Abstract
Background
Tuberculous pleurisy (TP) presents a diagnostic problem due to the limitations of traditional diagnostic methods. Different studies with the Xpert MTB/RIF assay have drawn variable conclusions about its values in TP diagnosis. We conducted a meta-analysis to assess whether the Xpert MTB/RIF assay is appropriate for the diagnosis of TP using pleural fluid samples.
Methods
A systematic search of four literature databases in English and Chinese language was performed to identify studies involving the use of Xpert MTB/RIF in patients with TP confirmed by plural biopsy and/or mycobacterial culture. Pooled sensitivity, specificity and accordance proportion were calculated, and the forest plots were generated to assess the accuracy of Xpert MTB/RIF for TP diagnosis.
Results
We identified 23 studies meeting our inclusion criteria. The pooled sensitivity and specificity of Xpert MTB/RIF were 30% (95% CI: 21–42%, I2 = 87.93%) and 99% (95% CI: 97–100%, I2 = 96.20%), respectively, and the area under the SROC curve (AUC) of Xpert MTB/RIF was 0.86 (95% CI: 0.83–0.89). Compared with drug susceptibility testing (DST), the pooled accordance rate of Xpert MTB/RIF in detecting rifampicin-susceptible cases and rifampicin-resistant cases was 99% (95% CI: 95–104%, I2 = 8.7%) and 94% (95% CI: 86–102%), respectively.
Conclusions
Our analysis suggests that the Xpert MTB/RIF assay is of limited value as a screening test for TP but has a high potential for confirming TP diagnosis and differentiating TP from non-TB diseases using pleural fluid samples.
Electronic supplementary material
The online version of this article (10.1186/s12879-018-3196-4) contains supplementary material, which is available to authorized users.
Keywords: Xpert MTB/RIF, Tuberculous pleurisy, Pleural fluid, Rifampicin resistance, Systematic review
Background
Tuberculosis (TB) remains a serious, life-threatening disease worldwide, with nearly 10.4 million cases and 1.7 million deaths reported in 2016 by the World Health Organization (WHO) [1]. While pulmonary TB is the most common presentation, extra-pulmonary TB is also an important clinical problem. One of the most common types of extra-pulmonary TB is tuberculous pleurisy (TP), which accounts for about one fourth of all TB cases [2]. At present, diagnosis of TP depends largely on detection of Mycobacterium tuberculosis in pleural fluid or pleura by microbiological culture, or demonstration of caseous granulomas in pleura by histopathological examination. However, these methods are invasive, laborious, time-consuming and insensitive, which often delay diagnosis and treatment.
Over the past several years, there has been a significant increase in using the Xpert MTB/RIF assay (also referred to as Xpert; Cepheid Inc., USA), which is an automated, cartridge-based nucleic acid amplification test for TB. This assay has the ability to simultaneously detect M. tuberculosis nucleic acid and resistance to rifampin (RIF) in less than 2 h. Due to its excellent performance, this assay has been recommended by WHO for diagnosing TB and detecting rifampicin resistance in pulmonary and extra-pulmonary TB in adults and children as well as for initial screening of individuals suspected of having multiple drug resistant-TB (MDR-TB) and HIV-co-infected TB cases [3, 4]. Most of the reported studies on Xpert MTB/RIF have been performed in sputum samples from pulmonary TB while there are relatively scarce reports using other types of samples from extra-pulmonary TB.
A limited number of studies have reported the utility of Xpert MTB/RIF in diagnosing TP, with highly variable sensitivity and specificity, ranging from 13 to 100%, between studies [5–7]. In one study carried out in pleural tissue samples from 17 patients with TP, Xpert MTB/RIF failed to detect any TP cases [8]. It is known that mycobacterial culture has limited ability to detect TP, when the study used only a culture reference standard and without histological biopsy, it is likely to overestimate the sensitivity of Xpert and underestimate the specificity [5, 9]. Although there is a published meta-analysis on the performance of Xpert MTB/RIF in diagnosing TP [10], this study has the limitations of using non-stringent inclusion criteria for TP patients (particularly without histopathological findings) and not including data on rifampicin-resistance. Therefore, the applicability of Xpert MTB/RIF to the diagnosis of TP as well as the detection of rifampicin-resistance in TP patients remains largely unclear.
To better understand the value of Xpert MTB/RIF in TP diagnosis, we conducted a comprehensive meta-analysis of literature published up to May 2018 involving the use of Xpert MTB/RIF for detecting TB and rifampicin resistance in TP patients.
Methods
Our meta-analysis was presented with reference to the recommendations from the PRISMA statement [11]. All data involved in this analysis were extracted from published articles, therefore, ethical approval was not applicable in this study.
Data sources and search strategy
A systematic search about studies of the accuracy of the Xpert MTB/RIF in diagnosing TP and rifampicin resistance was carried out. We searched the EMBASE, Cochrane, MEDLINE (PubMed) and China Science and Technology Journal (CSTJ) databases to identify original research articles and conference abstracts in English or Chinese language published on or before May 25, 2018. Search was implemented by using combinations of the following items: “pleural tuberculosis”, “tuberculous pleuritis”, “tuberculous pleural effusion”, “TPE”, “Xpert MTB/RIF”, “GeneXpert”, “Xpert”, and “TB/RIF”.
