Abstract
Responsive neurostimulation for epilepsy involves an implanted device that delivers direct electrical brain stimulation in response to detection of incipient seizures. Responsive neurostimulation is a safe and effective treatment for adults with drug-resistant epilepsy, but although novel treatments are critically needed for younger patients, responsive neurostimulation is currently not approved for children with drug-resistant epilepsy. Here, we report a 16-year-old patient with seizures arising from eloquent cortex, who was successfully treated with responsive neurostimulation. This case highlights the potential utility of this therapy for pediatric patients and underscores the need for larger studies.
Keywords: Neurostimulation, RNS system, Devices, Pediatric, Drug-resistant epilepsy
1. Introduction
Epilepsy is a common neurological disorder in children, and in about 20% of cases, seizures are not controlled by medications alone. Some drug-resistant patients are candidates for removal of epileptogenic brain tissue by open surgical resection or laser ablation. However, many children are not candidates for surgery because seizures arise from brain regions that cannot be safely removed. A recently approved implantable device (NeuroPace RNS® System, hereafter referred to as “RNS System”) allows chronic intracranial recordings of neural activity and provides direct, responsive brain stimulation to treat seizures [1]. Long-term safety and efficacy data have catalyzed widespread adoption of the RNS System as an adjunctive treatment in adults with drug-resistant focal epilepsy [2], [3], but the RNS System is currently approved only for patients 18 years of age or older. Although responsive brain stimulation has been proposed as a treatment option for pediatric epilepsy [4], [5], there are no published reports demonstrating feasibility of this treatment in children.
2. Materials and methods
The diagnostic evaluation and treatment plan for the patient described in this report were reviewed in a multidisciplinary epilepsy surgery case conference at the University of California, San Francisco. Approval for off-label RNS System implantation was obtained from the patient's insurance company by special appeal. Initial RNS System detection settings involved a line length detector applied to ECoG Channels 1 and 3. To detect low-voltage fast activity present at seizure onset, detection settings were switched at the first outpatient follow-up visit to a bandpass detector. Initial responsive stimulation settings were: 1.0 mA, 200 Hz, 160-μs pulse width, 100-ms burst duration. Current intensity was gradually increased to 3.0 mA over the first four months after implantation.
3. Case report
3.1. History
A 16-year-old right-handed girl presented with a 10-year history of drug-resistant seizures. Seizure semiology involved an indescribable aura followed by facial grimace, right arm tonic extension, right head turn, and left hand automatisms with impairment of awareness. Seizures occurred daily, often in clusters lasting several minutes. Since onset of epilepsy, she had progressive decline in academic performance, with mild neuropsychological deficits in auditory attention, phonological decoding, and verbal fluency and learning. Neurological examination was otherwise normal.
