Abstract
The aim of the present study was to investigate the key pathways and genes in the progression of cervical cancer. The gene expression profiles GSE7803 and GSE63514 were obtained from the Gene Expression Omnibus database. Differentially expressed genes (DEGs) were identified using GEO2R and the limma package, and Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses were conducted using the Database for Annotation, Visualization and Integrated Discovery. The hub genes were identified using Cytoscape and protein-protein interaction (PPI) networks were constructed using the STRING database. A total of 127 and 99 DEGs were identified in the pre-invasive and invasive stages of cervical cancer, respectively. GO enrichment analysis indicated that the DEGs in pre-invasive cervical cancer were primarily associated with the ‘protein binding’, ‘single-stranded DNA-dependent ATPase activity’, ‘DNA replication origin binding’ and ‘microtubule binding’ terms, whereas the DEGs in invasive cervical cancer were associated with the ‘extracellular matrix (ECM) structural constituent’, ‘heparin binding’ and ‘integrin binding’. KEGG enrichment analysis revealed that the pre-invasive DEGs were significantly enriched in the ‘cell cycle’, ‘DNA replication’ and ‘p53 signaling pathway’ terms, while the invasive DEGs were enriched in the ‘amoebiasis’, ‘focal adhesion’, ‘ECM-receptor interaction’ and ‘platelet activation’ terms. The PPI network identified 4 key genes (PCNA, CDK2, VEGFA and PIK3CA), which were hub genes for pre-invasive and invasive cervical cancer. In conclusion, bioinformatics analysis identified 4 key genes in cervical cancer progression (PCNA, CDK2, VEGFA and PIK3CA), which may be potential biomarkers for differentiating normal cervical epithelial tissue from cervical cancer.
Keywords: cervical cancer, differentially expressed genes, gene ontology, Kyoto Encyclopedia of Genes and Genomes, protein-protein interactions
Introduction
Cervical cancer is the fourth most common cancer in women worldwide, with an estimated 527,600 new cases and 265,600 deaths in 2012 (1). Although the association between persistent high-risk human papillomavirus (HR-HPV) infection and the development of cervical cancer has been demonstrated by molecular and functional studies, the specific molecular network mechanisms from HPV infection to tumorigenesis have not been fully elucidated. Therefore, investigating the potential mechanism underlying tumorigenesis may be crucial for prolonging patient survival.
Tumorigenesis is a complex pathological process involving a variety of genetic alterations, including the overexpression of oncogenes and/or the inactivation of tumor suppressor genes (2). The development of cervical cancer is a stepwise process from a low-grade cervical intraepithelial neoplasia (CIN1) to high-grade CIN (CIN2 and 3) that ultimately develops into carcinoma (3), involving multiple genetic and epigenetic events. The identification of dysregulated genes in cancer-associated pathways may shed light on the molecular mechanisms underlying tumorigenesis, thus helping to develop new strategies for tumor therapy.
Recently, gene analysis using the high-throughput platforms has been developed as a promising tool with various clinical applications, such as the molecular diagnosis and classification of cancers, and the prediction of tumor response and patient prognosis (4). Several gene expression profiles related to cervical carcinogenesis have been studied with microarray technology, revealing hundreds of differentially expressed genes (DEGs) that are involved in the process of tumorigenesis, serving a potential role in the identification of novel therapeutic targets (5). The present study applied bioinformatics analysis to identify DEGs involved in the progression from normal cervical epithelium tissue to high-grade CIN and cervical cancer, and explored the significant GO terms, KEGG pathways and protein-protein interaction (PPI) networks, with a particular focus on possible hub genes that are likely to play key roles in the progression of cervical cancer.
Materials and methods
Microarray datasets
The cervical cancer microarray datasets GSE7803 and GSE63514 were downloaded from the NCBI GEO database (http://www.ncbi.nlm.nih.gov/geo). The dataset GSE7803 was based on the GPL96 platform (Affymetrix Human Genome U133A Array; Thermo Fisher Scientific, Inc., Waltham, MA, USA), including 10 normal squamous cervical epithelium (NE), 7 high-grade squamous intraepithelial cervical lesion (HSIL), and 21 invasive squamous cell carcinoma (SCC) of the cervix samples. The dataset GSE63514, produced using the GPL570 Affymetrix Human Genome U133 Plus 2.0 Array, included 24 NE samples, 2 CIN2 lesion samples, 40 CIN3 lesion samples, and 28 cancer specimens. CIN2 and CIN3 were considered to be HSIL in our study.
