Abstract
Rationale: The effects of fluid administration during acute asthma exacerbation are likely unique in this patient population: highly negative inspiratory intrapleural pressure resulting from increased airway resistance may interact with excess fluid administration to favor the accumulation of extravascular lung water, leading to worse clinical outcomes.
Objectives: Investigate how fluid balance influences clinical outcomes in children hospitalized for asthma exacerbation.
Methods: We analyzed the association between fluid overload and clinical outcomes in a retrospective cohort of children admitted to an urban children’s hospital with acute asthma exacerbation. These findings were validated in two cohorts: a matched retrospective and a prospective observational cohort. Finally, ultrasound imaging was used to identify extravascular lung water and investigate the physiological basis for the inferential findings.
Measurements and Main Results: In the retrospective cohort, peak fluid overload [(fluid input − output)/weight] is associated with longer hospital length of stay, longer treatment duration, and increased risk of supplemental oxygen use (P values < 0.001). Similar results were obtained in the validation cohorts. There was a strong interaction between fluid balance and intrapleural pressure: the combination of positive fluid balance and highly negative inspiratory intrapleural pressures is associated with signs of increased extravascular lung water (P < 0.001), longer length of stay (P = 0.01), longer treatment duration (P = 0.03), and increased risk of supplemental oxygen use (P = 0.02).
Conclusions: Excess volume administration leading to fluid overload in children with acute asthma exacerbation is associated with increased extravascular lung water and worse clinical outcomes.
Keywords: severe asthma, asthma exacerbation, intrapleural pressure, extravascular lung water
At a Glance Commentary
Scientific Knowledge on the Subject
The factors that influence clinical outcomes of children hospitalized for acute asthma exacerbation are poorly understood. During severe acute asthma exacerbation, highly negative intrapleural pressures during the inspiratory phase of the respiratory cycle are generated to overcome the resistive load imposed by narrowed airways. Evidence in animal models suggests that such negative intrapleural pressures can promote the accumulation of extravascular lung water, which in turn could impact gas exchange, lung compliance, and work of breathing. In this context, administering excess fluids during treatment for acute asthma exacerbation may further augment the accumulation of extravascular lung water and lead to worse clinical outcomes. However, to date, there has been no study to directly investigate this possibility.
What This Study Adds to the Field
This study establishes that in a large retrospective cohort of children hospitalized with acute asthma exacerbation, fluid overload is associated with worse clinical outcome, namely hypoxia, duration of therapy, and hospital length of stay. These findings were validated in a large prospective observational cohort of children hospitalized with rhinovirus-triggered asthma exacerbation, providing evidence of generalizability to those with acute exacerbation. Finally, we provide a physiologic mechanism for the associations by demonstrating that the large negative intrapleural pressure swings that occur during asthma exacerbation interact with fluid overload to increase the risk of extravascular lung water accumulation. Our analysis suggests that future studies investigating fluid management in asthma exacerbation are a promising avenue to improve the hospital-based care of children with acute asthma exacerbation.
Although asthma exacerbation is characterized by incomplete lung emptying due to airway obstruction, increases in end-expiratory intrapleural pressure tend to be modest (1–4). Indeed, highly negative intrapleural pressures are generated during the inspiratory phase of the respiratory cycle to overcome the resistive load imposed by narrowed airways, and an overall decrease in mean intrapleural pressure has been observed during acute asthma exacerbation (1–3, 5). In animal models, such negative swings in intrapleural pressure generate a hydrostatic driving gradient favoring the movement of fluid out of the vascular compartment into the interstitium of the lung (5, 6). In this context, administering fluids to individuals with severe acute asthma exacerbation in excess of deficit replacement may further enhance the hydrostatic and oncotic gradients favoring the accumulation of extravascular lung water (5). Thus, we hypothesize that administering excess fluid during treatment for severe acute exacerbation may interact with underlying physiological derangements, leading to increased lung water and worse clinical outcomes. Such an association would have important implications for fluid management during asthma exacerbation. There have been few studies in humans that investigate fluid management during acute asthma exacerbation and how fluid overload may contribute to asthma outcomes (7). We sought to test the association between fluid balance and clinical outcomes in children with severe acute asthma exacerbation and then investigate how changes in intrapleural pressure during acute exacerbation interact with fluid balance.
Methods
Study Population and Procedures
We performed a retrospective cohort study of patients with asthma presenting to Boston Children’s Hospital. The findings were validated in a matched retrospective cohort and in a prospective observational cohort. The Boston Children’s Hospital institutional review board approved this study (protocol p00000084).
Retrospective cohort
Asthma exacerbation encounters were identified for the 7-year period beginning January 2010, on the basis of International Classification of Diseases billable codes (see online supplement). Exclusion criteria were: not admitted, absence of intravenous fluids within 24 hours of presentation, aged younger than 6 years, chronic lung disease, acute pneumonia, and absence of admission weight. From this cohort, individuals with a single hospital encounter recorded during the study period were included in the standard retrospective analysis (see Figure E1 in the online supplement). The study time frame was chosen because it coincided with the introduction of an asthma Standard Clinical Assessment and Management Plan (8), designed to reduce practice variation by matching treatment regimen to an internally validated acute asthma severity score similar to the Modified Pulmonary Index Score (9).
