Abstract
The etiology and pathogenesis of nutritional rickets are becoming progressively clearer. Vitamin D deficiency has generally been considered the major or only player in the pathogenesis of nutritional rickets. However, recent research into calcium deficiency has now provided clinicians with reasons to investigate and manage patients with nutritional rickets more appropriately.
The important question when assessing cases of nutritional rickets is: “Is it calcium or vitamin D deficiency or both that play a major role in the pathogenesis of the disease?”
The case presentation in this review highlights the risk factors, clinical presentation and pathophysiology of nutritional rickets in a young South African black child from a semi-urban area in Johannesburg, a city with abundant sunshine throughout the year.
Keywords: Nutritional rickets; Vitamin D; Dietary calcium; 1,25 dihydroxyvitamin D
Highlights
-
•
Vitamin D and calcium deficiencies play synergistic roles in nutritional rickets.
-
•
Dietary calcium intake of <300 mg/day increases the risk of nutritional rickets.
-
•
Vitamin D status is worsened by dietary calcium deficiency itself.
-
•
Marked elevation of 1,25-dihydroxyvitamin D is characteristic of calcium deficiency.
-
•
Preventative strategies needed to decrease the prevalence of nutritional rickets.
Case study: The patient, a 4 year old black African boy (Fig. 1), presented to a hospital in Johannesburg, South Africa (latitude 26 S) with a change in walking pattern. The initial presenting complaint from the parents was that they had noticed that the child had an “odd” or “different” walking pattern over the previous year. He had started walking late at the age of 2 years, although cognitive development was normal. The parents had also noted deformities of the lower limbs from 2 and half years of age, which they described as an “odd shape of his legs”. He had also become increasingly tired. The family is from a low socioeconomic community. When the child was still an infant, their home had burnt down and the young child had been placed in a children's home, while the parents re-established their lives. During this time, the child spent most of his time indoors. He was born by a normal vertex delivery with a birth weight 3.3 kg. The infant had not been breastfed but did receive vitamin D fortified formula feeds up to 6 months of age. On ceasing formula feeds, his diet on history was considered to be poor with negligible amounts of milk and dairy products (total dietary calcium intake estimated to be <200 mg/day), and no red meat.
Fig. 1.
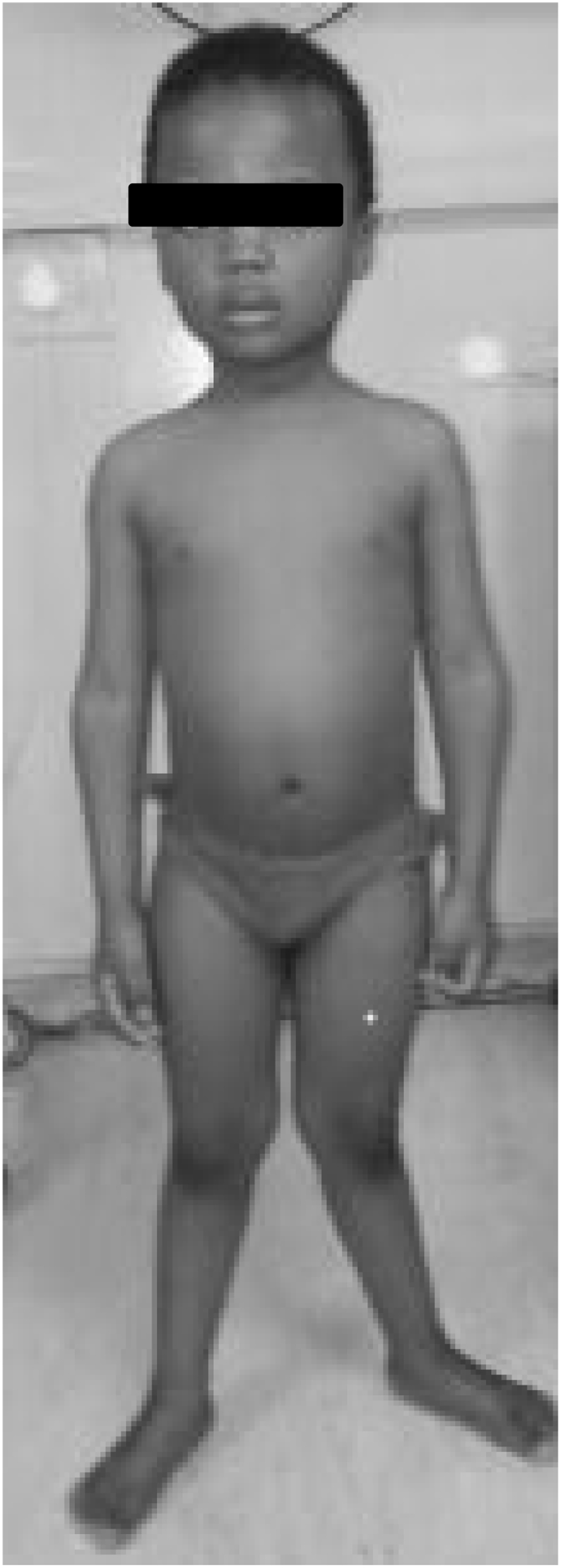
The index patient presented with genu valgus deformities of the lower limbs at the age of 4 years.
Our patient presented with stunting (height for age Z score = −3.65) and normal weight for height (Z score = 0.98). He had a valgus deformity of the knees, but did not have widened wrists or a rachitic rosary. He did have a prominent forehead and reversal of the carrying angle of the elbows.
Based on the history, presenting complaints and clinical features, he was considered to have rickets, due most likely to nutritional causes, although hypophosphataemic rickets and skeletal dysplasias were considered as other unlikely possibilities.
The radiological findings are shown in Fig. 2, Fig. 3, Fig. 4. The salient findings were those of sclerotic bands at the distal ends of the metaphyses of the radius and ulna. Growth plates were widened, and a deformity was present at the distal end of the right radius possibly due to an old healed fracture. Lateral bowing of the right femur and valgus deformity of the left knee were noted. A possible old healed fracture of the right midshaft femur was also present. There were no metaphyseal irregularities at the proximal ends of the tibias or widened growth plates, however there were mild irregularities and slightly widened growth plates at the medial and lateral aspects of the right and left distal ends of femurs respectively. The Thacher rickets severity score was assessed as being 1.
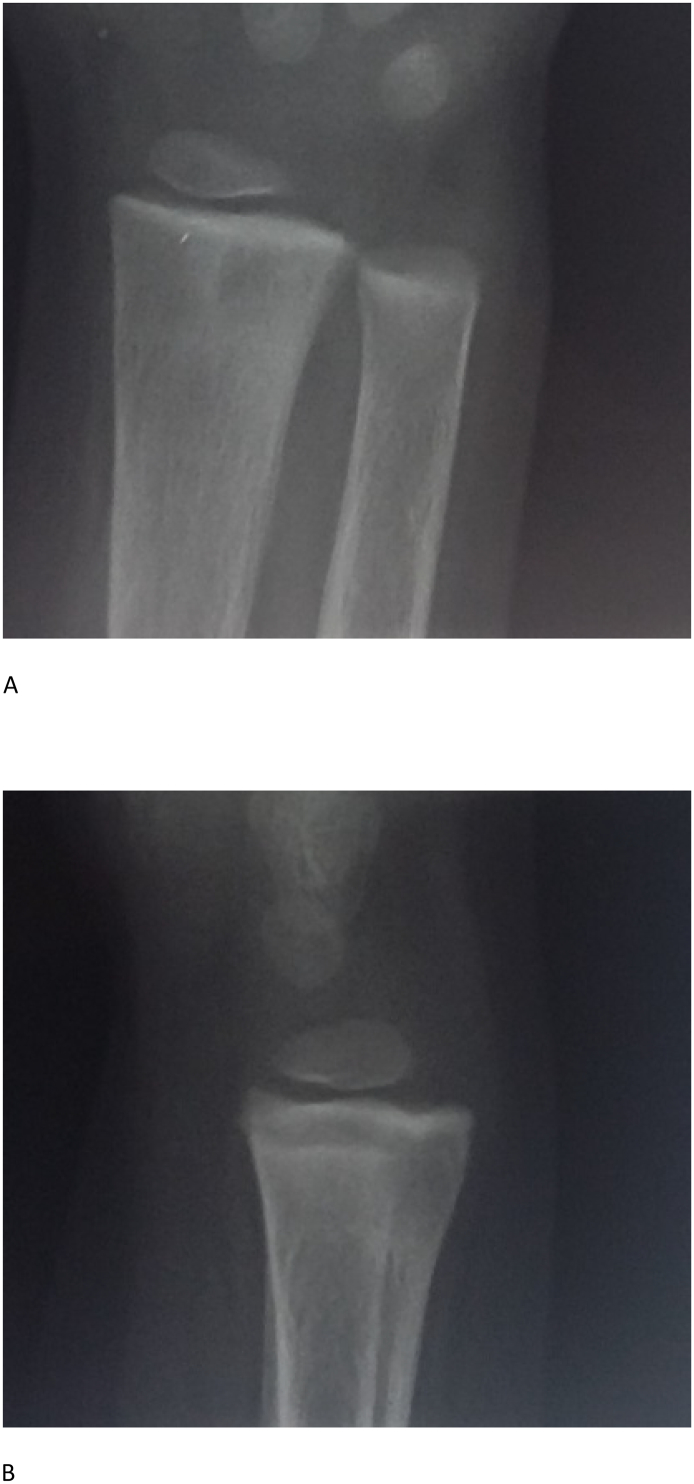
Fig. 2.
