Abstract
Introduction
Tumours involving the duodenum are usually treated with pancreaticoduodenectomy, which may be associated with considerable morbidity. Limited distal duodenal resection, a relatively smaller procedure, can be done in some of these patients. We describe our experience with this operation for such lesions.
Methods
We retrospectively analyzed, from prospectively collected data 10 consecutive patients who underwent limited duodenal and proximal jejunal resection between March 2011 and Nov 2015.
Results
There were 8 males and 2 females who had a median age of 47 years. Their common presentations were abdominal pain (50%) and upper gastrointestinal bleeding (40%). Five had malignancy (adenocarcinoma: 2, neuroendocrine tumours: 2, non Hodgkin's lymphoma 1). Three had gastrointestinal stromal tumours (GISTs) and 2 had other benign tumours (lipoma 1, ectopic pancreas 1). The 30-day post-operative morbidity rate was 60% (n = 6) with mostly minor complications (Clavien grade 1 or 2). Median post-operative stay was 9 (range, 6–13) days. All ten patients were alive without recurrence after a median follow up of 26.5 months.
Conclusion
Limited distal duodenal resection is a feasible surgical alternative to a pancreaticoduodenectomy in carefully selected patients with benign and some malignant tumours of the third and fourth part of the duodenum.
Keywords: Limited distal duodenectomy, Gastrointestinal stromal tumours, Duodenal adenocarcinoma, Segmental duodenectomy, Duodenal neuroendocrine tumours
Highlights
-
•
Pancreaticodudenctomy is the usual treatment for tumours of the duodenum, which still has 30–50% morbidity.
-
•
Limited resection of the duodenum without pancreatic head resection for benign as well carefully selected malignant tumours of the distal duodenum has considerably lesser morbidity.
-
•
Cattell and Braash maneuver and mobilization of the ligament of Treitz is very helpful during such resection.
-
•
The present study suggests the feasibility of performing such limited resection with equivalent survival and oncological outcomes.
1. Introduction
Resection of the duodenum with pancreaticoduodenectomy and bilioenteric, pancreaticoenteric and gastroenteric reconstruction still has a considerable morbidity (30–50%) [1]. Limited duodenal resection is an alternative to pancreaticoduodenectomy for benign and some malignant tumours of the duodenum with the hope of decreasing morbidity and achieving equivalent oncological outcomes [[2], [3], [4], [5],[7], [8], [9], [10], [11], [12], [13], [14], [15]]. Duodenal adenocarcinoma accounts for 45% of small bowel adenocarcinomas and 0.4% of all gastrointestinal malignancies [6]. The role of limited duodenal resection for malignant tumours of third (D3) and fourth (D4) part of duodenum without pancreatic and ampullary involvement has been evaluated in some recent studies [9,10,13,14]. We studied our experience with limited resection of the duodenum for selected malignant and benign distal duodenal and proximal jejunal tumours.
2. Methods
We retrospectively analyzed prospectively collected data of all patients who underwent limited resection of the duodenum from March 2011 to March 2016 in our unit. Ten patients underwent limited resection of the duodenum for tumours of its third and fourth part or the proximal jejunum. A limited resection of the duodenum i.e. either local wedge excision or segmental duodenectomy with duodenojejunal anastomosis with preservation of the pancreas was performed by a surgeon with at least five years experience in gastrointestinal surgery in a tertiary care academic institute. The patients' demographic data, clinical history, peri-operative details, post-operative outcomes (morbidity and 30 day mortality) and histopathological data were evaluated. Patients who underwent endoscopic excision, emergency surgical procedures, trans-duodenal ampullectomy/polypectomy or pancreatico-duodenectomy were excluded from the study. We have reported this work in line with the standard “preferred reporting of case series in surgery” (PROCESS) criteria [16].
