Abstract
Large post-traumatic osteochondral defects of the proximal tibia in young active patients can be challenging because total or partial arthroplasties are to be avoided. The use of a fresh osteochondral allograft including its meniscus is one of the few options to biologically treat these injuries. Although the use of a fresh allograft is not easily accessible in some places and carries considerable logistical limitations, it is an alternative that provides viable chondrocytes to the defect. The inclusion of the meniscus in the osteochondral graft improves the results but also makes the technique even more demanding. We present a thorough description of this allograft transplantation to make it as reproducible as possible.
Few surgical alternatives are suitable to treating post-traumatic large osteochondral defects of the tibial plateau in young active patients. Total or partial knee replacement is undoubtedly not advisable in this group of patients. Instead, an alternative biological repair method is highly preferred. However, techniques such as mosaicplasty or autologous chondrocyte implantation in large lesions have not turned out to be as positive for patients with tibial plateau injuries as it has been for patients with femoral condylar lesions.1, 2
The results from transplantation of the tibial plateau were first reported some decades ago.3 However, few study groups have reported on each specific surgical step of this demanding technique in detail. The success of this technique depends on several surgical details that have not been fully described. One of the most relevant steps is to include the meniscus together with the osteochondral graft.5 However, this increases the difficulty of the surgical technique.
The purpose of the description of this technique is to detail every step in the transplantation of a fresh osteochondral allograft with its meniscus to improve function and delay the need for a knee arthroplasty in young active patients with a large post-traumatic tibial plateau defect.
Indication
Fresh osteochondral tibial plateau and meniscus (FOTAM) transplantation is indicated in patients below 50 years of age with post-traumatic tibial plateau osteochondral defects measuring at least 30 mm in diameter and 10 mm in depth.
A kissing lesion is not a contraindication if the femoral condyle chondral or osteochondral injury is concomitantly addressed. The same is true for any axial malalignment of as little as 2° or more leading to overloading the index compartment or for ligament insufficiencies. The exclusion criteria are advanced osteoarthritis of other compartments of the knee, a body mass index greater than 30 as well as general conditions such as tumors, rheumatic diseases that are locally aggressive, infections, diabetes, and vasculitis. Smokers must commit to stopping smoking for at least the first 6 months after surgery.
Preoperative Study
All the patients undergo the following radiographic evaluation:
-
•
A long-standing radiograph to assess for axial malalignment
-
•
A magnetic resonance imaging (MRI) to assess for chondral/subchondral, ligaments, and meniscus concomitant injuries
-
•
A computed tomography (CT) scan (mandatory) to provide the most accurate information on bone loss and allow for measurement of the defect
Fresh Allograft Harvesting and Processing
The local authorized tissue bank supplied the allografts and performed all the preoperative graft processing. The osteochondral tissue should be obtained from donors below 45 years of age. Once a donor is available, harvesting of the grafts must be performed within the first 12 hours of death. An additional 12-hour window for harvesting can be gained if the donor's body is kept refrigerated at 4°C for the first 4 to 6 hours.
The osteochondral allograft including its corresponding meniscus is placed in a transport media (lactated Ringer's) and kept refrigerated between 4°C and 8°C. Once in the tissue bank, the graft is prepared and cleaned in a Class A clean room. The joint cartilage is first macroscopically evaluated. Next, there is the removal of the soft tissue and periosteum and high-pressure pulsatile lavage irrigation with sterile saline. The graft obtained is subjected to decontamination. It first consists of dry centrifugation followed by centrifugation with sterile phosphate buffered saline. Microbiological testing is carried out on both the graft and the last wash solution. Finally, the allograft is submerged in a solution with lactated Ringer's and an antibiotic cocktail that includes vancomycin (50 mg/mL), tobramycin (3 mg/mL), cotrimoxazole (160 mg/mL), and amphotericin (125 μg/mL). After 5 days, further microbiological testing is performed on both the preservation solution and the graft. From the time the graft arrives at the tissue bank until it is implanted in the patient, the allograft is maintained at between 4°C and 8°C. Currently, the estimated risk for infectious disease transmission is 1 in 420,000 donations for the hepatitis C virus, 1 case in 100,000 donations for the hepatitis B virus, and 1 case in 175,000 donations for HIV.6 Allograft sizing is performed in accordance with a preoperative CT scan (both in the patient and on the graft) and anthropometric agreement between the donor and recipient. Then, a CT scan of the graft is essential. We recommend having it performed immediately after harvesting to give more time for matching with the corresponding patient and enough time for surgical scheduling and planning.