Identification of DEGs
GEO2R, an interactive web tool for comparing two or more groups of samples, and identifying genes that are differentially expressed across experimental conditions, was used to identify DEGs in the GSE7803 and GSE63514 datasets with the limma package, which had been processed, normalized and transformed. An adjusted P-value was obtained by applying the Benjamini-Hochberg false discovery rate (FDR) correction on the original P-value, and a fold change threshold was selected based on our aim to focus on statistically significant DEGs (6). Only genes with a fold change >2 and adjusted P-value <0.05 were considered as statistically significant DEGs. In addition, the selected DEGs were divided into two groups: DEGs between the NE and HSIL samples were considered as pre-invasive DEGs, whereas DEGs between the HSIL and invasive SCC samples were considered as invasive DEGs. A heat map of the identified DEGs was also constructed, using an R package.
Gene ontology and pathway enrichment analysis of DEGs
In the present study, the significant enrichment analysis of the two groups of DEGs was assessed based on the Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) using the Database for Annotation, Visualization and Integrated Discovery (DAVID), an online tool for functional annotation analysis (7). GO analysis is a common, useful method for annotating genes and gene products, and for identifying characteristic biological attributes of high-throughput genome or transcriptome data (8), including 3 categories: Biological process (BP), cellular component (CC) and molecular function (MF). KEGG (http://www.genome.jp/) is a knowledge database for the assignment of specific pathways to sets of DEGs, thus linking-omics data with higher-order functional information (9). Comprehensively mapping genes to relevant biological annotations in databases such as DAVID is critical for the success of any high-throughput gene functional analysis. An FDR of <0.05 was set as the cut-off.
Construction of biological network
To evaluate the interactions among the two groups of identified DEGs, we mapped them to the STRING database, a database of known and predicted protein-protein interactions (PPIs), and constructed two PPI networks; only experimentally validated interactions with a combined score >0.7 were considered significant. Subsequently, the PPI networks were imported into Cytoscape, an open-source software platform for visualizing molecular interaction networks and integrating data, for further analysis (10). A plugin of Cytoscape, CytoHubba was used to predict and explore the important nodes and subnetworks in the network with 12 topological algorithms, including degree, edge percolated component (EPC), maximum neighborhood component (MNC) and density of maximum neighborhood component (DMNC), among others (11). CytoHubba was used to rank nodes in a network by their network features, select the top 10 genes from each method, and eliminate the duplicate genes. Finally, all the identified hub genes and imported into STRING to construct a complete PPI network.
Results
Identification of DEGs
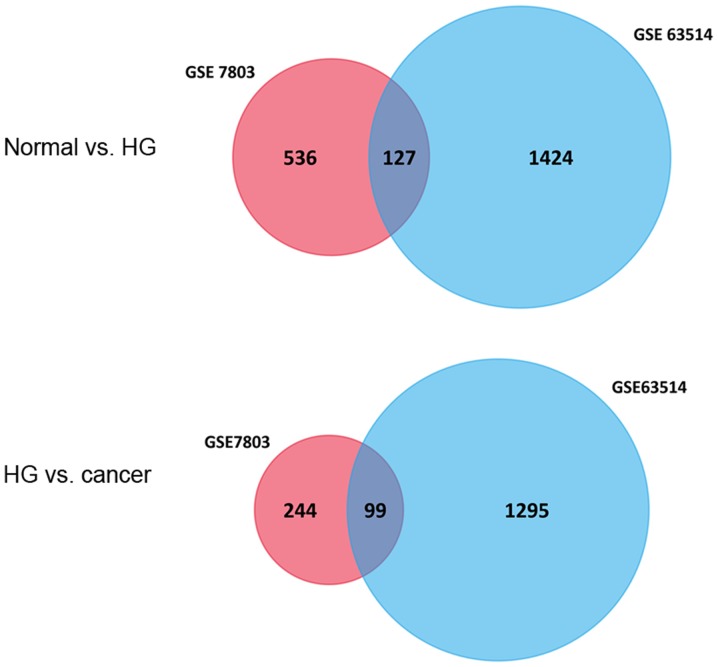
In the present study, a total of 663 and 1,551 genes were identified as the DEGs between NE and HSIL, among which 127 DEGs were co-expressed, of which 52 genes were upregulated and 75 were downregulated. Furthermore, 343 and 1,394 genes were identified as the DEGs between HSIL and SCC, with 99 DEGs overlapping, of which 32 were upregulated and 67 were downregulated (Fig. 1). A corresponding heat map is shown in Fig. 2.