A and B: X-ray of right wrist (AP and lateral views) showing sclerotic bands of healing at the distal metaphyses and possible healed distal radius fracture (evidenced by remodeling defect (tubulation defect) of the distal radius).
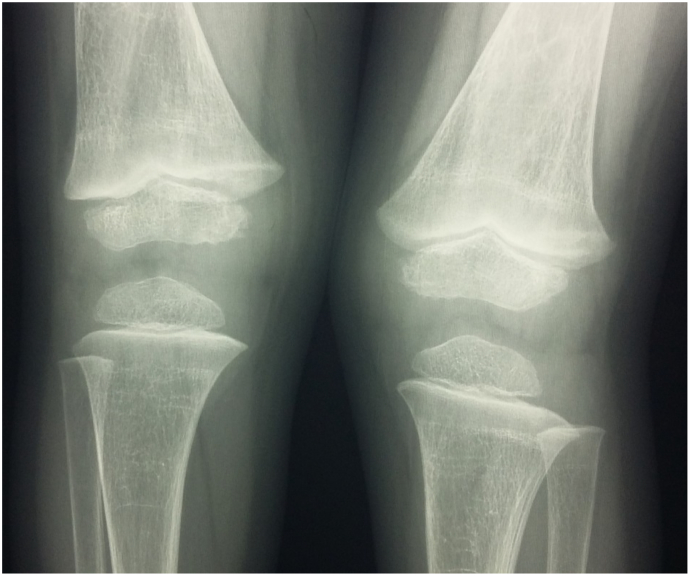
Fig. 3.
Radiological findings at the knees showing slight increased width of the growth plate at distal ends of right and left femurs.
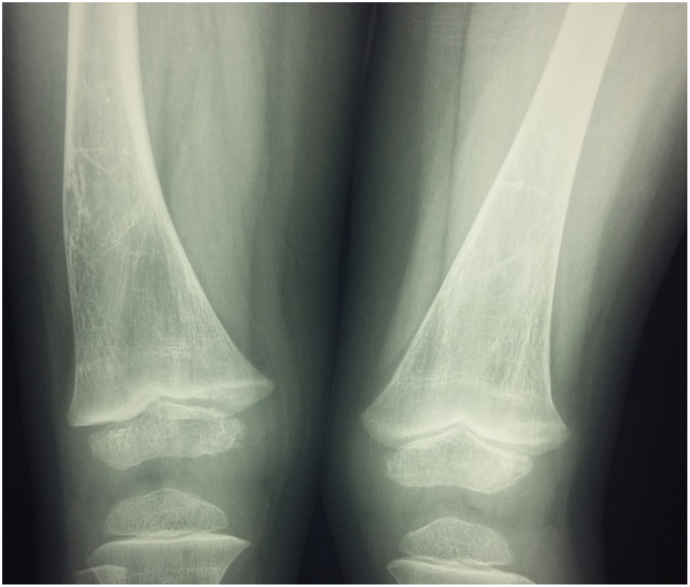
Fig. 4.
Bowing of right femur and possibly an old healed fracture at the distal midshaft femur with coarsened trabeculae and osteopenia.
Biochemical investigations were performed to establish whether or not rickets was present and if so the etiology of the disease, particular to determine if it was secondary to vitamin D or dietary calcium deficiency (Table 1). Based on the biochemical findings, the patient was commenced on vitamin D and calcium carbonate therapy.
Alt-text: Unlabelled Box
1. Introduction
Nutritional rickets remains a global public health concern, particularly in a number of lower–middle income countries (LMIC) and among at-risk immigrant communities in resource rich countries, as has been highlighted by a recent international consensus statement (Munns et al., 2016), despite a greater understanding of the etiology and pathogenesis of the condition. Vitamin D deficiency has generally been considered to be the primary cause of nutritional rickets, however more recent research into dietary calcium deficiency has now provided clinicians with reasons to investigate and manage patients with nutritional rickets differently. Although vitamin D deficiency is probably the commonest cause of nutritional rickets globally, dietary calcium deficiency has been identified as a common cause of nutritional rickets in a number of developing countries (Pettifor, 2014).
2. Definition of rickets
Rickets manifests as a defect in calcification of cartilage matrix at the epiphyseal growth plate due to defective chondrocyte differentiation and a delay in mineralization of newly formed osteoid (termed osteomalacia) at the trabecular bone surfaces; and endosteal and periosteal surfaces of cortical bone (Pettifor and Thandrayen, 2017). The failure of endochondral calcification results in widening and deformation of the growth plates and subsequent development of visible bony deformities, especially in weight bearing long bones.
3. What are the risk factors for nutritional rickets?
The two factors responsible to varying degrees and combinations for the development of nutritional rickets are vitamin D deficiency and dietary calcium deficiency. The vitamin D status of a subject is dependent on the amount of vitamin D synthesized in the skin and vitamin D content of the diet. Skin synthesis is dependent on the amount of UV radiation received by the skin, the surface area of skin that is exposed and the degree of melanin pigmentation of the skin. Thus the risk factors for vitamin D deficiency and nutritional rickets relate to those factors which reduce sunlight (UV radiation) exposure, such as duration and extent of skin exposure, season of the year, latitude, air-pollution, overcrowding, and increased skin pigmentation, and those factors influencing the dietary intake of vitamin D, such as whether or not foods are vitamin D fortified and their natural vitamin D content. Breastmilk normally contains only small amounts of vitamin D (40–60 IU/L) unless the mother is supplemented with high doses of vitamin D, thus all breastfed infants should be vitamin D supplemented (Wagner and Greer, 2008). Further a neonate's vitamin D status is dependent on that of the mother's at the time of delivery, thus babies born to vitamin D deficient mothers are deficient as well (Karras et al., 2014; Wagner et al., 2006; Wall et al., 2016). The latitude of the area of residence is also a major factor in determining the vitamin D status of unsupplemented children, especially during the winter months, as those residing at latitudes > 34° N or S have limited cutaneous vitamin D synthesis due to the negligible amounts of UV radiation reaching the earth during this period (Holick et al., 2007). Thus globally the two main causes of vitamin D deficiency are due to geographical and cultural factors, which limit skin exposure to UVB.
In comparison to vitamin D deficiency, the risk factors for dietary calcium deficiency rickets are poor dietary calcium intakes, and diets rich in high phytate cereals, or green leafy vegetables rich in calcium inhibitors such as oxalates. In lower socioeconomic communities, the high cost and relative unavailability of dairy products limits dairy product consumption in the child who has been weaned (Marwaha et al., 2005; Pettifor, 2014). Lactose intolerance is not thought to be an important deterrent to milk intake for African and Asian children, as it is infrequent in the paediatric age range (Thacher, 2003), however it can be a cause for low dietary calcium intake. There are no studies investigating the association between lactose intolerance and calcium intake in children presenting with rickets and this aspect requires further investigation. Low-lactose diets result in low dietary calcium intakes and low bone mineral content in pre-pubertal children with lactose-intolerance (Stallings et al., 1994) and lactose intolerance has been suggested to prevent the achievement of an adequate peak bone mass in young adults (Di Stefano et al., 2002).