2.1. Surgical technique
The surgical technique consisted of Kocherization of the duodenum along with the Cattell and Braash maneuver followed by mobilization of the ligament of Treitz [9]. The whole of the small bowel, ascending colon and proximal transverse colon were retracted cranially to expose the duodenojejunal junction. This junction was then mobilized dividing the ligament of Treitz. The jejunum was passed to the right side below the superior mesenteric vessels. Depending upon the location of the tumour, dissection of the third part of the duodenum from the uncinate process of the pancreas was carried out. Small and benign tumours were managed by wedge resection of the duodenum. T-tube stents were inserted depending upon the proximity of the resection margin to the ampulla. We did intra-operative endoscopy in two patients, one with a D3 adenocarcinoma to identify the ampulla and the other with a duodenal lipoma to rule out other lesions. Reconstruction was done by a gastrojejunostomy (with closure of the duodenal defect) in two patients, and side-to-side duodenojejunal anastomosis (Fig. 1, Fig. 2) in the rest. A feeding jejunostomy (FJ) was added depending upon the pre-operative and intra-operative assessment.
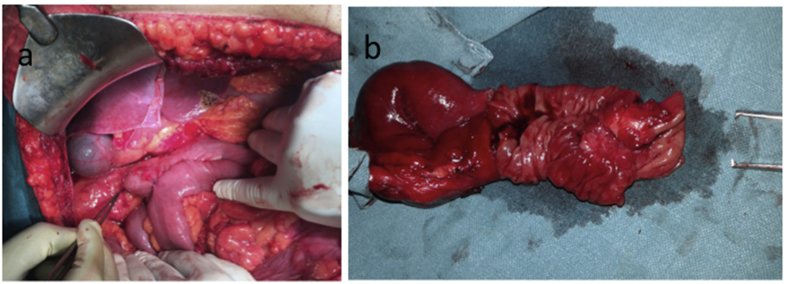
Fig. 1.
Intra-operative photos showing a) side-to-side duodenojejunal anastomosis after segmental duodenectomy and mobilization of duodenojejunal flexure in case no.5 (Forcep pointing towards proximal duodenal stump) and b) cut and open specimen of the duodenum and proximal jejunum in the same patient showing tumour in distal part of the duodenum.
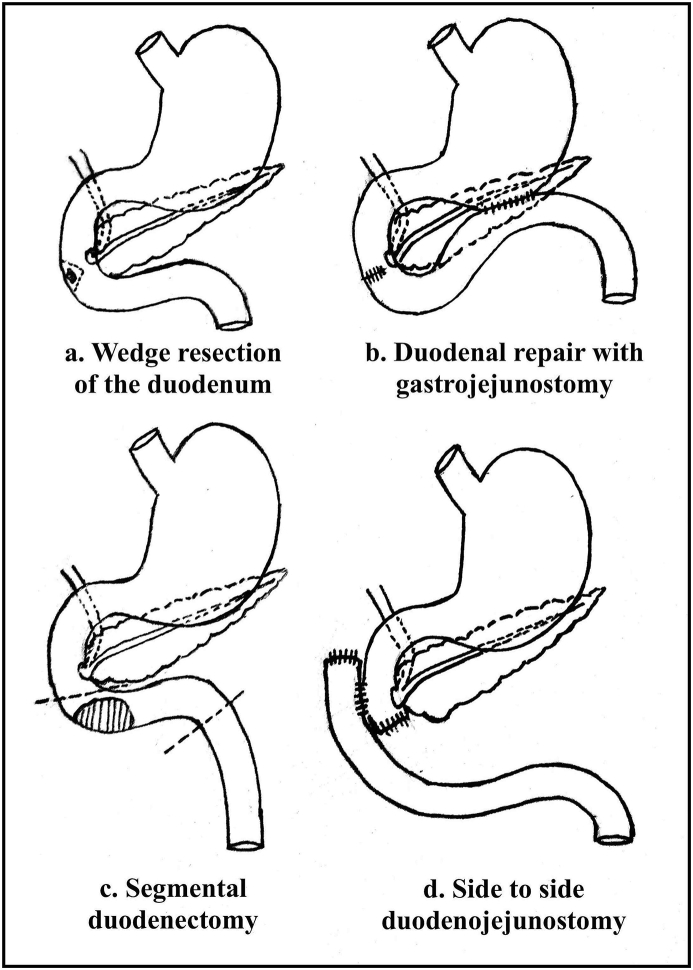
Fig. 2.