Quasi-experimental matched validation cohort
Individuals from the retrospective cohort with multiple hospital encounters recorded during the study period were included in the matched analysis, in which we created a quasi-treatment and a quasi-control group as follows: 1) quasi-treatment group, those with a clinically meaningful peak fluid overload greater than or equal to 7% (a threshold identified in the receiver operating characteristic [ROC] analysis, see below); and 2) quasi-control group, those with peak fluid overload less than 7% (summarized in Figure E1). Mahalanobis distance matching was used to reduce bias and balance covariate distributions on the basis of the following covariates: initial acute asthma severity score, serum bicarbonate, and median heart rate. Matching covariates were chosen because of a significant association with the amount of fluid administration (data not shown). Quasi-treatment and quasi-control events were paired based on the shortest Euclidean distance, and the remaining observations were eliminated from the analysis. Individuals were included in this analysis only if they had both a quasi-treatment encounter and a quasi-control encounter (maximum one pair per individual); hence, each treated individual served as their owned paired control.
Prospective validation cohort
Details of this cohort have been published previously (10, 11). Only subjects who were single positive for rhinovirus (out of 12 respiratory viruses tested) were included (see online supplement).
Evaluation of Fluid Overload and Other Measures
Fluid management decisions were not dictated by any protocol. For the retrospective cohort, fluid intake and output were abstracted from the electronic medical record for the initial 72 hours of hospitalization or until discharge. Fluid totals were recorded twice daily at times corresponding to hospital-wide change of nursing shift. Fluid intake included all enteral and intravenous fluids. Fluid output included all recorded output. For each nursing shift, fluid overload percent (FO%) was calculated as follows (12):
Note that this calculation does not account for the degree of dehydration at the time of admission. The peak fluid overload was defined as the highest cumulative fluid overload percent over the first 72 hours of hospitalization (or until discharge if this occurred before 72 h). For the prospective cohort, inputs and outputs during hospitalization were recorded prospectively per study protocol, and the fluid overload percent at the time of the outpatient follow-up visit was assumed to be zero.
Time to every-2-hour albuterol is the hours over which β-agonist therapy was weaned. A supplemental oxygen requirement was defined as oxygen in use for more than 2 hours and room air oxygen saturation less than 94%. Duration of supplemental oxygen was the time from first use until discontinuation for greater than 2 hours. A noninvasive positive pressure ventilation requirement was defined as the use of continuous positive airway pressure, biphasic positive airway pressure, or high-flow nasal cannula for 2 or more continuous hours.
Esophageal balloon catheters were placed for clinical indications only in patients who were intubated, and pressure measurements were recorded during spontaneous breathing, pressure support trials (prospective cohort). For each time point, the minimum esophageal pressure readings during the inspiratory phase of three respiratory cycles were averaged. Peak aortic velocity (see below) was measured at approximately the same time.
Ultrasound Imaging and Measurements
Ultrasound images were acquired in the prospective cohort using a GE Healthcare Logiq e System for the 5-year period beginning January 2012. B-lines (a comet tail artifact extending from the visceral pleura of the lung vertically to the edge of the screen) were visualized with a 12-MHz transducer as described (13), and the cumulative number of B-lines was scored by a blinded observer from four separate intercostal spaces per individual (13). An abnormal B-line pattern (indicative of extravascular lung water) was defined as follows: 1) three or more B-lines per intercostal space, and 2) positive findings in each of the four scanned intercostal spaces (14).
Cardiac ultrasound images were obtained in the prospective cohort using a 7-MHz transducer. Peak aortic velocity was measured from an apical five-chamber view by pulsed Doppler. Maximal and minimal values of peak aortic velocity (Vpeakmax and Vpeakmin) were determined over a single respiratory cycle, and the mean of three measurements was used to calculate percent variation in peak aortic velocity over the respiratory cycle (∆ peak aortic velocity %) as follows:
Aortic velocity measurements and lung ultrasound B-line measurements were recorded longitudinally over the course of the hospitalization and at follow-up clinic visits.
Statistical Analysis
For the standard retrospective cohort analysis, univariate and multivariable linear or logistic regression were used to investigate the associations between peak fluid overload percent and outcome measures. The following covariates were included: age, sex, white race, body mass index (BMI), use of high-dose inhaled corticosteroids (ICS), initial serum bicarbonate, season, and initial acute asthma severity score. Comparisons in the matched retrospective cohort were made using the paired Student’s t test. For the prospective cohort, univariate and multivariable linear or logistic regression was used to investigate the associations between fluid overload percent and outcome measures. Covariates for multivariable models included age, sex, white race, Composite Asthma Severity Index score (15), initial serum bicarbonate, and acute asthma severity score.
For the retrospective cohort (n = 1,175), ROC curve analysis was used to identify a clinically meaningful fluid overload percent cutoff for the risk of supplemental oxygen use. Multivariable linear or logistic regression was used to validate this cutoff against the outcome measures, adjusting for age, sex, race, BMI, use of high-dose ICS, initial serum bicarbonate, season, and the acute asthma severity score.
The multivariable linear regression models of the association between peak aortic velocity and the number of B-lines were adjusted for age, weight, length of stay, and acute asthma severity score at the time measurements were performed. Because ultrasound measurements were recorded longitudinally, a random effect for individuals was added to the models to account for this by-individual variation. Certain models included an interaction term to investigate how the association between peak aortic velocity and B-lines changed depending on fluid overload. A two-sided P value < 0.05 was considered statistically significant. All analyses were performed with Stata, v.13.