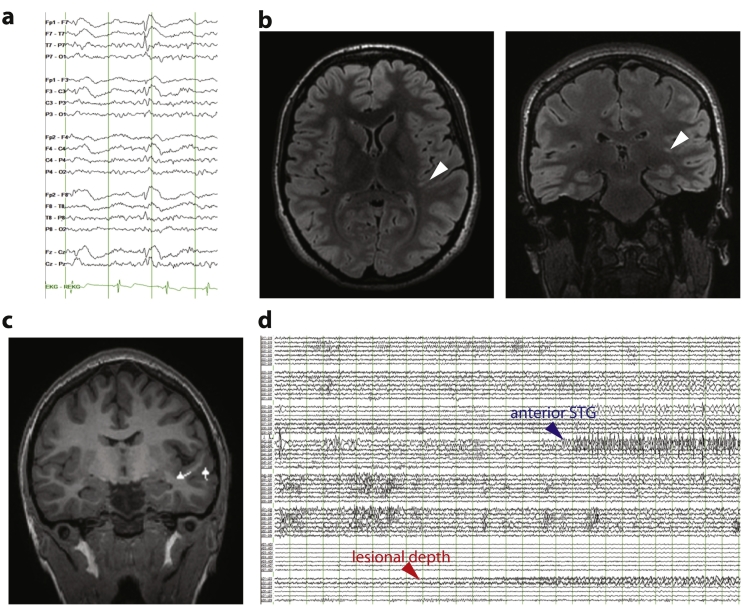
The patient's seizures had poor correlate on scalp electroencephalography (EEG). Interictal EEG findings included intermittent left temporal slowing and occasional left frontotemporal spikes (Fig. 1a). Brain MRI revealed a linear T2-hyperintense lesion in the left temporal lobe extending from the superior temporal gyrus (STG) to the left lateral ventricle (Fig. 1b), suggestive of transmantle focal cortical dysplasia (FCD). Magnetoencephalography (MEG) revealed spikes clustered in the region of the lesion (Fig. 1c). Positron emission tomography demonstrated subtle focal left posterior temporal hypometabolism. Functional magnetic resonance imaging confirmed left hemispheric lateralization of expressive language. To localize the seizure onset zone, left frontotemporal surface grid and lesional depth electrodes were implanted. Intracranial EEG recordings revealed seizure onset from the lesional depth electrodes, with rapid spread to the posterior STG and angular gyrus as well as an anatomically distinct focus in the anterior STG (Fig. 1d).
Fig. 1.
Diagnostic evaluation for seizure localization.
(a) Interictal scalp EEG showing a left temporal spike. Scalp EEG during seizures (not shown) did not reveal a clear ictal pattern. (b) 3 Tesla brain MRI with axial (left) and coronal (right) T2 FLAIR sequences showing suspected FCD (arrowheads) extending from the left transverse temporal gyrus to the lateral ventricle (full extent of lesion not appreciable in single slices). (c) MEG showing spike dipoles (triangles with lines) clustering near the lesion. (d) Intracranial recording of seizure onset showing initial emergence of low-voltage fast activity in the lesional depth electrode channels with early involvement of the anterior STG, an area that was subsequently resected. Other seizures started in the lesional depth electrode but showed early spread to cortex of the angular gyrus and posterior STG (not shown). Vertical lines in (a) and (d) are spaced by 1 s.
3.2. Treatment
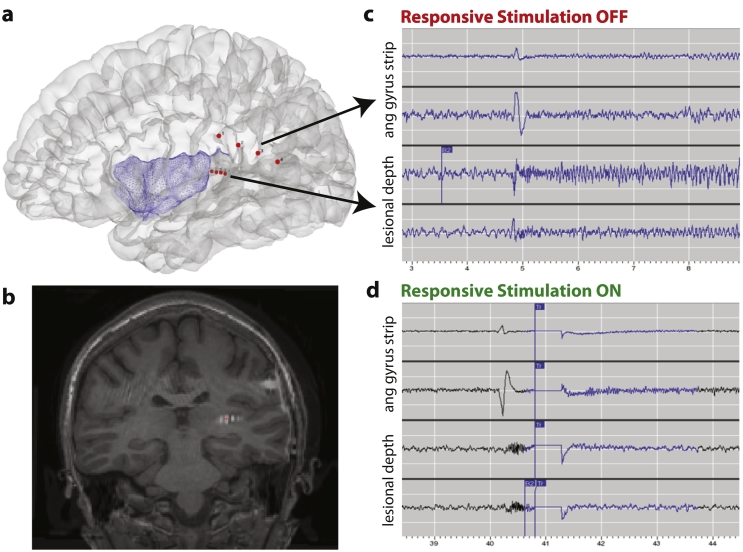
During bedside cortical mapping, stimulation of portions of the posterior STG and angular gyrus involved in seizure generation resulted in language disruption, whereas stimulation of the anterior STG did not. Given early ictal involvement of eloquent neocortex, complete resection of epileptogenic tissue was not possible. Therefore, she underwent resection of the portion of the anterior STG cleared for language. Surgical pathology analysis of resected tissue revealed FCD, type IIa. Patients with incomplete resection of FCD have low rates of seizure-freedom [6] and often require repeat resection [7], and seizures originating in the insula can persist after temporal resection [8], [9]. Therefore, at time of resection, the RNS System was implanted to treat residual epileptogenic tissue. The RNS neurostimulator was connected to a four-contact depth lead placed within the insular lesion and a four-contact cortical strip lead placed over the angular gyrus and posterior STG. Post-operative head computed tomography (CT) scan co-registered with pre-operative brain MRI confirmed expected RNS System electrode locations (Fig. 2a, b). The patient's hospital course was uncomplicated and she was discharged on her pre-operative antiepileptic drug regimen.
