Abstract
Rationale: Therapies for obstructive sleep apnea (OSA) could be administered on the basis of a patient’s own phenotypic causes (“traits”) if a clinically applicable approach were available.
Objectives: Here we aimed to provide a means to quantify two key contributors to OSA—pharyngeal collapsibility and compensatory muscle responsiveness—that is applicable to diagnostic polysomnography.
Methods: Based on physiological definitions, pharyngeal collapsibility determines the ventilation at normal (eupneic) ventilatory drive during sleep, and pharyngeal compensation determines the rise in ventilation accompanying a rising ventilatory drive. Thus, measuring ventilation and ventilatory drive (e.g., during spontaneous cyclic events) should reveal a patient’s phenotypic traits without specialized intervention. We demonstrate this concept in patients with OSA (N = 29), using a novel automated noninvasive method to estimate ventilatory drive (polysomnographic method) and using “gold standard” ventilatory drive (intraesophageal diaphragm EMG) for comparison. Specialized physiological measurements using continuous positive airway pressure manipulation were employed for further comparison. The validity of nasal pressure as a ventilation surrogate was also tested (N = 11).
Measurements and Main Results: Polysomnography-derived collapsibility and compensation estimates correlated favorably with those quantified using gold standard ventilatory drive (R = 0.83, P < 0.0001; and R = 0.76, P < 0.0001; respectively) and using continuous positive airway pressure manipulation (R = 0.67, P < 0.0001; and R = 0.64, P < 0.001; respectively). Polysomnographic estimates effectively stratified patients into high versus low subgroups (accuracy, 69–86% vs. ventilatory drive measures; P < 0.05). Traits were near-identical using nasal pressure versus pneumotach (N = 11, R ≥ 0.98, both traits; P < 0.001).
Conclusions: Phenotypes of pharyngeal dysfunction in OSA are evident from spontaneous changes in ventilation and ventilatory drive during sleep, enabling noninvasive phenotyping in the clinic. Our approach may facilitate precision therapeutic interventions for OSA.
Keywords: pathophysiology, personalized medicine, endotype
At a Glance Commentary
Scientific Knowledge on the Subject
Physiological studies demonstrate that the mechanisms of obstructive sleep apnea (OSA) vary greatly across patients and can predict responses to treatments. Precision treatment in the clinic relies on the development of new techniques to identify the pathophysiological mechanisms underlying OSA—including pharyngeal collapsibility and muscle compensation—from the wealth of underutilized data available in a patient’s own sleep study.
What This Study Adds to the Field
Our study shows that a patient’s phenotypic mechanisms of OSA are apparent from the spontaneous changes in ventilation and ventilatory drive during sleep. Thus, a patient’s phenotype can be identified from polysomnographic signals without the need for calibrated airflow or specialized interventions. We provide an automated technique to accurately yield underlying OSA phenotypes, opening the doors for precision treatment for OSA.
For the treatment of obstructive sleep apnea (OSA), there are a variety of alternatives to continuous positive airway pressure (CPAP) including oral appliances, surgery, hypoglossal nerve stimulation, supplemental oxygen, and novel medications. Each of these alternatives is effective in a subset of individuals characterized by several key pathophysiological traits, including pharyngeal collapsibility and compensatory pharyngeal dilator muscle responses (1–4) among others. For example, patients with a more collapsible airway are less responsive to nonmechanical (e.g., pharmaceutical) interventions, including the combination of oxygen and a hypnotic (5). Oral appliances are less effective in patients with more severe collapsibility and high loop gain (2, 6). Stimulating pharyngeal muscles pharmacologically with desipramine was most effective in patients with the poorest compensatory muscle responses (3). Thus, the means to estimate a patient’s individual OSA mechanisms may help clinicians choose which treatment will be most appropriate. Unfortunately, measurements of the phenotypic traits causing OSA to date have largely been invasive, specialized, and therefore limited to select physiological laboratories (7, 8).
To facilitate personalized OSA therapy, we have sought to develop noninvasive clinically applicable techniques to quantify OSA traits, using a ventilation signal collected in routine sleep studies (polysomnography). We have developed, validated, and automated a measure of ventilatory drive (i.e., “intended” ventilation) as a means to assess the ventilatory control contribution to OSA (loop gain and arousal threshold) (9). In principle, the ability to estimate ventilatory drive during spontaneous breathing makes it feasible to assess pharyngeal collapsibility and muscle compensation according to standard definitions (2, 5, 7, 8): Collapsibility is defined as the level of ventilation that can be achieved at eupneic ventilatory drive (Vpassive, the ventilatory equivalent of critical collapsing pressure); and muscle compensation is the increase in ventilation that occurs in conjunction with a rise in ventilatory drive (from eupneic levels to the level that triggers arousal from sleep).
Accordingly, we extended our approach for estimating loop gain and arousal threshold (10, 11) to also simultaneously quantify pharyngeal collapsibility and muscle compensation, and compared these values against “gold standards” (see Methods). To facilitate translation of our method to the clinical setting, we also compared traits obtained using nasal pressure (a clinical surrogate of ventilation) with values obtained when using ventilation assessed via a sealed mask and pneumotachograph. We note that our goal was not to provide a replacement for gold standard methods. Rather, we intended to provide a means to approximate the pharyngeal trait contributions to OSA in the clinical setting, which is otherwise unavailable. Some results have been reported previously in abstract form (10).
Methods
Participants
Patients diagnosed with moderate-to-severe OSA (apnea–hypopnea index [AHI] ≥ 15 events/h) were eligible to participate. Exclusion criteria included use of respiratory stimulants or depressants (including opioids, benzodiazepines), heart failure or lung diseases, central sleep apnea, and pregnancy. Participants provided written informed consent and approval was granted by the Partners’ Institutional Review Board.
Thirty-one patients were enrolled in the primary study to validate our phenotyping technique. One patient was enrolled but unable to tolerate esophageal catheter placement or sleep on CPAP and therefore provided no data for analysis. One individual exhibited an AHI less than 5 events/h on the study night despite the initial diagnosis and was excluded from analysis. Patients who exhibited milder OSA (5 ≤ AHI < 15) were not excluded from analysis.
Eleven patients completed a secondary validation study to confirm that measurements obtained using nasal pressure as a ventilation surrogate were equivalent to measurements obtained with a sealed mask and pneumotachograph (7 of 11 patients also participated in the primary study).