Schematic diagram showing techniques of limited distal duodenal resection.
3. Results
3.1. Demographics and operative details
The characteristics of the 10 patients are shown in Table 1, Table 2. There were 8 males and 2 females, who had a median age of 47 years (range, 35–70). The most common clinical presentations were abdominal pain (n = 5, 50%), and upper gastrointestinal bleeding (melaena n = 4, 40%), followed by vomiting (n-3, 30%). The diagnosis was a gastrointestinal stromal tumour (GIST) in 3 (30%); primary duodenal adenocarcinoma in 2 (20%); neuroendocrine tumour in 2 and duodenal and proximal jejunal non Hodgkin's lymphoma (NHL), lipoma of the duodenum with duodenal diverticulum and ectopic pancreatic tissue in the wall of D3 part of the duodenum in one each. Eight patients underwent segmental duodenal resection and the rest had excision of a small wedge of the duodenal wall. The patient with a NHL required en bloc resection of the proximal jejunum, fourth part of duodenum and splenic flexure of the colon for a large tumour involving all these structures.
Table 1.
Clinicopathological data of the patients.
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | Case 9 | Case 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Age (Years) | 52 | 70 | 40 | 43 | 52 | 37 | 35 | 44 | 50 | 58 |
| Sex | M | F | M | M | M | M | M | M | F | M |
| Symptoms | Upper GI bleed | Upper GI bleed | Abdominal pain | Upper GI bleed | Abdominal pain, Vomiting and fever | Upper GI bleed | Abdominal pain, recurrent fever, vomiting | Abdominal pain | Vomiting | Abdominal pain |
| Comorbidities | HTN | HTN, CLD | None | CLD | None | None | HIV | None | None | None |
| ASA score | II | III | II | II | I | I | III | I | I | I |
| Preopera- tive Diagnosis |
Tumour on D2 & D3 | Tumour D3 with duodenal diverticulum | Tumour D4 | Tumour D4 | Adenocarcinoma D3 | Tumour D4 | Proximal jejunal tumour | Multilocular cystic lesion of DJ flexure | Adenocarcinoma of D4 | Neuroendocrine tumour D3 with lymph nodal mass |
| Tumour Size (cm) |
3.5 × 3 | 11 × 1.5 | 3 × 2 | 4 × 2 | 4 × 2 | 4 × 2 | 8 × 6 | 11 × 10 | 3 × 2 | 2 × 2 |
| No. of segments resected | 0 | 1 | 1 | 1 | 2 | 0 | 1 | 1 | 2 | 2 |
Acronyms: CLD: Chronic liver disease, D2: second portion of the duodenum, D3: third portion of duodenum, D4: fourth portion of duodenum, DJ: Duodenojejunal flexure, HTN: systemic hypertension, HIV: Human immunodeficiency virus, M: Male, F: Female.
Table 2.
Operative data and postoperative outcomes.
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | Case 9 | Case 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Operating time (minutes) | 90 | 270 | 250 | 195 | 240 | 120 | 300 | 255 | 200 | 160 |
| Blood loss (ml) | 100 | 200 | 100 | 250 | 400 | 100 | 500 | 300 | 230 | 400 |
| Surgery | WR + GJ + FJ | SD + DJ | SD + DJ | SD + DJ + FJ | SD + DJ + FJ | WR + GJ + FJ | Enbloc SD + LHC + DJ + FJ | SD + DJ + FJ | SD + DJ | SD + DJ + FJ |
| Post-operative stay (Days) | 10 | 6 | 6 | 6 | 9 | 6 | 10 | 9 | 13 | 10 |
| Post operative complications | DGE Grade B | DGE Grade A | No | No | No | No | LRTI, ICU stay, DGE Grade B | DGE Grade A | DGE Grade B | DGE Grade B |
| Histopathology | GIST CD117 + Ki67 < 2% | Lipoma | Heterotrophic pancreas | GIST CD117 +, CD34 + Ki67 < 1% | Moderately differentiated adeno-carcinoma | GIST CD117 + Ki67 < 1% | Large B cell NHL CD 20 + LCA + | Grade 1 NET Ki67 < 3% LN metastases | Moderately differentiated adeno-carcinoma | Grade 1 NET Ki67 < 3% LN and Liver Metastases. |
| Adjuvant therapy | No | No | No | No | Chemo therapy | No | Chemo therapy | No | Chemo therapy | No |
| Follow up (months) | 58 | 52 | 24 | 14 | 4 | 3 | 45 | 29 | 47 | 3 |
Acronyms: CD: clusters of differentiation, DJ: duodenojejunostomy, DGE: delayed gastric emptying, FJ: Feeding jejunostomy, GJ: Gastrojejunostomy, GIST: Gastrointestinal stromal tumours, LCA: Leucocyte common antigen, LHC: Left hemicolectomy, LN: lymph node, LRTI: Lower respiratory tract infection, NHL: non Hodgkin Lymphoma, SD: segmental duodenectomy, WR: wedge resection.