Reference standard and study selection
Our literature search was restricted to studies involving the use of Xpert MTB/RIF in patients diagnosed as TP according to the current gold standard: a combination of histopathological examination and mycobacterial culture [2, 12]. That is: the reference standard of confirmed TP should include positive Mycobacterium tuberculosis culture from pleural fluid or tissue, or/ and histological manifestations of granulomas in pleural tissue.
As the first step, two investigators (ZY.Huo and L.Peng) independently screened for articles containing the defined items in the title and abstract. All articles reporting the performance of Xpert MTB/RIF in diagnosing TP and rifampicin resistance on pleural fluid samples were retrieved for full-text review. Through full-text review, articles were excluded if they met any of the following criteria: 1) Experiments were not performed with a commercial Xpert MTB/RIF assay; 2) The performance of Xpert MTB/RIF was not evaluated; 3) TP cases were not defined using a combination of histopathological examination and mycobacterial culture; 4) Xpert MTB/RIF was performed using blood or non-pleural fluid specimens; 5) Duplicated reports from the same research group; 6) Reports of systematic reviews or meta-analysis. After full-text review, the two researchers met together to compare the retrieved articles meeting the inclusion criteria. In case of any discrepancy, a third person (physician from our hospital) was invited to discuss and resolve the discrepancy.
Data extraction and quality assessment
Two researchers (ZY.Huo and L.Peng) independently extracted and crosschecked the data from all included articles. The following information was retrieved from all included articles: 1) The first author, publication year and study location; 2) The number and age of the enrolled participants; 3) The proportion of HIV-seropositive participants; 4) The specimen types and diagnostic standard; 5) The statistics of positive and negative results of the Xpert MTB/RIF assay; 6) The statistics of rifampicin sensitive and resistant cases for the Xpert MTB/RIF assay. The quality of all included articles was evaluated based upon the recommendations from QUADAS-2 checklist [13]. Based on the QUADAS-2 system, concerns with respect to applicability and risk of bias in meta-analysis were verbalized as “high”, “unclear” or “high”. Two researchers (ZY.Huo and L.Peng) independently scored and recorded all included references using the QUADAS-2 tool, and then reviewed the results together. Next, IBM SPSS software 19.0 (SPSS Inc., USA) was used to calculate the kappa statistic for consistency check. In case of discrepancy, a third person (statistician from our university) was invited to re-evaluate the data and solve the disagreement.
Statistical analysis
We performed descriptive statistics and analyses adopting the recommended methods for assessment of diagnostic trials in meta-analysis [14, 15]. All data analysis including the pooled sensitivity, specificity, and accordance proportion with corresponding 95% confidence intervals (95% CI), and the I2 statistic test was performed using the Stata/MP 13.1 (Stata Corp., USA). The results were summarized and synthesized by using forest plots. A symmetric receiver operator characteristic (SROC) curve was made to present the individual assessment of sensitivity and specificity for each study [16–18]. The publication bias of inclusive researches was evaluated by Deeks’ funnel plot asymmetry test.
Results
Literature search results and characteristics of published studies
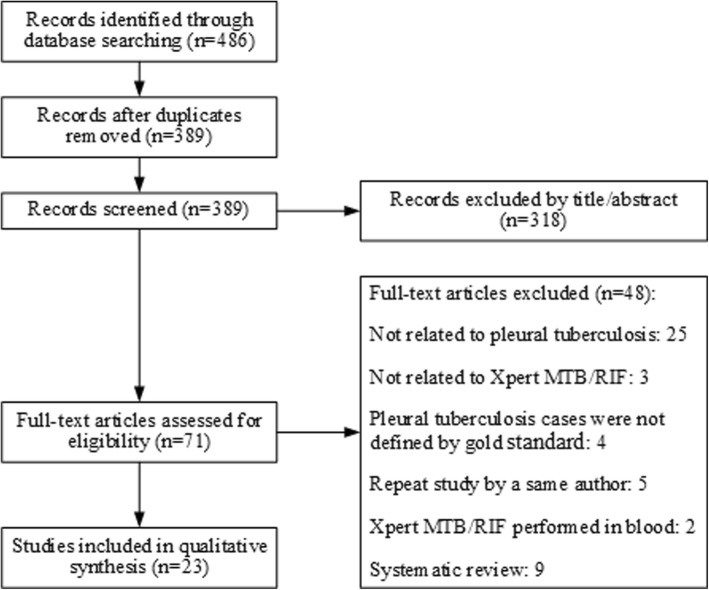
Initial literature search resulted in a total of 486 unique studies. Following full-text review, we identified 23 studies that met all search criteria and were suitable for meta-analysis [5–9, 19–36]. Details of the literature selection process are illustrated in Fig. 1. The characteristics of the 23 included studies are summarized in Table 1. All these 23 studies demonstrated the performance of Xpert MTB/RIF in detecting TB or rifampicin resistance in TP patients confirmed following the gold standard involving a combination of mycobacterial culture and histopathological examination of pleural samples. Publication years ranged from 2011 to 2018 (Table 1). Study locations included 11 countries across Europe, South America, Asia and Africa. There was a total of 2646 individuals with pleural effusion, including 1194 (45.1%) with confirmed TP and 1452 (54.9%) without TP. Of the 23 studies, 7 reported the utility of Xpert MTB/RIF in HIV-associated TB patients, and 2 reported results of Xpert MTB/RIF in detection of rifampicin resistance in comparison with drug susceptibility testing (DST) [9, 31] (Table 2).
Fig. 1.
Flowchart diagram of the literature search process
Table 1.