Figure 1.
Venn diagram illustrating the number of DEGs in the mRNA microarray expression profile datasets GSE7803 and GSE63514, as determined with the GEO2R tool. Thresholds were set as adjusted P<0.05 and fold change >2. HG, high-grade squamous intraepithelial cervical lesion. DEGs, differentially expressed genes.
Figure 2.
Heat map of DEGs from the (A and B) GSE7803 and (C and D) GSE63514 data sets. The group is indicated at the top of the figure (A and C) by orange (normal) or blue (HSIL) and (B and D) by blue (HSIL) or red (SCC). Red squares, upregulated genes; green squares, downregulated genes. DEG, differentially expressed gene; HSIL, high-grade squamous intraepithelial cervical lesion; SCC, squamous cell carcinoma.
Function and pathway enrichment analysis
To uncover the biological significance of the screened DEGs in the progression of cervical cancer, GO functional and KEGG pathway enrichment analyses were performed using the DAVID database. As shown in Fig. 3, for pre-invasive DEGs, ‘nucleoplasm’, ‘nucleus’, ‘spindle’, and ‘midbody’ were enriched from the CC category; enriched BP terms included ‘cell division’, ‘DNA replication’, ‘cell cycle’ and ‘transcription regulation’; enriched MF terms included ‘protein binding’, ‘single-stranded DNA-dependent ATPase activity’, ‘DNA replication origin binding’ and ‘microtubule binding’. Based on KEGG pathway enrichment analysis (Fig. 4), the pre-invasive DEGs were significantly associated with the cell cycle, DNA replication and p53 signaling pathways.
Figure 3.
Gene ontology enrichment analysis. The left histograms represent the pre-invasive DEGs, while the right represent the invasive DEGs. MF, molecular function; CC, cellular components; BP, biological process; DEG, differentially expressed gene.
Figure 4.
The expression change of hub genes from normal tissue, squamous cervical epithelium to cervical cancer. SCC, squamous cell carcinoma; HG, high-grade squamous intraepithelial cervical lesion.
For the invasive DEGs, CC terms were mainly enriched in ‘extracellular space’, ‘extracellular exosome’, ‘extracellular region’ and ‘extracellular matrix’; BP terms included ‘extracellular matrix organization’, ‘epithelial cell differentiation’ and ‘collagen fibril organization’; the identified MF terms included ‘extracellular matrix structural constituent’, ‘heparin binding’ and ‘integrin binding’. The significantly enriched KEGG pathways included amoebiasis, focal adhesion, ECM-receptor interaction and platelet activation.