Fig. 2.
RNS System implantation and recordings.
(a) 3D reconstruction showing location of RNS System electrodes (red dots). Insular cortex is colored in blue for reference. (b) Coronal T1 brain MRI co-registered with post-operative head CT to show location of RNS depth lead in the region of the presumed FCD (compare Fig. 1b). (c) RNS System ECoG capturing a seizure before responsive stimulation was enabled. (d) RNS System ECoG showing putative seizure termination by responsive stimulation. As in (c), low-voltage fast activity arises from the lesional depth electrode channels but, following delivery of responsive therapy (vertical blue line), an ictal pattern does not develop.
3.3. Follow-up
The patient returned to clinic on post-operative day 12. She denied pain or other symptoms. Electrocorticograms (ECoGs) stored by the RNS System revealed several electrographic seizures (Fig. 2c) which correlated well with the patient's clinical seizure diary. Detection settings were optimized and responsive stimulation was enabled on post-operative day 12. Stimulation was imperceptible to the patient. By the second post-operative follow-up appointment two months later, several instances of putative seizure termination by responsive stimulation were evident on stored ECoGs (Fig. 2d). The patient reported that daily seizures continued but with significantly reduced intensity and duration. She denied new neurological symptoms or device-related adverse events. By six months post-implant, she reported persistent auras several times per week but no seizures that progressed to impairment of awareness.
4. Discussion
Here, we describe use of an implanted brain responsive neurostimulator in a child with seizures arising from eloquent regions of dominant temporal neocortex. This case serves as proof-of-principle that the RNS System, currently approved only for use in adults, can be safe, well-tolerated, and effective in pediatric patients. In clinical trials, acute reduction in seizure frequency was observed in adults following RNS System implantation [1], but our patient experienced durable benefit extending beyond the typical “implant effect” window [10]. Still, larger studies with longer duration of follow-up will be necessary to corroborate this initial experience. To our knowledge, this case is also the first report in any age group of concurrent brain resection and RNS System implantation. Combination of resective and neuromodulatory therapies is appealing in complex cases involving non-eloquent and eloquent epileptogenic tissue, respectively, and this strategy also merits further study. Although use of two concurrent therapeutic interventions in our case limits ascertainment of cause and effect relationships regarding clinical outcome, ECoGs showing putative seizure termination by responsive neurostimulation and the patient's steady clinical improvement both suggest significant impact of the RNS System.
There are several potential limitations of the RNS System in pediatric populations. The RNS System is palliative and relatively few adult patients become seizure-free. The neurostimulator is typically implanted in a full-thickness craniectomy, and there may be anatomic constraints associated with this approach in children with smaller and/or growing skulls. MRI is contraindicated in patients implanted with the RNS System, and so the likelihood of future neuroimaging in children will need to be considered. Finally, as the RNS System is not currently approved for use in patients under the age of 18, insurers may deny coverage. In this case, special approval from the insurance company was secured in advance through an appeal based on medical necessity.
The seizure improvement our patient experienced in the first six months after RNS System implantation is expected to continue for some time. A feature common to several neurostimulation devices is that, unlike anti-seizure drugs, efficacy for seizure control tends to increase over months to years [11]. This is true even when stimulation settings are held constant, an observation which suggests that the therapeutic mechanism of neurostimulation involves neuroplasticity. Given the plasticity of the developing brain, a speculative possibility is that children might be particularly sensitive to adaptive changes in brain networks induced by direct electrical stimulation. Future investigations into this treatment modality will help define which age groups are most likely to benefit.