Most of India, the Middle East and Africa have abundance of sunshine but children residing in these countries generally have diets low in calcium (Pettifor, 2014). Studies done in the late 20th century in rural South African black children and Nigerian children have highlighted the importance of low dietary intakes of calcium in the pathogenesis of hypocalcaemia and rickets (Eyberg et al., 1986; Oginni et al., 1996; Pettifor et al., 1978; Thacher et al., 1999). Thacher et al. (2000a) questioned the factors associated with nutritional rickets in Nigerian children and postulated that neither vitamin D deficiency nor low dietary calcium intake alone accounted for the development of rickets, but that insufficient dietary calcium intake might interact with genetic, hormonal and other nutritional factors to cause rickets in susceptible children. In a case-control study, they found no difference in calcium intake between Nigerian children with rickets and control subjects (217 mg/day vs 214 mg/day) (Thacher et al., 2000a), and suggested that genetic determinants of rickets (allelic variations in vitamin D receptor and 25-hydroxylation) together with low dietary calcium intakes underlie the pathophysiology of the disease. However, in a South African study, children with rickets had significantly lower calcium intakes than control subjects from the same community (Eyberg et al., 1986).
More recent studies in Indian, Mongolian and European children have proposed that nutritional rickets is secondary to both low dietary calcium intake and a low or borderline vitamin D nutritional status (Aggarwal et al., 2012; Uush, 2014; Voloc et al., 2010). In an Indian study of children with rickets, 25(OH)D levels were ≤20 ng/mL (50 nmol/L) but not significantly different from controls. However mean calcium intakes in the rachitic children were approximately half that of controls and similar to levels found in Nigerian and South African rachitic children (≈200 mg/day) (Aggarwal et al., 2012). Calcium intakes but not 25(OH)D levels were inversely associated with the severity of rickets and parathyroid hormone levels. In a European study, Moldovan children with low dietary calcium intakes in combination with low 25(OH)D levels were associated with a high prevalence of leg deformities, raised alkaline phosphatase levels and low serum calcium concentrations (Voloc et al., 2010).
Thus, a combination of associated risk factors can be accountable for the presentation of nutritional rickets in children residing in different countries worldwide with diverse cultural backgrounds.
4. Clinical presentation of nutritional rickets
The peak prevalence of vitamin D deficiency rickets occurs between 6 months and 2 years of age, when the infant is being breastfed and yet to become independently ambulatory. In some communities, the prevalence peaks again during late childhood and adolescence probably due to the increased growth rate and social customs, which prevent adequate skin exposure to sunlight. Rickets related to dietary calcium deficiency tends to occur later than vitamin D deficiency. In South Africa, the age of affected children varied between 4 and 16 years, while in Nigeria the average age at presentation was approximately 4 years.
The clinical presentation of nutritional rickets is dependent on the age of the child (Table 2). In the first 6 months of life, symptoms and signs frequently relate to hypocalcaemia (seizures, apnoeic attacks, stridor and tetany) as a consequence of vitamin D deficiency. The other clinical findings in infants include delayed closure of fontanelles, craniotabes and delayed dentition. In older infants and toddlers, the most common clinical findings are widened wrists, rachitic rosary and frontal bossing (Soliman et al., 2012; Thacher et al., 2002; Agaba et al., 2016). Other features include frontal bossing and macrocephaly in toddlers and Harrison's sulcus and other chest wall deformities in severe rickets. Further hypotonia, excessive sweating and bone tenderness may be present in severely affected infants. In Nigerian children with rickets (older than 18 months of age), costochondral and wrist enlargement were the two clinically independent features with the highest positive predictive value for active rickets (Thacher et al., 2002). In older children and adolescents, <50% of patients with rickets present with lower limb deformities (Voloc et al., 2010). Genu varum tends to present in infants with rickets when they start to weight-bear or walk. Knock-knees (genu valgum) and wind-swept deformities tend to manifest in older children as exemplified in our case report and are more common in children with phosphopenic rickets (Agaba et al., 2016). In adolescents, proximal muscle weakness, presenting as difficulty rising from sitting, may be apparent. In severe nutritional rickets, fractures, especially of the long bones, may occur with minimal trauma.
Table 2.
Age-related changes of the clinical features of rickets.
| Age | Clinical presentation |
|---|---|
| <6 months | Hypocalcaemia Craniotabes |
| >6 months | More common:
|
| >1 year | On weight bearing:
|
| At any age | Severe rickets can present with:
|
Results in bold are "abnormal results, out of the normal reference range".
Our index patient presented with features that are in keeping with less severe rickets or possibly healing rickets. In addition, his radiological findings (described below) were also more in keeping with healing rickets and thus the initial clinical diagnosis by the referring orthopaedic surgeon was that of a skeletal dysplasia.
5. Radiological confirmation of rickets
The earliest radiographic sign of rickets in young infants is a generalized demineralization of the skeleton, but its detection is rather subjective depending on the quality of the radiographs. Early rickets is manifest as a loss of the provisional zone of calcification of its metaphyseo-physis border (seen best at the wrist, the distal femur and proximal tibial metaphyses). More severe signs of active rickets are cupping, fraying or splaying of the metaphyseal ends of the long bones together with increased longitudinal width of the growth plates (normal distance = 1 mm) at the wrists and knees. The 10-point Thacher score (radiographic severity score) is a reliable scoring system to assess the severity of active rickets and the improvement in radiological findings while patients are on treatment (Thacher et al., 2000b). The Thacher score was initially validated in children with dietary calcium deficiency rickets over the age of 12 months, however more recently it has been validated for nutritional rickets by Chatterjee et al. (2014). When using the Thacher score, Chatterjee et al. found that the distal femur is a better indicator of healing in radiologically severe nutritional rickets and when resolution is delayed, it is the last bone to heal (Chatterjee et al., 2014). In our index case, the distal femur still had radiographic changes of active rickets at the metaphysis, supporting the findings of Chatterjee et al. (2014) as the other bones appeared to show sclerotic metaphyseal bands indicative of healing.
6. Pathophysiology of nutritional rickets
The important question when assessing cases of nutritional rickets is: “What roles do vitamin D deficiency and/or low dietary calcium intakes play in the pathogenesis of the disease?”
The case report highlights the presentation of calcium deficiency rickets with concomitant vitamin D deficiency in a young South African black child from a semi-urban area in Johannesburg, a city with a latitude of 26° S and abundant sunshine throughout the year (an average of nearly 9 h/day of sunshine over the year). Despite the copious sunlight, it is likely that the index case suffered from vitamin D deficiency while he was cloistered in the children's home, which manifested as a delay in motor milestones. Once he was reunited with his family, it is likely that a poor dietary calcium intake became a more prominent factor in maintaining or aggravating his bone disease. Although dietary calcium deficiency (Eyberg et al., 1986; Pettifor et al., 1978) or vitamin D deficiency (Munns et al., 2012; Al-Mustafa et al., 2007) may individually be responsible for the pathogenesis of nutritional rickets, recent studies (Thacher et al., 1999; Voloc et al., 2010; Aggarwal et al., 2013) have indicated that both dietary calcium and vitamin D deficiencies probably play synergistic roles in the pathogenesis of nutritional rickets in children after infancy, when breastfeeding is uncommon and dairy product intake small.
According to the Institute of Medicine (IOM) guidelines and the recent global consensus recommendations on prevention and management of nutritional rickets, vitamin D deficiency is regarded to be present at 25(OH)D levels <30 nmol/L, insufficiency at between 30 and 50 nmol/L and sufficiency > 50 nmol/L (Munns et al., 2016; Ross et al., 2011). In classical vitamin D deficiency, the risk of developing nutritional rickets with abnormalities of skeletal mineralization and mineral ion metabolism, increases when serum levels of 25(OH)D fall below 30 nmol/L (Munns et al., 2012; Ross et al., 2011; Majid Molla et al., 2000). Several studies have also confirmed that in the face of normal 25(OH)D levels, nutritional rickets can present as a result of inadequate dietary calcium intake (Thacher et al., 1999; Aggarwal et al., 2012; Balasubramanian et al., 2003). A dietary calcium intake of <300 mg/day increased the risk of nutritional rickets independent of 25(OH)D levels. The global consensus guidelines on prevention and management of nutritional rickets for children over 12 months of age, has classified dietary calcium intake into three categories: sufficiency >500 mg/day, insufficiency between 300 and 500 mg/day and deficiency <300 mg/day (Munns et al., 2016). Nutritional rickets does not occur in the presence of vitamin D sufficiency and calcium intakes of >500 mg/day (Eyberg et al., 1986; Pettifor et al., 1978; Aggarwal et al., 2012; Balasubramanian et al., 2003; Legius et al., 1989; Okonofua et al., 1991).