Surgical Technique
Patients are placed in the supine position with a foot support and a lateral support for the thigh to maintain the knee at around 90° of flexion. A thigh tourniquet is used that is preferably inflated after the sterile field is fully prepared to save time for an expected lengthy procedure. The contralateral limb is placed in extension. In the case of a lateral FOTAM procedure, this allows for the figure-of-four position without the need for an assistant. Ideally, the surgical team comprises 4 surgeons. Two of them handle graft preparation and the remaining 2 prepare the recipient area, where the graft is to be implanted.
An arthroscopic evaluation is first performed to reconfirm that FOTAM transplantation is suitable and that there are no excluding criteria that may have been overlooked preoperatively.
When measuring the biomechanical axis, any degree of deformity toward the injured tibiofemoral compartment must be corrected.7 It is crucial to protect the delicate osteochondral allograft with an osteotomy. This can be performed concomitantly with the FOTAM procedure.
Recipient Site Preparation
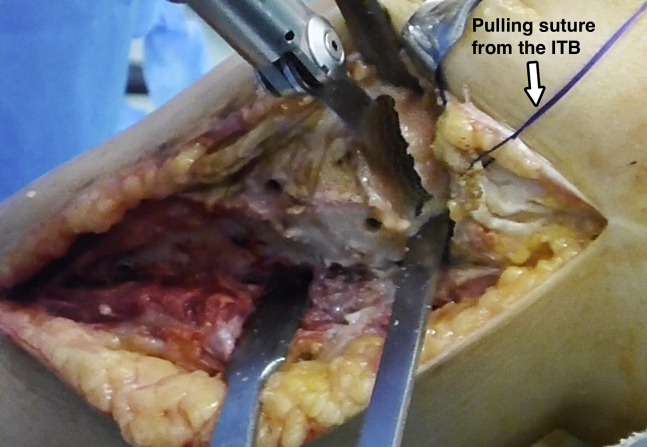
A longitudinal midline incision is used in cases of medial FOTAM transplantation. However, a longitudinal anterolateral approach is preferred in lateral cases. To start, a vertical bone cut is performed in the center of the corresponding tibial spine. Then, the horizontal osteotomy is performed. Only the minimum amount of bone that makes for obtaining a flat surface with healthy bleeding cancellous bone is resected (Fig 1). This step is crucial because the thicker the bone tissue of the graft, the greater the chances of an immune reaction and bone resorption. Bone integration is only achieved in the first 8- to 12-mm layer of bone tissue.8 Because the thickness of the osteochondral allograft will be no more than 10 mm, bone autografting obtained from the iliac crest is recommended in cases where the defect is larger. In cases of central bone loss, it can be filled with a cancellous bone graft. If the defect affects the whole metaphyseal bone, a bicortical autograft is preferable. During the osteotomy, special care is taken to preserve all the ligament and tendon attachments and avoid injury to the popliteal vessels. On the medial side, this is accomplished with a retractor placed deep to the medial collateral ligament and around the posteromedial aspect of the tibial plateau with the knee at 90° of flexion. On the lateral side, the iliotibial band is detached and retracted proximally with 1 or 2 pulling sutures with the knee at 90° of knee flexion in the figure-of-four position to open the lateral compartment. Then, a wide curved retractor is placed posterolaterally to prevent the saw and osteotome going beyond the posterior border of the tibial plateau.
