The mammalian gastrointestinal (GI) tract houses hundreds of species of microbes from all domains of life—Archaea, Bacteria, and Eukarya. As in many environments, bacteria are the most abundant members of this complex microbial community. Hundreds of metabolites attributed to the gut microbial community circulate in the mammalian bloodstream,1 extending the reach of the microbiome to every host cell. It is no wonder that the GI tract has been described as the body’s largest endocrine organ.2 Given the role, contributions, and potential of the gut microbiome, it would be valuable to know how environmental conditions in the gut influence the composition and function of its microbiota. In this issue, Albenberg et al3 unveiled new evidence supporting a dynamic mammalian microbiome by exploring the potential for molecular oxygen (O2) to shape the structure of the gut microbiome and offer a potential mechanism of the benefit of hyperbaric O2 treatment in intestinal diseases.4,5
O2 in the environment has a dramatic influence on the evolution of life6 and its availability remains an important driver of organismal physiology and ecology, particularly in the microbial world. As a potent electron acceptor, microbes that can utilize O2 for respiration are at a tremendous selective advantage in environments containing O2. The consequences of this selective force are particularly strong in environments rich in organic compounds but with few alternative electron acceptors for microbial respiration, that is, any place where fermentation is the primary, energy-yielding form of metabolism. The digestive tracts of most animals have regions that fit this description precisely: A regular input of food supporting dense communities of fermentative microbes.
The availability of electron acceptors—in particular nitrate and tetrathionate—in the anoxic environment of mammalian colon leads to blooms of bacteria able to respire these compounds. Nitrate (NO3 −) is generated as a metabolic byproduct of the host inflammatory response and fuels blooms of Escherichia coli in the inflamed colons of mice.7 Similarly, populations of Salmonella enterica thrive in the inflamed gut by triggering a cascade of events that leads to the production of tetrathionate (S4O62−), another potent electron acceptor that can be respired by populations of Salmonella.8 It has been suggested that infusion of O2 after gastric bypass surgery9 may lead to the observed enrichments of Proteobacteria in the colon associated with this procedure.
Another consequence of O2 in the gut was revealed in a recent study of gene expression in Shigella flexneri. Coordinated expression of virulence determinants, including genes involved in the type III secretion systems, were observed in response to a zone of oxygenation adjacent to the mucosa of the GI tract.10 This microoxic zone was attributed to diffusion of O2 from the capillary network at the tips of villi and extended approximately 70 μm into the lumen.10
One limitation that we have in understanding the full impact of O2 on the structure and function of the microbiota is the lack of accurate measurements O2 concentrations that microbes experience. As reported in this issue of Gastroenterology, Albenberg et al met this challenge with the development of a phosphorescent probe, OxyphorMicro, that was distributed throughout the GI tract of mice by mixing the probe with mouse chow. Phosphorescence of the probes, which is quenched proportionately with the concentration of O2, was measured with a specially designed fiberoptic detector. The instrument was inserted after laparotomy to measure phosphorescence of the probe in the organs of interest. OxyphorMicro is a particularly appropriate probe to use because it is insensitive to the presence of solutes in the aqueous environment of the intestines, thus providing sensitive and accurate measurements of O2.
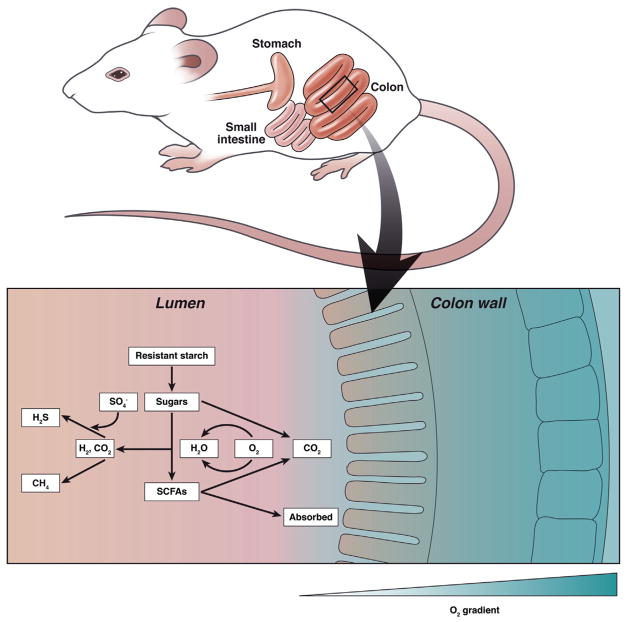
In agreement with previous measurements in the cecum,11 luminal concentrations of O2 were approximately 40 mmHg. That concentration increased after inhalation of pure O2, reflecting diffusion of O2 from the tissue into the cecum lumen. Based on the compilation of O2 concentrations throughout experimental manipulations, the authors conclude that there is a steep, radial gradient of O2 in the intestines (Figure 1). O2 diffusing into the lumen is consumed by mucosal-associated microbes resulting in extremely low concentrations of O2 in the intestinal lumen (<1 mmHg). The microbiota in the mucosa are clearly important to the health of the host, and until now we know little about this community of microbes and how it is structured by the chemical features of the environment. Albenberg et al showed that mucosa-associated bacteria are not static under physiologic conditions and are highly influenced by the O2 tension in mucosa layer. The dynamic shifts in the microbiome in turn may affect the functional status of the mucosal layer contributing to mucosal homeostasis or pathology.