Procedure
The primary study involved a single overnight polysomnographic study with additional physiological measurements (see below). The first and last thirds of the night were reserved for spontaneous breathing during sleep (off CPAP) to witness respiratory events without interruption. From these data, our novel method estimated pharyngeal traits based on breath-by-breath measurement of ventilation (tidal volume × respiratory rate) and a model-based calculation of ventilatory drive (using ventilation, scored arousals, and a best-fit chemoreflex model; see below). Ventilation data were mean-normalized such that the technique does not require a calibrated ventilation signal. Results were compared with traits also measured under spontaneous breathing conditions via the same approach but with the use of gold standard ventilatory drive measurement based on intraesophageal diaphragm EMG. For additional comparison, the middle third of the night was used to measure OSA traits via an established approach employing CPAP manipulation (3, 7, 8, 12). Patients slept supine for the duration of each study.
Polysomnographic Setup
In addition to routine polysomnographic measures (electroencephalography, electro-oculography, electrocardiography, thoracoabdominal movements, oximetry), ventilatory flow was assessed via a pneumotachograph (Hans Rudolph; Validyne Engineering) and a sealed oronasal mask (AirFit small [ResMed Inc.]; see the online supplement). To assess ventilatory drive we measured intraesophageal diaphragm EMG (Servo-i ventilator; Maquet Getinge Group). In brief, a catheter (diameter, 2.7 mm) was placed through a lidocaine-anesthetized nostril such that the center of its electrode array (nine circumferential electrodes, 16 mm apart) lay at the level of the crural diaphragm. Raw EMG was root-mean-squared and smoothed (160 ms, low pass) to provide an integrated diaphragm EMG signal for analysis. Sleep, arousals, and respiratory events were scored according to standard criteria (hypopneas: 30% reduction in flow with ≥3% desaturation or arousal) (13, 14).
Novel Method to Quantify the Pathophysiological Traits by Polysomnography
Our method assessed periods of the night off CPAP to quantify the OSA traits (9–11).
Chemical drive
Phenotypic traits were quantified by first estimating “ventilatory drive,” that is, the intended ventilation that would be observed during sleep if the pharyngeal airway was not obstructed. Conceptually, ventilatory drive can be witnessed when the airway is unobstructed, that is, between obstructive respiratory events. As described previously (9), ventilatory drive was calculated using a ventilation signal (breath-to-breath tidal volume × respiratory rate, mean-normalized) input to a chemoreflex feedback control model (gain, response time, delay), which outputs a ventilatory drive signal; parameters are adjusted to best fit the drive signal to the ventilation signal during breaths when the airway is open (i.e., the ventilation between scored obstructive events, least squares). For example, the reduction in ventilation during apnea/hypopnea yields a subsequent increase in ventilatory drive that is fit to the time course of the postevent ventilatory overshoot. Importantly, ventilatory drive changes during each event can be estimated, when these were otherwise unknown. Models were fit separately to each available 7-minute window (9) containing non-REM sleep. In addition to the chemoreflex model, the ventilatory response to arousal (i.e., the average additional increase in ventilation that accompanies the scored EEG arousals in each window, independent of chemical drive) was accounted for via an additional parameter fit to the data (9).
Upper airway pathophysiology
Pharyngeal collapsibility is defined here as the ventilation at normal/eupneic ventilatory drive (Vpassive) (7, 8), where a more collapsible airway is captured by a lower ventilation. Pharyngeal muscle compensation is taken as the change in ventilation that accompanies an increase in ventilatory drive. Specifically, compensation was taken as the simple difference between Vactive and Vpassive (3, 7, 8), where Vactive is the level of ventilation at maximum drive (i.e., ventilatory drive at the arousal threshold; see below). This difference is the ventilatory equivalent of the active minus passive critical collapsing pressures (8, 15, 16). To make these measurements, we first estimated the arousal threshold based on the median value of ventilatory drive immediately preceding scored EEG arousals (7). Second, a novel breath-by-breath “phenotype plot” of ventilation versus ventilatory drive during sleep was constructed (see Results—Figure 1A, right) (7). Breath-by-breath values of ventilation and ventilatory drive during sleep, from all windows analyzed above, were tabulated and pooled (breaths during arousals and ≤2 breaths after sleep onset, i.e., end of arousals, were excluded). Ventilatory drive data were sorted into 10 bins (deciles), and median values of ventilation and ventilatory drive were obtained for each decile. Third, linear interpolation between bins was used to find 1) the median ventilation at eupneic ventilatory drive (i.e., Vpassive) and 2) the median value of ventilation at the arousal threshold (i.e., Vactive). Analysis was fully automated and performed with in-house software (Phenotyping Using Polysomnography, written using MATLAB [MathWorks]). All analytic steps were visually inspected.
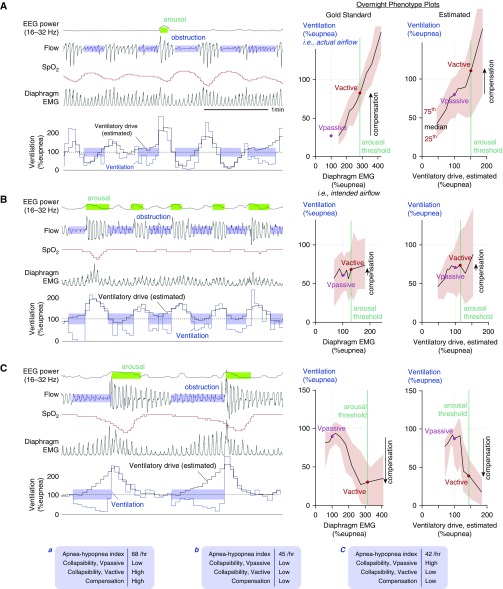
Figure 1.