The median duration of surgery was 245 (range, 90–300) minutes (Table 3). The median intra-operative blood loss was 240 (range, 100–500) ml. Four patients who had presented with upper gastrointestinal bleeding required peri-operative blood transfusion of anaemia.
Table 3.
Preoperative data and postoperative outcomes.
| n = 10 | ||
|---|---|---|
| Age | 35-70 years (median 47) | |
| Sex | Male | Female |
| 8 | 2 | |
| Presentation | Pain in abdomen | 5 (50%) |
| UGI bleeding | 4 (40%) | |
| Vomiting | 3 (30%) | |
| Surgery done | ||
| SD + DJ + FJ | 4 | |
| SD + DJ | 3 | |
| Enbloc | ||
| SD + LHC + DJ + FJ | 1 | |
| WR + GJ + FJ | 2 | |
| Duration of surgery | 90–300 min (median 245 min) | |
| Blood loss | 100-500 ml (median 245 ml) | |
| Complications | 6 (60%) | |
| 1.DGE | 6 (60%) | |
| Grade A | 2 (33.3%) | |
| Grade B | 4 (66.6%) | |
| 2. LRTI | 1 (10%) | |
| Length of stay | 6-13 days (median 9 days) | |
| Follow up | 3-58 months (median 26.5 months) |
Acronyms: DJ: duodenojejunostomy, DGE: delayed gastric emptying, FJ: feeding jejunostomy, GJ: Gastrojejunostomy, LHC: Left hemicolectomy, LRTI: lower respiratory tract inflammation, SD: segmental duodenectomy, WR: wedge resection.
3.2. Morbidity
Postoperative morbidity was graded as per Clavien Dindo classification (grade I minor deviation form normal postoperative course, grade II complications requiring therapeutic drugs outside of those allowed in grade I, grade III requiring surgical, endoscopic or radiological intervention, grade IV life threatening complications) [17]. Complications occurred in 6 (60%) patients, grade 1 or 2 in 5 (50%) patients and Clavien Grade IV in 1 (10%) patient who developed lower respiratory infection with respiratory distress requiring ICU stay. Delayed gastric emptying (DGE) was seen in six (60%) patients. Grade A and B DGE was seen in two (33.3%) and four (66.6%) patients respectively. There were no other complications. An oral gastrografin follow through study was done between post-operative day 4 and 7 in all patients except the one with respiratory distress who underwent contrast enhanced computed tomography (CECT) to confirm the anastomotic integrity. One patient had mechanical obstruction due to narrowing at the FJ site. DGE improved with conservative management in all patients with a high nasogastric aspirate. There was no anastomotic leak. The median postoperative hospital stay was 9 days (range, 6–13 days).
3.3. Follow up and outcomes
All patients had R0 resections. All three patients with GISTs had low risk features for recurrence and did not receive any adjuvant treatment. Both patients with adenocarcinoma received adjuvant chemotherapy. They were asymptomatic and free of recurrence at a follow up duration of 47 months and 6 months respectively. All ten patients are alive at a median follow up period of 26.5 (range, 3–58) months.