PPI network construction
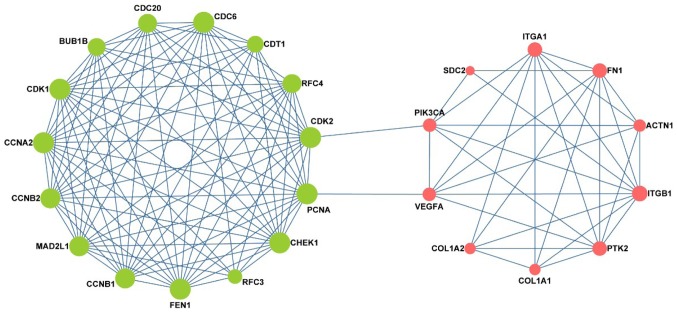
The screened DEGs were used to construct PPI networks. CytoHubba was used to rank nodes by their network features, select the top 10 genes from each methods, and eliminate duplicate genes. From the pre-invasive DEGs, we screened 23 hub genes, while from the invasive DEGs, we screened 21 hub genes. Finally, all the hub genes were summarized and imported into STRING software to construct the PPI network., there were 25 nodes and 128 edges in the network. The hub genes are listed in Table I, among which BUB1B, MAD2L1, CHEK1, CCNB1, CCNB2, CDC20, CDC6, CCNA2 and PCNA were associated with the cell cycle, RFC3, RFC4, FEN1 and PCNA were associated with DNA replication, and PIK3CA, VEGFA, ITGA1, PTK2, ITGB1, ACTN1, FN1, COL1A1 and COL1A2 were associated with focal adhesion (Table II). As shown Fig. 4, the expression of CDC6, CDT1, CHEK1 were significantly increased from normal tissue to SIL, while FN1, ITGB1 were significantly increased from SIL to cancer. Interestingly, as shown in Fig. 5 the network consisted of two clusters: The left network was composed of pre-invasive DEGs, while the right was composed of invasive DEGs; moreover, these two parts were connected by 4 key nodes, including PCNA, CDK2, VEGFA and PIK3CA. Notably, the left network was predominantly associated with the cell cycle and DNA replication, while the right was mainly associated with focal adhesion.
Table I.
Whole hub genes screened by Cytoscape.
| Pre-invasive hub genes | Invasive hub genes |
|---|---|
| BUB1B, CDC20, CDC6 | ACTN1, COL1A1, COL1A2 |
| CDT1, RFC4, CDK2 | FN1, ITGA1, ITGB1 |
| PCNA, CHEK1, RFC3 | PIK3CA, PTK2, SDC2 |
| FEN1, CCNB1, MAD2L1 | VEGFA |
CCNB2, CCNA2, CDK1
Table II.
Kyoto Encyclopedia of Genes and Genomes pathway analysis of differentially expressed hub genes associated with cervical cancer.
| Pathway ID | Name | Gene count | FDR | Genes |
|---|---|---|---|---|
| 4110 | Cell cycle | 12 | 2.38×10−17 | BUB1B, CCNA2, CCNB1, CCNB2, CDC20, CDC6, CHEK1, MAD2L1, PCNA |
| 3030 | DNA replication | 6 | 8.80×10−10 | FEN1, PCNA, RFC3, RFC4 |
| 4510 | Focal adhesion | 9 | 8.80×10−10 | ACTN1, COL1A1, COL1A2, FN1, ITGA1, ITGB1, PIK3CA, PTK2, VEGFA |
FDR, false discovery rate.
Figure 5.
A protein-protein interaction network was constructed based on the connections with the hub genes. The green nodes represent the pre-invasive genes, while the red nodes represent the invasive genes. The edges represent interactions between genes. The size of each node represents the repeat count. As the repeat count increases, the size of the node increases.
Discussion
Malignant transformation in tumor progression is caused by a series of genetic alterations. To better understand the genetic alterations occurring during cervical cancer progression, bioinformatics methods were used to extract data from the GSE7803 and GSE63514 gene expression profiles. In this study, we identified 127 DEGs between normal squamous cervical epithelium and HSIL, while 99 DEGs were identified between HSIL and invasive SCC of the cervix. Functional analysis demonstrated that these DEGs were mainly involved in the cell cycle, DNA replication, p53 signaling and focal adhesion pathways.
From the PPI network constructed from the DEGs, we found that the network was composed of two clusters. Notably, the left cluster consisted of pre-invasive DEGs, suggesting that these genes were involved in the progression to HSIL. GO term analysis revealed that the pre-invasive DEGs were mainly involved in cell division, DNA replication, the cell cycle and transcription regulation, among which BUB1B, MAD2L1, CHEK1, CCNB1, CCNB2, CDC20, CDC6, CCNA2 and PCNA were involved in the cell cycle, whereas RFC3, RFC4, FEN1 and PCNA were involved in DNA replication.