Attempts have been made to define vitamin D deficiency based on the 25(OH)D level at which PTH concentrations starts to rise. There are, however, several draw backs to this method: 1) the variation in the association between PTH and 25(OH)D is large thus making it difficult to detect an inflection point and 2) the relationship is influenced by dietary calcium intakes and requirements (Djennane et al., 2014; Aloia et al., 2006; Patel et al., 2016). Many studies conducted in adults and adolescents have shown an inverse relationship between 25(OH)D concentrations and PTH, however the actual point of inflection at which PTH starts to rise is a point of considerable debate (Aloia et al., 2006; Guillemant et al., 1999; Harkness and Cromer, 2005). In children results have been similarly variable; a study of children with vitamin D insufficiency (<50 nmol/L) in Northern Spain showed no relationship between 25(OH)D and PTH concentrations (Alonso et al., 2015) while in another study by Atapattu et al. (2013), 25(OH)D levels < 34 nmol/L were associated with an increased release of PTH. In another study of Chinese adolescents who had poor vitamin D status and low calcium intakes, 25(OH)D thresholds were demonstrated at which bone mineralization and turnover were affected (Wu et al., 2015). Below these thresholds (in boys at 33–39 nmol/L and in girls at 20–37 nmol/L), 25(OH)D levels were positively associated with total BMD, and inversely associated with serum PTH and TRAP5b; whereas above these thresholds no relationships were found (Wu et al., 2015). The influence of dietary calcium intake on the relationship between serum 25(OH)D concentrations and PTH levels was shown in healthy Indian adolescents, in whom those with higher than median dietary calcium intakes but with similar 25(OH)D concentrations to the control group had lower PTH concentrations while those with lower dietary calcium intake had higher PTH concentrations (Patel et al., 2016). Similarly, Djennana et al. emphasized the significant influence of a low calcium diet on the increase in PTH concentrations in children with 25(OH)D levels >50 nmol/L (Djennane et al., 2014). Similarly Aggarwal et al. (2012) reported that in children with similar 25(OH)D levels, those with rickets had very much higher PTH levels than controls, due most likely to associated dietary calcium deficiency in the rachitic children. Indian, Nigerian and South African children with dietary calcium deficiency rickets have 25(OH)D levels that are generally low normal. It is suggested that the vitamin D status is worsened by calcium deficiency itself through the latter inducing secondary hyperparathyroidism and increasing 25(OH)D catabolism (Aggarwal et al., 2012; Pettifor, 1994; Clements et al., 1987). As suggested by Thacher and Abrams (2010), the proportion of intestinal calcium absorption that is active is greater in calcium deprivation states which increases the requirements for vitamin D.
The salient and characteristic biochemical feature that is indicative of calcium deficiency rather than vitamin D deficiency is the marked elevation of 1,25-dihydroxyvitamin D (1,25(OH)2D) found prior to treatment, with concentrations being generally 1.5–2.0 times higher than normal controls (Glorieux and Pettifor, 2014). These levels were further increased by nearly 2 fold in Nigerian children with calcium deficiency rickets who were given an oral bolus of vitamin D, an increase not seen in control children (Thacher et al., 2006; Thacher et al., 2010). Thacher and Abrams (2010) suggest that the increase in 1,25(OH)2D in calcium deficiency rickets implies that vitamin D status is not optimal, despite elevated baseline 1,25(OH)2D concentrations. This biochemical finding is also clearly shown in our case report (Table 1). PTH is increased in vitamin D deficiency rickets and is usually increased in calcium deficiency rickets. The increased PTH levels in vitamin D deficiency rickets are due to the direct effects of reduced 25(OH)D production and the subsequent decrease in 1,25(OH)2D concentrations, reducing intestinal calcium absorption and serum calcium concentrations. In calcium deficiency rickets, the inadequate absorption of calcium from the gastrointestinal tract results in an increase in PTH levels that causes an increase in the 1α hydroxylase activity thus increasing levels of 1,25(OH)2D; improving the fractional absorption of calcium.
Table 1.
Biochemistry and treatment administered to patient over the first 12 months.
| Clinic visits/follow-ups | Initial presentation | 3 month follow-up | 7–8 month follow-up | 10 month follow-up |
|---|---|---|---|---|
| Serum biochemistry | ||||
| Calcium (mmol/L) | 1.86 | 2.25 | 2.6 | 2.3 |
| Phosphate (mmol/L) | 1.29 | 1.07 | 1.38 | 1.45 |
| Alkaline phosphatase (U/L) (N < 350 IU/L) | 691 | 396 | 473 | 363 |
| Parathyroid hormone (N = 1.6–6.9 pmol/L) | 21.5 | 4 | 6 | 6.1 |
| 25 hydroxyvitamin D (nmol/L) | 28.2 | 49.0 | ||
| 1.25 dihydroxyvitamin D (N = 43–168 pmol/L) | 379 | 404 | ||
| Urine calcium/creatinine ratio (mmol/mmol) | 0.14 | |||
| Treatment | ||||
| Vitamin D (calciferol) | 5000 IU | 2500 IU | – | – |
| Calcium carbonate | 500 mg (despite 1000 mg being prescribed) | 1000 mg | 1000 mg | 1000 mg |
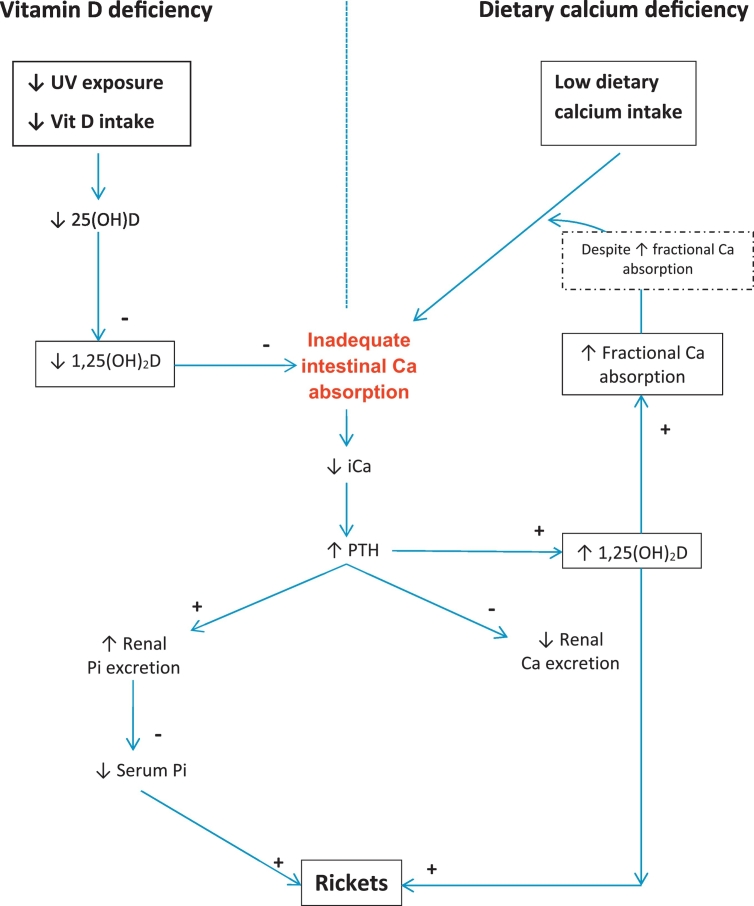
A further feature which distinguishes vitamin D deficiency rickets from that due to dietary calcium deficiency is the difference in fractional intestinal calcium absorption between the two conditions. Studies in Nigeria have found markedly increased fractional calcium absorption (mean 61%) in children with active rickets due to dietary calcium deficiency prior to treatment (Thacher and Abrams, 2010), which contrasts with the suppressed calcium absorption in vitamin D deficiency. The pathogenesis of vitamin D and calcium deficiency rickets is shown in Fig. 5 (Pettifor, 2014). The main role of vitamin D is to optimize intestinal calcium absorption to meet the needs of the growing child, thus maintaining serum calcium concentrations. It is this role that is thought to be pivotal in the pathogenesis of vitamin D deficiency rickets as inadequate calcium absorption leads to secondary hyperparathyroidism and hypophosphataemia, the latter resulting in inhibition of apoptosis of hypertrophic chondrocytes at the epiphyseal growth plate and impaired endochondral calcification leading to the features of rickets. In dietary calcium deficiency rickets the key mechanism in its pathogenesis is similar to that in vitamin D deficiency – inadequate intestinal calcium absorption to meet the needs of the organism, however an added pathogenetic factor in dietary calcium deficiency is the high 1,25(OH)2D concentrations, which in animal studies have been shown to directly inhibit bone matrix mineralization and redirect skeletal calcium toward the serum; helping to maintaining normocalcaemia when intestinal calcium transport is inadequate, but exacerbating the features of osteomalacia (Lieben et al., 2012).