Characteristics of 23 published studies included for meta-analysis and the primary results of Xpert MTB/RIF test
| First author | Year | Country | Patients enrolled | Mean age (years)a | HIV-infection prevalence (%) | Specimen types | Reference standard | Results | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TP | FP | FN | TN | ||||||||
| Causse | 2011 | Spain | 34 | 45(5–83) | NR | Pleural fluid | Culture | 4 | 0 | 0 | 30 |
| Friedrich | 2011 | South Africa | 25 | NR | NR | Pleural fluid | Culture | 5 | 0 | 15 | 5 |
| Malbruny | 2011 | France | 12 | 52 | 4.3% | Pleural fluid | Culture | 0 | 0 | 2 | 10 |
| Moure | 2012 | Spain | 31 | NR | NR | Pleural fluid | Culture | 7 | 0 | 19 | 5 |
| Tortoli | 2012 | Italy | 330 | NR | NR | Pleural fluid | Culture | 5 | 3 | 10 | 312 |
| Christopher | 2013 | India | 91 | 46(33–57) | NR | Pleural fluid | Culture and biopsy | 4 | 0 | 26 | 61 |
| Porcel | 2013 | Spain | 67 | 50 | 0% | Pleural fluid | Culture and biopsy | 5 | 0 | 28 | 34 |
| Zmak | 2013 | Croatia | 42 | NR | NR | Pleural fluid | Culture | 0 | 0 | 1 | 41 |
| Javed | 2014 | Pakistan | 25 | NR | NR | Pleural fluid | Biopsy | 2 | 0 | 12 | 11 |
| Lusiba | 2014 | Uganda | 116 | 34 ± 13 | 44.8% | Pleural fluid | Culture and biopsy | 25 | 1 | 62 | 28 |
| Meldau | 2014 | South Africa | 88 | 51 | 10.2% | Pleural fluid | Culture and biopsy | 9 | 1 | 31 | 47 |
| Scott | 2014 | South Africa | 528 | 39 | NR | Pleural fluid | Culture | 227 | 3 | 255 | 43 |
| Sharma SK | 2014 | India | 364 | NR | NR | Pleural fluid | Culture | 37 | 8 | 54 | 265 |
| Theron | 2014 | South Africa | 76 | 55(38–65) | 17% | Pleural fluid | Culture | 5 | 6 | 11 | 54 |
| Trajman | 2014 | Brazil | 59 | 50 | 5% | Pleural fluid | Culture and biopsy | 1 | 0 | 32 | 26 |
| Coleman | 2015 | Malawi | 50 | 32 | 100% | Pleural fluid | Culture | 9 | 0 | 4 | 18 |
| Liu | 2015 | China | 126 | 38.6 ± 13.2 | 4.0% | Pleural fluid | Culture | 24 | 1 | 31 | 70 |
| Rufai | 2015 | India | 162 | 41.6 ± 19 | 0% | Pleural fluid | Culture | 23 | 0 | 19 | 119 |
| Wang | 2015 | China | 125 | 43 | NR | Pleural fluid | Culture | 13 | 47 | 0 | 65 |
| Che | 2017 | China | 78 | 44 (18–83) | 1.3% | Pleural fluid | Culture and biopsy | 12 | 0 | 48 | 18 |
| Li | 2018 | China | 70 | 42 ± 20 | 0% | Pleural fluid | Culture and biopsy | 6 | 0 | 39 | 25 |
| Sharma S | 2018 | India | 37 | 39 | 0% | Pleural fluid | Culture and biopsy | 5 | 0 | 2 | 30 |
| Christopher | 2018 | India | 130 | 50.9 ± 14.1 | 0% | Pleural fluid | Biopsy | 9 | 0 | 56 | 65 |
TP, true positive. FP, false positive. FN, false negative. TN, true negative. NR, not reported in the study
a The values represent the means±SD or medians with corresponding interquartile ranges (IQRs)
Table 2.
Characteristics and results of Xpert MTB/RIF studies on rifampicin resistance
| First author | Year | Country | Samples enrolled | Xpert MTB/RIF | DST | ||
|---|---|---|---|---|---|---|---|
| Rifampicin sensitive | Rifampicin resistant | Rifampicin sensitive | Rifampicin resistant | ||||
| Liu | 2015 | China | 43 | 32 | 11 | 33 | 10 |
| Wang | 2015 | China | 13 | 13 | 0 | 13 | 0 |
DST, drug susceptibility testing
Quality assessment of the included studies
Based on quality assessment by the QUADAS-2 tool, 11 of the 23 included studies showed a high risk of bias, whereas 5 of them had high applicability concerns. The kappa statistic between the primary results of two researchers (ZY.Huo and L.Peng) was 0.787 (P<0.01), which suggested a good inter-rater reliability of consistency. Additional information about the ratings of risk of bias and applicability concerns was provided in Additional file 1.
