Abstract
Bariatric surgery has proven to be a valuable treatment option for morbid obesity. However, these procedures can lead to impaired intestinal absorption of calcium and vitamin D, thereby challenging calcium homeostasis and possibly contributing to bone loss leading to an increased fracture risk. Besides calcium and vitamin D malabsorption, hormonal changes occurring after surgery can also be the source of observed bone loss. In this review, first, a case report will be discussed, highlighting the relevance of this topic. Afterwards, changes in bone density and fracture risk, after the two most performed types of bariatric surgery, Sleeve Gastrectomy (SG) and Roux-en-Y Gastric Bypass (RYGB) will be discussed. In addition, we discuss the putative underlying mechanisms leading to bone changes based on both preclinical and clinical observations. Nonetheless, it is clear further research is needed to further elucidate the exact mechanisms of bone loss following bariatric surgery and subsequently identify potential treatment options for bone preservation.
Abbreviations: SG, Sleeve Gastrectomy; RYGB, Roux-en-Y Gastric Bypass
Keywords: Bariatric surgery, Sleeve Gastrectomy, Roux-en-Y Gastric Bypass, Calcium, Vitamin D, Bone mineral density (BMD), Fractures
Highlights
-
•
Bariatric surgery induces bone loss and leads to increased fracture risk.
-
•
Bone resorption increases after both SG and, more strongly, after RYGB.
-
•
Malabsorption and adipose tissue-related hormones likely contribute to bone loss.
1. Case report
A 41-year-old non-smoking male patient was referred to our outpatient clinic because he collapsed on the street and was unable to stand up again. He had been complaining of increasing back pain for the last months. An X-ray revealed multiple vertebral compression fractures (thoracic vertebrae 10, 11, 12 and all lumbar vertebrae) and a fracture of the sixth left rib. Two years earlier, he had undergone a surgical procedure known as a biliopancreatic diversion according to Scopinaro, which is a combination of a restrictive and malabsorptive surgery, in order to lose weight. Blood tests revealed an extremely low 25-hydroxyvitamin D (25(OH)D) level at 3.8 μg/L and a low serum calcium and phosphorus level at 6.18 mg/dl and 2.11 mg/dl, respectively. The protein serum level was also decreased at 51 g/l. The serum level of parathyroid hormone was elevated at 151.4 ng/L. A bone density measurement revealed a T-score of −3.69 at the lumbar level (L2–L4) and −2.37 at the femoral neck. Initially, he was supplemented with oral vitamin D without success and subsequently intramuscular injections of Cholecalciferol were given in order to restore normal vitamin D levels. A 72 h faeces collection revealed extreme steatorrhea with a total faeces volume of 756 g/day with 76.8 g of fat/day. Consequently, treatment of pancreatic exocrine insufficiency was started with lipase, amylase and protease. This restored serum levels of calcium, phosphorus and vitamin D with a beneficial effect on bone mineral density (BMD).
This case study illustrates an important complication after bariatric surgery, often misdiagnosed and not linked to the surgery that was performed a few years earlier. This can lead to wrong treatments, such as bisphosphonates, inhibiting bone resorption, which in this case could lead to hypocalcemia. This highlights the need for knowledge on long-term complications arising after bariatric surgery and the need for post-surgical follow-up and attention for these issues.
2. Introduction
Obesity and overweight are defined as abnormal or excessive fat accumulation that often impairs health. Frequent health consequences are cardiovascular diseases, diabetes, musculoskeletal disorders and cancers. Over the last decades, obesity has risen to epidemic proportions and poses a health threat for the individual patient as well as a major socio-economic burden (World Health Organization, 2017). Bariatric or weight-loss surgery has proven to be a valuable treatment option for morbid obesity as it leads to manifest and sustainable weight reduction and improves more than 40 obesity-related diseases or conditions (Buchwald et al., 2004; American Society for Metabolic and Bariatric Surgery, 2010). Historically, bariatric surgeries have been divided into three categories: restrictive, malabsorptive and the combination of these two. Restrictive procedures, such as Sleeve Gastrectomy (SG), intentionally alter the anatomy of the gastrointestinal tract to reduce the amount of food intake. Following malabsorptive surgery, the amount of lipids and other nutrients that can be absorbed is reduced. Roux-en-Y Gastric Bypass (RYGB) is a combination of restrictive and malabsorptive surgery. Because of the limited food intake and/or malabsorption, bariatric surgery can indirectly induce nutritional deficiencies. The most common nutritional deficiencies after bariatric surgery include calcium, copper, folate, iron, vitamin B12, vitamin D and zinc. These deficiencies can lead to various complications such as osteoporosis, anemia, neurologic problems, fatigue and generalized weakness. In particular, changes in calcium and vitamin D handling following bariatric surgery can induce bone loss, eventually resulting in higher fracture risk (Rousseau et al., 2016).
Bone loss is observed to some extent after every type of bariatric surgery, and several mechanisms have been proposed, which are mutually non-exclusive. The most important mechanisms are mechanical unloading of the skeleton, intestinal malabsorption of calcium and vitamin D (Wucher et al., 2008) and hormonal changes in response to the reduced caloric intake and the resulting energy deficit after surgery. These hormonal changes include reduced sex steroid production (testosterone and oestradiol) (Sainsbury and Zhang, 2012) and alterations in the secretion of gut-derived (Glucagon-like peptide 1 (GLP-1), peptide YY (PYY) and ghrelin) and adipocytic (leptin and adiponectin) hormones (Sainsbury and Zhang, 2012; Brzozowska et al., 2013).
In this review, we will provide a comprehensive overview of alterations in bone metabolism following the currently most performed bariatric procedures, namely SG and RYGB. First, we will discuss the consequences of SG and RYGB on bone mass and risk of fractures, based on pre-clinical and clinical data. Next, we will discuss whether changes in bone resorption or rather formation lead to bone loss after surgery and what the possible underlying mechanisms are.
3. Effect of bariatric surgery on BMD and fractures
Several clinical studies have reported lower BMD after bariatric surgery, in particular when the duodenum is bypassed, like in RYGB (Fig. 1). Indeed, the duodenum is the site where active calcium transport can be enhanced to achieve sufficient calcium absorption when dietary calcium intake is low. After RYGB, bone loss is consistently observed during the first two years (Campanha-Versiani et al., 2017; Frederiksen et al., 2016; Obinwanne et al., 2014; Casagrande et al., 2012; Shanbhogue et al., 2017; Yu et al., 2015; Bazzocchi et al., 2015; Kaulfers et al., 2011; Maghrabi et al., 2015; Carrasco et al., 2014; Vilarrasa et al., 2013; Muschitz et al., 2016; Hsin et al., 2015; Muschitz et al., 2015; Bredella et al., 2017). Longer follow-up for three to six years still showed persistent bone loss, although the number of studies is limited (Rousseau et al., 2016; Vilarrasa et al., 2013; Elias et al., 2014; Raoof et al., 2016). After SG, which only reduces the stomach volume, it is generally considered that the effects on the skeleton would be less pronounced than after RYGB. However, recent studies seem to refute this hypothesis as not only RYGB, but also SG appears to have detrimental effects on bone density. It is, however, important to note that at this moment the studies on SG are still limited, both in terms of duration of follow-up and sample size (Maghrabi et al., 2015; Carrasco et al., 2014; Vilarrasa et al., 2013; Muschitz et al., 2016; Hsin et al., 2015; Muschitz et al., 2015; Pluskiewicz et al., 2012; Adamczyk et al., 2015a; Adamczyk et al., 2015b; Ruiz-Tovar et al., 2013). Nevertheless, most studies on SG reported decreased BMD (Maghrabi et al., 2015; Carrasco et al., 2014; Vilarrasa et al., 2013; Muschitz et al., 2016; Hsin et al., 2015; Muschitz et al., 2015; Pluskiewicz et al., 2012; Adamczyk et al., 2015a), whereas two studies described an opposite trend with increased BMD (Adamczyk et al., 2015b; Ruiz-Tovar et al., 2013). A possible explanation for this discrepancy is that Adamczyk et al. investigated solely men, and this observation might indicate sex-dependent responses (Adamczyk et al., 2015b).