DNA replication is a key process for cell proliferation; however, the abnormal proliferation of tumor cells may be characterized by irregularities in pathways involved in DNA replication, cell cycle, apoptosis resistance and metabolic capacity, with significant implications in tumorigenesis. The cell cycle is a series of events leading to DNA division and replication to produce two daughter cells. Enhanced cell proliferation capacity is the hallmark of cancer. We observed that the biological processes of DNA replication and cell cycle transition were significantly increased in cervical cancer tissues. To maintain a hyperproliferative state, cervical cancer cells upregulate a group of genes that control multiple steps of DNA replication (12). The mitotic spindle checkpoint Bub1 is involved in monitoring the assembly of the mitotic spindle, which ensures the accurate segregation of sister chromatids during mitosis (13). Bub1 was found to be mutated in human cancers, such as colorectal cancer, which is characterized by chromosomal instability and increased aneuploidy (14). The cyclin proteins CCNA2 and CCNB1 and their associated kinases CHEK1 and CDK1 were significantly upregulated in cervical cancer tissue; these proteins promote cell cycle transition from the G1 to the S phase, and from the G2 to the M phase. Furthermore, PCNA was also found to be upregulated in cervical cancer tissues (12). CDC20 is upregulated in HSIL as well as SCC of the uterine cervix (15). Replication factor C (RFC) is important for DNA replication and cell cycle control (16). RFC3 and RFC4 were reported to promote tumor cell proliferation, and the high expression of RFC3 was associated with poor prognosis in a variety of cancers (17,18).
The right cluster consisted of invasive DEGs, which were involved in biological processes such as ECM organization, epithelial cell differentiation and collagen fibril organization, suggesting that these genes were involved in the progression of SCC. This is in accord with established paradigm that the dysfunction of cell proliferation and cell cycle regulation is the primary cause of tumor development (19). Among the identified DEGs, PIK3CA, VEGFA, ITGA1, PTK2, ITGB1, ACTN1, FN1, COL1A1, COL1A2 and SDC2 were associated with focal adhesion. Focal adhesions are large macromolecular assemblies through which mechanical force and regulatory signals are transmitted between the ECM and interacting cells. Focal adhesion kinase (FAK) is the key enzyme in regulating the formation of focal adhesions, and a key regulator of survival, proliferation, migration and invasion, which endows cells with higher motility (20). Indeed, FAK overexpression has been identified in aggressive cervical cancer (21). FAK was recently established as a cardinal controller of cell migration, particularly during tumor metastasis (22). In human cervical cancer samples, the high expression or phosphorylation of FAK is associated with an aggressive phenotype (23). Overall, FAK is crucial for cervical cancer metastasis.
Co-expressed genes are a group of genes with similar expression profiles that are often involved in parallel biological processes. By constructing a PPI network from the DEGs, we found that the two clusters were connected by 4 key genes, namely PCNA, CDK2, VEGFA and PIK3CA.
From the GO analysis, it was observed that most pre-invasive DEGs were enriched in the nucleoplasm, cell division and protein binding, while most invasive DEGs were enriched in the extracellular space, ECM organization and structural constituents.
Proliferating cell nuclear antigen (PCNA) is reported as an important marker of the progression of tumors, which acts as a central coordinator of DNA transactions by providing interaction surface for factors involved in DNA replication, repair, chromatin dynamics and cell cycle regulation (24). In a systematic review by Lv et al, PCNA upregulation was found to be significantly associated with poor 5-year survival, advanced disease stage and higher WHO grade in cervical cancer, suggesting that PCNA may be a useful prognostic and diagnostic biomarker in cervical cancer (25). Kim et al considered PCNA to be a biomarker to reflect cellular proliferation, and PCNA protein immunostaining enhanced the diagnostic accuracy for HSIL, indicating that PCNA may act as a key gene mediating the progression from HSIL to cervical cancer (26).