Sensitivity and specificity of Xpert MTB/RIF
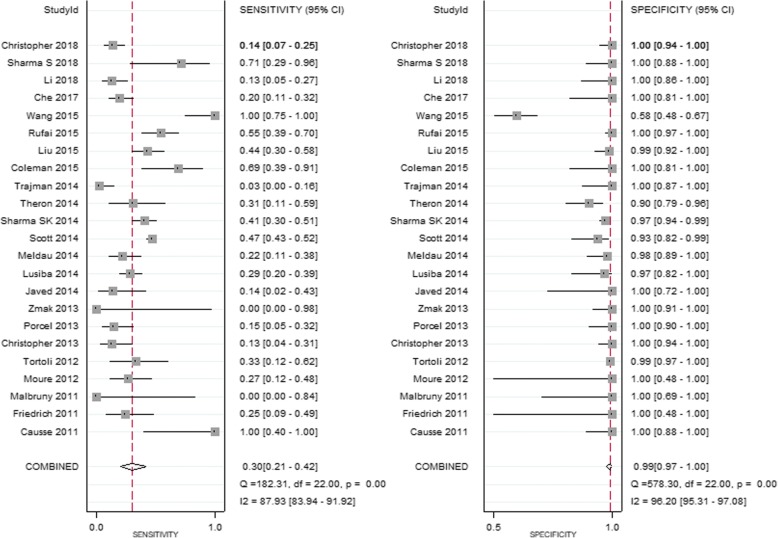
A total of 2646 eligible participants were used to evaluate the performance of Xpert MTB/RIF for TP diagnosis, including 1194 participants confirmed to be TP by mycobacterial culture and/or histopathological examination, and 1452 participants not diagnosed as TP by the same criteria. For all these participants, pleural fluid samples were used in the Xpert MTB/RIF assay. The pooled sensitivity of Xpert MTB/RIF was 30% (95% CI: 21–42%, I2 = 87.93%), while the pooled specificity was 99% (95% CI: 97–100%, I2 = 96.20%, Fig. 2). The SROC curve for Xpert MTB/RIF was situated near the desirable upper left corner of the plot (Fig. 3), and the area under the SROC curve (AUC) was 0.86 (95% CI: 0.83–0.89).
Fig. 2.
Forest plots of the performance of Xpert MTB/RIF in diagnosing TP. See references [5–9, 19–36] for details
Fig. 3.
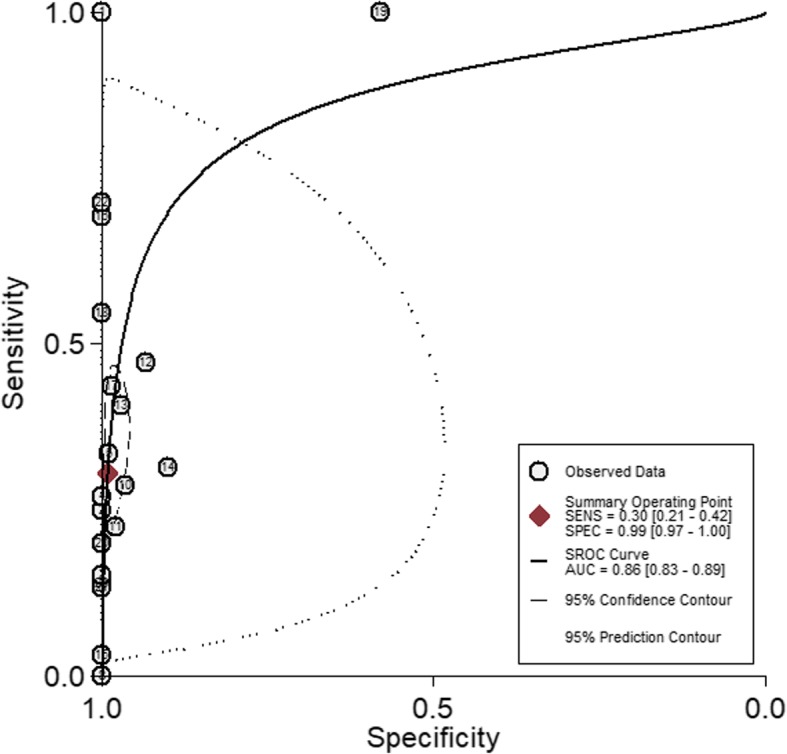
Symmetric receiver operator characteristic (SROC) curve for Xpert MTB/RIF assay. The SROC curve was derived by Stata/MP 13.1
Accordance rate of rifampicin-susceptible and rifampicin-resistant cases
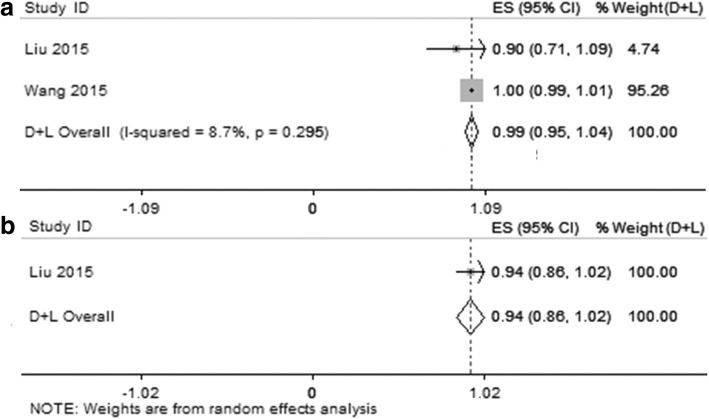
Two of the 23 included studies presented the performance of Xpert MTB/RIF for the detection of rifampicin susceptibility and resistance in TP patients [9, 31]. Comparison of the results between Xpert MTB/RIF and DST revealed a pooled accordance rate of was 99% (95% CI, 95–104%, I2 = 8.7%) in rifampicin-susceptible cases, and 94% (95% CI, 86–102%) in rifampicin-resistant cases (Fig. 4). In one of these two studies [31], there was no rifampicin resistant case for either Xpert MTB/RIF or DST (Table 2), and thus the accordance rate was not calculated (Fig. 4b). These results indicate a trend of high concordance of Xpert MTB/RIF with DST for detection of rifampicin susceptibility and resistance, although the limited number of inclusive studies.