Fig. 1.
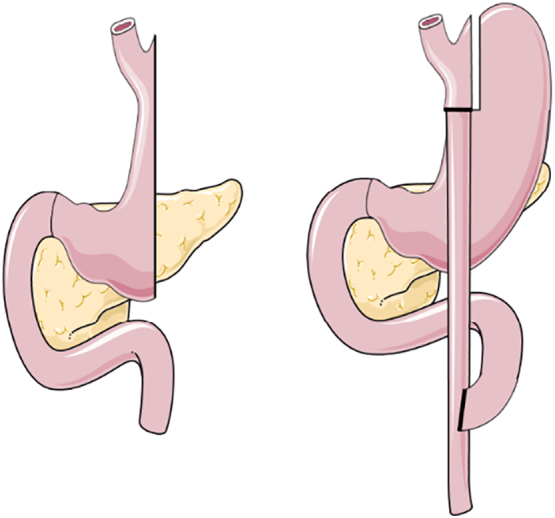
Sleeve Gastrectomy (left) and Roux-en-Y Gastric Bypass (right).
At present, most studies focused on BMD and much less on fracture risk, which is, however, an important clinical outcome. Only a limited number of studies have followed up fracture risk after bariatric surgery on the long-term, until 12 to 14 years (Rousseau et al., 2016; Nakamura et al., 2014; Lu et al., 2015). Although all three studies used different control groups, comprising comparison to the general population (Nakamura et al., 2014), obese individuals (Lu et al., 2015) or both obese and non-obese persons (Rousseau et al., 2016), they consistently showed an increased fracture risk ranging from 1.2 to 2.3 fold. Interestingly, two studies that followed mainly patients after adjusted gastric banding for 4 to 5 years, found no relation with fracture risk or only an increased trend, when compared to a matched control group (Lalmohamed et al., 2012) or obese control group (Douglas et al., 2015). These findings indicate that the restrictive procedure of gastric banding likely induces less harmful effects than malabsorption procedures (Lalmohamed et al., 2012; Douglas et al., 2015).
Research in animal models supports the findings in humans. Studies in rats (Abegg et al., 2013; Stemmer et al., 2013; Canales et al., 2014) and mice (Yu et al., 2016a) found manifest bone loss after RYGB, even when comparing to body weight-matched animals (Abegg et al., 2013). With respect to SG, relatively little is known in animals, although some data suggests limited to no bone loss, in contrast to RYGB (Stemmer et al., 2013).
Taken together, most clinical and preclinical studies point towards bone loss and increased fracture risk after RYGB and SG, which is, however, less pronounced after purely restrictive procedures such as banding.
4. Bone loss after bariatric surgery: resorption versus formation
Bone is continuously remodeled during adult life by local cycles of bone resorption followed by bone formation. In order to preserve bone mass, bone resorption has to be balanced by bone formation and bone loss occurs when bone resorption outpaces bone formation. Each of these two processes can be assessed by analyzing specific bone turnover markers in serum. To assess bone formation, osteocalcin (OC) and N-terminal propeptide of type 1 procollagen (P1NP) are used as biomarkers. OC is a bone-specific protein produced by active, mature osteoblasts when they synthesize bone matrix, and increased OC levels therefore correlate with bone formation (Hauschka et al., 1989). Although OC remains a good marker for bone formation, it primarily functions as a hormone by stimulating β-cells to release insulin and white adipocytes to produce adiponectin, which on its turn increases the sensitivity to insulin (Karsenty and Ferron, 2012). The bone formation marker P1NP also reflects bone matrix formation, as it is cleaved from type 1 procollagen during its extracellular processing and assembling into collagen fibrils, which are then incorporated into the bone matrix. A useful marker for bone resorption is C-terminal telopeptide of type I collagen (CTX-1), as it is formed by proteolytic cleavage of collagen when osteoclasts degrade the bone matrix (Szulc et al., 2017). Another marker of bone resorption is Tartrate-resistant acid phosphatase (TRACP) 5b. This form of TRACP enzyme is highly expressed in osteoclasts (Halleen et al., 2006). These serum markers help to evaluate the degree of bone resorption versus bone formation after bariatric surgery and assist to determine the types of changes in bone turnover that lead to bone loss. For RYGB, numerous clinical studies showed major increases in bone resorption markers such as CTX and TRACP 5b, but only small increases in bone formation markers P1NP and OC (Obinwanne et al., 2014; Casagrande et al., 2012; Shanbhogue et al., 2017; Yu et al., 2015; Muschitz et al., 2015; Bredella et al., 2017; Elias et al., 2014; Yu et al., 2016b; Ivaska et al., 2017; Stein et al., 2013; von Mach et al., 2004; Coates et al., 2004; Riedt et al., 2006; Riedl et al., 2008; Fleischer et al., 2008; Carlin et al., 2009; Bruno et al., 2010; Yu et al., 2014; Sinha et al., 2011; Yu, 2014; Biagioni et al., 2017; Hofsø et al., 2016). After SG, an increase in resorption markers has also been noticed, however this was less pronounced than after RYGB (Muschitz et al., 2015; Bredella et al., 2017; Ivaska et al., 2017; Stein et al., 2013; Schollenberger et al., 2015). Taken together, the present studies point towards a high bone turnover with unbalanced increased bone resorption after bariatric surgery.
5. Potential mechanisms underlying bone loss after bariatric surgery and possible therapeutic targets
In the next section, four possible mechanisms underlying bone loss after bariatric surgery will be discussed. First, the effect of mechanical unloading will be considered as weight loss is substantial after surgery, thereby decreasing mechanical loading. Secondly, the contribution of calcium and vitamin D malabsorption, with effects on parathyroid hormone (PTH) will be discussed. Thirdly, the role of the local Wnt signaling on bone mass will be considered. Lastly, the connection with adipose tissue, either through changes in adipokines or local alterations in the bone marrow, will be reviewed.