5. Conclusions
Responsive neurostimulation may be a viable therapeutic option for some pediatric patients with drug-resistant epilepsy who are not candidates for resective epilepsy surgery. Larger studies are needed to establish the safety and efficacy of responsive neurostimulation in this age group.
Acknowledgments
Acknowledgments
The authors are grateful to Emily Mirro and Dr. Archana Pasupuleti for assistance with clinical management of the patient in this report.
Submitting author's declaration
I acknowledge that all co-authors have been substantially involved in the study and/or preparation of the manuscript; no undisclosed groups or persons have had a primary role in the study and/or in the manuscript preparation; and all co-authors have seen and approved the submitted version of the paper and accept responsibility for its content.
Disclosure of conflicts of interest
V.R.R. has served as a paid consultant for NeuroPace, Inc. but declares no targeted funding or compensation for this study. A.L.N. has received speaker honoraria from LivaNova, Inc. The remaining authors have no conflicts of interest.
References
- 1.Morrell M.J., Group RNSSiES Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology. 2011;77:1295–1304. doi: 10.1212/WNL.0b013e3182302056. [DOI] [PubMed] [Google Scholar]
- 2.Bergey G.K., Morrell M.J., Mizrahi E.M., Goldman A., King-Stephens D., Nair D. Long-term treatment with responsive brain stimulation in adults with refractory partial seizures. Neurology. 2015;84:810–817. doi: 10.1212/WNL.0000000000001280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heck C.N., King-Stephens D., Massey A.D., Nair D.R., Jobst B.C., Barkley G.L. Two-year seizure reduction in adults with medically intractable partial onset epilepsy treated with responsive neurostimulation: final results of the RNS system pivotal trial. Epilepsia. 2014;55:432–441. doi: 10.1111/epi.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karsy M., Guan J., Ducis K., Bollo R.J. Emerging surgical therapies in the treatment of pediatric epilepsy. Transl Pediatr. 2016;5:67–78. doi: 10.21037/tp.2016.04.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ravindra V.M., Sweney M.T., Bollo R.J. Recent developments in the surgical management of paediatric epilepsy. Arch Dis Child. 2017;102:760–766. doi: 10.1136/archdischild-2016-311183. [DOI] [PubMed] [Google Scholar]
- 6.Hauptman J.S., Mathern G.W. Surgical treatment of epilepsy associated with cortical dysplasia: 2012 update. Epilepsia. 2012;53(Suppl. 4):98–104. doi: 10.1111/j.1528-1167.2012.03619.x. [DOI] [PubMed] [Google Scholar]
- 7.Spencer S., Huh L. Outcomes of epilepsy surgery in adults and children. Lancet Neurol. 2008;7:525–537. doi: 10.1016/S1474-4422(08)70109-1. [DOI] [PubMed] [Google Scholar]
- 8.Isnard J., Guenot M., Ostrowsky K., Sindou M., Mauguiere F. The role of the insular cortex in temporal lobe epilepsy. Ann Neurol. 2000;48:614–623. [PubMed] [Google Scholar]
- 9.Harroud A., Bouthillier A., Weil A.G., Nguyen D.K. Temporal lobe epilepsy surgery failures: a review. Epilepsy Res Treat. 2012;2012:201651. doi: 10.1155/2012/201651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun F.T., Arcot Desai S., Tcheng T.K., Morrell M.J. Changes in the electrocorticogram after implantation of intracranial electrodes in humans: the implant effect. Clin Neurophysiol. 2017;129:676–686. doi: 10.1016/j.clinph.2017.10.036. [DOI] [PubMed] [Google Scholar]
- 11.Ben-Menachem E. Neurostimulation-past, present, and beyond. Epilepsy Curr. 2012;12:188–191. doi: 10.5698/1535-7511-12.5.188. [DOI] [PMC free article] [PubMed] [Google Scholar]