Results
Fluid Balance and Clinical Outcomes
Retrospective cohort
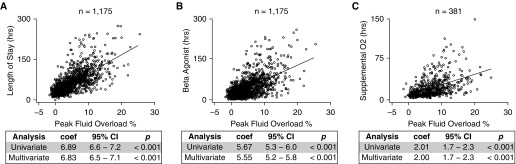
We analyzed 1,175 encounters to determine the association between fluid balance and clinical outcomes in children with acute asthma exacerbation. This cohort represented 8% of total asthma exacerbation encounters (n = 15,992), and 23.1% of all inpatient admissions (n = 5,068) during the study time frame (Figure E1). The demographics for this retrospective cohort are summarized in Table 1. We found that a positive fluid balance was significantly associated with longer length of stay and longer duration of β-agonist therapy and supplemental oxygen (Figure 1): on average a 1% increase in fluid overload is associated with ∼7-hour increase in hospital length of stay, ∼6-hour increase in the duration of β agonist, and ∼2-hour increase in the duration of supplemental oxygen (all P values < 0.001). These relationships remained significant after adjusting for age, sex, race, BMI, use of high-dose ICS, initial serum bicarbonate, season, and the acute asthma severity score (Figure 1 and summarized in Table E1).
Table 1.
Characteristics of Retrospective Cohort
| Variable | Value |
|---|---|
| n | 1,175 |
| Age, yr, mean (SD) | 10.5 (3.9) |
| Male, n (%) | 689 (59) |
| Ethnicity, n (%) | |
| African American | 404 (34) |
| White | 427 (36) |
| Hispanic | 267 (23) |
| Other | 77 (7) |
| BMI, kg/m2, mean (SD) | 21.6 (6.2) |
| Season, n (%) | |
| Spring | 356 (30) |
| Summer | 271 (23) |
| Fall | 381 (32) |
| Winter | 167 (15) |
| High-dose ICS, n (%)* | 243 (21) |
| Heart rate, mean (SD)† | 115.6 (19.5) |
| Bicarbonate, mean (SD)‡ | 19.0 (2.4) |
| ICU admission, n (%) | 741 (63) |
Definition of abbreviations: BMI = body mass index; ICS = inhaled corticosteroids.
High dose according to Expert Panel Report 3 (33).
During first 12 hours of hospital encounter.
First measured value for hospital encounter.
Figure 1.
Fluid overload is associated with worse clinical outcomes in the retrospective cohort. For individuals with a single recorded admission during the study time frame, cumulative peak fluid overload percent is directly related to (A) hospital length of stay, (B) duration of β-agonist therapy, and (C) duration of supplemental oxygen. The linear regression coefficient (coef), 95% confidence interval (CI), and P value are shown (multivariable analysis adjusted for age, sex, race, body mass index, use of high-dose inhaled corticosteroids, initial serum bicarbonate, season, and acute asthma severity score).
An ROC analysis identified a peak fluid overload percent of greater than or equal to 7% as clinically meaningful: those with fluid overload greater than or equal to 7% had significantly increased risk of requiring supplemental oxygen (odds ratio [OR], 12.3; 95% confidence interval [CI], 8.7–17.3; P < 0.001) and noninvasive positive pressure ventilation (OR, 15.4; 95% CI, 10.7–22.2; P < 0.001) (Table E2 and Figure E2) even after adjustment. The area under the ROC curve was 77%.
Validation in quasi-experimental matched retrospective cohort
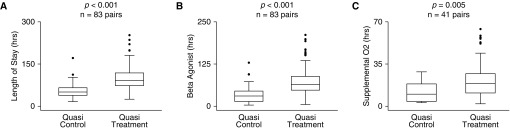
We validated the relationship between fluid balance and clinical outcomes using a quasi-experimental approach in which individuals with multiple asthma admissions during the study time frame were assigned to a quasi-treatment group (fluid overload ≥ 7%) and a quasi-control group (fluid overload < 7%) (see Methods). In 83 well-balanced, paired encounters (demographics summarized in Table 2), the average peak fluid overload for the quasi-control group was 2.86 ± 1.81%, and the average for the quasi-treatment group was (mean ± SD) 8.9 ± 2.1% (P < 0.001). The mean length of stay was greater in the quasi-treatment group (101.8 ± 44.1 h) than in the quasi-control group (53.6 ± 22.5 h; P < 0.001), the mean duration of β-agonist therapy was greater in the quasi-treatment group (76.4 ± 44.2 h) than in the quasi-control group (32.5 ± 23.5 h; P < 0.001), and the mean duration of supplemental oxygen was greater in the quasi-treatment group (22.0 ± 15.4 h) than in the quasi-control group (12.4 ± 8.3 h; P < 0.001) (Figure 2 and summarized in Table E1). The quasi-treatment group had a significantly increased risk of requiring supplemental oxygen (OR, 5.95; 95% CI, 2.9–12.2; P < 0.001).
Table 2.
Characteristics of Matched Cohort
| Variable | Quasi-control* | Quasi-treatment† | Standardized Difference | P Value‡ |
|---|---|---|---|---|
| n | 83 | 83 | n/a | n/a |
| Fluid overload %, mean (SD) | 2.86 (1.8) | 8.9 (2.1) | 1.67 | <0.001 |
| Age, yr, mean (SD) | 12.7 (3.9) | 12.1 (4.0) | 0.15 | 0.217 |
| BMI, kg/m2, mean (SD) | 24.3 (7.4) | 23.7 (8.1) | 0.08 | 0.635 |
| Season, n (%) | ||||
| Spring | 31 (37) | 32 (39) | n/a | 0.999 |
| Summer | 17 (20) | 19 (23) | n/a | 0.851 |
| Fall | 25 (30) | 21 (25) | n/a | 0.603 |
| Winter | 10 (12) | 11 (13) | n/a | 0.999 |
| High-dose ICS, n (%)§ | 61 (73) | 60 (72) | n/a | 0.999 |
| Heart rate, median (SD)|| | 110.1 (17.0) | 112.8 (14.8) | −0.13 | 0.268 |
| Bicarbonate, mean (SD)¶ | 19.0 (1.8) | 18.7 (2.1) | 0.15 | 0.266 |
| Severity score, mean (SD)** | 9.0 (1.7) | 9.1 (1.5) | −0.06 | 0.64 |
| ICU admission, n (%) | 53 (64) | 57 (69) | n/a | 0.623 |
Definition of abbreviations: BMI = body mass index; ICS = inhaled corticosteroids; n/a = not applicable.