Vascular endothelial growth factor A (VEGFA) is a significant biomarker that elicits tumor angiogenesis, a BP crucial for primary tumor growth and metastasis. The overexpression of VEGFA is associated with poor survival in a variety of cancers, such as lung, colorectal and cervical cancer (27–29), suggesting that VEGFA is significantly involved in cervical tumorigenesis. Combined with the KEGG pathway analysis of the hub genes, which indicated that PCNA was involved in the cell cycle and DNA replication, and VEGFA in focal adhesion, we may infer that the effect of PCNA upregulation on the cell cycle promoted the connection between cells and the ECM by focal adhesions, thus activating extracellular angiogenesis, which promoted the transition from HSIL to cervical cancer.
Cyclin-dependent kinases (CDKs) play key roles in cell proliferation, and have attracted considerable attention in the study of tumor growth. CDK2 is a member of the CDK family, which associates with cyclin A or cyclin E, and is considered to be essential in the cell cycle, driving cells through the S phase by binding with cyclin A (30).
PIK3CA is a part of the PI3K/AKT/mTOR pathway, a pathway that is disrupted in several types of cancer with high frequency and is involved in the regulation of cell growth, proliferation, differentiation, glucose metabolism, protein synthesis and apoptosis (31). Somatic mutations in PIK3CA have been detected in a variety of human malignant solid tumors, including cervical cancer (32). Chung et al performed whole-exome sequencing in 15 paired cervical adenocarcinoma and peripheral leukocyte DNA samples, and identified specific PIK3CA aberrations in cervical cancer (33). In addition, Cui et al (34) analyzed PIK3CA mutations in CIN3 lesions and cervical carcinomas, and identified somatic mutations in 8.15% of cervical carcinomas, whereas there were no mutations in CIN3 cases, suggesting that genetic alterations of PIK3CA are late events during cervical carcinogenesis. Hence, we hypothesized that the upregulation of CDK2 promoted the cell cycle and DNA replication in cervical epithelial cells, leading to the development of HSIL, which, following PIK3CA mutation and focal adhesion dysregulation, ultimately developed into cervical cancer.
In summary, a comprehensive bioinformatics analysis of DEGs that may be involved in cervical cancer development is provided by the present study. Furthermore, a series of useful targets for the future study of biomarkers and molecular mechanisms were identified. Further molecular biological experiments, however, are required to confirm the role of the identified genes in cervical cancer.
Acknowledgements
Not applicable.
Funding
This study was supported by the Zhongnan Hospital of Wuhan University Science, Technology and Innovation Seed Fund (grant no. znpy2016040).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
KW and YY performed the experiments and wrote the paper. FL, WW and YC analyzed the data. WZ designed the study and reviewed the manuscript. All authors discussed the results and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10:789–799. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 3.Wu NL, Huang DY, Tsou HN, Lin YC, Lin WW. Syk mediates IL-17-induced CCL20 expression by targeting Act1-dependent k63-linked ubiquitination of TRAF6. J Invest Dermatol. 2015;135:490–498. doi: 10.1038/jid.2014.383. [DOI] [PubMed] [Google Scholar]
- 4.Kulasingam V, Diamandis EP. Strategies for discovering novel cancer biomarkers through utilization of emerging technologies. Nat Clin Pract Oncol. 2008;5:588–599. doi: 10.1038/ncponc1187. [DOI] [PubMed] [Google Scholar]