Fig 1.
Left knee, lateral view. The least amount of bone necessary to obtain a flat, bleeding bone is removed. The iliotibial band (ITB) is retracted proximally to improved visualization with a pulling suture.
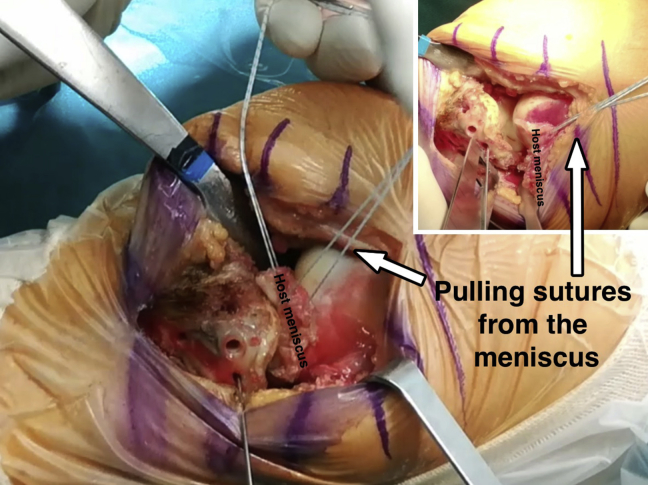
In almost all the cases, the native meniscus is resected. Preserving the native meniscal attachments of the allograft is preferable. It has shown better clinical outcomes than transplanting the osteochondral graft without the meniscus.5 In those few cases in which the patient's meniscus is preserved, traction sutures pull it proximally to allow for improved visualization (Fig 2). The posterior horn of the patient's meniscus is sutured with a whipstitch technique using a No. 2 high-strength suture. Alternatively, the posterior horn can be sutured with the help of an Autopass suture passer (Spectrum Conmed, Largo, FL). This suture will be later introduced through a hole drilled at the footprint of the posterior horn of the meniscus in the osteochondral allograft.
Fig 2.
Left knee, lateral view. If the patient's meniscus is being preserved, traction sutures pull it proximally to allow for improved visualization.
Donor Graft Preparation
The preparation of the graft is performed according to the dimensions previously measured in the recipient area. Although preoperative CT and MRI evaluation allow for approximate measurements, it must be confirmed intraoperatively. Graft preparation is a delicate step and it is advisable to always err on the side of oversizing. This can always be corrected with further graft trimming. In cases in which the native meniscus is preserved, a 3- to 4-mm hole is drilled at the footprint of the posterior horn. The suture of the posterior horn of the patient's meniscus will be passed through this tunnel.