5.1. Mechanical unloading
After bariatric surgery, body weight decreases up to 30%, indicating that the mechanical load on the skeleton is also reduced. Theoretically, this lower mechanical load can lead to less bone formation, increased bone resorption and thus decreased BMD, as has been observed in other models of mechanical unloading (Komori, 2015). However, weight loss-induced bone loss is an adaptive response and might thus not evolve to a pathological level. In addition, even when mechanical loading decreases BMD, it might not result in increased fracture risk as the load on the skeleton also decreases. Pre-clinical data also question the contribution of weight loss-related mechanical unloading, since animals after bariatric surgery have a lower bone mass even when compared to weight-matched animals, indicating that the weight loss per se is not responsible for the lower bone mass (Abegg et al., 2013). Therefore, it is unlikely that unloading-induced bone loss is avoidable or even necessary to circumvent.
5.2. Calcium and vitamin D handling
5.2.1. Intestinal malabsorption of calcium and vitamin D
Because of the alterations in intestinal anatomy induced by bariatric surgery, malabsorption of calcium and vitamin D is assumed, an effect that may contribute to bone loss. Calcium plays an essential role in processes such as muscle contraction, protein secretion, blood clotting and neuronal excitability. Serum calcium levels are therefore very tightly regulated by an interplay between the intestine, kidneys, parathyroid glands and the skeleton. The intestine is responsible for adequate absorption of calcium after oral intake, whereas the kidneys contribute to serum calcium levels by the reabsorption of filtered calcium. When intestinal and renal calcium (re)absorption is insufficient, calcium will be released from the skeleton, as it functions as a calcium reservoir, in order to maintain normal serum calcium levels. Unfortunately, the increased bone resorption needed to preserve serum calcium homeostasis will deteriorate bone quality and mass, with increased fracture risk as a consequence.
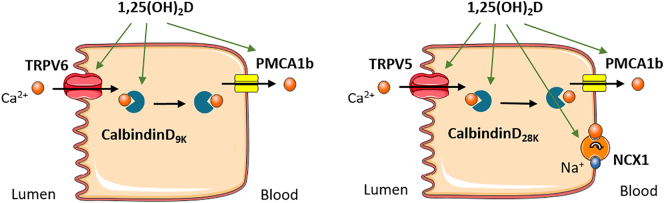
Intestinal calcium transport occurs through an active, saturable, transcellular, energy-dependent pathway as well as a passive, non-saturable, paracellular, diffusional pathway (Fleet and Schoch, 2010). In case of high calcium intake, absorption mainly occurs through passive transport, viewed as calcium diffusing through tight junctions between the cells. The amount of calcium absorbed by this pathway is directly related to the calcium concentration within the intestinal lumen and the contact time between calcium and intestinal cells (Fleet and Schoch, 2010). However, when calcium intake is normal to low, the active transcellular transport predominates. This transport mechanism consists of three steps. First, calcium enters into the cell through the transient receptors potential vanilloid type 6 (TRPV6). Subsequently, calcium is transported across the cytosol by binding to calbindin-D9k and finally, calcium moves out the cell through the plasma membrane calcium ATPase (PMCA1b) into the blood (Fig. 2, left) (Bronner et al., 1986; Christakos et al., 2016). This active calcium transport is regulated by 1,25-dihydroxyvitamin D (1,25(OH)2D), the active form of vitamin D. Renal calcium transport occurs in a similar manner, but with different transporters (Fig. 2, right).
Fig. 2.
Intestinal (left) and renal (right) calcium (re)absorption. Calcium is taken up by the cell through TRPV6 or TRPV5, transported in the cytoplasm bound to calbindinD9k and transported across the basolateral membrane into the blood by PMCA1b or NCX1.
Since bariatric surgery alters the anatomy of the gastrointestinal tract, it has a major influence on the biological availability of all nutrients, including calcium and vitamin D. Both, SG and RYGB lead to decreased acid secretion, which influences the disintegration and solubility of nutritional components. Additionally, RYGB influences the absorption capacity for calcium since the duodenum is bypassed and most of the active transporters for calcium are located in the duodenum and jejunum (Fleet and Schoch, 2010). Also after SG, the contact time with the intestinal mucosa is shortened, which thus may hamper nutrient absorption (Fig. 1) (Chakhtoura et al., 2016; Aarts et al., 2011). In addition, food and supplements do not mix with bile and pancreatic enzymes until the intestines join in the common channel after RYGB. This altered anatomy has major consequences for the absorption of vitamin D as it is a fat-soluble hormone and hence needs biliary acids and digestive enzymes for uptake. Moreover, vitamin D deficiency is extremely prevalent in candidates for bariatric surgery, and this pre-surgery deficiency further worsens after RYGB and according to some studies also after SG (Chakhtoura et al., 2016; Aarts et al., 2011; Van der Schueren et al., 2011).
To investigate the effect of the surgery-induced new anatomical situation on calcium absorption, fractional calcium absorption is used. This technique measures the percentage of an orally given dose of calcium that is absorbed. Schafer et al. recently investigated calcium absorption 6 months after RYGB surgery using dual stable isotope methodology (Schafer et al., 2015a). Despite maintaining 25(OH)D serum levels above 30 ng/ml and calcium intake at 1200 mg daily, fractional calcium absorption decreased from 33 ± 14% before surgery to 7 ± 4% after surgery, indicating a manifest reduction in the absolute amount of calcium that is daily absorbed, decreasing from 392 ± 168 mg to 82 ± 45 mg (Schafer et al., 2015a). Accordingly, 24 hour urinary calcium decreased from 191 mg to 109 mg.
In an effort to elucidate intestinal molecular mechanisms after bariatric surgery, Elias et al. investigated jejunal mucosa biopsies in patients 6 to 8 months after RYGB and vertical banded gastroplasty, who did not receive calcium supplements (Elias et al., 2014). This study revealed decreased expression of TRPV6, possibly due to reduced protein levels of heat-shock protein 90β, a co-activator of the vitamin D receptor, whereas vitamin D receptor levels were increased. These findings are consistent with the reduced calcium absorption observed at early time points after surgery (6 months, (Schafer et al., 2015a)). Nonetheless, over time, the gut may compensate for the decreased ability to absorb calcium. Indeed, TRPV6 mRNA levels were strongly increased in the jejunum and ileum of rats, 16 weeks after RYGB, which is a rather late time point (Abegg et al., 2013).
Taken together, these data suggest that adaptations in intestinal absorption occur after SG and RYGB and that it is important to elucidate the mechanisms underlying these changes. Investigating intestinal calcium absorption in detail is difficult in patients, as only minimally invasive techniques are available. Animal models can likely provide more thorough mechanistic insight into the temporal changes in intestinal calcium and vitamin D absorption that occur after bariatric surgery and that may contribute to bone loss.
