To the Editor:
Over 60% of pediatric tuberculosis cases are undetected by healthcare services in low-income settings (1). Untreated children with tuberculosis have fatality rates of >20%, reaching above 40% in children <5 years old (2). Specific, effective, and validated interventions to increase case detection in children are urgently needed.
Household contact tracing has been widely recommended to increase detection of childhood tuberculosis cases. Although it can provide high yields (3, 4), child contact tracing has rarely been implemented in high-burden settings (5). Methods are urgently needed to incentivize contact tracing for national tuberculosis programs in low-income settings. A clinical risk score was recently proposed in Taiwan (a high-income, low-prevalence setting) to increase the effectiveness of child contact tracing (6). This algorithm uses a combination of contact, index, and environmental characteristics to allocate child contacts into high- and low-risk groups for secondary disease. External validation in high-burden settings has not been performed. To investigate whether this score (6) is useful for detecting tuberculosis in exposed children from Sub-Saharan Africa, we used data from a large Ugandan child contact cohort.
This was a prospective cohort study of household contacts of tuberculosis cases; the study has been described previously (7–9). Briefly, we identified adult patients in Kampala, Uganda, with a new diagnosis of tuberculosis. Index cases were microbiologically confirmed through a positive culture test and evaluated through physical examination and medical history. Households were visited by field workers within 2 weeks of diagnosis, and contacts were defined as spending at least 7 consecutive days in the index’s household 3 months before diagnosis.
Coprevalent tuberculosis was defined as tuberculosis within 3 months of baseline. Contacts were evaluated for tuberculosis through a medical examination, posteroanterior chest radiographs, specimen microscopy, and mycobacterial culture. Contacts without coprevalent tuberculosis were followed for incident tuberculosis for 2 years.
Contacts were grouped by age (<13 yr old) and disease status to categorize the data according to the scoring algorithm (6). Contacts were given a point score based on the algorithm (6): contact tuberculin skin test (TST) induration (2 points if 10–14 mm, 3 points if 15–20 mm, and 4 points if ≥20 mm), index smear result (1 point if smear positive), index sex (1 point if female), and burden of residence area (2 points if index lived in a high-incidence area). Because all index cases in the Ugandan cohort lived in a high-incidence area, all contacts were automatically given 2 points.
The score’s discrimination and classification accuracy was compared with results from the original derivation and internal validation populations. Cochran-Armitage tests were used to test for trends between disease outcomes and multiple point categories in the risk score.
The institutional review boards at the Uganda National Council for Science and Technology, the Uganda National AIDS Research Subcommittee, University Hospitals Cleveland Medical Center, and Makerere University approved this study. Informed consent was obtained for index cases, and parents of contacts provided verbal assent for their children. Nine months of isoniazid prophylaxis was offered to child contacts if they were <5 years old, HIV-infected, or TST-positive.
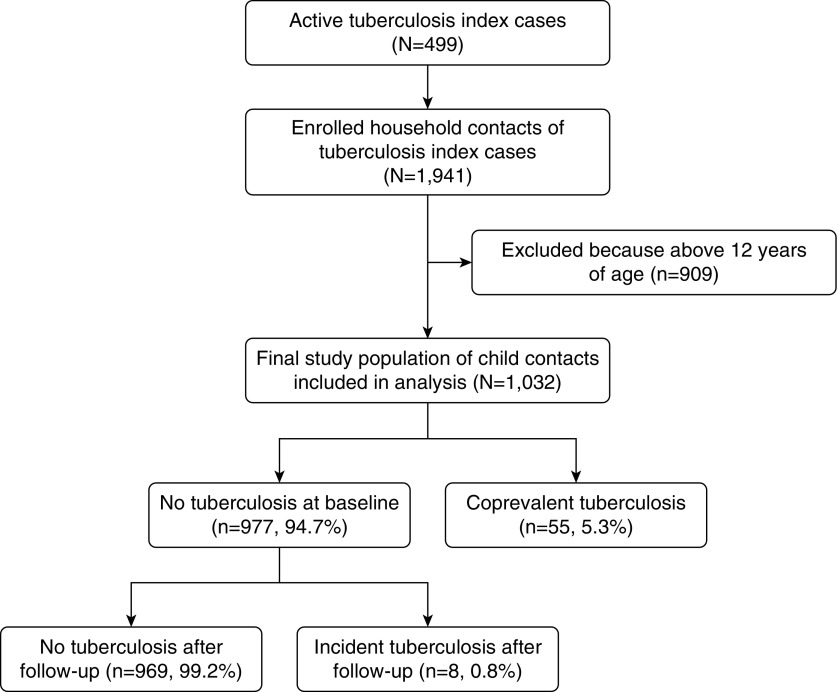
Overall, 1,941 household contacts were enrolled; 1,032 of these contacts were <13 years old and included in this analysis (Figure 1). The median contact age was 6 years. The majority had a positive TST (n = 586), and 63 had tuberculosis (55 subjects [5.3%] had tuberculosis at baseline and 8 [0.8%] developed tuberculosis over 2 yr). Tuberculosis was microbiologically confirmed in 65.1% of these cases. Isoniazid preventive therapy was started in 274 children who were free of tuberculosis at baseline.