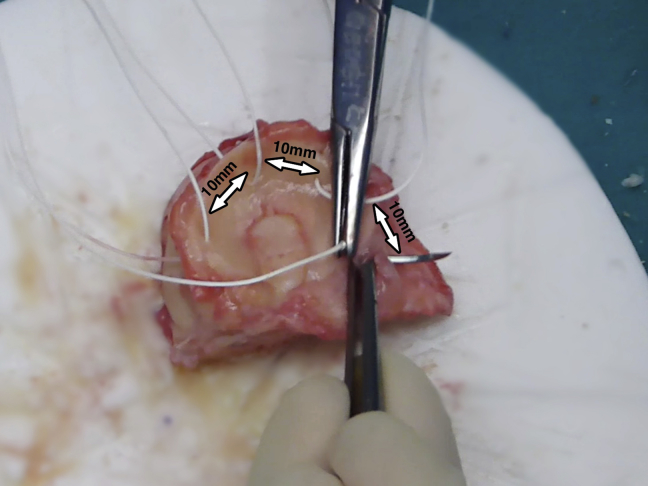
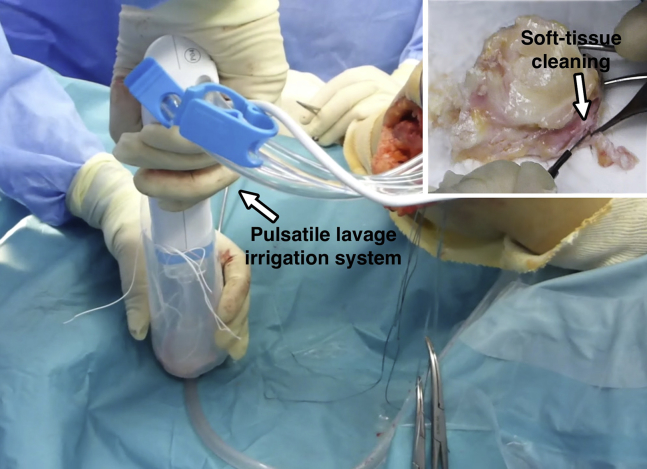
Once the appropriate size and shape of the osteochondral allograft has been obtained, the graft is placed in the defect and visually observed and checked under a fluoroscope. If no further undersizing or reshaping of the graft is needed, the graft is taken out and vertical sutures every 10 mm are placed along the meniscus (Fig 3). Either absorbable or nonabsorbable No. 0 to No. 2 sutures can be used. All the soft tissue other than the meniscus is resected. The prepared graft is now thoroughly washed with 6 L of saline solution using high-pressure pulsatile lavage irrigation. These 2 later steps help in decreasing the number of living cells and bone marrow elements9 other than chondrocytes, thus decreasing immunogenicity (Fig 4).
Fig 3.
Vertical sutures every 10 mm are placed along the meniscus for later fixation to the patient's capsule.
Fig 4.
Left knee, lateral view. The graft is being washed with a pulsatile lavage irrigation system inside a 0.5 L saline solution recipient. All the unnecessary soft tissue is resected. Both actions help to decrease the graft's immunogenicity.
Graft Implantation and Fixation
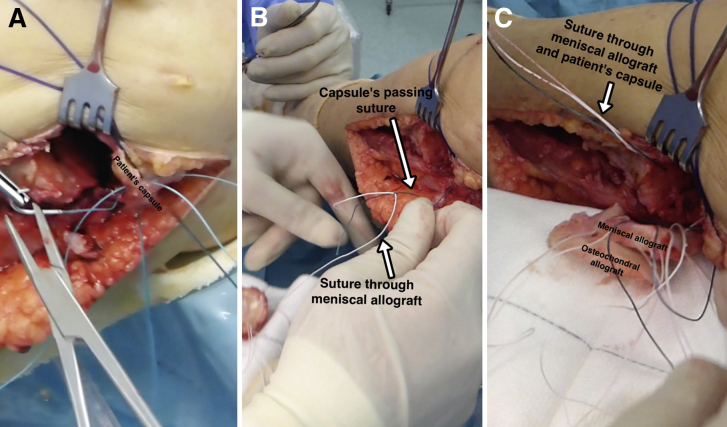
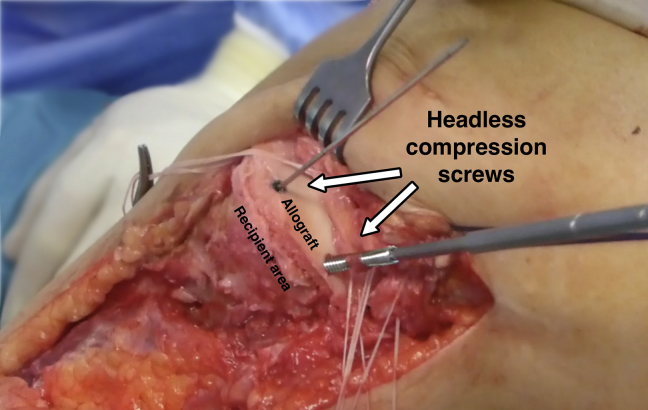
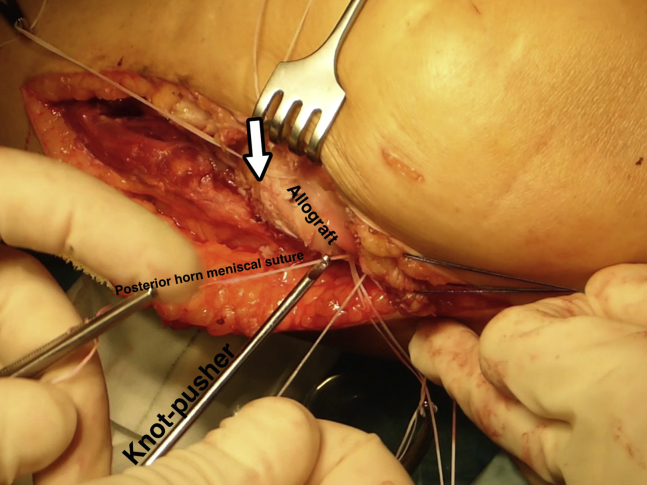
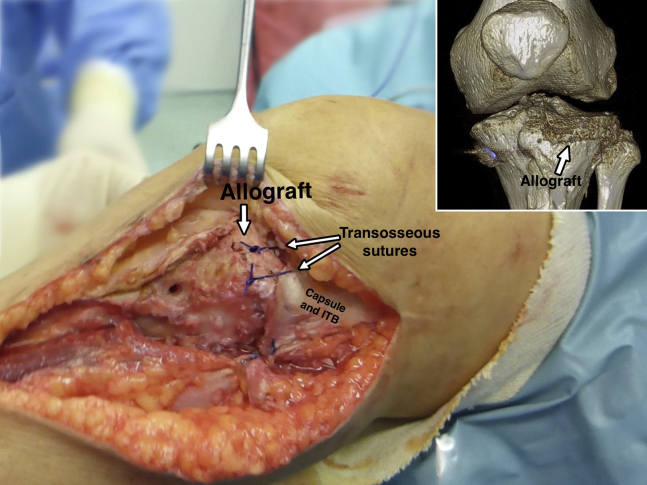