5.2.2. PTH and 1,25(OH)2D
Since normal serum calcium levels are critical for several processes, they are tightly regulated involving mainly PTH and 1,25(OH)2D signaling (Fig. 3). When serum calcium levels drops, the parathyroid glands will secrete PTH. Subsequently, PTH stimulates the osteoblasts to produce the osteoclastogenic factor Receptor activator of nuclear factor kappa-β ligand (RANKL), which binds to RANK expressed on osteoclasts, thereby promoting osteoclast differentiation and survival. Osteoclasts will resorb the bone matrix and release calcium to maintain serum calcium levels in the normal range, but this occurs at the expenses of bone mass (Christakos et al., 2016). In addition, PTH activates the enzyme 1α-hydroxylase (CYP27B1) in the kidneys, which hydroxylates the inactive form 25(OH)D to its active form 1,25(OH)2D (Christakos et al., 2016; Jones et al., 2014; Holick, 2016). 1,25(OH)2D mediates its actions through binding to the Vitamin D receptor (VDR). As a negative feedback, 1,25(OH)2D suppresses PTH synthesis and reduces its own production by inhibiting CYP27B1. It also stimulates the enzyme CYP24A1, which degrades 1,25(OH)2D and thus limits the amount of free circulating active vitamin D. In the intestine, 1,25(OH)2D stimulates calcium absorption by increasing the expression of mainly TRPV6, calbindin-D9K and possibly PMCA1b (Fig. 2). When the supply of calcium is normal, 1,25(OH)2D has no major effect on bone mass. Besides the reciprocal regulation between 1,25(OH)2D and PTH, a comparable interaction exists between 1,25(OH)2D and Fibroblast growth factor 23 (FGF23). Indeed, 1,25(OH)2D induces FGF23 expression by osteocytes and osteoblasts. FGF23 acts as an endocrine factor by promoting renal phosphate excretion which in turn can influence PTH and calcium serum levels (Christakos et al., 2016). FGF23 signaling also inhibits CYP27B1 expression and induces CYP24A1, thereby decreasing 1,25(OH)2D levels and avoiding calcium levels to rise too high (Hu et al., 2013). Together, these mechanisms allow a tight regulation of serum calcium levels.
Fig. 3.
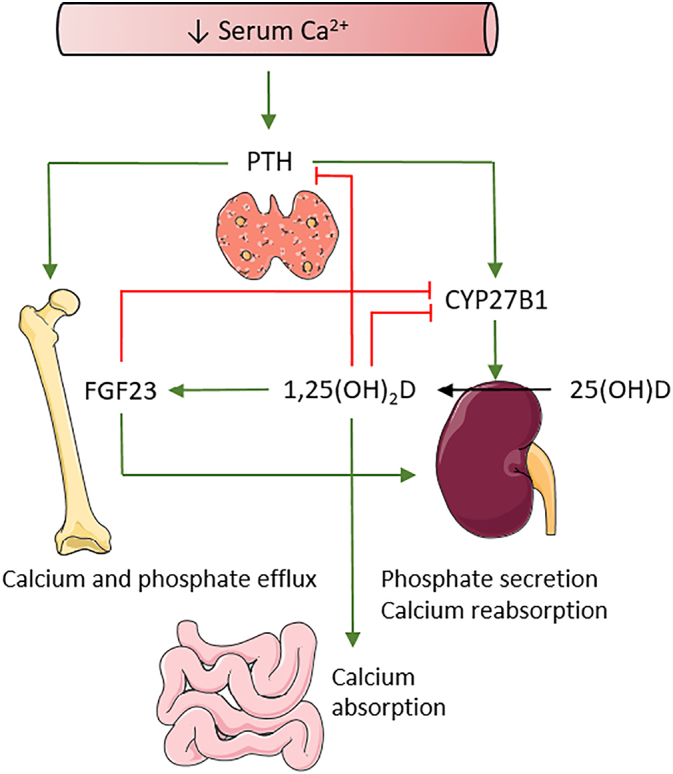
Calcium and vitamin D homeostasis. PTH and 1,25(OH)2D tightly regulate serum calcium levels. When calcium levels drop, PTH is secreted by the parathyroid glands. PTH will have its effects on kidneys and bone to increase calcium levels in the serum. Red lines indicate inhibitory effects, green lines indicate stimulatory effects.
Several clinical studies show a high prevalence of secondary hyperparathyroidism (SHPT) after bariatric surgery, which is believed to be caused by intestinal calcium malabsorption (Ybarra et al., 2005; Youssef et al., 2007; DiGiorgi et al., 2008; Clements et al., 2008; Compher et al., 2008; Valderas et al., 2009; Signori et al., 2010; Søvik et al., 2011; Grethen et al., 2011; Hewitt et al., 2013). However, the control groups in those studies often consist of normal weight individuals and not weight-matched controls which may also show high PTH levels. Indeed, a few of these studies report high PTH levels already before surgery, which would suggest that it could be obesity per se, more than the malabsorption that induces SHPT. Additionally, declines in BMD after surgery have been reported in multiple studies, even without increased PTH levels (Shanbhogue et al., 2017; Yu et al., 2015; Vilarrasa et al., 2013; Bredella et al., 2017; von Mach et al., 2004; Yu et al., 2014), further questioning whether SHPT is truly responsible for bone loss following bariatric surgery. Information on surgery-induced changes in FGF23 levels is very limited with only one study showing an increase in FGF23 after bariatric surgery in women (Grethen et al., 2012).
Animal studies did not yet increase our insight in the importance and contribution of SHPT in the observed bone loss following surgery. Rodent studies did not find significant differences in PTH levels between bariatric groups and controls (Abegg et al., 2013; Stemmer et al., 2013; Canales et al., 2014; Yu et al., 2016a). On the other hand, Abegg et al. observed a strong induction of CYP27B1 and inhibition of CYP24A1 expression, which resulted in increased 1,25(OH)2D levels, which theoretically may suppress PTH and avoid SHPT (Abegg et al., 2013).
Taken together, these findings suggest that although intestinal calcium absorption is decreased after bariatric surgery, other mechanisms than increased PTH levels seem to contribute to the observed bone loss, but the exact mechanisms remain elusive. It would be interesting to follow PTH levels longitudinally, starting from the pre-operative stage and analyzing several time points after surgery to exclude or not contribution of PTH to bone loss. At this moment, simply targeting PTH levels to prevent bone loss or titrate supplementation of calcium and vitamin D might therefore not be sufficient.
5.3. Wnt-signaling
The function of skeletal cells is not only regulated by hormones, but also by locally produced factors like regulators of Wnt signaling. This pathway affects osteoblast number, maturation and differentiation, and is inhibited by osteoblast or osteocyte secreted factors such as sclerostin and Dickkopf-1 (DKK-1). Sclerostin, encoded by the SOST gene, is produced by osteocytes and inhibits osteoblast differentiation (Muschitz et al., 2015; Viapiana et al., 2013). In mice, Sclerostin levels are down-regulated by mechanical loading and by PTH (Bellido et al., 2005; Jia et al., 2017). DKK-1 has been associated with bone resorption and osteopenia (Li et al., 2006; Li et al., 2011). Studies examining the effect of bariatric surgery on Wnt-signaling, show conflicting results. Two studies reporting decreased BMD, also found an increase of sclerostin, but, surprisingly, also a decrease of DKK-1 after SG or RYGB (Muschitz et al., 2016; Muschitz et al., 2015). Another study did not find different sclerostin levels after RYGB (Grethen et al., 2012), but BMD was not measured. Taken together, although Wnt signaling is an important pathway regulating bone mass, its contribution to bariatric surgery-induced bone loss remains elusive.