Fig. 5.
Schematic representation of the pathogenesis of vitamin D deficiency and dietary calcium deficiency rickets. + = stimulatory effect; − = suppressive effect; Ca = calcium; iCa = ionized calcium; Pi = inorganic phosphate; PTH = parathyroid hormone; Vit D = vitamin D.
A unique finding has been reported in children from The Gambia with bone deformities in keeping with dietary calcium deficiency. Serum fibroblast growth factor 23 (FGF23) levels were elevated in a large proportion of the cases, and were inversely associated with plasma haemoglobin concentrations in the 5 year follow-up children with more marked rachitic-like bone deformities (Braithwaite et al., 2012; Prentice et al., 2008). The lower haemoglobin levels in these Gambian children suggest a lower iron status as iron deficiency is endemic in Gambia and the inverse relationship supports the involvement of iron in FGF23 metabolism. Iron is thought to play a role in the clearance of FGF23 fragments by the kidney and to inhibit cleavage of intact FGF23. Increased FGF23 levels are characteristically associated with certain forms of genetic or acquired (tumour-induced osteomalacia with increased production of FGF23) hypophosphataemic rickets. Despite elevated FGF23 levels in the Gambian children, 1,25(OH)2D levels were increased in response to their low dietary calcium intake and may have contributed to the elevated FGF23 levels (Prentice et al., 2008). Osteoblastic FGF23 expression is stimulated by 1,25(OH)2D (Kolek et al., 2005). The increase in FGF23 reduces renal phosphate reabsorption through decreased production of sodium-phosphate cotransporters IIa and inhibits 1,25(OH)2D production through down-regulation of 1α-hydroxylase. This negative feedback loop maintains phosphate homeostasis and prevents hyperphosphataemic spikes (Prentice et al., 2008). Whether or not elevated FGF23 levels are found in children with rachitic-like bone disease from other communities requires further study.
Apart from the increase in 1,25(OH)2D, which differentiates calcium from vitamin D deficiency rickets, other biochemical differences and similarities between these two nutritional deficiencies are outlined and summarized in Table 3. In both these deficiencies, serum calcium and phosphate levels are typically low. Secondary hyperparathyroidism is present in both deficiencies as a consequence of hypocalcaemia. Phosphate levels may be normal in the early or mild stages of the disease but as time progresses and secondary hyperparathyroidism develops, hypophosphataemia become progressively more apparent. 25(OH)D levels are very low (frequently < 10–15 nmol/L) in vitamin D deficiency rickets but can be normal or borderline low in calcium deficiency rickets. Alkaline phosphatase is significantly raised in both deficiencies. Evaluating these differences will help in differentiating between these two deficiencies but most often patients present with a combination of the two deficiencies and the biochemical differences are generally similar.
Table 3.
Biochemical differences between vitamin D and calcium deficiency rickets.
| Biochemistry | Vitamin D deficiency rickets | Calcium deficiency rickets |
|---|---|---|
| Calcium | ↓/N | ↓↓/N |
| Phosphate | N/↓ | N/↓ |
| Alkaline phosphatase | ↑↑ | ↑↑ |
| Parathyroid hormone | ↑ | N/↑ |
| 25 hydroxyvitamin D | ↓↓ (<30 nmol/L) | N/borderline low |
| 1.25 dihydroxyvitamin D | N/↓ | ↑↑ |
It is important to consider nutritional causes of hypophosphataemic rickets as a differential diagnosis but this generally manifests in premature infants. Nutritional deficiency of phosphate in older children is rare as phosphate is ubiquitous in food, making deficiency a rarity. The biochemical findings would be of low phosphate but normal PTH levels.
7. Treatment of nutritional rickets
Preventative strategies are the first and foremost approach to decreasing the prevalence and occurrence of nutritional rickets especially in high risk communities. Opportunities to provide education on adequate sun-exposure especially in areas with abundant sunshine are mandatory, although concerns about the relationship between sun exposure and skin cancers have resulted in recommendations being made that infants should avoid direct sun exposure (“Ultraviolet light: a hazard to children. American Academy of Pediatrics. Committee on Environmental Health,” 1999) and sunshine exposure should be restricted in older children. The average fair-skinned individual (Fitzpatrick (1988) skin type II) with ¼ of their skin exposed to sunlight is able to produce 1000 IU vitamin D within 14–30 min while the dark-skinned type IV individual requires 57–58 min or nearly a 2–4 fold longer duration of sun exposure (Gill and Kalia, 2015). Food fortification with vitamin D (mainly staple foods) and routine vitamin D supplementation are the priorities for preventing vitamin D deficiency rickets. Margarine, cereals, cow's milk (in some countries) and milk formulas are fortified but the fortification standards may vary across countries worldwide. The global consensus recommendations on prevention and management of nutritional rickets are that all infants up to 12 months receive oral vitamin D supplementation (400 IU/day) regardless of feeding method or rickets risk and that high risk groups continue supplementation thereafter (Munns et al., 2016). In order to implement routine vitamin D supplementation, it should be part of the routine well-baby clinic visits and possibly be incorporated into the vaccination schedules for infants. The consensus statements also advocated that all pregnant women receive 600 IU/day of vitamin D supplementation together with adequate calcium intakes to prevent congenital rickets (Munns et al., 2016). Lactating mothers should meet the dietary recommendations of 600 IU of vitamin D for their own needs as there is evidence to suggest that there is no extra benefit to the infant from extra vitamin D supplementation to the lactating mother (Munns et al., 2016). Infants of mothers supplemented with 2000 IU/day or more have similar 25(OH)D levels as infants receiving 400 IU/day and for safety reasons, current recommendations suggest infant supplementation rather than high dose maternal supplementation.
Diets rich in dairy products should be recommended to prevent calcium deficiency rickets but this might be difficult in less affluent communities. Lactose intolerance and avoidance of milk in children result in low calcium absorption. Lactose-free and lactose-reduced milk can be substituted for normal formula feeds in lactose intolerant infants but breastfed infants should be continued on human milk. Tolerance to milk products may be partial so small amounts of lactose spaced throughout the day with meals can be tolerated and two glasses of milk may be tolerated without problems (Heyman, 2006; Heaney, 2013).
Despite the simplicity of the prevention and treatment of vitamin D deficiency, many studies have confirmed poor patient treatment adherence, more so in preventative strategies (Rodd et al., 2011; Grant et al., 2014). The option of stoss therapy or single large dose vitamin D treatment to combat the problems associated with treatment adherence has been recommended. Studies have shown that a single high dose of 300,000–600,000 IU vitamin D2 or D3 given intramuscularly or orally is effective but hypercalcaemia or hypercalciuria as a side effect has been encountered (Shah and Finberg, 1994; Mittal et al., 2014; Cesur et al., 2003). Lower doses of 150,000 or 200,000 IU have been shown to be effective as well without the adverse effects (Cesur et al., 2003; Emel et al., 2012).
Despite the effectiveness of vitamin D, given either as a daily dose or as a bolus, in the management of vitamin D deficiency rickets, several studies have highlighted the need to include calcium supplements to manage hypocalcaemia in severe vitamin D deficiency, in young infants with symptomatic hypocalcaemia and in children with suspected dietary calcium deficiency.
Table 4 provides the vitamin D treatment options (daily and stoss therapy) for nutritional rickets and the supplementation doses following completion of treatment doses (Munns et al., 2016).
Table 4.
Treatment doses of vitamin D for nutritional rickets.
| Age | Daily dose for 90 days, IU | Single dose, IU | Maintenance daily dose, IU |
|---|---|---|---|
| <3 months | 2000 | N/A | 400 |
| 3–12 months | 2000 | 50,000 | 400 |
| >12 months to 12 years | 3000–6000 | 150,000 | 600 |
| >12 years | 6000 | 300,000 | 600 |
Abbreviation: N/A, not available. Reassess response to treatment after 3 months as further treatment may be required. Ensure a daily calcium intake of at least 500 mg. For conversion from IU to μg, divide by 40.