(A–C) Example illustrations of phenotypic traits in three patients with severe obstructive sleep apnea. Left: Signals shown include ventilation, diaphragm EMG (gold standard ventilatory drive; unseen clinically), and our novel flow-based estimate of ventilatory drive. EEG power (arousal) and oxygen saturation (SpO2) are shown to provide clinical context. In each example patient (A–C), note that the model-estimated ventilatory drive varies in concordance with diaphragm EMG. Right: Gold standard phenotype plots illustrate distinct relationships between actual airflow (ventilation; y-axis) and intended airflow (diaphragm EMG; x-axis) during sleep. These plots reveal the phenotypes: collapsibility (Vpassive) and muscle compensation (Vactive − Vpassive; arrow). Far right: Estimated phenotype plots, based on estimated ventilatory drive, present an accurate picture as to why each patient has obstructive sleep apnea. Each overnight phenotype plot represents all non-REM sleep (black lines represent median values, shaded region represents interquartile range). Bottom: Summary data describing the phenotypes of the patients in (A–C). Low/high classifications, based on group medians, were accurately classified by our method. Patient A has poor collapsibility (low Vpassive) but high compensation. Patient B has borderline poor collapsibility (low Vpassive) and poor compensation. Patient C has mild collapsibility (high Vpassive) but “negative” compensation (low), that is, increased drive yields reduced ventilation. SpO2 = oxygen saturation as measured by pulse oximetry; Vactive = ventilation at maximum ventilatory drive without arousal; Vpassive = ventilation at normal ventilatory drive.
Gold Standard Method to Quantify Traits under Spontaneous Conditions, Using Diaphragm EMG
To test whether our novel method encapsulates the phenotypic pattern of ventilation and ventilatory drive during sleep, we reexamined the aforementioned periods (9–11) by the same approach but instead used diaphragm EMG to provide a gold standard measure of ventilatory drive. For each breath, the peak diaphragm EMG—minus the value at the onset of inspiration—was recorded and normalized to represent ventilatory drive as follows: Breaths recorded during wakefulness (≥4 breaths away from sleep) were identified and the ratio of ventilation (Ve) to peak diaphragm EMG (EMGdi) was measured for each breath; the median ratio Y = Ve/EMGdi was taken to reflect normal respiratory mechanics (in L⋅min/μV). All overnight breath-by-breath diaphragm EMG data were then multiplied by this value to provide a ventilatory drive signal (Vdrive = EMGdi × Y) with units of ventilation, such that ventilatory drive = ventilation when the pharyngeal airway is patent.
Gold Standard Method to Quantify Traits under Controlled Conditions, Using CPAP Drops
CPAP drops were also performed to provide an established gold standard measure of collapsibility and compensatory responses for comparison (2, 3, 7, 8, 12, 16–19). In brief, patients were placed on a therapeutic level of CPAP (to resolve flow limitation), from which CPAP was lowered abruptly to assess the ventilation at normal ventilatory drive at atmospheric pressure (Vpassive, the ventilatory equivalent to the critical collapsing pressure [Pcrit]). We aimed to assess Vpassive rather than Pcrit because 1) Vpassive is the more relevant determinant of the ventilatory pattern manifest at atmospheric pressure, and 2) Vpassive can be feasibly assessed without CPAP manipulation, the primary goal of the study. Breaths 2–4 after each drop were used (20). In practice, many patients exhibit apnea at atmospheric pressure (Pcrit > 0); thus, CPAP was dropped to a range of levels and Vpassive was taken as the x-intercept of a plot of ventilation versus CPAP (5, 7). Subsequently, CPAP was lowered gradually (≤1 cm H2O/min) to a level that raises ventilatory drive close to the arousal threshold (and thereby increases pharyngeal dilator muscle activity). Abrupt drops to 0 cm H2O from this CPAP level were made to quantify the ventilation (Vactive) that occurs at elevated drive. This ventilation value was compared with Vpassive and the difference was quantified as the pharyngeal compensation (3, 12, 16, 18). Values were expressed as a percentage of eupneic ventilation, taken from manually selected periods on therapeutic CPAP. Procedures were repeated as often as possible in the allocated period. In two patients, CPAP drops could not be performed because of insufficient sleep.
Use of Nasal Pressure as a Ventilation Surrogate
In a separate secondary protocol (N = 11) we estimated the pathophysiological traits using nasal pressure as a clinically applicable ventilation signal. Overnight polysomnography was performed while simultaneously recording nasal pressure via nasal cannula and ventilatory flow (pneumotachograph, as described above). Nasal pressure was referenced to mask pressure to reflect the pressure difference inside versus outside both nares to mimic the signal available clinically. Routine care was taken to ensure high-quality nasal pressure signals were recorded: A cannula without evidence of signal smoothing effects was selected (3.5-mm-diameter prongs; Hudson RCI “over-the-ear” cannula; Teleflex), signal clipping was avoided, the cannula was taped in place onto the face under the mask, and the recorded signal was unfiltered (DC coupled). The nasal pressure signal was square-root transformed (9, 21, 22) to provide an uncalibrated surrogate of ventilatory flow that was then used to estimate the traits as described above.
Statistical Analysis
Values obtained from our method were correlated with gold standards. Correlations were used rather than Bland-Altman comparisons because the clinical applicability of our approach was considered not to hinge on whether our measures exactly equaled gold standard values (i.e., y = x, without calibration) but rather the capacity to explain variability across subjects. Nonlinear regressions were used where appropriate (piecewise linear or square-root) and compared with linear models via Fisher F tests. Piecewise regression equations were given by y = ax + b where y(x ≤ b) = 0 (lower breakpoint at x = b) and y(x ≥ c) = a(c − b) (upper breakpoint at x = c); parameters a, b, and c were fit using least-squares minimization. Correlation coefficients (R values) between 0.4 and 0.7 were considered modest, and above 0.7 were considered strong. Classification analysis was used to find a cutoff value for each polysomnographic trait that optimally identifies higher-than-median or lower-than-median gold standard traits (maximizing sensitivity plus specificity) (23); leave-one-out cross-validation assessed classification performance more conservatively. Significance was accepted at P < 0.05.
Results
Baseline characteristics are detailed in Table 1. Patients exhibited a broad range of OSA severities (6–91 events/h) on the study night.
Table 1.