5.4. Link with adipose tissues
5.4.1. Adipokines
Bariatric surgery has a profound impact on the body fat mass and will consequently affect adipocyte-secreted hormones, i.e. adipokines. Leptin can affect bone mass by locally inducing bone formation and by a central nervous relay that activates the sympathetic nervous system (Wucher et al., 2008) and cocaine- and amphetamine-regulated transcript (CART) signaling in the hypothalamus. Studies on the role of leptin in mice resulted in discordant observations (Karsenty and Oury, 2012). A possible explanation to reconcile the data is the model stating that leptin has a complex, dual effect on the skeleton leading to increased cortical bone, but to decreased trabecular bone (Hamrick and Ferrari, 2008). In humans, a positive association of leptin and a negative association of adiponectin with BMD have been demonstrated (Biver et al., 2011; Blain et al., 2002; Pasco et al., 2001). Recent studies in mice have further elucidated that adipokines not only affect the function of skeletal cells and thereby regulate bone mass, but also control systemic mineral homeostasis. Indeed, leptin can directly stimulate FGF23 synthesis in osteocytes, whereas adiponectin decreases FGF23 release (Fig. 4) (Tsuji et al., 2010; Rutkowski et al., 2017). On the other hand, 1,25(OH)2D signals to adipocytes and can enhance the production of leptin and adiponectin. Consistent herewith, vitamin D3 deficiency is associated with low leptin levels (Wagner et al., 2017). After SG and RYGB, serum leptin levels decrease due to loss of adipose tissue (Shanbhogue et al., 2017; Biagioni et al., 2017; Grethen et al., 2012), whereas adiponectin levels increase (Shanbhogue et al., 2017; Carrasco et al., 2014; Biagioni et al., 2017; Grethen et al., 2012; Carrasco et al., 2009). BMD was decreased in the limited number of studies in which BMD and adipokines were measured together (Shanbhogue et al., 2017; Carrasco et al., 2014; Carrasco et al., 2009). Taken together, some correlations between levels of adipokines and bone mass after surgery have been reported, but further research is needed to explore the contribution of adipokines to surgery-induced bone loss and to hormonal changes related to mineral homeostasis.
Fig. 4.
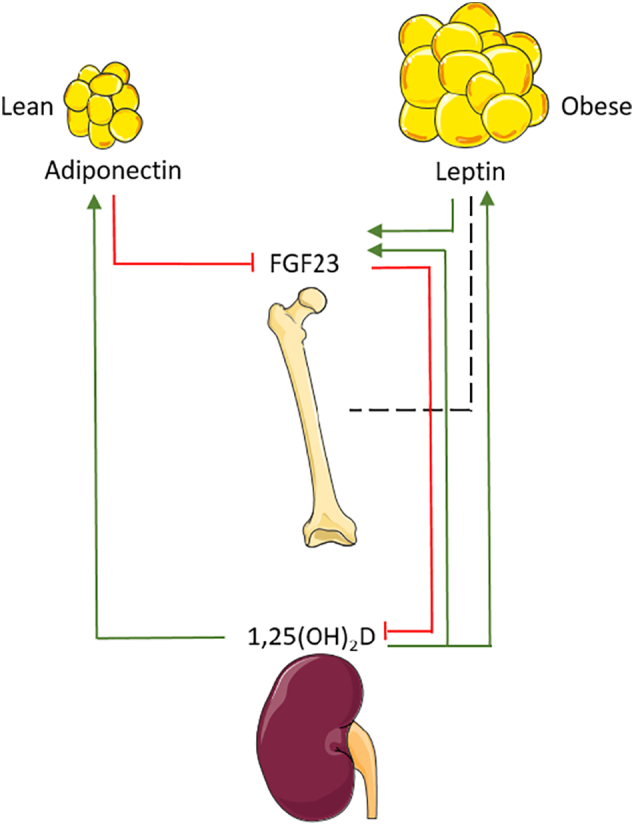
The relationship between adipose tissue and bone homeostasis. Lean white adipose tissue produces more adiponectin. With obesity, adipose tissue expands and more leptin will be produced. These hormones are influenced by factors from the bone homeostasis and vice versa, as indicated by the lines. Red lines indicate inhibitory effects, green lines indicate stimulatory effects. The black dashed line indicates the relationship between bone and leptin, which is not entirely clear yet.
5.4.2. Paracrine effects: local interaction between bone marrow adipose tissue and bone cells
Recently, another fat depot than white adipose tissue (WAT), has gained attention as a potential mediator of bone loss after bariatric surgery: bone marrow adipose tissue (BMAT). This hypothesis is based on research in women with anorexia nervosa in which higher levels of BMAT have been found compared to controls, although peripheral WAT is lost (Bredella et al., 2009). In mice, caloric restriction resulted in high levels of BMAT as well (Devlin et al., 2010). These findings indicate that BMAT is differentially regulated from visceral WAT and led to the hypothesis that it is used as an energy depot in the setting of starvation (Scheller and Rosen, 2014). Over the last years, BMAT has been suggested to negatively affect bone strength (Schellinger et al., 2001). Six months after RYGB, 6 diabetic and 5 nondiabetic patients showed decreased BMD with no differences between diabetic and nondiabetic patients (Schafer et al., 2015b). Interestingly, BMAT was decreased in diabetic patients, but not in the nondiabetic patients, although the overall fat mass decreased in all patients. Two other studies did not find any difference in BMAT 6 and 12 months after RYGB (Bredella et al., 2017; Ivaska et al., 2017), although BMD was decreased after 12 months (Bredella et al., 2017). One year after SG, an increase in BMAT was observed associated with a decrease in BMD (Bredella et al., 2017), while, another study did not report these effects after 6 months (Ivaska et al., 2017). More extensive research is necessary to unravel the effect of bone marrow fat after surgery.
6. Conclusion
Over the last decades, bariatric surgery has become a very important treatment for obesity. It leads to sustained weight loss and strongly reduces the risk for obesity-associated comorbidities. However, it can also lead to nutritional deficiencies of which the long-term consequences are not fully known yet. Bone loss and increased fracture risk is often observed after bariatric surgery and requires adequate therapy. Mechanistically, calcium malabsorption and vitamin D deficiency may contribute to bone loss by inducing secondary hyperparathyroidism that increases bone resorption. However, increased PTH levels are not always detected, suggesting that also other mechanisms are involved. Possible other mechanisms that need to be explored include Wnt signaling and the connection with adipose tissue. In addition, future research needs to focus on the long-term adaptations of the body in response to the anatomical and physiological changes of bariatric surgery. Hopefully, a better understanding of the impact of bariatric surgery on the bone will indicate how to prevent or cure the bone loss.
Conflict of interest statements
The authors do not have any conflicts of interests to disclose.
Funding sources
This work was supported by the FWO (Flemish Research Council) 1S27317N and 1802714N.