Combined poor vitamin D status and low dietary calcium intakes are becoming recognized more commonly, especially in low and middle income countries. A recent Indian study of children with nutritional rickets found that the participants had a mean dietary calcium intake of 204 mg/day and mean 25(OH)D level of 15.9 ng/mL. These children responded better to a combination of vitamin D (600,000 IU single intramuscular injection) and calcium (75 mg/kg/day elemental calcium orally) than to calcium alone with 50% in the former group responding within 12 weeks compared to 15.7% in calcium alone group (Aggarwal et al., 2013). Similar results were found in a Nigerian study, in which rachitic children on combination therapy (vitamin D and calcium) had a more rapid initial decline in serum alkaline phosphatase and improvement in radiological score compared to those on calcium alone (Thacher et al., 1999).
Thacher et al. (2014) further investigated the response of rickets in Nigerian children to calcium treatment administered as limestone with and without vitamin D supplementation and found that vitamin D once again facilitated more rapid healing and improved vitamin D status. The response to treatment was independent of baseline 25(OH)D concentrations. An additional study in Nigerian children with rickets, comparing treatment with limestone to ground fish, found similar healing effects in the two groups (Thacher et al., 2015). These studies (Thacher et al., 2014; Thacher et al., 2015) recommend that limestone or ground fish mixed with food or porridge (provided the child completes the meal) can be used as inexpensive sources of calcium in low-income countries where low calcium intakes and rickets are prevalent.
The reasons why vitamin D enhances healing of rickets in children with dietary calcium deficiency is unclear. It has been shown that vitamin D does not improve the already established maximal fractional calcium absorption in Nigerian children with rickets and that the response to therapy is unrelated to initial 25(OH)D concentrations (Thacher et al., 2009). As has been reported by Thacher, administration of a bolus of vitamin D in children with active rickets leads to a rapid nearly two fold elevation in 1,25(OH)2D levels above the already elevated levels prior to treatment. It appears that vitamin D metabolites may have a direct or indirect effect on bone mineralization by mechanisms other than calcium absorption (Anderson et al., 2012). As explained earlier under the pathophysiology of calcium deficiency, Lieben et al. described the effects of high 1,25(OH)2D-mediated regulation of the bone mineralization inhibitors whose role it is to maintain normocalcaemia but the authors emphasized that there are other modulators and/or post-translational modifications that cannot be excluded as possible co-mechanisms for bone remodeling (Lieben et al., 2012).
What is the optimal dose of calcium for treatment of nutritional rickets secondary to calcium deficiency? The calcium requirements in children with healing rickets may be greater than those of normal children because of “hungry bones”. A daily dose of 1000 mg is optimal for treatment of children with a customary dietary calcium intake < 300 mg/day and some children required treatment for >24 weeks for complete healing of rickets (Thacher et al., 2016). Fractional calcium absorption is greatest with calcium intakes of <500 mg/day and the total calcium absorption may not be significantly greater with a 2000 mg dose compared with a 1000 mg dose (Thacher et al., 2016).
The global consensus recommendations on prevention and management of nutritional rickets recommends that oral calcium (500 mg/day) either as dietary intake or supplements, should be routinely used in conjunction with vitamin D in the treatment regardless of age and weight.
As documented in this case presentation, the patient responded well to at least three months of oral calcium of 500 mg/day and vitamin D of 5000 IU/day (Table 1).
8. Conclusion
Nutritional rickets is the most common metabolic bone disorder globally despite its ease of prevention and treatment. A thorough detailed history of diet and associated risk factors, together with the clinical features of rickets should lead the attending clinician to the underlying diagnosis, be it calcium deficiency or vitamin D deficiency or a combination of both. The important biochemical clues to the underlying dietary calcium deficiency will be that of a low calcium, high 1,25(OH)2D and concomitant low-normal vitamin D status whereas untreated vitamin D deficiency rickets will have low 25(OH)D levels usually in the range <10 nmol/L. In resource constrained settings, the global consensus recommendations for the management and treatment of nutritional rickets should be followed, which recommend the administration of at least 500 mg of calcium per day and vitamin D therapy according to age. Response to therapy should be reassessed after 3 months; if clinical, biochemical and radiographic evidence of improvement has occurred, treatment should be maintained for a further three months, while if no evidence of improvement has occurred, it is imperative that adherence to the treatment regimen be assessed before investigating the rarer often genetic causes of calciopenic rickets further.
References
- Agaba F., Pettifor J.M., Thandrayen K. Comparative study of children with calciopenic and phosphopenic rickets seen at Chris Hani Baragwanath Hospital. SA Orthop. J. 2016;15:37–42. [Google Scholar]
- Aggarwal V., Seth A., Aneja S., Sharma B., Sonkar P., Singh S., Marwaha R.K. Role of calcium deficiency in development of nutritional rickets in Indian children: a case control study. J. Clin. Endocrinol. Metab. 2012;97:3461–3466. doi: 10.1210/jc.2011-3120. [DOI] [PubMed] [Google Scholar]
- Aggarwal V., Seth A., Marwaha R.K., Sharma B., Sonkar P., Singh S., Aneja S. Management of nutritional rickets in Indian children: a randomized controlled trial. J. Trop. Pediatr. 2013;59:127–133. doi: 10.1093/tropej/fms058. [DOI] [PubMed] [Google Scholar]
- Al-Mustafa Z.H., Al-Madan M., Al-Majid H.J., Al-Muslem S., Al-Ateeq S., Al-Ali A.K. Vitamin D deficiency and rickets in the Eastern Province of Saudi Arabia. Ann. Trop. Paediatr. 2007;27:63–67. doi: 10.1179/146532807X170529. [DOI] [PubMed] [Google Scholar]
- Aloia J.F., Talwar S.A., Pollack S., Feuerman M., Yeh J.K. Optimal vitamin D status and serum parathyroid hormone concentrations in African American women. Am. J. Clin. Nutr. 2006;84:602–609. doi: 10.1093/ajcn/84.3.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso M.A., Pallavicini Z.F., Rodriguez J., Avello N., Martinez-Camblor P., Santos F. Can vitamin D status be assessed by serum 25OHD in children? Pediatr. Nephrol. 2015;30:327–332. doi: 10.1007/s00467-014-2927-z. [DOI] [PubMed] [Google Scholar]
- Anderson P.H., Turner A.G., Morris H.A. Vitamin D actions to regulate calcium and skeletal homeostasis. Clin. Biochem. 2012;45:880–886. doi: 10.1016/j.clinbiochem.2012.02.020. [DOI] [PubMed] [Google Scholar]
- Atapattu N., Shaw N., Hogler W. Relationship between serum 25-hydroxyvitamin D and parathyroid hormone in the search for a biochemical definition of vitamin D deficiency in children. Pediatr. Res. 2013;74:552–556. doi: 10.1038/pr.2013.139. [DOI] [PubMed] [Google Scholar]
- Balasubramanian K., Rajeswari J., Govil Y.C., Agarwal A.K., Kumar A., Bhatia V. Varying role of vitamin D deficiency in the etiology of rickets in young children vs. adolescents in northern India. J. Trop. Pediatr. 2003;49:201–206. doi: 10.1093/tropej/49.4.201. [DOI] [PubMed] [Google Scholar]