Fig. 4.
Forest plots of the pooled accordance rate of the Xpert MTB/RIF and DST test results for rifampicin-susceptible cases (a) and rifampicin-resistant cases (b). See references [9, 31] for details
Publication bias
Based on Deeks’ funnel plot asymmetry test, there was no significant asymmetry for the 23 studies on Xpert MTB/RIF (P = 0.54) included in our meta-analysis, suggesting a low risk for publication bias (Fig. 5).
Fig. 5.
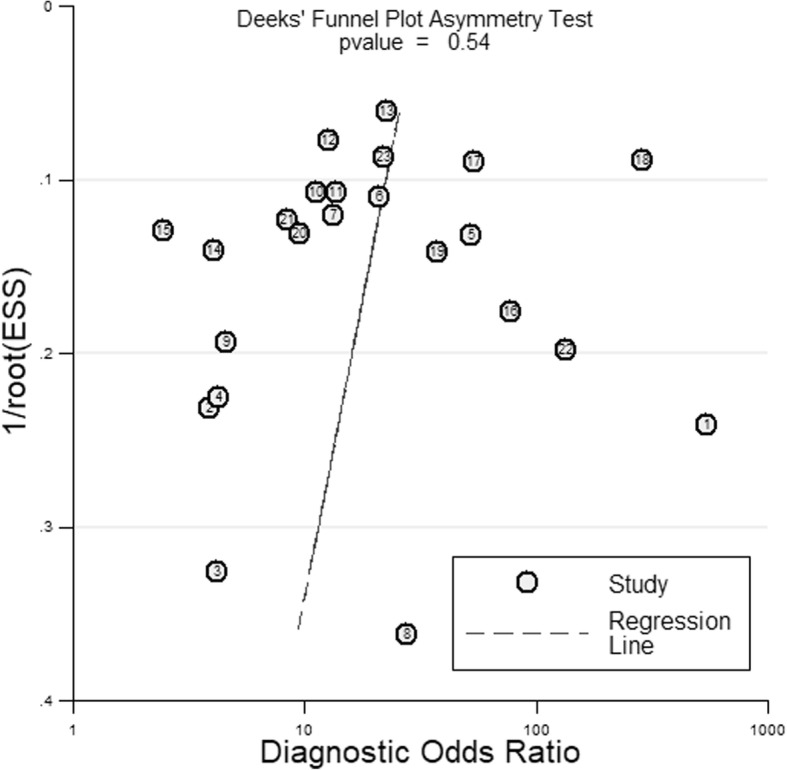
A Deeks’ funnel plot asymmetry test for evaluation of potential publication bias in Xpert MTB/RIF studies. This plot indicated a low risk of publication bias
Discussion
Despite an increasing and widespread use of Xpert MTB/RIF for the diagnosis of pulmonary and extra-pulmonary TB worldwide, it remains unclear how valuable this method is for diagnosing TB and detecting rifampicin resistance in TP patients using pleural fluid samples. To summarize the results of and to overcome the limitations of small samples size among existing individual studies, we conducted this systemic review and meta-analysis to assess the performance of Xpert MTB/RIF in diagnosing TP and detecting rifampicin susceptibility or resistance using pleural fluid specimens. In order to produce the best possible results, we adopted a combination of histopathological examination and mycobacterial culture as the reference standard, which is the current gold standard for the diagnosis of TP. Our meta-analysis involves a total of 2646 patients with pleural effusion from 23 eligible studies published between 2011 and 2018 among 11 countries across Europe, South America, Asia and Africa (Table 1).
Based on our meta-analysis, the overall sensitivity and specificity of Xpert MTB/RIF in diagnosing TP from 23 eligible studies were 30 and 99%, respectively, indicating a low sensitivity and a high specificity. While the high specificity of this assay suggests it to be an excellent rule-in test (confirming TP diagnosis), the low sensitivity suggests a limited rule-out value (ruling out TP diagnosis). The ability of Xpert MTB/RIF to detect rifampicin susceptibility or resistance was evaluated in two small studies, which showed a trend of high concordance with DST, suggesting its potential usefulness for detection of MDR-TB in TP patients.
A similar meta-analysis has been previously reported by Sehgal et al. [10], which reported a pooled sensitivity and specificity of 51.4 and 98.6%, respectively, with culture used as a reference standard, and 22.7 and 99.8%, respectively, with a composite reference standard (CRS) used as the benchmark. The specificity in this report is similar to that in our analysis (99%) while the sensitivity in this report appears to be different compared to our analysis (30%). The exact reasons for the difference in sensitivity are unclear. One possible explanation is the lack of histopathological examination in the reference standard in the report of Sehgal et al. [10], which may lead to different results in sensitivity compared to our analysis. We used a combination of mycobacterial culture and histopathological examination as the reference standard, and excluded 9 studies included in the report of Sehgal et al. [10], which did not meet our reference standard while adding 8 new studies [9, 23, 30, 31, 33–36] in our analysis. Given that the reference standard we used is the current gold standard, it is likely that the results of our analysis more reliable.