Peak fluid overload less than 7%.
Peak fluid overload greater than or equal to 7%.
Paired t test or chi-square test for continuous or categoric variables, respectively.
High dose according to Expert Panel Report 3 (33).
During first 12 hours of hospital encounter.
First measured value for hospital encounter.
Hospital Asthma Severity Score, see Methods.
Figure 2.
Validation in a quasi-experimental matched cohort. For individuals with multiple admissions during the study time frame, a data-matching approach was used to construct a quasi-control group (cumulative peak fluid overload percent <7%) and a quasi-treatment group (cumulative peak fluid overload percent ≥7%). Encounters were matched based on median initial heart rate, initial serum bicarbonate, and acute asthma severity score. Individuals were included in this analysis only if they had both a quasi-treatment encounter and a quasi-control encounter; hence, each treated individual served as their owned paired control (n = 83 individuals; 166 paired encounters). (A) Length of stay, (B) duration of β-agonist therapy, and (C) duration of supplemental oxygen were all significantly greater in the quasi-treatment group compared with the quasi-control group. Box-plots split datasets into quartiles, with outliers represented by solid circles. Paired t test P values are shown.
Validation in prospective observational cohort
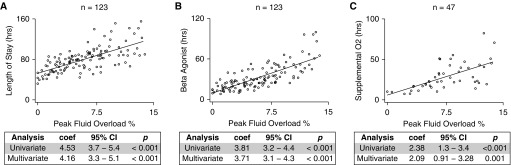
We also validated our findings in a large prospective observational cohort of children with acute asthma exacerbation triggered by rhinovirus (10). The demographics for this prospective observational cohort are summarized in Table 3. Positive fluid balance was significantly associated with longer length of stay and longer duration of β-agonist therapy and supplemental oxygen (Figure 3). These relationships remained significant after adjusting for age, sex, race, Composite Asthma Severity Index (15), serum bicarbonate, and acute asthma severity score (Figure 3 and Table E1). We found that individuals with a peak fluid overload greater than or equal to 7% had significantly increased risk of requiring supplemental oxygen after adjusting for the same covariates (OR, 2.3; 95% CI, 1.02–5.2; P = 0.044).
Table 3.
Characteristics of Prospective Cohort
| Variable | Value |
|---|---|
| n | 123 |
| Age, yr, mean (SD) | 10.0 (3.2) |
| Male, n (%) | 82 (66.7) |
| Ethnicity, n (%) | |
| African American | 59 (48) |
| White | 29 (24) |
| Hispanic | 28 (23) |
| Other | 7 (5) |
| BMI, kg/m2, mean (SD) | 21.9 (5.6) |
| Season, n (%) | |
| Spring | 36 (29.3) |
| Summer | 17 (13.8) |
| Fall | 49 (39.8) |
| Winter | 21 (17.1) |
| High-dose ICS, n (%)* | 43 (34.9) |
| Heart rate, mean (SD)† | 117.3 (20.1) |
| Bicarbonate, mean (SD) ‡ | 18.9 (2.3) |
| ICU admission, n (%) | 80 (65.0) |
Definition of abbreviations: BMI = body mass index; ICS = inhaled corticosteroids.
High dose according to Expert Panel Report 3 (33).
During first 12 hours of hospital encounter.
First measured value for hospital encounter.
Figure 3.
Validation in a prospective observational cohort. For individuals with rhinovirus-triggered asthma exacerbation, cumulative peak fluid overload percent is directly related to (A) hospital length of stay, (B) duration of β-agonist therapy, and (C) duration of supplemental oxygen. The linear regression coefficient (coef), 95% confidence interval (CI), and P value are shown (multivariable analysis adjusted for age, sex, race, composite asthma severity index score, initial serum bicarbonate, and acute asthma severity score).
Physiologic Validation
In the prospective observational cohort, we found a modest but significant association between fluid overload greater than or equal to 7% and the presence of an abnormal number of ultrasound B-lines (a sensitive and specific proxy for the presence of extravascular lung water [13, 16, 17]) (OR, 2.71; 95% CI, 1.62–4.5; P < 0.001).
We also observed that intubated but spontaneously breathing subjects with acute asthma exacerbation generated significantly more negative inspiratory intrapleural pressures (measured with esophageal manometry) than control patients without evidence of airway obstruction (asthma [mean ± SD], −18.1 ± 7.97 cm H2O, n = 24 time points in four individuals; control [mean ± SD], −8.3 ± 2.99 cm H2O, n = 17 time points in three individuals; P < 0.001 Student’s t test). This led us to hypothesize that the highly negative intrapleural pressures that occur during asthma exacerbation could interact with positive fluid balance to enhance the hydrostatic driving gradient favoring the movement of fluid out of the vascular compartment and into the lung interstitium (2, 5, 18, 19).