Two patients had nonfunctioning duodenal neuroendocrine tumours. One of the patients with a neuroendocrine tumours had metastasis in a single lymph node while another patient had metastasis in a single liver nodule as well in one out of twelve lymph nodes resected and both the patients were asymptomatic at a follow up of 29 and 3 months respectively. The patient with a NHL had presented with upper gastrointestinal bleeding with a large mass in the upper abdomen. Pre-operative biopsy was suggestive of a granulomatous inflammation. Postoperative histopathological analysis however showed a diffuse large B cell type NHL with CD 20 and leucocyte common antigen (LCA) positive tumour cells. The patient received chemotherapy postoperatively and was recurrence free at 45 months of follow up. The two patients with an ectopic pancreas in the duodenum and a duodenal lipoma were asymptomatic at follow up of 24 and 52 months respectively.
4. Discussion
The duodenum has a complex anatomy with its retroperitoneal location adjacent to major vascular structures and therefore, duodenal resection is a surgically challenging procedure. Limited resection for duodenal tumours can be divided into a pancreas sparing total or subtotal duodenectomy, pancreas sparing proximal duodenectomy and a pancreas sparing distal duodenenctomy [7]. A pancreas sparing subtotal duodenectomy involves resection of the whole duodenum except the gastric pylorus and the duodenal bulb. A pancreas sparing proximal duodenectomy entails resection of the first and proximal second portion of the duodenum while in distal duodenenctomy the third, fourth and distal second portion of the duodenum are resected [7].
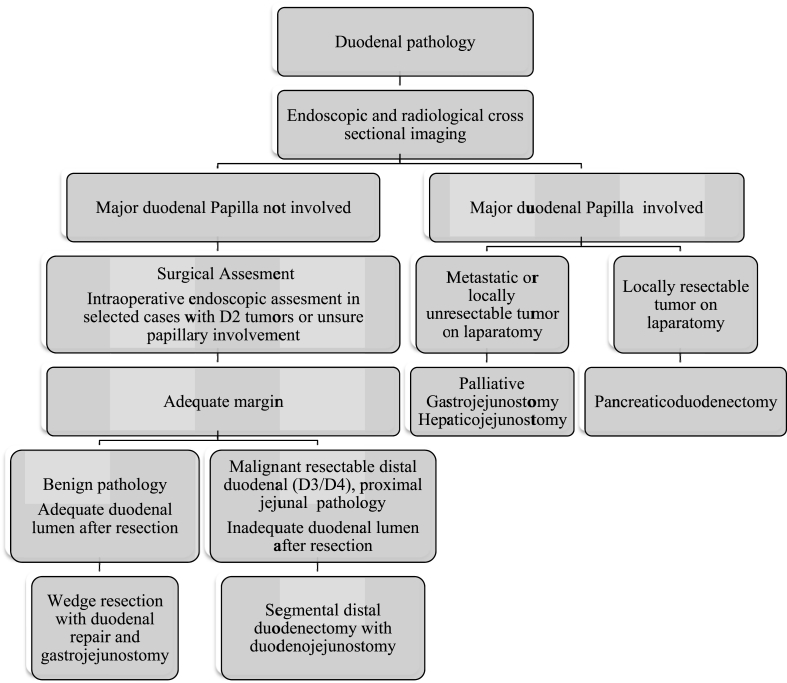
Limited resection of the distal duodenum may entail either a local wedge resection or segmental resection. Our protocol for evaluation and management of patients with distal duodenal pathology is briefly presented in Fig. 3. If a duodenal pathology is suspected on clinical examination, we perform detailed upper gastrointestinal endoscopic examination including side-viewing endoscopy and endoscopic ultrasound as indicated along with cross sectional imaging with multi-detector CECT (Fig. 4). In patients with obvious involvement of the major duodenal papilla, we perform pancreaticoduodenctomy after thorough assessment for ruling out metastatic disease. If the major duodenal papilla is free or if there is ambiguity after imaging and endoscopic evaluation, we do thorough surgical assessment including intra-operative endoscopic evaluation. For small benign tumours of the third and fourth part of duodenum with adequate residual lumen after resection, we perform wedge resection of the lesions with duodenal repair. The defect after a wedge resection may be closed primarily, by applying jejunal patch or with a Roux en Y duodenojejunal anastomosis [8,14]. It is our practice to perform the gastrojejunostomy in such patients to minimize the effects of small duodenal leak and stenosis. For benign tumours of second part of the duodenum, we perform wedge resection only if adequate margin of resection without threatening the major papilla can be achieved. We do segmental duodenal resection for selected malignant distal duodenal and duodenojejunal flexure tumours as well as benign tumours in which wedge resection will likely cause duodenal narrowing. An extended Kocher maneuver with full mobilization of the duodenum and proximal jejunum is important for creation of a tension free duodenojejunal anastomosis. We used the Cattell and Braash maneuver for distal duodenal exposure in 8 out of ten patients in our series and we feel it is extremely useful for this purpose as has also been described previously [9,10].