First, the passing sutures that had been passed through the capsule allow the sutures previously inserted in the meniscus of the allograft to be retrieved to perform the meniscocapsular fixation (Fig 5). Next, the whole graft is positioned in the prepared zone. Clinical and fluoroscopic evaluation aid in obtaining the desired position of the graft. Osteochondral fixation is ideally achieved with 2 headless titanium compression screws with a variable threaded pitch (Acutrak Standard; Acumed, Hillsboro, OR), which provides strong compression (Fig 6). Alternatively, 4-mm cancellous screws can be used. The meniscal sutures are now tightened to the capsule. Those corresponding to the posterior half of the meniscus are tightened with sliding knots pushed back with a shoulder knot-pusher (Fig 7). Intra-articular drainage is left in place and standard closure is performed (Fig 8). A step-by-step summary of the technique is observed in Table 1. The knee is finally immobilized in a brace, locked in full extension. Table 2 provides pearls and pitfalls to performing this surgical procedure. The whole technique is detailed described in Video 1.
Fig 5.
Left knee, lateral view. The passing sutures that had been passed through the patient's capsule (A) allows the sutures previously inserted in the meniscus of the allograft to be retrieved (B, C) to perform the meniscocapsular fixation.
Fig 6.
Left knee, lateral view. The osteochondral graft is being fixed with 2 titanium headless compression screws (Acutrak Standard; Acumed).
Fig 7.
Left knee, lateral view. Those sutures placed in the posterior half of the meniscus are tightened with sliding knots pushed back with a shoulder knot-pusher.
Fig 8.
Left knee, lateral view. Final clinical and 3-dimensional computed tomography aspect of the fresh osteochondral lateral tibial plateau and meniscal transplantation. Transosseous sutures help to properly close the joint.
Table 1.