To test this hypothesis in the larger prospective observational cohort of nonintubated patients with asthma, we measured the variation in peak aortic blood flow velocity over the respiratory cycle using Doppler echocardiography, a noninvasive and accurate proxy for intrapleural pressure: the drop in inspiratory intrapleural pressure resulting from airway obstruction in spontaneously breathing individuals causes a proportional drop in peak aortic blood flow velocity due to heart–lung interactions (2, 20–24). Hence, more negative intrapleural pressure (e.g., during asthma exacerbation) is reflected by increased variation in peak aortic velocity over the respiratory cycle. This is the same mechanism that accounts for the occurrence of pulsus paradoxus during asthma exacerbation (19). We confirmed that variation in peak aortic velocity is a close proxy for intrapleural pressure in a subset of the prospective observational cohort (R2 = 83%; Figure E3).
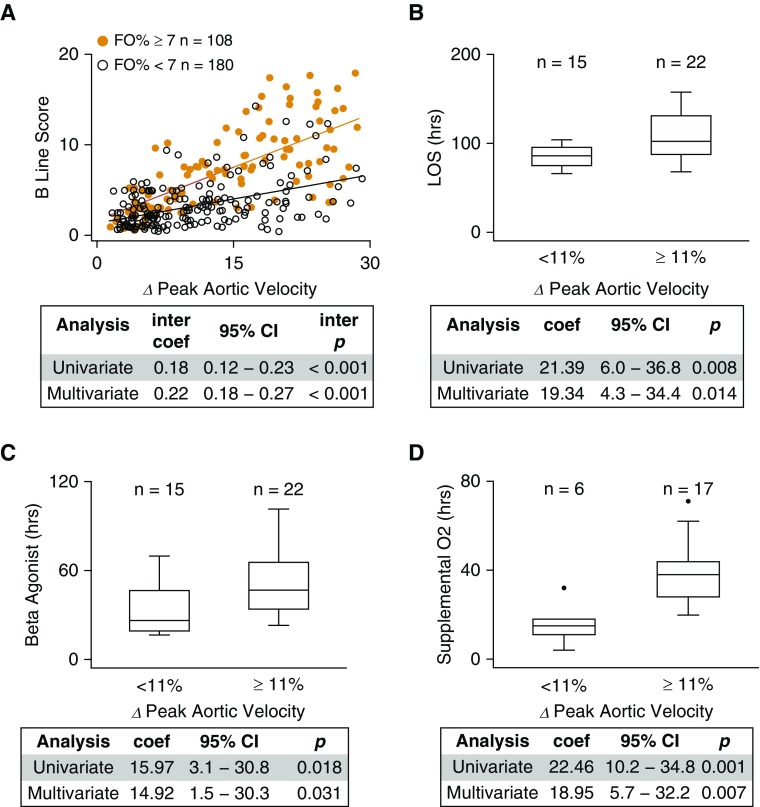
There is a robust interaction between fluid overload and respiratory variation in peak aortic velocity with respect to signs of extravascular lung water. As respiratory variation in peak aortic velocity increased (indicating more negative swings in inspiratory intrapleural pressure), those individuals with clinically meaningful fluid overload (≥7%) were significantly more likely to exhibit ultrasound signs of extravascular lung water compared with individuals with less fluid overload (<7%), even after adjusting for age, weight, length of stay, and acute asthma severity score (Figure 4A).
Figure 4.
Interaction between fluid overload and intrapleural pressure is associated with evidence of increased extravascular lung water. (A) For individuals with rhinovirus-triggered asthma exacerbation (n = 61 individuals with 288 measurements), as percent variation in peak aortic velocity over the respiratory cycle (∆ peak aortic velocity %) increased, those individuals with clinically meaningful fluid overloaded (fluid overload percent [FO%] ≥ 7, orange; n = 108 observations) were significantly more likely to exhibit ultrasound signs of extravascular lung water (B-lines) compared with individuals with FO% less than 7 (black; n = 180 observations). The linear regression interaction coefficient (inter coef), 95% confidence interval (CI), and interaction P (inter p) value are shown (multivariable analysis adjusted for age, weight, time from hospital admission to ultrasound measurements, and acute asthma severity score at the time of ultrasound measurements). The combination of clinically meaningful fluid overload (FO% ≥ 7) and high respiratory variation in peak aortic velocity (≥11%) was significantly associated with (B) longer length of stay (LOS), (C) longer duration of β-agonist therapy, and (D) longer duration of supplemental oxygen when compared with patients who had clinically meaningful fluid overload but low respiratory variation in peak aortic velocity. Box-plots split datasets into quartiles, with outliers represented by solid circles. The regression coefficient (coef), 95% CI, and P value are shown (multivariable analysis adjusted for age, sex, race, composite asthma severity index score, initial serum bicarbonate, and acute asthma severity score).