- Braithwaite V., Jarjou L.M., Goldberg G.R., Jones H., Pettifor J.M., Prentice A. Follow-up study of Gambian children with rickets-like bone deformities and elevated plasma FGF23: possible aetiological factors. Bone. 2012;50:218–225. doi: 10.1016/j.bone.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesur Y., Caksen H., Gundem A., Kirimi E., Odabas D. Comparison of low and high dose of vitamin D treatment in nutritional vitamin D deficiency rickets. J. Pediatr. Endocrinol. Metab. 2003;16:1105–1109. doi: 10.1515/jpem.2003.16.8.1105. [DOI] [PubMed] [Google Scholar]
- Chatterjee D., Gupta V., Sharma V., Sinha B., Samanta S. A reliable and cost effective approach for radiographic monitoring in nutritional rickets. Br. J. Radiol. 2014;87 doi: 10.1259/bjr.20130648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements M.R., Johnson L., Fraser D.R. A new mechanism for induced vitamin D deficiency in calcium deprivation. Nature. 1987;325:62–65. doi: 10.1038/325062a0. [DOI] [PubMed] [Google Scholar]
- Di Stefano M., Veneto G., Malservisi S., Cecchetti L., Minguzzi L., Strocchi A., Corazza G.R. Lactose malabsorption and intolerance and peak bone mass. Gastroenterology. 2002;122:1793–1799. doi: 10.1053/gast.2002.33600. [DOI] [PubMed] [Google Scholar]
- Djennane M., Lebbah S., Roux C., Djoudi H., Cavalier E., Souberbielle J.C. Vitamin D status of schoolchildren in Northern Algeria, seasonal variations and determinants of vitamin D deficiency. Osteoporos. Int. 2014;25:1493–1502. doi: 10.1007/s00198-014-2623-7. [DOI] [PubMed] [Google Scholar]
- Emel T., Dogan D.A., Erdem G., Faruk O. Therapy strategies in vitamin D deficiency with or without rickets: efficiency of low-dose stoss therapy. J. Pediatr. Endocrinol. Metab. 2012;25:107–110. doi: 10.1515/jpem-2011-0368. [DOI] [PubMed] [Google Scholar]
- Eyberg C.J., Pettifor J.M., Moodley G. Dietary calcium intake in rural black South African children. The relationship between calcium intake and calcium nutritional status. Hum. Nutr. Clin. Nutr. 1986;40:69–74. [PubMed] [Google Scholar]
- Fitzpatrick T.B. The validity and practicality of sun-reactive skin types i through vi. Arch. Dermatol. 1988;124:869–871. doi: 10.1001/archderm.124.6.869. [DOI] [PubMed] [Google Scholar]
- Gill P., Kalia S. Assessment of the feasibility of using sunlight exposure to obtain the recommended level of vitamin D in Canada. C. Open. 2015;3:E258–63. doi: 10.9778/cmajo.20140037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glorieux F.H., Pettifor J.M. Vitamin D/dietary calcium deficiency rickets and pseudo-vitamin D deficiency rickets. Bonekey Rep. 2014;3 doi: 10.1038/bonekey.2014.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant C.C., Stewart A.W., Scragg R., Milne T., Rowden J., Ekeroma A., Wall C., Mitchell E.A., Crengle S., Trenholme A., Crane J., Camargo C.A., Jr. Vitamin D during pregnancy and infancy and infant serum 25-hydroxyvitamin D concentration. Pediatrics. 2014;133:e143–53. doi: 10.1542/peds.2013-2602. [DOI] [PubMed] [Google Scholar]
- Guillemant J., Taupin P., Le H.T., Taright N., Allemandou A., Peres G., Guillemant S. Vitamin D status during puberty in French healthy male adolescents. Osteoporos. Int. 1999;10:222–225. doi: 10.1007/s001980050219. [DOI] [PubMed] [Google Scholar]
- Harkness L., Cromer B. Low levels of 25-hydroxy vitamin D are associated with elevated parathyroid hormone in healthy adolescent females. Osteoporos. Int. 2005;16:109–113. doi: 10.1007/s00198-004-1656-8. [DOI] [PubMed] [Google Scholar]
- Heaney R.P. Dairy intake, dietary adequacy, and lactose intolerance. Adv. Nutr. An. Int. Rev. J. 2013;4:151–156. doi: 10.3945/an.112.003368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyman M.B. Lactose intolerance in infants, children, and adolescents. Pediatrics. 2006;118:1279–1286. doi: 10.1542/peds.2006-1721. [DOI] [PubMed] [Google Scholar]
- Holick M.F., Chen T.C., Lu Z., Sauter E. Vitamin D and skin physiology: a D-lightful story. J. Bone Miner. Res. 2007;22(Suppl. 2):V28–33. doi: 10.1359/jbmr.07s211. [DOI] [PubMed] [Google Scholar]
- Karras S.N., Anagnostis P., Bili E., Naughton D., Petroczi A., Papadopoulou F., Goulis D.G. Maternal vitamin D status in pregnancy and offspring bone development: the unmet needs of vitamin D era. Osteoporos. Int. 2014;25:795–805. doi: 10.1007/s00198-013-2468-5. [DOI] [PubMed] [Google Scholar]
- Kolek O.I., Hines E.R., Jones M.D., LeSueur L.K., Lipko M.A., Kiela P.R., Collins J.F., Haussler M.R., Ghishan F.K. 1Alpha,25-dihydroxyvitamin D3 upregulates FGF23 gene expression in bone: the final link in a renal-gastrointestinal-skeletal axis that controls phosphate transport. Am. J. Physiol. Gastrointest. Liver Physiol. 2005;289:G1036–42. doi: 10.1152/ajpgi.00243.2005. [DOI] [PubMed] [Google Scholar]
- Legius E., Proesmans W., Eggermont E., Vandamme-Lobaerts R., Bouillon R., Smet M. Rickets due to dietary calcium deficiency. Eur. J. Pediatr. 1989;148:784–785. doi: 10.1007/BF00443112. [DOI] [PubMed] [Google Scholar]
- Lieben L., Masuyama R., Torrekens S., Van Looveren R., Schrooten J., Baatsen P., Lafage-Proust M.H., Dresselaers T., Feng J.Q., Bonewald L.F., Meyer M.B., Pike J.W., Bouillon R., Carmeliet G. Normocalcemia is maintained in mice under conditions of calcium malabsorption by vitamin D-induced inhibition of bone mineralization. J. Clin. Invest. 2012;122:1803–1815. doi: 10.1172/JCI45890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majid Molla A., Badawi M.H., al-Yaish S., Sharma P., el-Salam R.S., Molla A.M. Risk factors for nutritional rickets among children in Kuwait. Pediatr. Int. 2000;42:280–284. doi: 10.1046/j.1442-200x.2000.01230.x. [DOI] [PubMed] [Google Scholar]
- Marwaha R.K., Tandon N., Reddy D.R., Aggarwal R., Singh R., Sawhney R.C., Saluja B., Ganie M.A., Singh S. Vitamin D and bone mineral density status of healthy schoolchildren in northern India. Am. J. Clin. Nutr. 2005;82:477–482. doi: 10.1093/ajcn.82.2.477. [DOI] [PubMed] [Google Scholar]
- Mittal H., Rai S., Shah D., Madhu S.V., Mehrotra G., Malhotra R.K., Gupta P. 300,000 IU or 600,000 IU of oral vitamin D3 for treatment of nutritional rickets: a randomized controlled trial. Indian Pediatr. 2014;51:265–272. doi: 10.1007/s13312-014-0399-7. [DOI] [PubMed] [Google Scholar]
- Munns C.F., Simm P.J., Rodda C.P., Garnett S.P., Zacharin M.R., Ward L.M., Geddes J., Cherian S., Zurynski Y., Cowell C.T. Incidence of vitamin D deficiency rickets among Australian children: an Australian Paediatric Surveillance Unit study. Med. J. Aust. 2012;196:466–468. doi: 10.5694/mja11.10662. [DOI] [PubMed] [Google Scholar]
- Munns C.F., Shaw N., Kiely M., Specker B.L., Thacher T.D., Ozono K., Michigami T., Tiosano D., Mughal M.Z., Makitie O., Ramos-Abad L., Ward L., DiMeglio L.A., Atapattu N., Cassinelli H., Braegger C., Pettifor J.M., Seth A., Idris H.W., Bhatia V., Fu J., Goldberg G., Savendahl L., Khadgawat R., Pludowski P., Maddock J., Hypponen E., Oduwole A., Frew E., Aguiar M., Tulchinsky T., Butler G., Hogler W. Global consensus recommendations on prevention and management of nutritional rickets. J. Clin. Endocrinol. Metab. 2016;101:394–415. doi: 10.1210/jc.2015-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oginni L.M., Worsfold M., Oyelami O.A., Sharp C.A., Powell D.E., Davie M.W. Etiology of rickets in Nigerian children. J. Pediatr. 1996;128:692–694. doi: 10.1016/s0022-3476(96)80137-5. [DOI] [PubMed] [Google Scholar]
