. [Google Scholar]
- Volkow N. D., Baler R. D., Compton W. M., Weiss S. R. B. Adverse health effects of marijuana use. The New England Journal of Medicine. 2014;370:2219–2227. doi: 10.1056/NEJMra1402309. doi:10.1056/NEJMra1402309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N. D., Koob G. F., McLellan A. T. Neurobiologic advances from the brain disease model of addiction. The New England Journal of Medicine. 2016;374:363–371. doi: 10.1056/NEJMra1511480. doi:10.1056/NEJMra1511480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D. D., Roffman R. A., Stephens R. S., Wakana K., Berghuis J. Motivational enhancement therapy for adolescent marijuana users: A preliminary randomized controlled trial. Journal of Consulting and Clinical Psychology. 2006;74:628–632. doi: 10.1037/0022-006X.74.3.628. doi:10.1037/0022-006X.74.3.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters K. C., Tanner-Smith E. E., Bresani E., Meyers K. Current advances in the treatment of adolescent drug use. Adolescent Health, Medicine and Therapeutics. 2014;5:199–210. doi: 10.2147/AHMT.S48053. doi:10.2147/AHMT.S48053. [DOI] [PMC free article] [PubMed] [Google Scholar]