We identified a significance threshold in the association between respiratory variation in peak aortic velocity and B-lines when variation in aortic velocity was greater than or equal to 11% (Figure E4). Individuals who had both variation in peak aortic velocity above this threshold and clinically meaningful fluid overload (FO% ≥ 7) had significantly greater odds of an abnormal number of B-lines compared with patients with fluid overload greater than or equal to 7% but low variation in peak aortic velocity, even after adjustment (OR, 41.7; 95% CI, 9.1–192.3; P = 0.001). Furthermore, we found that the combination of fluid overload (FO% ≥ 7) and high respiratory variation in peak aortic velocity (≥11%) were significantly associated with longer length of stay and longer duration of β-agonist therapy and supplemental oxygen than those with high fluid overload but low respiratory variation in peak aortic velocity: on average, hospitalizations with both high fluid overload and high respiratory variation in peak aortic velocity were associated with ∼19-hour increased length of stay, ∼15-hour increased duration of β agonist, and ∼19-hour increase in the duration of supplemental oxygen. These relationships remain significant after adjusting for age, sex, race, Composite Asthma Severity Index score, initial serum bicarbonate, and acute asthma severity score (Figures 4B–4D). Individuals with high fluid overload (FO% ≥ 7) and high respiratory variation in peak aortic velocity (≥11%) were also significantly more likely to have used supplemental oxygen than those who had high fluid overload but low respiratory variation in peak aortic velocity after adjusting for the same covariates (OR, 5.1; 95% CI, 1.2–21.4; P = 0.026; n = 37).
Discussion
We demonstrate for the first time that positive fluid balance is associated with worse clinical outcomes in children hospitalized for severe acute asthma exacerbation. We also demonstrate that fluid overload is directly related to markers of increased extravascular lung water. Finally, we show a robust interaction between fluid overload and a proxy measure for intrapleural pressure: individuals with the combination of fluid overload and high respiratory variation in peak aortic velocity had lung ultrasound findings more consistent with lung water and had worse clinical outcomes than individuals who were fluid overloaded but had low respiratory variation in peak aortic velocity. These data are consistent with a model in which administering excess fluids during the course of treatment, combined with the highly negative inspiratory intrapleural pressure resulting from increased airway resistance, favors the movement of fluid from the vascular compartment to the lung interstitium, leading to decreased lung compliance, increased work of breathing, and impaired gas exchange (5, 6). These data suggest that fluid management is an important modifiable risk factor: those without fluid overload face a relatively low risk of extravascular lung water, even in the face of highly negative intrapleural pressure (Figure 4).
Our study is consistent with others that document highly negative swings in intrapleural pressures during an acute asthma exacerbation (2, 18, 19) and with those showing that negative pressure pulmonary edema results from hydrostatic forces that favor the accumulation of extravascular lung water (25). Our study further corroborates a recent report showing children with asthma exacerbation have abnormal lung ultrasound findings consistent with increased lung water (26) and supports that the interaction between fluid overload and asthma exacerbation physiology in humans is similar to animal models (5). Most importantly, our findings are broadly consistent with studies demonstrating that fluid overload is associated with poor clinical outcomes for a number of critical conditions in pediatrics (12, 27–29).
This study has multiple limitations. First, fluid balance may simply be a marker of illness severity rather than a cause of worse clinical outcomes (30). We do not favor this explanation because 1) our regression models were adjusted for illness severity using a clinical scoring tool that specifically quantifies the severity of acute asthma exacerbation; 2) we did not observe this relationship in patients with either acute appendicitis or with status epilepticus (see online supplement), arguing that fluid overload is not simply a nonspecific marker of disease severity; 3) we present evidence for a mechanistic basis for our inferential statistical findings (i.e., increased extravascular lung water); and 4) fluid resuscitation is not commonly used as a therapeutic goal in the management of acute asthma exacerbation, hence the relationship between severity and fluid balance is not as clear as it is for diseases such as septic shock. The second limitation is that fluid overload percent as calculated in our definition relies on admission weight and thus will exaggerate fluid overload in a population of patients, such as patients with asthma, who are likely to be dehydrated at admission. Third, measuring input and output in a retrospective cohort is imprecise, and fluid overload percent might be overestimated in cases where output was missed. However, our results were replicated both when we examine a subset of patients who had indwelling urinary catheter (presumably allowing more accurate output measurements; see online supplement) and in the prospective observational cohort. Fourth, outcome measures were not dictated by a study protocol; thus, bias could have been introduced through variation in individual provider practices. However, the majority of individuals were managed according to a Standard Clinical Assessment and Management Plan, which reduces practice variation (8). Fifth, this study was conducted at a single center and needs to be replicated by others. Sixth, the relationship between peak aortic velocity and intrapleural pressure is confounded because peak aortic velocity is also influenced by hydration status (31). Thus, in patients who are dehydrated, we may overestimate the degree of negative intrapleural pressure generated during inspiration. However, the regression model is adjusted for serum bicarbonate, a marker of dehydration (32). Furthermore, our data suggest that variation in peak aortic velocity is more closely correlated with intrapleural pressure, as we demonstrate a robust relationship between aortic velocity and the presence of lung water, a finding that seems biologically less plausible in the setting of substantial dehydration. Finally, because this was not a prospective randomized trial, we cannot make definitive conclusions about fluid administration protocols in patients with acute severe asthma.
In summary, this study establishes a relationship between positive fluid balance and worse clinical outcomes in children with severe acute asthma exacerbation and suggests a mechanistic basis for this relationship: fluid overload interacts with the highly negative swings in intrapleural pressure that occur with obstructed airways to drive the accumulation of extravascular lung water. These observations raise critical management questions regarding volume administration protocols and the use of maintenance fluids in pediatric patients with severe asthma exacerbation. Our results suggest that clinicians should be judicious in their approach to administering fluids to patients with asthma and that future studies investigating fluid management hold promise for improving the hospital-based care of children with severe acute asthma exacerbation.
Acknowledgments
Acknowledgment
We thank Dr. Robert Tasker for helpful comments.