Fig. 3.
Algorithm for evaluation and management of distal duodenal pathology.
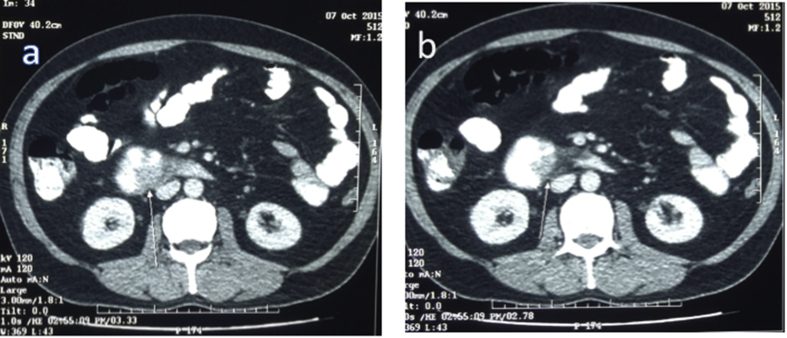
Fig. 4.
Computed tomography images in case no. 5 showing growth in the third part of duodenum (Arrows pointing towards growth).
Although a duodenojejunal anasomosis may be performed in an end-to-end, side-to-side or an end-to-side fashion [10,11], we prefer a side-to-side duodenojejunal anastomosis. Dorcaratto et al. [13] reported more complications and longer postoperative stay in patients with end to side compared to end-to-end anastomoses. There are limited studies to address this issue, and hence definitive conclusions regarding choice of anastomotic technique cannot be drawn [[10], [11], [12], [13]].
The median operative time and blood loss (245 min and 240 ml respectively) in our study were lower compared to most of the previously reported series (range of 130–300 min and 160–1100 ml respectively) [9,10,12,13]. DGE (60%) was the most common post-operative complication. Although anastomotic leak, bleeding and post-operative pancreatitis, have been reported in patients undergoing limited duodenal resection, we did not encounter these complications [13,14].
Surgical resection is the only curative treatment for GISTs and a limited duodenal resection is a good option for duodenal GISTs [15]. These tumours are usually well encapsulated, lymphatic spread is rare and a small margin of clearance is sufficient [18,19]. Routine lymphadenectomy is not recommended for GISTs unless the lymph nodes are grossly involved [11]. Even though the literature on limited duodenal resection describes only small numbers of patients, equivalent disease free and overall survival have been reported with limited duodenal resection as compared to pancreaticoduodenectomy [4,5,11,13]. Our results show that the limited resection of duodenal GISTs is a feasible option and good long-term disease free survival can be achieved.
Adenocarcinoma involving the fourth part of duodenum and proximal jejunum can also be treated with a limited duodenal resection. In a study by Onkendi et al. [14] even though the average lymph nodes sampled in segmental duodenectomy group were 8 compared to 12 in the pancreaticoduodenectomy group, there was no survival difference between the two groups. Also, a couple of other studies have reported similar survival outcomes and negligible morbidity with limited duodenal resection in patients with duodenal adenocarcinoma involving the distal part of the duodenum [9,20]. These reports and our study show that the limited distal duodenal resection can be a feasible option for malignant distal duodenal tumours with comparable survival outcomes to pancreaticoduodenctomy [9,14,20].