- Okonofua F., Gill D.S., Alabi Z.O., Thomas M., Bell J.L., Dandona P. Rickets in Nigerian children: a consequence of calcium malnutrition. Metabolism. 1991;40:209–213. doi: 10.1016/0026-0495(91)90177-x. [DOI] [PubMed] [Google Scholar]
- Patel P., Mughal M.Z., Patel P., Yagnik B., Kajale N., Mandlik R., Khadilkar V., Chiplonkar S.A., Phanse S., Patwardhan V., Patel A., Khadilkar A. Dietary calcium intake influences the relationship between serum 25-hydroxyvitamin D3 (25OHD) concentration and parathyroid hormone (PTH) concentration. Arch. Dis. Child. 2016;101:316–319. doi: 10.1136/archdischild-2015-308985. [DOI] [PubMed] [Google Scholar]
- Ultraviolet light: a hazard to children. American Academy of Pediatrics. Committee on Environmental HealthPediatrics. 1999;104:328–333. [PubMed] [Google Scholar]
- Pettifor J.M. Privational rickets: a modern perspective. J. R. Soc. Med. 1994;87:723–725. [PMC free article] [PubMed] [Google Scholar]
- Pettifor J.M. Calcium and vitamin d metabolism in children in developing countries. Ann. Nutr. Metab. 2014;64(Suppl. 2):15–22. doi: 10.1159/000365124. [DOI] [PubMed] [Google Scholar]
- Pettifor J.M., Thandrayen K. Nutritional rickets and vitamin D deficiency. In: de Pee S., Taren D., Bloem M.W., editors. Nutrition and Health in a Developing World. Springer Science+Business Media; New York: 2017. pp. 297–319. [Google Scholar]
- Pettifor J.M., Ross P., Wang J., Moodley G., Couper-Smith J. Rickets in children of rural origin in South Africa: is low dietary calcium a factor? J. Pediatr. 1978;92:320–324. doi: 10.1016/s0022-3476(78)80035-3. [DOI] [PubMed] [Google Scholar]
- Prentice A., Ceesay M., Nigdikar S., Allen S.J., Pettifor J.M. FGF23 is elevated in Gambian children with rickets. Bone. 2008;42:788–797. doi: 10.1016/j.bone.2007.11.014. [DOI] [PubMed] [Google Scholar]
- Rodd C., Jean-Philippe S., Vanstone C., Weiler H. Comparison of 2 vitamin D supplementation modalities in newborns: adherence and preference. Appl. Physiol. Nutr. Metab. 2011;36:414–418. doi: 10.1139/h11-018. [DOI] [PubMed] [Google Scholar]
- Ross A.C., Manson J.E., Abrams S.A., Aloia J.F., Brannon P.M., Clinton S.K., Durazo-Arvizu R.A., Gallagher J.C., Gallo R.L., Jones G., Kovacs C.S., Mayne S.T., Rosen C.J., Shapses S.A. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J. Clin. Endocrinol. Metab. 2011;96:53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah B.R., Finberg L. Single-day therapy for nutritional vitamin D-deficiency rickets: a preferred method. J. Pediatr. 1994;125:487–490. doi: 10.1016/s0022-3476(05)83303-7. [DOI] [PubMed] [Google Scholar]
- Soliman A., De Sanctis V., Adel A., El Awwa A., Bedair S. Clinical, biochemical and radiological manifestations of severe vitamin d deficiency in adolescents versus children: response to therapy. Georgian Med. News. 2012:58–64. [PubMed] [Google Scholar]
- Stallings V.A., Oddleifson N.W., Negrini B.Y., Zemel B.S., Wellens R. Bone mineral content and dietary calcium intake in children prescribed a low-lactose diet. J. Pediatr. Gastroenterol. Nutr. 1994;18:440–445. doi: 10.1097/00005176-199405000-00006. [DOI] [PubMed] [Google Scholar]
- Thacher T.D. Calcium deficiency rickets. In: Hochberg Z., editor. Vitamin D and Rickets. Karger, Endocr Dev; Basel: 2003. pp. 105–125. [DOI] [PubMed] [Google Scholar]
- Thacher T.D., Abrams S.A. Relationship of calcium absorption with 25(OH)D and calcium intake in children with rickets. Nutr. Rev. 2010;68:682–688. doi: 10.1111/j.1753-4887.2010.00338.x. [DOI] [PubMed] [Google Scholar]
- Thacher T.D., Fischer P.R., Pettifor J.M., Lawson J.O., Isichei C.O., Reading J.C., Chan G.M. A comparison of calcium, vitamin D, or both for nutritional rickets in Nigerian children. N. Engl. J. Med. 1999;341:563–568. doi: 10.1056/NEJM199908193410803. [DOI] [PubMed] [Google Scholar]
- Thacher T.D., Fischer P.R., Pettifor J.M., Lawson J.O., Isichei C.O., Chan G.M. Case-control study of factors associated with nutritional rickets in Nigerian children. J. Pediatr. 2000;137:367–373. doi: 10.1067/mpd.2000.107527. [DOI] [PubMed] [Google Scholar]
- Thacher T.D., Fischer P.R., Pettifor J.M., Lawson J.O., Manaster B.J., Reading J.C. Radiographic scoring method for the assessment of the severity of nutritional rickets. J. Trop. Pediatr. 2000;46:132–139. doi: 10.1093/tropej/46.3.132. [DOI] [PubMed] [Google Scholar]
- Thacher T.D., Fischer P.R., Pettifor J.M. The usefulness of clinical features to identify active rickets. Ann. Trop. Paediatr. 2002;22:229–237. doi: 10.1179/027249302125001525. [DOI] [PubMed] [Google Scholar]
- Thacher T.D., Fischer P.R., Isichei C.O., Pettifor J.M. Early response to vitamin D2 in children with calcium deficiency rickets. J. Pediatr. 2006;149:840–844. doi: 10.1016/j.jpeds.2006.08.070. [DOI] [PubMed] [Google Scholar]
- Thacher T.D., Obadofin M.O., O'Brien K.O., Abrams S.A. The effect of vitamin D2 and vitamin D3 on intestinal calcium absorption in Nigerian children with rickets. J. Clin. Endocrinol. Metab. 2009;94:3314–3321. doi: 10.1210/jc.2009-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thacher T.D., Fischer P.R., Obadofin M.O., Levine M.A., Singh R.J., Pettifor J.M. Comparison of metabolism of vitamins D2 and D3 in children with nutritional rickets. J. Bone Miner. Res. 2010;25:1988–1995. doi: 10.1002/jbmr.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thacher T.D., Fischer P.R., Pettifor J.M. Vitamin D treatment in calcium-deficiency rickets: a randomised controlled trial. Arch. Dis. Child. 2014;99:807–811. doi: 10.1136/archdischild-2013-305275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thacher T.D., Bommersbach T.J., Pettifor J.M., Isichei C.O., Fischer P.R. Comparison of limestone and ground fish for treatment of nutritional rickets in children in Nigeria. J. Pediatr. 2015;167:148–154.e1. doi: 10.1016/j.jpeds.2015.02.008. [DOI] [PubMed] [Google Scholar]
- Thacher T.D., Smith L., Fischer P.R., Isichei C.O., Cha S.S., Pettifor J.M. Optimal dose of calcium for treatment of nutritional rickets: a randomized controlled trial. J. Bone Miner. Res. 2016;31:2024–2031. doi: 10.1002/jbmr.2886. [DOI] [PubMed] [Google Scholar]
- Uush T. Calcium intake and serum calcium status in Mongolian children. J. Steroid Biochem. Mol. Biol. 2014;144(Pt A):167–171. doi: 10.1016/j.jsbmb.2014.01.010. [DOI] [PubMed] [Google Scholar]
- Voloc A., Esterle L., Nguyen T.M., Walrant-Debray O., Colofitchi A., Jehan F., Garabedian M. High prevalence of genu varum/valgum in European children with low vitamin D status and insufficient dairy products/calcium intakes. Eur. J. Endocrinol. 2010;163:811–817. doi: 10.1530/EJE-10-0434. [DOI] [PubMed] [Google Scholar]
- Wagner C.L., Greer F.R. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122:1142–1152. doi: 10.1542/peds.2008-1862. [DOI] [PubMed] [Google Scholar]
- Wagner C.L., Hulsey T.C., Fanning D., Ebeling M., Hollis B.W. High-dose vitamin D3 supplementation in a cohort of breastfeeding mothers and their infants: a 6-month follow-up pilot study. Breastfeed. Med. 2006;1:59–70. doi: 10.1089/bfm.2006.1.59. [DOI] [PubMed] [Google Scholar]
- Wall C.R., Stewart A.W., Camargo C.A., Jr., Scragg R., Mitchell E.A., Ekeroma A., Crane J., Milne T., Rowden J., Horst R., Grant C.C. Vitamin D activity of breast milk in women randomly assigned to vitamin D3 supplementation during pregnancy. Am. J. Clin. Nutr. 2016;103:382–388. doi: 10.3945/ajcn.115.114603. [DOI] [PubMed] [Google Scholar]
- Wu F., Laslett L.L., Zhang Q. Threshold effects of vitamin D status on bone health in Chinese adolescents with low calcium intake. J. Clin. Endocrinol. Metab. 2015;100:4481–4489. doi: 10.1210/jc.2015-2849. [DOI] [PubMed] [Google Scholar]