References
- Aarts E.O., Janssen I.M., Berends F.J. The gastric sleeve: losing weight as fast as micronutrients? Obes. Surg. 2011;21(2):207–211. doi: 10.1007/s11695-010-0316-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abegg K., Gehring N., Wagner C.A., Liesegang A., Schiesser M., Bueter M. Roux-en-Y gastric bypass surgery reduces bone mineral density and induces metabolic acidosis in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013;305(9):R999–R1009. doi: 10.1152/ajpregu.00038.2013. [DOI] [PubMed] [Google Scholar]
- Adamczyk P., Bužga M., Holéczy P., Švagera Z., Zonča P., Sievänen H. Body size, bone mineral density, and body composition in obese women after laparoscopic sleeve gastrectomy: a 1-year longitudinal study. Horm. Metab. Res. 2015;47(12):873–879. doi: 10.1055/s-0035-1555758. [DOI] [PubMed] [Google Scholar]
- Adamczyk P., Bužga M., Holéczy P., Švagera Z., Šmajstrla V., Zonča P. Bone mineral density and body composition after laparoscopic sleeve gastrectomy in men: a short-term longitudinal study. Int. J. Surg. 2015;23(Pt A):101–107. doi: 10.1016/j.ijsu.2015.09.048. [DOI] [PubMed] [Google Scholar]
- American Society for Metabolic and Bariatric Surgery Fact Sheet: Metabolic and Bariatric Surgery. 2010. http://asmbs.org/
- Bazzocchi A., Ponti F., Cariani S., Diano D., Leuratti L., Albisinni U. Visceral fat and body composition changes in a female population after RYGBP: a two-year follow-up by DXA. Obes. Surg. 2015;25(3):443–451. doi: 10.1007/s11695-014-1422-8. [DOI] [PubMed] [Google Scholar]
- Bellido T., Ali A.A., Gubrij I., Plotkin L.I., Fu Q., O'Brien C.A. Chronic elevation of parathyroid hormone in mice reduces expression of sclerostin by osteocytes: a novel mechanism for hormonal control of osteoblastogenesis. Endocrinology. 2005;146(11):4577–4583. doi: 10.1210/en.2005-0239. [DOI] [PubMed] [Google Scholar]
- Biagioni M.F.G., Mendes A.L., Nogueira C.R., Leite C.V., Gollino L., Mazeto G.M. Bariatric Roux-en-Y gastric bypass surgery: adipocyte proteins involved in increased bone remodeling in humans. Obes. Surg. 2017;27(7):1789–1796. doi: 10.1007/s11695-017-2546-4. [DOI] [PubMed] [Google Scholar]
- Biver E., Salliot C., Combescure C., Gossec L., Hardouin P., Legroux-Gerot I. Influence of adipokines and ghrelin on bone mineral density and fracture risk: a systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2011;96(9):2703–2713. doi: 10.1210/jc.2011-0047. [DOI] [PubMed] [Google Scholar]
- Blain H., Vuillemin A., Guillemin F., Durant R., Hanesse B., de Talance N. Serum leptin level is a predictor of bone mineral density in postmenopausal women. J. Clin. Endocrinol. Metab. 2002;87(3):1030–1035. doi: 10.1210/jcem.87.3.8313. [DOI] [PubMed] [Google Scholar]
- Bredella M.A., Fazeli P.K., Miller K.K., Misra M., Torriani M., Thomas B.J. Increased bone marrow fat in anorexia nervosa. J. Clin. Endocrinol. Metab. 2009;94(6):2129–2136. doi: 10.1210/jc.2008-2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredella M.A., Greenblatt L.B., Eajazi A., Torriani M., Yu E.W. Effects of Roux-en-Y gastric bypass and sleeve gastrectomy on bone mineral density and marrow adipose tissue. Bone. 2017;95:85–90. doi: 10.1016/j.bone.2016.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronner F., Pansu D., Stein W.D. An analysis of intestinal calcium transport across the rat intestine. Am. J. Phys. 1986;250(5 Pt 1):G561–9. doi: 10.1152/ajpgi.1986.250.5.G561. [DOI] [PubMed] [Google Scholar]
- Bruno C., Fulford A.D., Potts J.R., McClintock R., Jones R., Cacucci B.M. Serum markers of bone turnover are increased at six and 18 months after Roux-en-Y bariatric surgery: correlation with the reduction in leptin. J. Clin. Endocrinol. Metab. 2010;95(1):159–166. doi: 10.1210/jc.2009-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzozowska M.M., Sainsbury A., Eisman J.A., Baldock P.A., Center J.R. Bariatric surgery, bone loss, obesity and possible mechanisms. Obes. Rev. 2013;14(1):52–67. doi: 10.1111/j.1467-789X.2012.01050.x. [DOI] [PubMed] [Google Scholar]
- Buchwald H., Avidor Y., Braunwald E., Jensen M.D., Pories W., Fahrbach K. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292(14):1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- Campanha-Versiani L., Pereira D.A., Ribeiro-Samora G.A., Ramos A.V., de Sander Diniz M.F., De Marco L.A. The effect of a muscle weight-bearing and aerobic exercise program on the body composition, muscular strength, biochemical markers, and bone mass of obese patients who have undergone gastric bypass surgery. Obes. Surg. 2017;27(8):2129–2137. doi: 10.1007/s11695-017-2618-5. [DOI] [PubMed] [Google Scholar]
- Canales B.K., Schafer A.L., Shoback D.M., Carpenter TO Gastric bypass in obese rats causes bone loss, vitamin D deficiency, metabolic acidosis, and elevated peptide YY. Surg. Obes. Relat. Dis. 2014;10(5):878–884. doi: 10.1016/j.soard.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin A.M., Rao D.S., Yager K.M., Parikh N.J., Kapke A. Treatment of vitamin D depletion after Roux-en-Y gastric bypass: a randomized prospective clinical trial. Surg. Obes. Relat. Dis. 2009;5(4):444–449. doi: 10.1016/j.soard.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Carrasco F., Ruz M., Rojas P., Csendes A., Rebolledo A., Codoceo J. Changes in bone mineral density, body composition and adiponectin levels in morbidly obese patients after bariatric surgery. Obes. Surg. 2009;19(1):41–46. doi: 10.1007/s11695-008-9638-0. [DOI] [PubMed] [Google Scholar]
- Carrasco F., Basfi-Fer K., Rojas P., Valencia A., Csendes A., Codoceo J. Changes in bone mineral density after sleeve gastrectomy or gastric bypass: relationships with variations in vitamin D, ghrelin, and adiponectin levels. Obes. Surg. 2014;24(6):877–884. doi: 10.1007/s11695-014-1179-0. [DOI] [PubMed] [Google Scholar]
- Casagrande D.S., Repetto G., Mottin C.C., Shah J., Pietrobon R., Worni M. Changes in bone mineral density in women following 1-year gastric bypass surgery. Obes. Surg. 2012;22(8):1287–1292. doi: 10.1007/s11695-012-0687-z. [DOI] [PubMed] [Google Scholar]