Patient Characteristics
| Characteristic | Comparison with Gold Standards (N = 29) | Nasal Pressure versus Pneumotach (N = 11) |
|---|---|---|
| Demographics | ||
| Age, yr | 57 ± 9 | 57 ± 8 |
| Sex, M:F | 21:8 | 9:2 |
| Race, black:white:Asian:other | 9:19:0:1 | 1:10:0:0 |
| Body mass index, kg/m2 | 31.9 ± 5.6 | 29.8 ± 6.0 |
| Neck circumference, cm | 41.6 ± 4.3 | 41.7 ± 3.0 |
| Currently treated, CPAP:oral appliance:untreated | 11:2:16 | 2:1:8 |
| Polysomnography | ||
| OSA severity, mild:moderate:severe | 9:4:16 | 4:1:6 |
| Apnea–hypopnea index, total, events/h | 40.9 ± 28.0 | 41.6 ± 38.3 |
| Apnea–hypopnea index, non-REM, events/h | 40.7 ± 28.5 | 41.7 ± 38.8 |
| Central events, non-REM, % respiratory events | 0.0 ± 0.0 | 2.5 ± 8.2 |
| Hypopneas, non-REM, % respiratory events | 57.6 ± 30.9 | 64.7 ± 36.4 |
| Arousal index, non-REM, events/h | 55.3 ± 25.6 | 50.3 ± 26.4 |
| Total sleep time, min | 221 ± 100 | 254 ± 102 |
| Sleep time, spontaneous breathing off CPAP, min | 124 ± 86 | 210 ± 126 |
| Sleep time, CPAP dial downs, min | 98 ± 62 | N/A |
| Non-REM 1, % total sleep time | 38 ± 19 | 30 ± 14 |
| Non-REM 2, % total sleep time | 49 ± 15 | 54 ± 14 |
| Non-REM 3, % total sleep time | 5 ± 5 | 8 ± 13 |
| REM, % total sleep time | 8 ± 8 | 7 ± 6 |
Definition of abbreviations: CPAP = continuous positive airway pressure; N/A = not applicable; OSA = obstructive sleep apnea.
Values represent means ± SD, unless specified otherwise. Polysomnographic respiratory event data refers to the period off CPAP.
Example Traces
Differences in the phenotypic pattern of ventilation and ventilatory drive across subjects were noteworthy. Three illustrative examples are shown (Figure 1).
Comparison with Gold Standards
Correlations
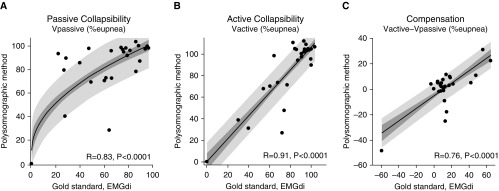
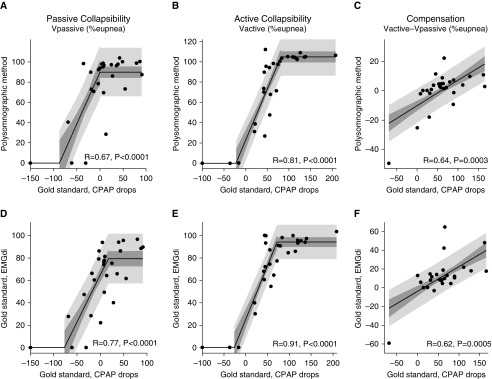
Polysomnographic measures of pharyngeal collapsibility and compensation correlated well with the same traits quantified under spontaneous conditions using diaphragm EMG (N = 29; Figure 2). Our novel collapsibility and compensation measures also correlated favorably with those measured using CPAP manipulation (N = 28; Figures 3A–3C; see also Figure E1 in the online supplement). Values of ventilation at eupneic ventilatory drive (Vpassive) were substantially greater during spontaneous breathing than immediately after CPAP drops.
Figure 2.
Our novel measures of (A and B) pharyngeal collapsibility and (C) compensatory responses compare favorably with traits measured using gold standard ventilatory drive (diaphragm EMG) during spontaneous breathing off continuous positive airway pressure. Shading shows the SE (dark) and SD (light) of each regression. Regression equations (coefficient ± SEM) are as follows: (A) y = (9.1 ± 1.2)x0.5 + (11.5 ± 9.6), P = 0.002 versus linear model (linear R = 0.74, P < 0.0001); (B) y = (1.06 ± 0.10)x + (0.8 ± 7.9); and (C) y = (0.51 ± 0.09)x − (5.1 ± 2.2). R values rather than R2 values are shown. Note there are three data points at (0,0) in panels A and B. EMGdi = intraesophageal diaphragm EMG; Vactive = ventilation at maximum ventilatory drive without arousal; Vpassive = ventilation at normal ventilatory drive.
Figure 3.
Our polysomnographic measures of (A and B) pharyngeal collapsibility and (C) compensatory responses also compare favorably with traits measured using gold standard continuous positive airway pressure (CPAP) drop techniques. (D–F) Gold standard traits under spontaneous conditions (via ventilation vs. EMGdi plots) versus gold standard CPAP drop traits; concordance validates the essential underlying concept that measuring ventilation and ventilatory drive during spontaneous breathing in sleep reveals the phenotypic pathophysiology in obstructive sleep apnea. Note that the spontaneous measures of passive collapsibility indicate reduced collapsibility (higher Vpassive) versus CPAP drop measures. Shading shows the SE (dark) and SD (light) of each regression. Piecewise regressions were chosen because polysomnographic values (A, B, D, and E) are physiologically bounded (minimum of zero, maximum typically 100%) but CPAP-derived measures are not. R values rather than R2 values are shown. EMGdi = intraesophageal diaphragm EMG; Vactive = ventilation at maximum ventilatory drive without arousal; Vpassive = ventilation at normal ventilatory drive.
Identifying high versus low values
Polysomnographic measures were able to identify subgroups of patients with high versus low pharyngeal collapsibility or compensation (Table 2); cross-validated results confirmed significant discriminative capacity for all measures except CPAP drop compensation.
Table 2.
Identifying High versus Low Subgroups by the Polysomnographic Method
| All Data |
Cross-Validation |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Trait† | High/Low Definition (Gold Standard Median)‡ | PSG Cutoff§ | Accuracy (% ± SEM) | Sensitivity, Specificity (%) | Gold Standard Values within PSG Subgroups* |
Accuracy (% ± SEM) | Sensitivity, Specificity (%) | Gold Standard Values within PSG Subgroups* |
||
| High | Low | High | Low | |||||||
| Diaphragm EMG | ||||||||||
| Collapsibility, Vpassive | 66.4 | 86.6 | 83 ± 7|| | 93, 73 | 75 ± 5 | 41 ± 9¶ | 72 ± 8¶ | 71, 73 | 73 ± 7 | 49 ± 8** |
| Collapsibility, Vactive | 85.9 | 81.7 | 86 ± 6|| | 100, 73 | 92 ± 2 | 45 ± 10|| | 86 ± 6|| | 100, 73 | 92 ± 2 | 45 ± 10|| |
| Compensation, Vactive − Vpassive | 10.9 | 8.0 | 69 ± 9** | 40, 100 | 36 ± 9 | 7 ± 4¶ | 69 ± 9** | 40, 100 | 36 ± 9 | 7 ± 4¶ |
| CPAP drops | ||||||||||
| Collapsibility, Vpassive | 7.7 | 95.5 | 71 ± 9** | 64, 79 | 29 ± 10 | −10 ± 13** | 68 ± 9** | 64, 71 | 27 ± 9 | −11 ± 14** |
| Collapsibility, Vactive | 61.0 | 96.5 | 93 ± 5|| | 93, 93 | 108 ± 10 | 25 ± 12|| | 93 ± 5|| | 93, 93 | 108 ± 10 | 25 ± 12|| |
| Compensation, Vactive − Vpassive | 60.2 | 0.9 | 70 ± 9** | 85, 57 | 81 ± 9 | 25 ± 14¶ | 52 ± 10 | 46, 57 | 75 ± 10 | 49 ± 14 |
Definition of abbreviations: CPAP = continuous positive airway pressure; PSG = polysomnographic method; Vactive = ventilation at maximum ventilatory drive without arousal; Vpassive = ventilation at normal ventilatory drive.