Figure 1.
Study profile of the Kawempe Community Health Study and participants included in the primary analysis.
In a univariate analysis, no variable included in the risk score was a statistically significant predictor of coprevalent, incident, or any tuberculosis. The proposed algorithm had low predictive power (C-statistic, 0.54). Coprevalent tuberculosis prevalence varied from 4.8% for contacts with a score of 4 to 12.9% with a score of 8, but there was no association between the score and disease prevalence (Ptrend = 0.528; Table 1). Similarly, for contacts with any disease, the observed proportion varied from 3.8% for contacts with a score of 7 to 12.9% with a score of 8, and there was no point score trend (Ptrend = 0.212). There were eight incident cases, five of which occurred in contacts with a score of 7. Compared with children with a 0–4 score, children with a 5–8 score did not have statistically more coprevalent (P = 0.405), incident (P = 0.237), or any tuberculosis event (P = 0.227).
Table 1.
Scores Implemented in a Ugandan Cohort of Household Child Contacts of Tuberculosis Cases (N = 1,032)
| Proposed Score | No. of Contacts with Each Score | Percent Risk of All Disease Among Contacts (n Events/n Total)* | Percent Risk of Coprevalent Disease Cases (n Events/n Total)* | Percent Risk of Incident Disease Cases (n Events/n Total)* |
|---|---|---|---|---|
| 8 | 31 | 12.9 (4/31) | 12.9 (4/31) | 0 (0/27) |
| 7 | 159 | 6.9 (11/159) | 3.8 (6/159) | 3.3 (5/153) |
| 6 | 240 | 5.8 (14/240) | 5.8 (14/240) | 0 (0/226) |
| 5 | 133 | 7.5 (10/133) | 6.8 (9/133) | 0.8 (1/124) |
| 4 | 187 | 4.8 (9/187) | 4.3 (8/187) | 0.6 (1/179) |
| 3 | 224 | 5.4 (12/224) | 4.9 (11/224) | 0.5 (1/213) |
| 2 | 58 | 5.2 (3/58) | 5.2 (3/58) | 0 (0/55) |
| 1 | 0 | — | — | — |
| 0 | 0 | — | — | — |
| P for trend† | 0.212 | 0.528 | 0.071 | |
| High (5–8)‡ | 563 | 6.9 (39/563) | 5.9 (33/563) | 1.1 (6/530) |
| Low (0–4) | 469 | 5.1 (24/469) | 4.7 (22/469) | 0.5 (2/447) |
| P for trend† | 0.227 | 0.405 | 0.237 | |
| High (5–7)‡ | 532 | 6.6 (35/532) | 5.5 (29/532) | 1.2 (6/503) |
| Low (0–4) | 469 | 5.1 (24/469) | 4.7 (22/469) | 0.5 (2/447) |
| P for trend† | 0.327 | 0.585 | 0.21 |
Coprevalent tuberculosis disease was defined as the identification of tuberculosis disease at or within 3 months of the baseline household visit. Incident tuberculosis disease was defined as diagnosis of tuberculosis disease at subsequent household follow-up visits, conducted at 6-month intervals for 2 years. Individuals with coprevalent disease were excluded from analyses of incident disease. “All disease” indicates the combination of both coprevalent and incidence tuberculosis disease.
The Cochran-Armitage test was used to evaluate trends within groups.
Score cutoffs were chosen as shown in Reference 6 to provide proper comparison between studies.
Making a diagnosis of tuberculosis in children remains a clinical and programmatic challenge. To guide clinicians in identifying high-risk children, a new risk score was recently derived and implemented in Taiwan (6). Although it was highly predictive among Taiwanese child contacts (C-statistic, 0.87), this algorithm had low predictive power in our large, prospective child contact cohort from Uganda.