Footnotes
Supported by NIH grants T32 HD040128, K12 HD047349, and K23 HL138162 (D.B.K.); U01 AI 110397, R01 AI073964, and K24 AI106822 (W.P.); the American Medical Association Seed Grant (D.B.K.); and the American Asthma Foundation (J.N.H.). This work was conducted with support from Harvard Catalyst–The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, NIH Award UL1 TR001102) and financial contributions from Harvard University and its affiliated academic healthcare centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University or its affiliated academic healthcare centers, or the NIH.
Author Contributions: D.B.K., W.P., and J.N.H.: Study conceptualization, design, and implementation; data acquisition, statistical analysis, and interpretation; manuscript preparation and critical revision; and agreed to the final version of the manuscript and to be held accountable for all aspects of the work. E.L.H. and D.Z.: Statistical analysis and interpretation, manuscript preparation and critical revision, and agreed to the final version of the manuscript and to be held accountable for all aspects of the work. M.C.M., J.G., T.B., N.S., and C.D.S.: Data acquisition, manuscript revision, and agreed to the final version of the manuscript and to be held accountable for all aspects of the work. K.A.N.: Study conceptualization, design, and implementation; data acquisition and manuscript revision; and agreed to the final version of the manuscript and to be held accountable for all aspects of the work.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201709-1860OC on January 9, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Permutt S. Relation between pulmonary arterial pressure and pleural pressure during the acute asthmatic attack. Chest. 1973;63:25S–28S. doi: 10.1378/chest.63.4_supplement.25s. [DOI] [PubMed] [Google Scholar]
- 2.Scharf SM. Cardiovascular effects of airways obstruction. Hai. 1991;169:1–23. doi: 10.1007/BF02714137. [DOI] [PubMed] [Google Scholar]
- 3.Freedman S, Tattersfield AE. Pride NB. Changes in lung mechanics during asthma induced by exercise. J Appl Physiol. 1975;38:974–982. doi: 10.1152/jappl.1975.38.6.974. [DOI] [PubMed] [Google Scholar]
- 4.Holmes PW, Campbell AH, Barter CE. Acute changes of lung volumes and lung mechanics in asthma and in normal subjects. Thorax. 1978;33:394–400. doi: 10.1136/thx.33.3.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stalcup SA, Mellins RB. Mechanical forces producing pulmonary edema in acute asthma. N Engl J Med. 1977;297:592–596. doi: 10.1056/NEJM197709152971107. [DOI] [PubMed] [Google Scholar]
- 6.Mellins RB, Levine OR, Skalak R, Fishman AP. Interstitial pressure of the lung. Circ Res. 1969;24:197–212. doi: 10.1161/01.res.24.2.197. [DOI] [PubMed] [Google Scholar]
- 7.Potter PC, Klein M, Weinberg EG. Hydration in severe acute asthma. Arch Dis Child. 1991;66:216–219. doi: 10.1136/adc.66.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farias M, Jenkins K, Lock J, Rathod R, Newburger J, Bates DW, et al. Standardized Clinical Assessment And Management Plans (SCAMPs) provide a better alternative to clinical practice guidelines. Health Aff (Millwood) 2013;32:911–920. doi: 10.1377/hlthaff.2012.0667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carroll CL, Sekaran AK, Lerer TJ, Schramm CM. A modified pulmonary index score with predictive value for pediatric asthma exacerbations. Ann Allergy Asthma Immunol. 2005;94:355–359. doi: 10.1016/S1081-1206(10)60987-8. [DOI] [PubMed] [Google Scholar]
- 10.Kantor DB, Stenquist N, McDonald MC, Schultz BJ, Hauptman M, Smallwood CD, et al. Rhinovirus and serum IgE are associated with acute asthma exacerbation severity in children. J Allergy Clin Immunol. 2016;138:1467–1471.e9. doi: 10.1016/j.jaci.2016.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kantor DB, McDonald MC, Stenquist N, Schultz BJ, Smallwood CD, Nelson KA, et al. Omalizumab is associated with reduced acute severity of rhinovirus-triggered asthma exacerbation. Am J Respir Crit Care Med. 2016;194:1552–1555. doi: 10.1164/rccm.201606-1145LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arikan AA, Zappitelli M, Goldstein SL, Naipaul A, Jefferson LS, Loftis LL. Fluid overload is associated with impaired oxygenation and morbidity in critically ill children. Pediatr Crit Care Med. 2012;13:253–258. doi: 10.1097/PCC.0b013e31822882a3. [DOI] [PubMed] [Google Scholar]
- 13.Enghard P, Rademacher S, Nee J, Hasper D, Engert U, Jörres A, et al. Simplified lung ultrasound protocol shows excellent prediction of extravascular lung water in ventilated intensive care patients. Crit Care. 2015;19:36. doi: 10.1186/s13054-015-0756-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Volpicelli G, Mussa A, Garofalo G, Cardinale L, Casoli G, Perotto F, et al. Bedside lung ultrasound in the assessment of alveolar-interstitial syndrome. Am J Emerg Med. 2006;24:689–696. doi: 10.1016/j.ajem.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 15.Wildfire JJ, Gergen PJ, Sorkness CA, Mitchell HE, Calatroni A, Kattan M, et al. Development and validation of the Composite Asthma Severity Index--an outcome measure for use in children and adolescents. J Allergy Clin Immunol. 2012;129:694–701. doi: 10.1016/j.jaci.2011.12.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agricola E, Bove T, Oppizzi M, Marino G, Zangrillo A, Margonato A, et al. “Ultrasound comet-tail images”: a marker of pulmonary edema: a comparative study with wedge pressure and extravascular lung water. Chest. 2005;127:1690–1695. doi: 10.1378/chest.127.5.1690. [DOI] [PubMed] [Google Scholar]