Gold standard values (mean ± SEM) within high and low polysomnographic subgroups were compared by Student’s t tests. Classification analyses were repeated using leave-one-out cross-validation (right side of table) for a more conservative estimate of accuracy (the selected PSG cutoff was redefined for each subject based on all the other subjects). Note that CPAP drop compensation accuracy was reduced to chance levels with the cross-validation testing (optimal threshold fluctuated between 0.9 and 5.4; both of these thresholds separately yielded 70% accuracy [P < 0.05] using all data).
Traits are presented in units of ventilation as a percentage of eupneic levels.
Patients were defined as having a high versus low value of a given trait, based on the median gold standard value (shown).
The optimal polysomnographic cutoff used to identify high versus low subgroups was determined by maximizing sensitivity plus specificity (23); this cutoff was used to define the polysomnographic subgroups (left side of table).
Accuracy is the sum of correct predictions divided by total number of patients (%): P < 0.05 for accuracy (better than chance, normal approximation method) or high versus low subgroups (Student’s t test).
Accuracy is the sum of correct predictions divided by total number of patients (%): P < 0.01 for accuracy (better than chance, normal approximation method) or high versus low subgroups (Student’s t test).
Accuracy is the sum of correct predictions divided by total number of patients (%): P < 0.001 for accuracy (better than chance, normal approximation method) or high versus low subgroups (Student’s t test).
Additional details
On average, 64 ± 39 windows of polysomnographic data off CPAP were analyzed per patient (mean ± SD). Within-subject standard errors of Vpassive and Vactive, based on our novel method, were just 2.2 ± 2.1% and 2.9 ± 3.7% of eupneic ventilation, respectively. Intrasubject standard errors of Vpassive and Vactive based on diaphragm EMG were 2.4 ± 2.3% and 2.4 ± 2.4% of eupneic ventilation, respectively. On average, 16 ± 7 and 18 ± 10 CPAP drops were analyzed under passive and active conditions, respectively; standard errors for Vpassive and Vactive averaged 13 ± 14% and 8 ± 5% of eupneic ventilation (equivalent to 0.6 and 0.4 cm H2O error in “Pcrit” within subjects; see the online supplement).
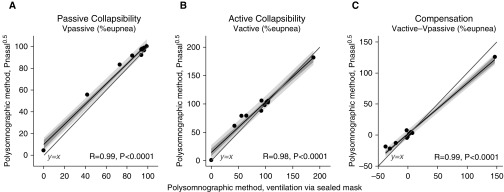
Use of Nasal Pressure as a Ventilation Surrogate
Almost identical values of the traits were obtained when using nasal pressure as a clinical surrogate for ventilation (R > 0.9, all traits; P < 0.0001; N = 11) (Figure 4; see also Figures E2 and E3).
Figure 4.
Comparisons of our novel measures of collapsibility taken using nasal pressure (Pnasal, square-root transformed)—a clinical surrogate of ventilation—and gold standard ventilation measured via a pneumotachograph and sealed oronasal mask. Note the excellent correlations observed. Shading shows the SE (dark) and SD (light) of each regression. Mean bias (±SD) = 4.0 ± 4.6%, 4.6 ± 10.0%, and 0.6 ± 10.2% for Vpassive, Vactive, and compensation, respectively. Mean absolute error = 4.3, 7.5, and 7.2%, respectively. R values rather than R2 values are shown. There is only one data point at (0,0) in panel B. Vactive = ventilation at maximum ventilatory drive without arousal; Vpassive = ventilation at normal ventilatory drive.
Discussion
The current study showed that two key pathophysiological traits causing OSA—namely collapsibility and compensatory responses—are evident from the breathing pattern during sleep and can be estimated noninvasively. We also demonstrate that nasal pressure, when recorded carefully, provides the information necessary to obtain these phenotypes. In combination with our measures of loop gain and the arousal threshold (10–11), we now have a means to estimate the four key traits causing OSA. Application of this approach in the clinical arena has the potential to facilitate judicious patient selection for personalized therapeutic interventions.
Physiological Insight
Our study demonstrates that the underlying phenotypic traits causing OSA can be measured from the dynamic pattern of ventilation and ventilatory drive observed spontaneously during sleep. For the first time, we have shown the capacity to measure the causes of OSA in a patient without first resolving it (Figure 1). The comparison of gold standard traits, using diaphragm EMG and CPAP dial-downs (Figures 3D–3F; Figure E1), supports this point. Specifically, our work shows that patients with a reduced ventilation at eupneic drive—in the midst of the cyclic respiratory events that define OSA—have a more collapsible upper airway, and patients whose ventilation most profoundly rises and falls in concert with ventilatory drive during sleep have a greater pharyngeal compensation. Although the link between the underlying traits and the emergent pattern of breathing is intuitive, no study has previously provided decisive evidence of this point. There is, however, supportive evidence in the literature: The proportion of obstructive hypopneas versus apneas—a surrogate of obstructive event depth—is associated with pharyngeal collapsibility assessed under active conditions (active critical collapsing pressure or “active Pcrit”) (24). Our work has shown that peak inspiratory flow measured during sleep is associated with active Pcrit (15). Overall, such knowledge relating the ventilatory patterns characterizing OSA to the underlying pathophysiological mechanisms is important for two key reasons: First, understanding this link enables the mechanisms to be measured noninvasively. Second, the heterogeneity in OSA expression has implications for adverse outcomes (25). Because OSA heterogeneity in the form of the patterns of ventilation versus ventilatory drive is clearly linked to the underlying pathophysiology, we therefore speculate that such outcomes could be related to the mechanisms of OSA.