Nevertheless, among the 23 studies included in our analysis there was substantial heterogeneity (I2 = 87.93 and 96.20% for sensitivity and specificity, respectively). One prominent example is the study by Wang et al. [9], which showed an exceptionally high sensitivity but a very low specificity as clearly shown in the forest plots in Fig. 2. The reason for this observation is uncertain but could be related to the use of mycobacterial culture alone as the reference standard in this study. Since the mycobacterial culture method is known to have low sensitivity in TB diagnosis, evaluation of Xpert MTB/RIF using samples from patients confirmed by culture alone is likely to overestimate the sensitivity and underestimate the specificity, as has been reported previously [5, 9].
Although there is apparent variation in the sensitivity of Xpert MTB/RIF among different studies on TP patients, the overwhelming trend is a low sensitivity (around 30%). When this assay is used as a diagnostic test, approximately 70% of TP patients could be misdiagnosed, suggesting that this assay is not appropriate as an initial screening test for suspected TP patients in countries with high TB burden. The reasons for this low sensitivity remain poorly understood, but could be due to the presence of PCR inhibitors in pleural fluid samples, and use of inappropriate or inefficient sampling methods [37]. Clearly, further research is needed to optimize the sample processing in order to improve the sensitivity of Xpert MTB/RIF.
Despite a low sensitivity for diagnosing TP, Xpert MTB/RIF consistently showed an excellent specificity (99%), which is attributed largely to the use of highly specific genetic target in this test. The high specificity suggests its high value in confirming TP diagnosis and differentiating TP from non-TB diseases, especially in countries with low or intermediate TB burden.
For the Xpert MTB/RIF assay in detection of rifampicin resistance in pleural fluid samples, the pooled accordance rate of rifampicin-susceptible cases and rifampicin-resistant cases was 99 and 94%, separately. The high concordance between the Xpert MTB/RIF and DST indicated good efficiency for rifampicin resistance detection, which was similar to the previous studies [38–40]. Although the number of inclusive studies in our meta-analysis is limited, the result indicated the Xpert MTB/RIF could rapidly detect patients suffered from MDR-TB and give them rapid initiation of anti-MDR-TB therapy.
The Deeks’ funnel plot asymmetry test was performed to evaluate and analyze the publication bias among 23 inclusive studies (Fig. 5). The plot showed no significant asymmetry for the Xpert MTB/RIF (P = 0.54), and there was no evidence of potential risk of publication bias.
Conclusions
In summary, the results of our meta-analysis suggest that the Xpert MTB/RIF assay is of limited value as a screening test for TP but has a high potential for confirming TP diagnosis and differentiating TP from non-TB diseases. The Xpert MTB/RIF assay showed high concordance with DST, suggesting its usefulness for detection of MDR-TB, which may help early decision making in anti-MDR-TB therapy.
Additional file
Methodological quality evaluation results of 23 studies sorted using the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) tool. The ratings of risk of bias and applicability concerns were provided. (TIF 3222 kb)
Acknowledgements
We sincerely thank all authors who provided published data for our meta-analysis, and we also acknowledge the editors and reviewers for insightful suggestions on this work. We thank Prof. Bin Peng and Dr. Youlun Li for their help in statistics and diagnostic criteria.
Funding
This work was supported by the National Key Clinical Specialist Construction Programs of China [2012] NO. 649. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
We declare that the data supporting the conclusions of this article are fully described within the article.
Abbreviations
- 95%CI
95% confidence intervals
- AUC
Area under the SROC curve
- DST
Drug susceptibility testing
- MDR-TB
Multiple drug resistant-TB
- PCR
Polymerase chain reaction
- PRISMA
Preferred reporting items for systematic reviews and meta-analyses
- QUADAS-2
Quality Assessment of Diagnostic Accuracy Studies-2
- RIF
Rifampin
- SROC
Symmetric receiver operator characteristic
- TB
Tuberculosis
- TP
Tuberculous pleurisy
- WHO
World Health Organization
Authors’ contributions
ZH and LP conceived and designed the experiments. ZH and LP analyzed the data. ZH and LP performed the experiments. ZH and LP wrote the paper. Both authors contributed equally to preparing the manuscript and approve of the content.
Ethics approval and consent to participate
All analyses were based on previous published studies, thus no ethical approval and patient consent are required.
Consent for publication
Not applicable.
Competing interests
All authors declare no competing interests. No competing interests exists in this study due to commercial or other associations (e.g., pharmaceutical stock ownership, consultancy, advisory board membership, relevant patents, or research funding). No competing interests exists in the submission of this manuscript, and the manuscript is approved by all of the authors for publication, all authors that the work described was original research that has not been published previously and is not under consideration for publication elsewhere in whole or in part.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s12879-018-3196-4) contains supplementary material, which is available to authorized users.
Contributor Information
Zhen-yu Huo, Email: 313879819@qq.com.
Li Peng, Phone: +86 23 89012806, Email: pli1228@163.com.
References
- 1.World Health Organization. Global tuberculosis report 2017. Available: http://apps.who.int/iris/bitstream/10665/259366/1/9789241565516-eng.pdf. Accessed 26 May 2018.