Step-by-Step Fresh Osteochondral Tibial Plateau and Meniscal Transplantation
| Step | Description |
|---|---|
| 1 | With the patient in the supine position, hold the knee at 90° of flexion with the help of a distal foot support and a lateral thigh support |
| 2 | An 8 to 10 cm longitudinal medial or anterolateral approach for the medial and lateral tibial plateaus, respectively, is performed |
| 3 | An osteotomy is performed using saws and chisels with special care given to resecting the least amount of bone necessary to obtain a flat surface |
| 4 | In case of a deeper defect, supplement the void with cancellous or corticocancellous bone autograft from the iliac crest |
| 5 | The graft is cut to a 10 mm thickness, matching the prepared recipient area relative to the mediolateral and anterior-posterior measures |
| 6 | Vertical sutures are passed all along the meniscal graft every 10 mm |
| 7 | Similarly, passing sutures are passed through the capsule every 10 mm at the joint line. They will guide the meniscal sutures to being positioned at the meniscocapsular junction |
| 8 | Resect all the unnecessary soft-tissue of the graft and then wash it with a high-pressure pulsatile system |
| 9 | The graft is introduced. Fix the osteochondral part of the graft with 2 cancellous screws and the meniscus with its sutures |
| 10 | The approach is closed in the usual manner. Intra-articular drainage is recommended |
Table 2.
Pearls, Pitfalls and Risks
|
|
CT, computed tomography.
Rehabilitation Protocol
Immediate quadriceps and hamstring muscle exercises as well as continuous passive motion from 0° to 90° are initiated. After 6 weeks, range of motion progresses gradually depending on patient compliance. Partial weight bearing with a knee immobilizer is allowed after 8 weeks, depending on the radiographic and CT scan aspect of the graft. Full weight bearing is generally allowed after 3 months. Patients return to a normal workload by the fifth month after surgery. Running and high impact sports are discouraged.
Radiologic Evaluation
Postoperative radiographic assessment includes anterior-posterior standard radiography. We strongly recommend a CT scan at 10 weeks after surgery to assess bone integration and recommend the degree of weight bearing accordingly. A long-standing radiograph to assess the lower limb alignment and an MRI at 6 and 12 months to assess the cartilage and the meniscus of the allograft are also part of the routine postoperative evaluation protocol. After 12 months, yearly clinical and radiologic evaluation with a long-standing radiograph and MRI are recommended.
Treatment failure is considered in any of the following scenarios:
-
•
The need for a partial or total knee arthroplasty.
-
•
Low-to-moderate scores calculated in functional tests after 1 year of the surgery.
-
•
The need for surgical allograft revision.
Discussion
Osteochondral tibial plateau transplantation is one of the few reliable treatment alternatives for large post-traumatic osteochondral defects in young patients other than total and partial knee arthroplasties.
Fresh-frozen and cryopreservation storage techniques are widely available and permit graft preservation for several months. However, those techniques have also been shown to affect the biomechanics and viability of the chondral layer of the graft in an irreversible manner.10 Conversely, fresh osteochondral allografts retain viable chondrocytes and are biomechanically and histologically comparable with autografts.11 On the downside, it carries logistical limitations that can make the planning and scheduling of the surgery a challenge. A very well-organized tissue bank with a well-trained team responsible for harvesting the grafts from the donor is requisite. The team must also be knowledgeable of the differences here from harvesting graft for standard storage techniques. Having the CT scan of the graft performed immediately after harvesting may also help speed up this process to some degree. Direct contact of the surgeons with this team involved in harvesting from the donor is also of utmost importance in saving valuable time.
Fresh osteochondral allograft transplantation in the treatment of osteochondral defects is not a new procedure, and it has already shown good to excellent results at more than 20 years of follow-up.4, 5 Although only a few groups have made all the available published studies, none of them have described the surgical technique in detail. Because of the fact that this demanding surgery is indicated in only a few cases, this more detailed description will aid surgeons with less experience in performing it.