Our study also provides a novel general framework for assessing OSA phenotypes without intervention beyond the current specific methodological implementation. Future techniques to measure/estimate ventilatory drive (26) will be able to employ this framework with ease to estimate phenotypes. Importantly, our approach makes use of the data during unstable breathing at the very time that it is most important. By contrast, phenotypic trait measurement using CPAP manipulation (2, 7, 8, 27) represents OSA pathophysiology during times of stable established sleep, that is, when traits are at their best (28–31).
Clinical Implications
Our study provides proof-of-principle that it is possible to identify OSA phenotypes using signals available from a polysomnographic sleep study (nasal pressure ventilation and scored arousals/events). At present, sleep clinicians test OSA therapies in individual patients without the ability to objectively consider the underlying causes of OSA. Thus, our approach to phenotyping patients with OSA using available data may enable clinicians to direct patients to the most optimal alternative treatment, based on the underlying pathophysiology. For example, interventions such as oral appliances and surgery that have scope for a modest improvement in pharyngeal anatomy/collapsibility may be most effective in patients with milder collapsibility (2, 6). A number of novel pharmaceutical targets for OSA are under investigation, but evidence repeatedly indicates that pharmaceutical therapies are effective only in a subgroup of patients. For example, the combination of a hypnotic and supplemental oxygen was particularly effective in patients with milder collapsibility (5), and noradrenergic activation of upper airway muscles (via desipramine) was exclusively effective in patients with poor muscle compensation (3, 12). Further investigation is underway to confirm that the pharyngeal phenotypes described here predict responses to interventions. Data indicate results are likely to be promising (ClinicalTrials.gov, accession No. NCT01751971 [32]).
Strengths and Limitations
Our approach to identifying OSA phenotypes using polysomnography employs an analytic technique that requires no invasive measurements or CPAP manipulations and is fully automated with the exception of clinical scoring. Thus, our approach has excellent potential for implementation in clinical settings where nasal pressure signals are carefully measured alongside EEG assessment of sleep and arousals. The impact of common clinical causes of reduced signal integrity (signal clipping; baseline drift due to high-pass filtering, mouth breathing) remains a subject of investigation. It may also be possible to use other ventilation surrogates (e.g., respiratory inductance plethysmography) in our approach. Importantly, our method is based on established physiological principles, which gives us confidence that it will apply beyond the patients studied here.
There are several limitations. First, the primary study presented here measured the traits using gold standard ventilation assessed via a sealed oronasal mask, raising concern regarding extrapolating these correlations to the use of nasal pressure in the clinic. To overcome this concern, we illustrated that traits are nearly identical when measured using nasal pressure versus gold standard ventilation (Figure 4). Nonetheless, future work should test a subject’s “phenotype” when derived from a nasal cannula without a mask as conventionally collected. Additional analysis to assess the appropriateness and necessity of the nasal pressure square-root transform (Figure E2) illustrates that this transform (exponent = 0.5) reduces error associated with the untransformed nasal pressure, but an alternative transform (exponent = ⅔) yields an optimal match to the gold standard ventilation values (Figure E3). Second, we normalized ventilation data to present the ventilatory traits as a proportion of eupneic values, as used in physiological studies (2, 18). Such normalization is also essential to enable uncalibrated ventilation signals to be used to quantify the traits. We note that eupneic values were estimated on the basis of the mean ventilation in each window, assuming that mean alveolar/arterial Po2/Pco2 values are not greatly deranged in OSA (11); normalization was implemented separately for each 7-minute window of data to minimize the impact of nonphysiological time-dependent variation in signal amplitude (e.g., via movement of the cannula relative to the nares) and physiological variation in eupneic ventilation (e.g., accompanying metabolic rate). Nonetheless, in some circumstances, for example, severe sleep-dependent hypoventilation, this approach may be suboptimal. The development of novel strategies to estimate eupneic ventilation during periods of airflow obstruction might be of additional benefit (e.g., based on alveolar/arterial Po2/Pco2 levels). Third, we chose to use a single night to assess spontaneous breathing patterns and perform CPAP drops, to reduce the impact of potential day-to-day physiological variability and maintain constancy of experimental setup and equipment (3, 12). However, our use of a split-night protocol may have affected the robustness of both sets of values. We note that we have used this approach previously in studies that provided physiological insight (3, 12). Moreover, we would expect the observed correlations to improve rather than deteriorate if full-night data were available for analyses. Fourth, we also observed that collapsibility values observed under spontaneous conditions were considerably higher than those observed with CPAP dial downs (Figures 3A and 3D), consistent with a persistent CPAP-related dilator muscle hypotonia that is not apparent during spontaneous breathing off CPAP despite the same level of ventilatory drive (note the cutoff values for high and low Vpassive are 7.7% for CPAP drops and 95.5% for our polysomnographic estimate). The increase in ventilation off CPAP is reminiscent of the phenomenon of hysteresis observed during upward versus downward CPAP titrations (33) (see Figure E1). We emphasize that spontaneous measures of Vpassive should not truly reflect a hypotonic pharyngeal airway, but rather the collapsibility at the prevailing level of muscle tone, which we believe is the desirable determinant of the anatomical intervention necessary to ameliorate OSA. The tradeoff is that spontaneous collapsibility measurement is less likely to reflect purely anatomical aspects of the pharynx. Fifth, we also observed considerable variability in estimating muscle compensation from the polysomnographic method relative to the diaphragm EMG values around diaphragm EMG values of 20% (Figure 2C); 3 of 22 patients in particular had a substantially underestimated compensation, which is a concern for clinical applicability. We noticed that, during some obstructive hypopneas, while ventilation was falling, the actual diaphragm EMG was also falling (reported previously [34]), yet our calculated chemical drive was rising (as expected, based on rising Pco2). This discrepancy naturally yields different traits, for example, falling flow with falling EMG drive suggests a positive compensation, but a falling flow with rising drive (and presumably rising Pco2) suggests a poor compensation. Both may reflect different aspects of pathophysiology; of note, two of these three individuals also had poor CPAP-drop compensation consistent with polysomnographic categorization (Figure 3C). The development of alternative methods to noninvasively estimate ventilatory drive during sleep may be of value in these circumstances. Sixth, traits were assessed here exclusively in supine non-REM; however, the traits are known to vary with sleeping state and position (35); future studies are needed to decide how best to pool polysomnographic results from multiple states and positions to optimally predict responses to interventions. Lastly, trait measures can also vary within a night, physiologically and systematically (36) as well as via measurement error, with implications for reproducibility. We calculated that the minimum sleep time necessary to make a reasonable measurement (expected SEM = 5% eupnea) of collapsibility (Vpassive) and compensation was approximately 30 minutes and approximately 1 hour, respectively (extrapolating from the SEM of 2.2% eupnea for Vpassive and ∼3.6% eupnea for compensation from 2 h of sleep). Variability in trait measures within and across nights remains an important area for future investigation.