- 2.Light RW. Update on tuberculous pleural effusion. Respirology. 2010;15:451–458. doi: 10.1111/j.1440-1843.2010.01723.x. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resitance: Xpert MTB/RIF System. Available: http://whqlibdoc.who.int/publications/2011/9789241501545_eng.pdf. Accessed 26 May 2018. [PubMed]
- 4.World Health Organization. Automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: Xpert MTB/RIF system for the diagnosis of pulmonary and extrapulmonaryTB in adults and children. Available: http://apps.who.int/iris/bitstream/10665/112472/1/9789241506335_eng.pdf. Accessed 26 May 2018. [PubMed]
- 5.Causse M, Ruiz P, Gutiérrez-Aroca JB, Casal M. Comparison of two molecular methods for rapid diagnosis of extrapulmonary tuberculosis. J Clin Microbiol. 2011;49:3065–3067. doi: 10.1128/JCM.00491-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malbruny B, Le Marrec G, Courageux K, Leclercq R, Cattoir V. Rapid and efficient detection of Mycobacterium tuberculosis in respiratory and non-respiratory samples. Int J Tuberc Lung Dis. 2011;15:553–555. doi: 10.5588/ijtld.10.0497. [DOI] [PubMed] [Google Scholar]
- 7.Tortoli E, Russo C, Piersimoni C, Mazzola E, Dal Monte P, Pascarella M, et al. Clinical validation of Xpert MTB/RIF for the diagnosis of extrapulmonary tuberculosis. Eur Respir J. 2012;40:442–447. doi: 10.1183/09031936.00176311. [DOI] [PubMed] [Google Scholar]
- 8.Christopher DJ, Schumacher SG, Michael JS, Luo R, Balamugesh T, Duraikannan P. Performance of Xpert MTB/RIF on pleural tissue for the diagnosis of pleural tuberculosis. Eur Respir J. 2013;42:1427–1429. doi: 10.1183/09031936.00103213. [DOI] [PubMed] [Google Scholar]
- 9.Wang D, Chen Z, Chen Q, Zhang G. Evaluation of GeneXpert MTB/RIF assay for diagnosis of tuberculous pleurisy and detection of rifampin resistance. Int J Respir. 2015;35:1071–1073. [Google Scholar]
- 10.Sehgal IS, Dhooria S, Aggarwal AN, Behera D, Agarwal R. Diagnostic performance of Xpert MTB/RIF in tuberculous pleural effusion: systematic review and meta-analysis. J Clin Microbiol. 2016;54:1133–1136. doi: 10.1128/JCM.03205-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Udwadia ZF, Sen T. Pleural tuberculosis: an update. Curr Opin Pulm Med. 2010;16:399–406. doi: 10.1097/MCP.0b013e328339cf6e. [DOI] [PubMed] [Google Scholar]
- 13.Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 14.Devillé WL, Buntinx F, Bouter LM, Montori VM, de Vet HC, van der Windt DA, et al. Conducting systematic reviews of diagnostic studies: didactic guidelines. BMC Med Res Methodol. 2002;2:9. doi: 10.1186/1471-2288-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leeflang MM, Deeks JJ, Gatsonis C, Bossuyt PM. Cochrane diagnostic test accuracy working group. Systematic reviews of diagnostic test accuracy. Ann Intern Med. 2008;149:889–897. doi: 10.7326/0003-4819-149-12-200812160-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moses LE, Shapiro D, Littenberg B. Combining independent studies of a diagnostic test into a summary ROC curve: data-analytic approaches and some additional considerations. Stat Med 1993; 12: 1293–1316. PMID: 8210827. [DOI] [PubMed]
- 17.Irwig L, Macaskill P, Glasziou P, Fahey M. Meta-analytic methods for diagnostic test accuracy. J Clin Epidemiol. 1995;48:119–130. doi: 10.1016/0895-4356(94)00099-C. [DOI] [PubMed] [Google Scholar]
- 18.Vamvakas EC. Meta-analyses of studies of the diagnostic accuracy of laboratory tests: a review of the concepts and methods. Arch Pathol Lab Med. 1998;122:675–686. [PubMed] [Google Scholar]
- 19.Friedrich SO, Von Groote-Bidlingmaier F, Diacon AH. Xpert MTB/RIF assay for diagnosis of pleural tuberculosis. J Clin Microbiol. 2011;49:4341–4342. doi: 10.1128/JCM.05454-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moure R, Martín R, Alcaide F. Effectiveness of an integrated real-time PCR method for detection of the Mycobacterium tuberculosis complex in smear-negative extrapulmonary samples in an area of low tuberculosis prevalence. J Clin Microbiol. 2012;50:513–515. doi: 10.1128/JCM.06467-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Porcel JM, Palma R, Valdés L, Bielsa S, San-José E, Esquerda A. Xpert® MTB/RIF in pleural fluid for the diagnosis of tuberculosis. Int J Tuberc Lung Dis. 2013;17:1217–1219. doi: 10.5588/ijtld.13.0178. [DOI] [PubMed] [Google Scholar]
- 22.Zmak L, Jankovic M, Jankovic VK. Evaluation of Xpert MTB/RIF assay for rapid molecular diagnosis of tuberculosis in a two-year period in Croatia. Int J Mycobacteriol. 2013;2:179–182. doi: 10.1016/j.ijmyco.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Javed N, Aslam M, Mushtaq MA, Khan T, Shaheen MZ. Role of gene Xpert in diagnosis of tuberculous pleural effusion: comparison with pleural biopsy. Eur Respir J. 2014;44(Suppl 58):P2655. [Google Scholar]