Figure 1.
The radial O2 gradient in the mouse colon and an overview of metabolic pathways that could be altered by the presence of O2.
To examine the potential for O2 to shape the composition of the gut microbiome, mice were exposed to hyperbaric oxygen therapy. After 9 days of hyperbaric oxygen therapy, tissue oxygenation increased 5-fold and the microbial community surveyed in fecal samples also shifted in composition. These finding were extended to the human microbiome through molecular surveys of microbial communities generated from biopsies of the rectal mucosa. The relative abundance of aerotolerant bacteria, particularly members of the Proteobacteria and Actinobacteria phyla, were enriched in the mucosa compared with fecal samples, again suggesting that O2 is shaping this region of the gut microbiota.
The mammalian intestinal tract has traditionally been considered to be an anoxic environment that harbors an array of obligately and facultatively anaerobic bacteria, but potential roles of O2 in this ecosystem are being reexamined. We know that the capacity to harvest the low levels of O2 found in these environments, which is conferred by cytochrome oxidases with a high-affinity for O2, are encoded in phylogenetically diverse microbes.12 Furthermore, an estimated 30% of the microbes from the mammalian GI tract were found to have the metabolic potential to use low, even nanomolar concentrations of O212
Clinical literature has suggested potential benefits of hyperbaric O2 therapy in chronic intestinal disorders, such as radiation enteritis and inflammatory bowel disease.4,5 The mechanism is believed to be primarily an increased delivery of O2 up to the injured intestinal tissue to promote faster healing, enhance immunity, and prevent colonization of harmful bacteria.13 Albenberg et al demonstrated convincingly that hyperbaric O2 therapy also alters the host mucosal bacterial communities and may provide further protection against pathogenesis by potential pathobionts in the gut. More research is needed to assess the functional role of hyperbaric O2-induced microbiota and whether this therapy may be beneficial in the co-management of patients with chronic intestinal inflammation or injury.
Understanding the role of O2 in shaping the structure and function of microbial communities, and thus the interaction of these communities with the mucosa, will advance our ability to build predictive models of interactions between the host and microbiota. Such an understanding will provide information important not only in the GI tract, but at all mucosal surfaces where gradients of O2 exist.
Footnotes
Conflicts of interest
The authors disclose no conflicts.
Contributor Information
THOMAS M. SCHMIDT, Department of Internal Medicine and Department of Ecology and Evolutionary Biology
JOHN Y. KAO, Department of Internal Medicine, University of Michigan, Ann Arbor, Michigan
References
- 1.Wikoff WR, Anfora AT, Liu J, et al. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci U S A. 2009;106:3698–3703. doi: 10.1073/pnas.0812874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Devkota S, Chang EB. Nutrition, microbiomes, and intestinal inflammation. Curr Opin Gastroenterol. 2013;29:603–607. doi: 10.1097/MOG.0b013e328365d38f. [DOI] [PubMed] [Google Scholar]
- 3.Albenberg L, Esipova TV, Judge CP, et al. Correlation between intraluminal oxygen gradient and radial partitioning of intestinal microbiota. Gastroenterology. 2014;147:1055–1063. doi: 10.1053/j.gastro.2014.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marshall GT, Thirlby RC, Bredfeldt JE, et al. Treatment of gastrointestinal radiation injury with hyperbaric oxygen. Undersea Hyperb Med. 2007;34:35–42. [PubMed] [Google Scholar]
- 5.Dulai PS, Gleeson MW, Taylor D, et al. Systematic review: The safety and efficacy of hyperbaric oxygen therapy for inflammatory bowel disease. Aliment Pharmacol Ther. 2014;39:1266–1275. doi: 10.1111/apt.12753. [DOI] [PubMed] [Google Scholar]
- 6.Canfield DE. Oxygen: a four billion year history. Princeton: Princeton University Press; 2014. [Google Scholar]
- 7.Winter SE, Winter MG, Xavier MN, et al. Host-derived nitrate boosts growth of E. coli in the inflamed gut. Science. 2013;339:708–711. doi: 10.1126/science.1232467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winter SE, Thiennimitr P, Winter MG, et al. Gut inflammation provides a respiratory electron acceptor for Salmonella. Nature. 2010;467:426–429. doi: 10.1038/nature09415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang H, DiBaise JK, Zuccolo A, et al. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci U S A. 2009;106:2365–2370. doi: 10.1073/pnas.0812600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marteyn B, West NP, Browning DF, et al. Modulation of Shigella virulence in response to available oxygen in vivo. Nature. 2010;465:355–358. doi: 10.1038/nature08970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sheridan WG, Lowndes RH, Young HL. Intraoperative tissue oximetry in the human gastrointestinal tract. Am J Surg. 1990;159:314–319. doi: 10.1016/s0002-9610(05)81226-7. [DOI] [PubMed] [Google Scholar]
- 12.Morris RL, Schmidt TM. Shallow breathing: bacterial life at low O(2) Nat Rev Microbiol. 2013;11:205–212. doi: 10.1038/nrmicro2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodriguez ML, Martin MM, Padellano LC, et al. Gastrointestinal toxicity associated to radiation therapy. Clin Transl Oncol. 2010;12:554–561. doi: 10.1007/s12094-010-0553-1. [DOI] [PubMed] [Google Scholar]