Conclusions
Polysomnographic quantification of ventilation and ventilatory drive in patients with OSA reveals the underlying pathophysiological phenotype. Specifically, pharyngeal collapsibility and compensatory responses can be estimated from a routine sleep study without specialized equipment or interventions. Alongside our measures of loop gain and the arousal threshold, we have now provided a clinically applicable means to estimate four key traits causing OSA. Further research is needed to define phenotypic subgroups of patients who are most amenable to each available OSA therapy. This work represents a major step toward transforming OSA management from a one-size-fits-all reliance on CPAP into a more nuanced, mechanistic approach whereby patients are matched to the most appropriate therapies.
Acknowledgments
Acknowledgment
The authors thank the Maquet Getinge Group for the loan of the Servo-i ventilator to measure intraesophageal diaphragm EMG. The authors also thank Drs. Stephen Loring, James Butler, Atul Malhotra, and Robert Owens for insightful discussions.
Footnotes
Supported by the American Heart Association (15SDG25890059 [S.A.S.]), the NIH (R01HL102321, R01HL128658, P01HL094307, and P01HL095491 [A.W. and S.A.S.]), the National Health and Medical Research Council of Australia (1053201 [S.A.S.], 1035115 [B.A.E.], and 1064163 [P.I.T., S.A.S., A.W., and B.A.E.]), the R. G. Menzies Foundation (S.A.S.), the American Thoracic Society Foundation (S.A.S.), and the Heart Foundation of Australia (Future Leader Fellowship 101167 [B.A.E.]). This work was also supported by Harvard Catalyst (National Center for Research Resources and the National Center for Advancing Translational Sciences, NIH Award UL1TR001102).
Author Contributions: Conception: S.A.S.; study design: S.A.S., B.A.E., P.I.T., D.P.W., and A.W.; algorithm development: S.A.S., A.A., and P.I.T.; and data collection and analysis: S.A.S., L.T.-M, and L.B.H. All authors interpreted data, edited the manuscript for important intellectual content, and approved the final draft.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201707-1435OC on January 12, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Wellman A, Malhotra A, Jordan AS, Stevenson KE, Gautam S, White DP. Effect of oxygen in obstructive sleep apnea: role of loop gain. Respir Physiol Neurobiol. 2008;162:144–151. doi: 10.1016/j.resp.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edwards BA, Andara C, Landry S, Sands SA, Joosten SA, Owens RL, et al. Upper-airway collapsibility and loop gain predict the response to oral appliance therapy in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2016;194:1413–1422. doi: 10.1164/rccm.201601-0099OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taranto-Montemurro L, Sands SA, Edwards BA, Azarbarzin A, Marques M, de Melo CM, et al. Desipramine improves upper airway collapsibility and reduces obstructive sleep apnoea severity in patients with minimal muscle compensation. Eur Respir J. 2016;48:1340–1350. doi: 10.1183/13993003.00823-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joosten SA, Leong P, Landry SA, Sands SA, Terrill PI, Mann D, et al. Loop gain predicts the response to upper airway surgery in patients with obstructive sleep apnea. Sleep. 2017;40(7) doi: 10.1093/sleep/zsx094. [DOI] [PubMed] [Google Scholar]
- 5.Edwards BA, Sands SA, Owens RL, Eckert DJ, Landry S, White DP, et al. The combination of supplemental oxygen and a hypnotic markedly improves obstructive sleep apnea in patients with a mild to moderate upper airway collapsibility. Sleep. 2016;39:1973–1983. doi: 10.5665/sleep.6226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sutherland K, Phillips CL, Davies A, Srinivasan VK, Dalci O, Yee BJ, et al. CPAP pressure for prediction of oral appliance treatment response in obstructive sleep apnea. J Clin Sleep Med. 2014;10:943–949. doi: 10.5664/jcsm.4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wellman A, Eckert DJ, Jordan AS, Edwards BA, Passaglia CL, Jackson AC, et al. A method for measuring and modeling the physiological traits causing obstructive sleep apnea. J Appl Physiol. 2011;110:1627–1637. doi: 10.1152/japplphysiol.00972.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wellman A, Edwards BA, Sands SA, Owens RL, Nemati S, Butler J, et al. A simplified method for determining phenotypic traits in patients with obstructive sleep apnea. J Appl Physiol. 2013;114:911–922. doi: 10.1152/japplphysiol.00747.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Terrill PI, Edwards BA, Nemati S, Butler JP, Owens RL, Eckert DJ, et al. Quantifying the ventilatory control contribution to sleep apnoea using polysomnography. Eur Respir J. 2015;45:408–418. doi: 10.1183/09031936.00062914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sands SA, Edwards BA, Terrill PI, Taranto-Montemurro L, Azarbarzin A, Marques M, et al. Phenotyping sleep apnea using polysomnography: upper airway collapsibility and responsiveness [abstract] Am J Respir Crit Care Med. 2016;193:A6378. [Google Scholar]
- 11.Sands SA, Terrill PI, Edwards BA, Taranto-Montemurro L, Azarbarzin A, Marques M, et al. Quantifying the arousal threshold using polysomnography in obstructive sleep apnea. Sleep. 2018;41:zsx183. doi: 10.1093/sleep/zsx183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taranto-Montemurro L, Edwards BA, Sands SA, Marques M, Eckert DJ, White DP, et al. Desipramine increases genioglossus activity and reduces upper airway collapsibility during non-REM sleep in healthy subjects. Am J Respir Crit Care Med. 2016;194:878–885. doi: 10.1164/rccm.201511-2172OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring systems for sleep stages of human subjects. Los Angeles, CA: UCLA Brain Information Service/Brain Research Institute; 1968. [Google Scholar]