One of the most important aspects of osteochondral transplantation is that bone integration is carried out by a creeping phenomenon in the first few millimeters. Then, the graft must be as thin as possible. Usually, a graft thickness up to 10 mm is the goal.8 When bone loss is greater, a good alternative is to add cancellous or bicortical bone autograft from the patient's iliac crest.
As to whether to include the meniscus in the procedure or not, the most experienced group on this technique reported that the mean time from the index surgery to a conversion to total knee arthroplasty rose upward from 7.1 to 10.6 years when the meniscus was included.5 Although no statistical differences were observed in the functional scores or radiographic evaluation, it should be considered sufficient data to justify routinely including the meniscus as we do.
Alignment assessment is mandatory before planning a FOTAM technique. Any degree of malalignment overloading the affected compartment must be corrected.4, 5, 7 A varus knee may be better addressed with an open-wedge high tibial osteotomy when a medial procedure is being performed. Conversely, a distal femoral open-wedge varus osteotomy is recommended when a valgus knee is observed in a lateral FOTAM procedure. Previous series with many patients and long follow-ups have reported concomitant osteotomies in more than half of all the cases.5, 7
Potential problems inherent to the use of fresh allografts are the risk of infectious disease transmission and some degree of immune reaction. Although the risk of an infection caused by the graft itself has been established in other procedures,6 no study has reported it to have occurred in any fresh osteochondral allograft transplantation of the knee. Also, it has been historically said that a fresh osteochondral allograft does not trigger any immune reaction.12 However, some degree of immune reaction is due to HMC type I and II present in the surface of chondrocytes and osteocytes. In addition, a CD4+ and CD8+ lymphocyte-mediated failure mechanism has recently been reported to be responsible for failure in a fresh osteochondral allograft of the talus.13 However, no study has reported like rejection in the knee, and it seems that the subtle immune reaction does not carry any clinical consequence in most cases.14 In this sense, a careful resection of the unnecessary soft tissue and pulsed lavage cleansing9 of the graft for 5 to 10 minutes might help to decrease graft immunogenicity. A list including the main advantages and disadvantages of the described technique is provided in Table 3.
Table 3.
Advantages and Limitations
|
|
The technique described here of a fresh osteochondral allograft of the tibial plateau and meniscus transplantation is perhaps the only alternative to treating large post-traumatic defects in the tibial plateau other than knee arthroplasties. Although the technique is demanding, it can be performed perfectly well following the described steps. It does require working closely with a good local tissue bank and being ready to deal with planning and scheduling issues.
Footnotes
The authors report the following potential conflicts of interest or sources of funding: P.G. is the consultant for CONMED, and receives payment for lectures including service on speakers bureaus from CONMED. J.C.M. has grants/grants pending from Spanish Ministerio de Economia, Industria y Competitividad (National Programme for Research Aimed at the Challenges of Society), and receives payment for lectures including service on speakers bureaus from Smith & Nephew. Full ICMJE author disclosure forms are available for this article online, as supplementary material.
Supplementary Data
Left knee. The fresh osteochondral and meniscus allografting is a demanding surgical technique that can be considered the only treatment alternative in some cases with post-traumatic large tibial plateau defects in young patients. In this video, every step and all critical details are clearly seen. First, the host area is prepared and measured. Then, the osteochondral graft is trimmed and several sutures are placed through the meniscus. Finally, the prepared allograft is placed and fixed in the recipient area.