- 5.Wu SF, Qian WY, Zhang JW, Yang YB, Liu Y, Dong Y, Zhang ZB, Zhu YP, Feng YJ. Network motifs in the transcriptional regulation network of cervical carcinoma cells respond to EGF. Arch Gynecol Obstet. 2013;287:771–777. doi: 10.1007/s00404-012-2608-8. [DOI] [PubMed] [Google Scholar]
- 6.Luo Y, Wu Y, Peng Y, Liu X, Bie J, Li S. Systematic analysis to identify a key role of CDK1 in mediating gene interaction networks in cervical cancer development. Ir J Med Sci. 2016;185:231–239. doi: 10.1007/s11845-015-1283-8. [DOI] [PubMed] [Google Scholar]
- 7.Jiao X, Sherman BT, Huang da W, Stephens R, Baseler MW, Lane HC, Lempicki RA. DAVID-WS: A stateful web service to facilitate gene/protein list analysis. Bioinformatics. 2012;28:1805–1806. doi: 10.1093/bioinformatics/bts251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gene Ontology Consortium The Gene Ontology (GO) project in 2006. Nucleic Acids Res. 2006;34:D322–D326. doi: 10.1093/nar/gkj021. (Database Issue): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanehisa M, Goto S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chin CH, Chen SH, Wu HH, Ho CW, Ko MT, Lin CY. CytoHubba: Identifying hub objects and sub-networks from complex interactome. BMC Syst Biol. 2014;8(Suppl 4):S11. doi: 10.1186/1752-0509-8-S4-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng J, Lu X, Wang J, Zhang H, Duan P, Li C. Interactome analysis of gene expression profiles of cervical cancer reveals dysregulated mitotic gene clusters. Am J Transl Res. 2017;9:3048–3059. [PMC free article] [PubMed] [Google Scholar]
- 13.Cahill DP, Lengauer C, Yu J, Riggins GJ, Willson JK, Markowitz SD, Kinzler KW, Vogelstein B. Mutations of mitotic checkpoint genes in human cancers. Nature. 1998;392:300–303. doi: 10.1038/32688. [DOI] [PubMed] [Google Scholar]
- 14.Ru HY, Chen RL, Lu WC, Chen JH. Hbub1 defects in leukemia and lymphoma cells. Oncogene. 2002;21:4673–4679. doi: 10.1038/sj.onc.1205585. [DOI] [PubMed] [Google Scholar]
- 15.Kim Y, Choi JW, Lee JH, Kim YS. MAD2 and CDC20 are upregulated in high-grade squamous intraepithelial lesions and squamous cell carcinomas of the uterine cervix. Int J Gynecol Pathol. 2014;33:517–523. doi: 10.1097/PGP.0000000000000082. [DOI] [PubMed] [Google Scholar]
- 16.Masuda Y, Suzuki M, Piao J, Gu Y, Tsurimoto T, Kamiya K. Dynamics of human replication factors in the elongation phase of DNA replication. Nucleic Acids Res. 2007;35:6904–6916. doi: 10.1093/nar/gkm822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lockwood WW, Thu KL, Lin L, Pikor LA, Chari R, Lam WL, Beer DG. Integrative genomics identified RFC3 as an amplified candidate oncogene in esophageal adenocarcinoma. Clin Cancer Res. 2012;18:1936–1946. doi: 10.1158/1078-0432.CCR-11-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arai M, Kondoh N, Imazeki N, Hada A, Hatsuse K, Matsubara O, Yamamoto M. The knockdown of endogenous replication factor C4 decreases the growth and enhances the chemosensitivity of hepatocellular carcinoma cells. Liver Int. 2009;29:55–62. doi: 10.1111/j.1478-3231.2008.01792.x. [DOI] [PubMed] [Google Scholar]
- 19.Perez R, Wu N, Klipfel AA, Beart RW., Jr A better cell cycle target for gene therapy of colorectal cancer: Cyclin G. J Gastrointest Surg. 2003;7:884–889. doi: 10.1007/s11605-003-0034-8. [DOI] [PubMed] [Google Scholar]
- 20.van Nimwegen MJ, van de Water B. Focal adhesion kinase: A potential target in cancer therapy. Biochem Pharmacol. 2007;73:597–609. doi: 10.1016/j.bcp.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 21.Oktay MH, Oktay K, Hamele-Bena D, Buyuk A, Koss LG. Focal adhesion kinase as a marker of malignant phenotype in breast and cervical carcinomas. Hum Pathol. 2003;34:240–245. doi: 10.1053/hupa.2003.40. [DOI] [PubMed] [Google Scholar]