- 14.Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, et al. American Academy of Sleep Medicine; Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. J Clin Sleep Med. 2012;8:597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Azarbarzin A, Sands SA, Taranto-Montemurro L, Oliveira Marques MD, Genta PR, Edwards BA, et al. Estimation of pharyngeal collapsibility during sleep by peak inspiratory airflow. Sleep. 2017;40(1) doi: 10.1093/sleep/zsw005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shapiro SD, Chin CH, Kirkness JP, McGinley BM, Patil SP, Polotsky VY, et al. Leptin and the control of pharyngeal patency during sleep in severe obesity. J Appl Physiol. 2014;116:1334–1341. doi: 10.1152/japplphysiol.00958.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edwards BA, Sands SA, Eckert DJ, White DP, Butler JP, Owens RL, et al. Acetazolamide improves loop gain but not the other physiological traits causing obstructive sleep apnoea. J Physiol. 2012;590:1199–1211. doi: 10.1113/jphysiol.2011.223925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sands SA, Eckert DJ, Jordan AS, Edwards BA, Owens RL, Butler JP, et al. Enhanced upper-airway muscle responsiveness is a distinct feature of overweight/obese individuals without sleep apnea. Am J Respir Crit Care Med. 2014;190:930–937. doi: 10.1164/rccm.201404-0783OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Younes M. Role of respiratory control mechanisms in the pathogenesis of obstructive sleep disorders. J Appl Physiol. 2008;105:1389–1405. doi: 10.1152/japplphysiol.90408.2008. [DOI] [PubMed] [Google Scholar]
- 20.Patil SP, Punjabi NM, Schneider H, O’Donnell CP, Smith PL, Schwartz AR. A simplified method for measuring critical pressures during sleep in the clinical setting. Am J Respir Crit Care Med. 2004;170:86–93. doi: 10.1164/rccm.200309-1239OC. [DOI] [PubMed] [Google Scholar]
- 21.Thurnheer R, Xie X, Bloch KE. Accuracy of nasal cannula pressure recordings for assessment of ventilation during sleep. Am J Respir Crit Care Med. 2001;164:1914–1919. doi: 10.1164/ajrccm.164.10.2102104. [DOI] [PubMed] [Google Scholar]
- 22.Farré R, Montserrat JM, Navajas D. Noninvasive monitoring of respiratory mechanics during sleep. Eur Respir J. 2004;24:1052–1060. doi: 10.1183/09031936.04.00072304. [DOI] [PubMed] [Google Scholar]
- 23.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 24.Gleadhill IC, Schwartz AR, Schubert N, Wise RA, Permutt S, Smith PL. Upper airway collapsibility in snorers and in patients with obstructive hypopnea and apnea. Am Rev Respir Dis. 1991;143:1300–1303. doi: 10.1164/ajrccm/143.6.1300. [DOI] [PubMed] [Google Scholar]
- 25.Zinchuk AV, Jeon S, Koo BB, Yan X, Bravata DM, Qin L, et al. Polysomnographic phenotypes and their cardiovascular implications in obstructive sleep apnoea Thorax(In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Catcheside PG, Reynolds K, Stadler D, McEvoy D. Ventilatory effort versus output in obstructive sleep apnea assessed by the respiratory system equation of motion [abstract] Sleep Biol Rhythms. 2014;12:59. [Google Scholar]
- 27.Eckert DJ, White DP, Jordan AS, Malhotra A, Wellman A. Defining phenotypic causes of obstructive sleep apnea: identification of novel therapeutic targets. Am J Respir Crit Care Med. 2013;188:996–1004. doi: 10.1164/rccm.201303-0448OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ratnavadivel R, Chau N, Stadler D, Yeo A, McEvoy RD, Catcheside PG. Marked reduction in obstructive sleep apnea severity in slow wave sleep. J Clin Sleep Med. 2009;5:519–524. [PMC free article] [PubMed] [Google Scholar]
- 29.Ratnavadivel R, Stadler D, Windler S, Bradley J, Paul D, McEvoy RD, et al. Upper airway function and arousability to ventilatory challenge in slow wave versus stage 2 sleep in obstructive sleep apnoea. Thorax. 2010;65:107–112. doi: 10.1136/thx.2008.112953. [DOI] [PubMed] [Google Scholar]
- 30.Hicks A, Cori JM, Jordan AS, Nicholas CL, Kubin L, Semmler JG, et al. Mechanisms of the deep, slow-wave, sleep-related increase of upper airway muscle tone in healthy humans. J Appl Physiol. 2017;122:1304–1312. doi: 10.1152/japplphysiol.00872.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dunai J, Kleiman J, Trinder J. Ventilatory instability during sleep onset in individuals with high peripheral chemosensitivity. J Appl Physiol. 1999;87:661–672. doi: 10.1152/jappl.1999.87.2.661. [DOI] [PubMed] [Google Scholar]
- 32.Sands SA, Edwards BA, Terrill PI, Butler JP, Owens RL, Taranto-Montemurro L, et al. Phenotyping from polysomnography predicts obstructive sleep apnea responses to supplemental oxygen therapy [abstract] Am J Respir Crit Care Med. 2017;195:A2931. [Google Scholar]
- 33.Condos R, Norman RG, Krishnasamy I, Peduzzi N, Goldring RM, Rapoport DM. Flow limitation as a noninvasive assessment of residual upper-airway resistance during continuous positive airway pressure therapy of obstructive sleep apnea. Am J Respir Crit Care Med. 1994;150:475–480. doi: 10.1164/ajrccm.150.2.8049832. [DOI] [PubMed] [Google Scholar]
- 34.Xiao SC, He BT, Steier J, Moxham J, Polkey MI, Luo YM. Neural respiratory drive and arousal in patients with obstructive sleep apnea hypopnea. Sleep. 2015;38:941–949. doi: 10.5665/sleep.4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joosten SA, Edwards BA, Wellman A, Turton A, Skuza EM, Berger PJ, et al. The effect of body position on physiological factors that contribute to obstructive sleep apnea. Sleep. 2015;38:1469–1478. doi: 10.5665/sleep.4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.El-Chami M, Shaheen D, Ivers B, Syed Z, Badr MS, Lin HS, et al. Time of day affects the frequency and duration of breathing events and the critical closing pressure during NREM sleep in participants with sleep apnea. J Appl Physiol. 2015;119:617–626. doi: 10.1152/japplphysiol.00346.2015. [DOI] [PubMed] [Google Scholar]