Duodenal neuroendocrine tumours account for 2–3% of all gastrointestinal neuroendocrine tumours [21]. As indicated in few previous reports, ours study also found that these tumours can be managed by limited duodenal resection with satisfactory long-term survival rates even in the presence of lymph node metastases [22,23].
4.1. Limitations
The rarity of patients with disease amenable to this surgical procedure limits the size of our study. However our data, documents the feasibility and safety of performing a limited duodenal resection for benign and selected malignant tumours of the distal duodenum. A well-designed prospective study is needed to establish the oncological equivalence of this procedure to pancreaticoduodenctomy in patients with distal duodenal adenocarcinoma.
5. Conclusion
Limited duodenal resection is a feasible option for selected patients with tumours involving the distal duodenum and/or proximal jejunum with good short-and long-term outcomes.
Ethical approval
None.
Funding for your research
None.
Author contribution
-
1)
Ankush Golhar: Study design and planning, data acquisition, clinical work, drafted the manuscript.
-
2)
Vivek Mangla: Study design and planning, clinical work, data acquisition, manuscript revision.
-
3)
Siddharth Mehrotra: Data acquisition, clinical work and drafted the manuscript
-
4)
Shailendra Lalwani: Data acquisition, Clinical work and manuscript revision
-
5)
Naimish Mehta: Data acquisition, Clinical work and manuscript revision
-
6)
Samiran Nundy: Design of work, clinical work and manuscript revision.
Conflicts of interest
None.
Research registration number
Trial registry number
Not applicable
Guarantor
The Guarantor is the one or more people who accept full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish. Dr Ankush Golhar, Dr Vivek Mangla, Dr Samiran Nundy.
Grants
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
This work has been reported in line with the PROCESS criteria.
References
- 1.DeOliveira M.L., Winter J.M., Schafer M. Assessment of complications after pancreatic surgery: a novel grading system applied to 633 patients undergoing pancreaticoduodenectomy. Ann. Surg. 2006;244:931–939. doi: 10.1097/01.sla.0000246856.03918.9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gervaz P., Huber O., Morel P. Surgical management of gastrointestinal stromal tumours. Br. J. Surg. 2009;96:567–578. doi: 10.1002/bjs.6601. [DOI] [PubMed] [Google Scholar]
- 3.Chung J.C., Kim H.C., Hur S.M. Limited resections for duodenal gastrointestinal stromal tumours and their oncologic outcomes. Surg. Today. 2016;46:110–116. doi: 10.1007/s00595-015-1163-x. [DOI] [PubMed] [Google Scholar]
- 4.Zhou B., Zhang M., Wu J. Pancreaticoduodenectomy versus local resection in the treatment of gastrointestinal stromal tumours of the duodenum. World Journal of Surgical Oncology. 2013;11:1–6. doi: 10.1186/1477-7819-11-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chung J.C., Chu C.W., Cho G.S. Management and outcome of gastrointestinal stromal tumours of the duodenum. J. Gastrointest. Surg. 2010;14:880–883. doi: 10.1007/s11605-010-1170-6. [DOI] [PubMed] [Google Scholar]
- 6.Struck A., Howard T., Chiorean E.G. Non-ampullary duodenal adenocarcinoma: factors important for relapse and survival. J. Surg. Oncol. 2009;100:144–148. doi: 10.1002/jso.21319. [DOI] [PubMed] [Google Scholar]
- 7.Konishi M., Kinoshita T., Nakagohri T. Pancreas-sparing duodenectomy for duodenal neoplasms including malignancies. Hepato-Gastroenterology. 2007;54:753–757. [PubMed] [Google Scholar]