- Chakhtoura M.T., Nakhoul N.N., Shawwa K., Mantzoros C., El Hajj Fuleihan G.A. Hypovitaminosis D in bariatric surgery: a systematic review of observational studies. Metabolism. 2016;65(4):574–585. doi: 10.1016/j.metabol.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christakos S., Dhawan P., Verstuyf A., Verlinden L., Carmeliet G. Vitamin D: metabolism, molecular mechanism of action, and pleiotropic effects. Physiol. Rev. 2016;96(1):365–408. doi: 10.1152/physrev.00014.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements R.H., Yellumahanthi K., Wesley M., Ballem N., Bland K.I. Hyperparathyroidism and vitamin D deficiency after laparoscopic gastric bypass. Am. Surg. 2008;74(6):469–474. (discussion 74-5) [PubMed] [Google Scholar]
- Coates P.S., Fernstrom J.D., Fernstrom M.H., Schauer P.R., Greenspan S.L. Gastric bypass surgery for morbid obesity leads to an increase in bone turnover and a decrease in bone mass. J. Clin. Endocrinol. Metab. 2004;89(3):1061–1065. doi: 10.1210/jc.2003-031756. [DOI] [PubMed] [Google Scholar]
- Compher C.W., Badellino K.O., Boullata J.I. Vitamin D and the bariatric surgical patient: a review. Obes. Surg. 2008;18(2):220–224. doi: 10.1007/s11695-007-9289-6. [DOI] [PubMed] [Google Scholar]
- Devlin M.J., Cloutier A.M., Thomas N.A., Panus D.A., Lotinun S., Pinz I. Caloric restriction leads to high marrow adiposity and low bone mass in growing mice. J. Bone Miner. Res. 2010;25(9):2078–2088. doi: 10.1002/jbmr.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiGiorgi M., Daud A., Inabnet W.B., Schrope B., Urban-Skuro M., Restuccia N. Markers of bone and calcium metabolism following gastric bypass and laparoscopic adjustable gastric banding. Obes. Surg. 2008;18(9):1144–1148. doi: 10.1007/s11695-007-9408-4. [DOI] [PubMed] [Google Scholar]
- Douglas I.J., Bhaskaran K., Batterham R.L., Smeeth L. Bariatric surgery in the United Kingdom: a cohort study of weight loss and clinical outcomes in routine clinical care. PLoS Med. 2015;12(12) doi: 10.1371/journal.pmed.1001925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias E., Casselbrant A., Werling M., Abegg K., Vincent R.P., Alaghband-Zadeh J. Bone mineral density and expression of vitamin D receptor-dependent calcium uptake mechanisms in the proximal small intestine after bariatric surgery. Br. J. Surg. 2014;101(12):1566–1575. doi: 10.1002/bjs.9626. [DOI] [PubMed] [Google Scholar]
- Fleet J.C., Schoch R.D. Molecular mechanisms for regulation of intestinal calcium absorption by vitamin D and other factors. Crit. Rev. Clin. Lab. Sci. 2010;47(4):181–195. doi: 10.3109/10408363.2010.536429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischer J., Stein E.M., Bessler M., Della Badia M., Restuccia N., Olivero-Rivera L. The decline in hip bone density after gastric bypass surgery is associated with extent of weight loss. J. Clin. Endocrinol. Metab. 2008;93(10):3735–3740. doi: 10.1210/jc.2008-0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederiksen K.D., Hanson S., Hansen S., Brixen K., Gram J., Jørgensen N.R. Bone structural changes and estimated strength after gastric bypass surgery evaluated by HR-pQCT. Calcif. Tissue Int. 2016;98(3):253–262. doi: 10.1007/s00223-015-0091-5. [DOI] [PubMed] [Google Scholar]
- Grethen E., McClintock R., Gupta C.E., Jones R., Cacucci B.M., Diaz D. Vitamin D and hyperparathyroidism in obesity. J. Clin. Endocrinol. Metab. 2011;96(5):1320–1326. doi: 10.1210/jc.2010-2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grethen E., Hill K.M., Jones R., Cacucci B.M., Gupta C.E., Acton A. Serum leptin, parathyroid hormone, 1,25-dihydroxyvitamin D, fibroblast growth factor 23, bone alkaline phosphatase, and sclerostin relationships in obesity. J. Clin. Endocrinol. Metab. 2012;97(5):1655–1662. doi: 10.1210/jc.2011-2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halleen J.M., Tiitinen S.L., Ylipahkala H., Fagerlund K.M., Väänänen H.K. Tartrate-resistant acid phosphatase 5b (TRACP 5b) as a marker of bone resorption. Clin. Lab. 2006;52(9–10):499–509. [PubMed] [Google Scholar]
- Hamrick M.W., Ferrari S.L. Leptin and the sympathetic connection of fat to bone. Osteoporos. Int. 2008;19(7):905–912. doi: 10.1007/s00198-007-0487-9. [DOI] [PubMed] [Google Scholar]
- Hauschka P.V., Lian J.B., Cole D.E., Gundberg C.M. Osteocalcin and matrix Gla protein: vitamin K-dependent proteins in bone. Physiol. Rev. 1989;69(3):990–1047. doi: 10.1152/physrev.1989.69.3.990. [DOI] [PubMed] [Google Scholar]
- Hewitt S., Søvik T.T., Aasheim E.T., Kristinsson J., Jahnsen J., Birketvedt G.S. Secondary hyperparathyroidism, vitamin D sufficiency, and serum calcium 5 years after gastric bypass and duodenal switch. Obes. Surg. 2013;23(3):384–390. doi: 10.1007/s11695-012-0772-3. [DOI] [PubMed] [Google Scholar]
- Hofsø D., Bollerslev J., Sandbu R., Jørgensen A., Godang K., Hjelmesæth J. Bone resorption following weight loss surgery is associated with treatment procedure and changes in secreted Wnt antagonists. Endocrine. 2016;53(1):313–321. doi: 10.1007/s12020-016-0903-z. [DOI] [PubMed] [Google Scholar]
- Holick M.F. Biological effects of sunlight, ultraviolet radiation, visible light, infrared radiation and vitamin D for health. Anticancer Res. 2016;36(3):1345–1356. [PubMed] [Google Scholar]
- Hsin M.C., Huang C.K., Tai C.M., Yeh L.R., Kuo H.C., Garg A. A case-matched study of the differences in bone mineral density 1 year after 3 different bariatric procedures. Surg. Obes. Relat. Dis. 2015;11(1):181–185. doi: 10.1016/j.soard.2014.07.008. [DOI] [PubMed] [Google Scholar]
- Hu M.C., Shiizaki K., Kuro-o M., Moe O.W. Fibroblast growth factor 23 and Klotho: physiology and pathophysiology of an endocrine network of mineral metabolism. Annu. Rev. Physiol. 2013;75:503–533. doi: 10.1146/annurev-physiol-030212-183727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivaska K.K., Huovinen V., Soinio M., Hannukainen J.C., Saunavaara V., Salminen P. Changes in bone metabolism after bariatric surgery by gastric bypass or sleeve gastrectomy. Bone. 2017;95:47–54. doi: 10.1016/j.bone.2016.11.001. [DOI] [PubMed] [Google Scholar]