- 22.Fong YC, Liu SC, Huang CY, Li TM, Hsu SF, Kao ST, Tsai FJ, Chen WC, Chen CY, Tang CH. Osteopontin increases lung cancer cells migration via activation of the alphavbeta3 integrin/FAK/Akt and NF-kappaB-dependent pathway. Lung Cancer. 2009;64:263–270. doi: 10.1016/j.lungcan.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 23.Moon HS, Park WI, Choi EA, Chung HW, Kim SC. The expression and tyrosine phosphorylation of E-cadherin/catenin adhesion complex, and focal adhesion kinase in invasive cervical carcinomas. Int J Gynecol Cancer. 2003;13:640–646. doi: 10.1046/j.1525-1438.2003.13396.x. [DOI] [PubMed] [Google Scholar]
- 24.Srinivasan M, Jewell SD. Quantitative estimation of PCNA, c-myc, EGFR and TGF-alpha in oral submucous fibrosis-an immunohistochemical study. Oral Oncol. 2001;37:461–467. doi: 10.1016/S1368-8375(00)00115-9. [DOI] [PubMed] [Google Scholar]
- 25.Lv Q, Zhang J, Yi Y, Huang Y, Wang Y, Wang Y, Zhang W. Proliferating cell nuclear antigen has an association with prognosis and risks factors of cancer patients: A systematic review. Mol Neurobiol. 2016;53:6209–6217. doi: 10.1007/s12035-015-9525-3. [DOI] [PubMed] [Google Scholar]
- 26.Kim TH, Han JH, Shin E, Noh JH, Kim HS, Song YS. Clinical implication of p16, Ki-67, and proliferating cell nuclear antigen expression in cervical neoplasia: Improvement of diagnostic accuracy for high-grade squamous intraepithelial lesion and prediction of resection margin involvement on conization specime. J Cancer Prev. 2015;20:70–77. doi: 10.15430/JCP.2015.20.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gkiozos I, Tsagouli S, Charpidou A, Grapsa D, Kainis E, Gratziou C, Syrigos K. Levels of vascular endothelial growth factor in serum and pleural fluid are independent predictors of survival in advanced non-small cell lung cancer: Results of a prospective study. Anticancer Res. 2015;35:1129–1137. [PubMed] [Google Scholar]
- 28.Zhang J, Liu J, Zhu C, He J, Chen J, Liang Y, Yang F, Wu X, Ma X. Prognostic role of vascular endothelial growth factor in cervical cancer: A meta-analysis. Oncotarget. 2017;8:24797–24803. doi: 10.18632/oncotarget.15044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsai HL, Yang IP, Lin CH, Chai CY, Huang YH, Chen CF, Hou MF, Kuo CH, Juo SH, Wang JY. Predictive value of vascular endothelial growth factor overexpression in early relapse of colorectal cancer patients after curative resection. Int J Colorectal Dis. 2013;28:415–424. doi: 10.1007/s00384-012-1570-z. [DOI] [PubMed] [Google Scholar]
- 30.Tian RQ, Wang XH, Hou LJ, Jia WH, Yang Q, Li YX, Liu M, Li X, Tang H. MicroRNA-372 is down-regulated and targets cyclin-dependent kinase 2 (CDK2) and cyclin A1 in human cervical cancer, which may contribute to tumorigene. J Biol Chem. 2011;286:25556–25563. doi: 10.1074/jbc.M111.221564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koncar RF, Feldman R, Bahassi EM, Hashemi Sadraei N. Comparative molecular profiling of HPV-induced squamous cell carcinomas. Cancer Med. 2017;6:1673–1685. doi: 10.1002/cam4.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McIntyre JB, Wu JS, Craighead PS, Phan T, Köbel M, Lees-Miller SP, Ghatage P, Magliocco AM, Doll CM. PIK3CA mutational status and overall survival in patients with cervical cancer treated with radical chemoradiotherapy. Gynecol Oncol. 2013;128:409–414. doi: 10.1016/j.ygyno.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 33.Chung TK, van Hummelen P, Chan PK, Cheung TH, Yim SF, Yu MY, Ducar MD, Thorner AR, MacConaill LE, Doran G, et al. Genomic aberrations in cervical adenocarcinomas in Hong Kong Chinese women. Int J Cancer. 2015;137:776–783. doi: 10.1002/ijc.29456. [DOI] [PubMed] [Google Scholar]
- 34.Cui B, Zheng B, Zhang X, Stendahl U, Andersson S, Wallin KL. Mutation of PIK3CA: Possible risk factor for cervical carcinogenesis in older women. Int J Oncol. 2009;34:409–416. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.