- 17.Rossi P, Wanecek M, Rudehill A, Konrad D, Weitzberg E, Oldner A. Comparison of a single indicator and gravimetric technique for estimation of extravascular lung water in endotoxemic pigs. Crit Care Med. 2006;34:1437–1443. doi: 10.1097/01.CCM.0000215830.48977.29. [DOI] [PubMed] [Google Scholar]
- 18.Jardin F, Farcot JC, Boisante L, Prost JF, Gueret P, Bourdarias JP. Mechanism of paradoxic pulse in bronchial asthma. Circulation. 1982;66:887–894. doi: 10.1161/01.cir.66.4.887. [DOI] [PubMed] [Google Scholar]
- 19.Blaustein AS, Risser TA, Weiss JW, Parker JA, Holman BL, McFadden ER. Mechanisms of pulsus paradoxus during resistive respiratory loading and asthma. J Am Coll Cardiol. 1986;8:529–536. doi: 10.1016/s0735-1097(86)80179-6. [DOI] [PubMed] [Google Scholar]
- 20.Yuan L, Cao T, Duan Y, Yang G, Wang Z, Ruan L. Noninvasive assessment of influence of resistant respiration on blood flow velocities across the cardiac valves in humans--a quantification study by echocardiography. Echocardiography. 2004;21:391–398. doi: 10.1111/j.0742-2822.2004.03086.x. [DOI] [PubMed] [Google Scholar]
- 21.Iliceto S, Dambrosio M, Sorino M, D’Ambrosio G, Amico A, Fiore T, et al. Effects of acute intrathoracic pressure changes on left ventricular geometry and filling. Am Heart J. 1988;116:455–465. doi: 10.1016/0002-8703(88)90618-7. [DOI] [PubMed] [Google Scholar]
- 22.Andreas S, Werner GS, Sold G, Wiegand V, Kreuzer H. Doppler echocardiographic analysis of cardiac flow during the Mueller manoeuver. Eur J Clin Invest. 1991;21:72–76. doi: 10.1111/j.1365-2362.1991.tb01361.x. [DOI] [PubMed] [Google Scholar]
- 23.Innes JA, De Cort SC, Kox W, Guz A. Within-breath modulation of left ventricular function during normal breathing and positive-pressure ventilation in man. J Physiol. 1993;460:487–502. doi: 10.1113/jphysiol.1993.sp019483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Cort SC, Innes JA, Guz A. Effect of positive and negative step changes in intrathoracic pressure on left ventricular function in conscious man. J Physiol. 1993;472:513–520. doi: 10.1113/jphysiol.1993.sp019959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhattacharya M, Kallet RH, Ware LB, Matthay MA. Negative-pressure pulmonary edema. Chest. 2016;150:927–933. doi: 10.1016/j.chest.2016.03.043. [DOI] [PubMed] [Google Scholar]
- 26.Dankoff S, Li P, Shapiro AJ, Varshney T, Dubrovsky AS. Point of care lung ultrasound of children with acute asthma exacerbations in the pediatric ED. Am J Emerg Med. 2017;35:615–622. doi: 10.1016/j.ajem.2016.12.057. [DOI] [PubMed] [Google Scholar]
- 27.Hayes LW, Oster RA, Tofil NM, Tolwani AJ. Outcomes of critically ill children requiring continuous renal replacement therapy. J Crit Care. 2009;24:394–400. doi: 10.1016/j.jcrc.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 28.Maitland K, Kiguli S, Opoka RO, Engoru C, Olupot-Olupot P, Akech SO, et al. FEAST Trial Group. Mortality after fluid bolus in African children with severe infection. N Engl J Med. 2011;364:2483–2495. doi: 10.1056/NEJMoa1101549. [DOI] [PubMed] [Google Scholar]
- 29.Valentine SL, Sapru A, Higgerson RA, Spinella PC, Flori HR, Graham DA, et al. Pediatric Acute Lung Injury and Sepsis Investigator’s (PALISI) Network; Acute Respiratory Distress Syndrome Clinical Research Network (ARDSNet) Fluid balance in critically ill children with acute lung injury. Crit Care Med. 2012;40:2883–2889. doi: 10.1097/CCM.0b013e31825bc54d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schuller D, Mitchell JP, Calandrino FS, Schuster DP. Fluid balance during pulmonary edema: is fluid gain a marker or a cause of poor outcome? Chest. 1991;100:1068–1075. doi: 10.1378/chest.100.4.1068. [DOI] [PubMed] [Google Scholar]
- 31.Gan H, Cannesson M, Chandler JR, Ansermino JM. Predicting fluid responsiveness in children: a systematic review. Anesth Analg. 2013;117:1380–1392. doi: 10.1213/ANE.0b013e3182a9557e. [DOI] [PubMed] [Google Scholar]
- 32.Vega RM, Avner JR. A prospective study of the usefulness of clinical and laboratory parameters for predicting percentage of dehydration in children. Pediatr Emerg Care. 1997;13:179–182. doi: 10.1097/00006565-199706000-00001. [DOI] [PubMed] [Google Scholar]
- 33.National Heart, Lung, and Blood Institute 2007. National Asthma Education and Prevention Program. Expert panel report 3: guidelines for the diagnosis and management of asthma: full report 2007 [accessed 2018 Mar 28]. Available from: https://www.nhlbi.nih.gov/files/docs/guidelines/asthgdln.pdf.