- 24.Lusiba JK, Nakiyingi L, Kirenga BJ, Kiragga A, Lukande R, Nsereko M, et al. Evaluation of Cepheid's Xpert MTB/RIF test on pleural fluid in the diagnosis of pleural tuberculosis in a high prevalence HIV/TB setting. PLoS One. 2014;9:e102702. doi: 10.1371/journal.pone.0102702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meldau R, Peter J, Theron G, Calligaro G, Allwood B, Symons G, et al. Comparison of same day diagnostic tools including gene Xpert and unstimulated IFN-γ for the evaluation of pleural tuberculosis: a prospective cohort study. BMC Pulm Med. 2014;14:58. doi: 10.1186/1471-2466-14-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scott LE, Beylis N, Nicol M, Nkuna G, Molapo S, Berrie L, et al. Diagnostic accuracy of xpert MTB/RIF for extrapulmonary tuberculosis specimens: establishing a laboratory testing algorithm for South Africa. J Clin Microbiol. 2014;52:1818–1823. doi: 10.1128/JCM.03553-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharma SK, Kohli M, Chaubey J, Yadav RN, Sharma R, Singh BK, et al. Evaluation of Xpert MTB/RIF assay performance in diagnosing extrapulmonary tuberculosis among adults in a tertiary care Centre in India. Eur Respir J. 2014;44:1090–1093. doi: 10.1183/09031936.00059014. [DOI] [PubMed] [Google Scholar]
- 28.Theron G, Peter J, Calligaro G, Meldau R, Hanrahan C, Khalfey H, et al. Determinants of PCR performance (Xpert MTB/RIF), including bacterial load and inhibition, for TB diagnosis using specimens from different body compartments. Sci Rep. 2014;4:5658. doi: 10.1038/srep05658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trajman A, De Oliveira EF DSSK, Bastos ML, Belo Neto E, Silva EM, Da Silva Lourenço MC, et al. Accuracy of polimerase chain reaction for the diagnosis of pleural tuberculosis. Respir Med. 2014;108:918–923. doi: 10.1016/j.rmed.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 30.Coleman M, Finney LJ, Komrower D, Chitani A, Bates J, Chipungu GA, et al. Markers to differentiate between Kaposi's sarcoma and tuberculous pleural effusions in HIV-positive patients. Int J Tuberc Lung Dis. 2015;19:144–150. doi: 10.5588/ijtld.14.0289. [DOI] [PubMed] [Google Scholar]
- 31.Liu X, Huang Z, Du J. Rapid diagnosis of pleural tuberculosis by Xpert MTB/RIF assay. Zhonghua jie he he hu xi za zhi. 2015;38:741–745. [PubMed] [Google Scholar]
- 32.Rufai SB, Singh A, Kumar P, Singh J, Singh S. Performance of xpert MTB/RIF assay in diagnosis of pleural tuberculosis by use of pleural fluid samples. J Clin Microbiol. 2015;53:3636–3638. doi: 10.1128/JCM.02182-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Che N, Yang X, Liu Z, Li K, Chen X. Rapid detection of cell-free Mycobacterium tuberculosis DNA in tuberculous pleural effusion. J Clin Microbiol. 2017;55:1526–1532. doi: 10.1128/JCM.02473-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li XL, Chen HM, Zhang LQ, Ma LP, WU XG, MQ GAO. Diagnostic value of three kinds of pleural effusion detection methods for pleural tuberculous. Chinese J Med. 2018;53:134–138. [Google Scholar]
- 35.Sharma S, Dahiya B, Sreenivas V, Singh N, Raj A, Sheoran A, et al. Comparative evaluation of GeneXpert MTB/RIF and multiplex PCR targeting mpb64 and IS6110 for the diagnosis of pleural TB. Future Microbiol. 2018;13:407–413. doi: 10.2217/fmb-2017-0147. [DOI] [PubMed] [Google Scholar]
- 36.Christopher DJ, Dinakaran S, Gupta R, James P, Isaac B, Thangakunam B. Thoracoscopic pleural biopsy improves yield of Xpert MTB/RIF for diagnosis of pleural tuberculosis. Respirology. 2018; 10.1111/resp.13275. [DOI] [PubMed]
- 37.Weyer K, Mirzayev F, Migliori GB, Van Gemert W, D’Ambrosio L, Zignol M, et al. Rapid molecular TB diagnosis: evidence, policy-making and global implementation of Xpert MTB/RIF. Eur Respir J. 2013;42:252–271. doi: 10.1183/09031936.00157212. [DOI] [PubMed] [Google Scholar]
- 38.Chang K, Lu W, Wang J, Zhang K, Jia S, Li F, et al. Rapid and effective diagnosis of tuberculosis and rifampicin resistance with Xpert MTB/RIF assay: a meta-analysis. J Inf Secur. 2012;64:580–588. doi: 10.1016/j.jinf.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 39.Steingart KR, Schiller I, Horne DJ, Pai M, Boehme CC, Dendukuri N. Xpert® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev. 2014;21:CD009593. doi: 10.1002/14651858.CD009593.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang XW, Pappoe F, Huang Y, Cheng XW, Xu DF, Wang H, et al. Xpert MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in children: a meta-analysis. Clin Lab. 2015;61:1775–1785. doi: 10.7754/clin.lab.2015.150509. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Methodological quality evaluation results of 23 studies sorted using the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) tool. The ratings of risk of bias and applicability concerns were provided. (TIF 3222 kb)
Data Availability Statement
We declare that the data supporting the conclusions of this article are fully described within the article.