There are several possible reasons why this algorithm performed poorly in our cohort. First, low- and high-incidence areas may have different risk factors for tuberculosis. For example, the prevalence of HIV in Taiwan is significantly different from that in Uganda, and HIV plays a critical role in influencing the tuberculosis epidemic in Africa (7, 9, 10). This score may be most useful in high-income, low-tuberculosis-burden settings where HIV prevalence is low. External, prospective validations in such settings are necessary to address this issue. Second, case ascertainment bias is a concern because the original derivation-cohort study used retrospective programmatic data.
This study has certain limitations that should be mentioned. First, without molecular genotyping, we are unable to state with certainty that contacts acquired disease owing to household exposure. However, our aim was to evaluate the disease yield in our setting using the specified algorithm, not to measure household transmission. Second, because one variable in the derived score was “high-incidence area,” to evaluate this score in Sub-Saharan Africa, we had to assign 2 points to all contacts.
In conclusion, a previously derived risk score demonstrated poor predictive value for detecting coprevalent and incident tuberculosis in a large, prospective Ugandan child contact cohort. This score should be evaluated in low-burden settings to determine its effectiveness in settings similar to but outside of Taiwan. Additional clinical algorithms that can more efficiently detect tuberculosis in child contacts are urgently needed in Sub-Saharan Africa to improve case detection.
Acknowledgments
Acknowledgment
The authors thank the Uganda-CWRU Research Collaboration; the National Tuberculosis Treatment Centre, Mulago Hospital; and the Uganda Tuberculosis Investigation Bacteriological Unit, Wandegeya, for their contributions to this study.
Footnotes
Supported in part by the National Institute of Allergy and Infectious Diseases (NO1-AI-95383 and HHSN266200700022C/NO1-AI-70022), the Fogarty International Center (TW-00011), and the Center for AIDS Research (AI 36219).
Originally Published in Press as DOI: 10.1164/rccm.201706-1210LE on October 16, 2017
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Dodd PJ, Gardiner E, Coghlan R, Seddon JA. Burden of childhood tuberculosis in 22 high-burden countries: a mathematical modelling study. Lancet Glob Health. 2014;2:e453–e459. doi: 10.1016/S2214-109X(14)70245-1. [DOI] [PubMed] [Google Scholar]
- 2.Jenkins HE, Yuen CM, Rodriguez CA, Nathavitharana RR, McLaughlin MM, Donald P, et al. Mortality in children diagnosed with tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis. 2017;17:285–295. doi: 10.1016/S1473-3099(16)30474-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martinez L, Shen Y, Mupere E, Kizza A, Hill PC, Whalen CC. Transmission of Mycobacterium tuberculosis in households and the community: a systematic review and meta-analysis. Am J Epidemiol. 2017;185:1327–1339. doi: 10.1093/aje/kwx025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morrison J, Pai M, Hopewell PC. Tuberculosis and latent tuberculosis infection in close contacts of people with pulmonary tuberculosis in low-income and middle-income countries: a systematic review and meta-analysis. Lancet Infect Dis. 2008;8:359–368. doi: 10.1016/S1473-3099(08)70071-9. [DOI] [PubMed] [Google Scholar]
- 5.Hill PC, Rutherford ME, Audas R, van Crevel R, Graham SM. Closing the policy-practice gap in the management of child contacts of tuberculosis cases in developing countries. PLoS Med. 2011;8:e1001105. doi: 10.1371/journal.pmed.1001105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan PC, Shinn-Forng Peng S, Chiou MY, Ling DL, Chang LY, Wang KF, et al. Risk for tuberculosis in child contacts: development and validation of a predictive score. Am J Respir Crit Care Med. 2014;189:203–213. doi: 10.1164/rccm.201305-0863OC. [DOI] [PubMed] [Google Scholar]
- 7.Martinez L, Sekandi JN, Castellanos ME, Zalwango S, Whalen CC. Infectiousness of HIV-seropositive patients with tuberculosis in a high-burden African setting. Am J Respir Crit Care Med. 2016;194:1152–1163. doi: 10.1164/rccm.201511-2146OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whalen CC, Zalwango S, Chiunda A, Malone L, Eisenach K, Joloba M, et al. Secondary attack rate of tuberculosis in urban households in Kampala, Uganda. PLoS One. 2011;6:e16137. doi: 10.1371/journal.pone.0016137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jaganath D, Zalwango S, Okware B, Nsereko M, Kisingo H, Malone L, et al. Tuberculosis Research Unit. Contact investigation for active tuberculosis among child contacts in Uganda. Clin Infect Dis. 2013;57:1685–1692. doi: 10.1093/cid/cit645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaisson RE, Martinson NA. Tuberculosis in Africa: combating an HIV-driven crisis. N Engl J Med. 2008;358:1089–1092. doi: 10.1056/NEJMp0800809. [DOI] [PubMed] [Google Scholar]