References
- 1.Solheim E., Hegna J., Strand T., Harlem T., Inderhaug E. Randomized study of long-term (15-17 years) outcome after microfracture versus mosaicplasty knee articular cartilage defects. Am J Sport Med. 2018;46:826–831. doi: 10.1177/0363546517745281. [DOI] [PubMed] [Google Scholar]
- 2.Niemeyer P., Albrecht D., Andereya S. Autologous chondrocyte implantation (ACI) for cartilage defects of the knee: A guideline by the working group “Clinical Tissue Regeneration” of the German Society of Orthopaedics and Trauma (DGOU) Knee. 2016;23:426–435. doi: 10.1016/j.knee.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Gross A.E., Silverstein E.A., Falk J., Falk R., Langer F. The allotransplantation of partial joints in the treatment of osteoarthritis of the knee. Clin Orthop Relat Res. 1975;(108):7–14. doi: 10.1097/00003086-197505000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Chahal J., Gross A.E., Gross C. Outcomes of osteochondral allograft transplantation in the knee. Arthroscopy. 2013;29:575–588. doi: 10.1016/j.arthro.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Shasha N., Krywulak S., Backstein D., Pressman A., Gross A.E. Long-term follow-up of fresh tibial osteochondral allografts for failed tibial plateau fractures. J Bone Joint Surg Am. 2003;85(suppl 2):33–39. doi: 10.2106/00004623-200300002-00005. [DOI] [PubMed] [Google Scholar]
- 6.Zou S., Dodd R.Y., Stramer S.L., Strong D.M., Tissue Safety Study Group Probability of viremia with HBV, HCV, HIV, and HTLV among tissue donors in the United States. N Engl J Med. 2004;351:751–759. doi: 10.1056/NEJMoa032510. [DOI] [PubMed] [Google Scholar]
- 7.Gross A.E., Shasha N., Aubin P. Long-term follow-up of the use of fresh osteochondral allografts for post-traumatic knee defects. Clin Orthop Relat Res. 2005;435:79–87. doi: 10.1097/01.blo.0000165845.21735.05. [DOI] [PubMed] [Google Scholar]
- 8.Riberts T.T., Rosenbaum A.J. Bone grafts, bone substitutes and orthobiologics. The bridge between basic science and clinical advancements in fracture healing. Organogenesis. 2012;8:114–124. doi: 10.4161/org.23306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun Y., Jiang W., Cory E. Pulsed lavage cleansing of osteochondral grafts depends on lavage duration, flow intensity, and graft storage condition. PLoS One. 2017;12:e0176934. doi: 10.1371/journal.pone.0176934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pallante-Kichura A.L., Chen A.C., Temple-Wong M.M., Bugbee W.D., Sah R.L. In vivo efficacy of fresh versus frozen osteochondral allografts in the goat at 6 months is associated with PRG-4 secretion. J Orthop Res. 2013;31:880–886. doi: 10.1002/jor.22319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCarty E.C., Fader R.R., Mitchell J.J., Glenn R.E., Jr., Potter H.G., Spindler K.P. Fresh osteochondral allograft versus autograft: Twelve-month results in isolated canine knee defects. Am J Sport Med. 2016;44:2354–2365. doi: 10.1177/0363546516648700. [DOI] [PubMed] [Google Scholar]
- 12.Zukor D.J., Cameron J.C., Brooks P.J. The fate of human meniscal allografts. In: Ewing J.W., editor. Articular Cartilage and Knee Joint Function. Raven; New York: 1990. pp. 147–152. [Google Scholar]
- 13.Pomajzl R.J., Baker E.A., Baker K.C. Case series with histopathologic and radiographic analyses following failure of fresh osteochondral allograft of the talus. Foot Ankle Int. 2016;37:958–967. doi: 10.1177/1071100716651963. [DOI] [PubMed] [Google Scholar]
- 14.Kandel R.A., Gross A.E., Ganel A., McDermott A.G., Langer F., Pritzker K.P. Histopathology of failed osteoarticular shell allografts. Clin Orthop Relat Res. 1985;197:103–110. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Left knee. The fresh osteochondral and meniscus allografting is a demanding surgical technique that can be considered the only treatment alternative in some cases with post-traumatic large tibial plateau defects in young patients. In this video, every step and all critical details are clearly seen. First, the host area is prepared and measured. Then, the osteochondral graft is trimmed and several sutures are placed through the meniscus. Finally, the prepared allograft is placed and fixed in the recipient area.