- 8.Goh B.K., Chow P.K., Ong H.S., Wong W.K. Gastrointestinal stromal tumour involving the second and third portion of the duodenum: treatment by partial duodenectomy and Roux-en-Y duodenojejunostomy. J. Surg. Oncol. 2005;91:273–275. doi: 10.1002/jso.20311. [DOI] [PubMed] [Google Scholar]
- 9.Tocchi A., Mazzoni G., Puma F. Adenocarcinoma of the third and fourth portions of the duodenum: results of surgical treatment. Arch. Surg. 2003;138:80–85. doi: 10.1001/archsurg.138.1.80. [DOI] [PubMed] [Google Scholar]
- 10.García-Molina F.J., Mateo-Vallejo F., de Dios Franco-Osorio J., Esteban-Ramos J.L., Rivero-Henández I. Surgical approach for tumours of the third and fourth part of the duodenum. Distal pancreas-sparing duodenectomy. Int. J. Surg. 2015;18:143–148. doi: 10.1016/j.ijsu.2015.04.051. [DOI] [PubMed] [Google Scholar]
- 11.Liang X., Yu H., Zhu L.H., Wang X.F., Cai X.J. Gastrointestinal stromal tumours of the duodenum: surgical management and survival results. World Journal of Gastroenterology. 2013;19:6000–6010. doi: 10.3748/wjg.v19.i36.6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spalding D.R., Isla A.M., Thompson J.N., Williamson R.C. Pancreas-sparing distal duodenectomy for infrapapillary neoplasms. Ann. R. Coll. Surg. Engl. 2007;89:130–135. doi: 10.1308/003588407X155815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dorcaratto D., Heneghan H.M., Fiore B. Segmental duodenal resection: indications, surgical techniques and postoperative outcomes. J. Gastrointest. Surg. 2015;19:736–742. doi: 10.1007/s11605-015-2744-0. [DOI] [PubMed] [Google Scholar]
- 14.Onkendi E.O., Boostrom S.Y., Sarr M.G. 15-year experience with surgical treatment of duodenal carcinoma: a comparison of periampullary and extra-ampullary duodenal carcinomas. J. Gastrointest. Surg. 2012;16:682–691. doi: 10.1007/s11605-011-1808-z. [DOI] [PubMed] [Google Scholar]
- 15.Connolly E.M., Gaffney E., Reynolds J.V. Gastrointestinal stromal tumours. Br. J. Surg. 2003;90:1178–1186. doi: 10.1002/bjs.4352. [DOI] [PubMed] [Google Scholar]
- 16.Agha R.A., Fowler A.J., Rajmohan S., Barai I., Orgill D.P. PROCESS Group. Preferred reporting of case series in surgery; the PROCESS guidelines. Int. J. Surg. 2016;36:319–323. doi: 10.1016/j.ijsu.2016.10.025. [DOI] [PubMed] [Google Scholar]
- 17.Dindo D., Demartines N., Clavien P.A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004;240:205. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bucher P., Egger J.F., Gervaz P. An audit of surgical management of gastrointestinal stromal tumours (GIST) Eur. J. Surg. Oncol. 2006;32:310–314. doi: 10.1016/j.ejso.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 19.Gold J.S., DeMatteo R.P. Combined surgical and molecular therapy: the gastrointestinal stromal tumour model. Ann. Surg. 2006;244:176–184. doi: 10.1097/01.sla.0000218080.94145.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bakaeen F.G., Murr M.M., Sarr M.G. What prognostic factors are important in duodenal adenocarcinoma? Arch. Surg. 2000;135:635–642. doi: 10.1001/archsurg.135.6.635. [DOI] [PubMed] [Google Scholar]
- 21.Hoffmann K.M., Furukawa M., Jensen R.T. Duodenal neuroendocrine tumours: classification, functional syndromes, diagnosis and medical treatment. Best Pract. Res. Clin. Gastroenterol. 2005;19:675–697. doi: 10.1016/j.bpg.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 22.Pitt S.C., Pitt H.A., Baker M.S. Small pancreatic and periampullary neuroendocrine tumours: resect or enucleate? J. Gastrointest. Surg. 2009;13:1692–1698. doi: 10.1007/s11605-009-0946-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jordan P.H. A personal experience with pancreatic and duodenal neuroendocrine tumours. J. Am. Coll. Surg. 1999;189:470–482. doi: 10.1016/s1072-7515(99)00162-3. [DOI] [PubMed] [Google Scholar]