- Jia H., Ma X., Wei Y., Tong W., Tower R.J., Chandra A. Loading-Induced Reduction in Sclerostin as a Mechanism of Subchondral Bone Plate Sclerosis in Mouse Knee Joints During Late-Stage Osteoarthritis. Arthritis Rheum. 2018;70(2):230–241. doi: 10.1002/art.40351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G., Prosser D.E., Kaufmann M. Cytochrome P450-mediated metabolism of vitamin D. J. Lipid Res. 2014;55(1):13–31. doi: 10.1194/jlr.R031534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsenty G., Ferron M. The contribution of bone to whole-organism physiology. Nature. 2012;481(7381):314–320. doi: 10.1038/nature10763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsenty G., Oury F. Biology without walls: the novel endocrinology of bone. Annu. Rev. Physiol. 2012;74:87–105. doi: 10.1146/annurev-physiol-020911-153233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaulfers A.M., Bean J.A., Inge T.H., Dolan L.M., Kalkwarf H.J. Bone loss in adolescents after bariatric surgery. Pediatrics. 2011;127(4):e956–61. doi: 10.1542/peds.2010-0785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori T. Animal models for osteoporosis. Eur. J. Pharmacol. 2015;759:287–294. doi: 10.1016/j.ejphar.2015.03.028. [DOI] [PubMed] [Google Scholar]
- Lalmohamed A., de Vries F., Bazelier M.T., Cooper A., van Staa T.P., Cooper C. Risk of fracture after bariatric surgery in the United Kingdom: population based, retrospective cohort study. BMJ. 2012;345 doi: 10.1136/bmj.e5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Sarosi I., Cattley R.C., Pretorius J., Asuncion F., Grisanti M. Dkk1-mediated inhibition of Wnt signaling in bone results in osteopenia. Bone. 2006;39(4):754–766. doi: 10.1016/j.bone.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Li X., Grisanti M., Fan W., Asuncion F.J., Tan H.L., Dwyer D. Dickkopf-1 regulates bone formation in young growing rodents and upon traumatic injury. J. Bone Miner. Res. 2011;26(11):2610–2621. doi: 10.1002/jbmr.472. [DOI] [PubMed] [Google Scholar]
- Lu C.W., Chang Y.K., Chang H.H., Kuo C.S., Huang C.T., Hsu C.C. Fracture risk after bariatric surgery: a 12-year nationwide cohort study. Medicine (Baltimore) 2015;94(48) doi: 10.1097/MD.0000000000002087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Mach M.A., Stoeckli R., Bilz S., Kraenzlin M., Langer I., Keller U. Changes in bone mineral content after surgical treatment of morbid obesity. Metabolism. 2004;53(7):918–921. doi: 10.1016/j.metabol.2004.01.015. [DOI] [PubMed] [Google Scholar]
- Maghrabi A.H., Wolski K., Abood B., Licata A., Pothier C., Bhatt D.L. Two-year outcomes on bone density and fracture incidence in patients with T2DM randomized to bariatric surgery versus intensive medical therapy. Obesity (Silver Spring) 2015;23(12):2344–2348. doi: 10.1002/oby.21150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschitz C., Kocijan R., Marterer C., Nia A.R., Muschitz G.K., Resch H. Sclerostin levels and changes in bone metabolism after bariatric surgery. J. Clin. Endocrinol. Metab. 2015;100(3):891–901. doi: 10.1210/jc.2014-3367. [DOI] [PubMed] [Google Scholar]
- Muschitz C., Kocijan R., Haschka J., Zendeli A., Pirker T., Geiger C. The impact of vitamin D, calcium, protein supplementation, and physical exercise on bone metabolism after bariatric surgery: the BABS study. J. Bone Miner. Res. 2016;31(3):672–682. doi: 10.1002/jbmr.2707. [DOI] [PubMed] [Google Scholar]
- Nakamura K.M., Haglind E.G., Clowes J.A., Achenbach S.J., Atkinson E.J., Melton L.J. Fracture risk following bariatric surgery: a population-based study. Osteoporos. Int. 2014;25(1):151–158. doi: 10.1007/s00198-013-2463-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obinwanne K.M., Riess K.P., Kallies K.J., Mathiason M.A., Manske B.R., Kothari S.N. Effects of laparoscopic Roux-en-Y gastric bypass on bone mineral density and markers of bone turnover. Surg. Obes. Relat. Dis. 2014;10(6):1056–1062. doi: 10.1016/j.soard.2014.06.018. [DOI] [PubMed] [Google Scholar]
- Pasco J.A., Henry M.J., Kotowicz M.A., Collier G.R., Ball M.J., Ugoni A.M. Serum leptin levels are associated with bone mass in nonobese women. J. Clin. Endocrinol. Metab. 2001;86(5):1884–1887. doi: 10.1210/jcem.86.5.7417. [DOI] [PubMed] [Google Scholar]
- Pluskiewicz W., Bužga M., Holéczy P., Bortlík L., Šmajstrla V., Adamczyk P. Bone mineral changes in spine and proximal femur in individual obese women after laparoscopic sleeve gastrectomy: a short-term study. Obes. Surg. 2012;22(7):1068–1076. doi: 10.1007/s11695-012-0654-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raoof M., Näslund I., Rask E., Szabo E. Effect of gastric bypass on bone mineral density, parathyroid hormone and vitamin D: 5 years follow-up. Obes. Surg. 2016;26(5):1141–1145. doi: 10.1007/s11695-016-2114-3. [DOI] [PubMed] [Google Scholar]
- Riedl M., Vila G., Maier C., Handisurya A., Shakeri-Manesch S., Prager G. Plasma osteopontin increases after bariatric surgery and correlates with markers of bone turnover but not with insulin resistance. J. Clin. Endocrinol. Metab. 2008;93(6):2307–2312. doi: 10.1210/jc.2007-2383. [DOI] [PubMed] [Google Scholar]
- Riedt C.S., Brolin R.E., Sherrell R.M., Field M.P., Shapses S.A. True fractional calcium absorption is decreased after Roux-en-Y gastric bypass surgery. Obesity (Silver Spring) 2006;14(11):1940–1948. doi: 10.1038/oby.2006.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau C., Jean S., Gamache P. Change in fracture risk and fracture pattern after bariatric surgery: nested case-control study. BMJ. 2016;354 doi: 10.1136/bmj.i3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Tovar J., Oller I., Priego P., Arroyo A., Calero A., Diez M. Short- and mid-term changes in bone mineral density after laparoscopic sleeve gastrectomy. Obes. Surg. 2013;23(7):861–866. doi: 10.1007/s11695-013-0866-6. [DOI] [PubMed] [Google Scholar]
- Rutkowski J.M., Pastor J., Sun K., Park S.K., Bobulescu I.A., Chen C.T. Adiponectin alters renal calcium and phosphate excretion through regulation of klotho expression. Kidney Int. 2017;91(2):324–337. doi: 10.1016/j.kint.2016.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainsbury A., Zhang L. Role of the hypothalamus in the neuroendocrine regulation of body weight and composition during energy deficit. Obes. Rev. 2012;13(3):234–257. doi: 10.1111/j.1467-789X.2011.00948.x. [DOI] [PubMed] [Google Scholar]
- Schafer A.L., Weaver C.M., Black D.M., Wheeler A.L., Chang H., Szefc G.V. Intestinal calcium absorption decreases dramatically after gastric bypass surgery despite optimization of vitamin D status. J. Bone Miner. Res. 2015;30(8):1377–1385. doi: 10.1002/jbmr.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer A.L., Li X., Schwartz A.V., Tufts L.S., Wheeler A.L., Grunfeld C. Changes in vertebral bone marrow fat and bone mass after gastric bypass surgery: a pilot study. Bone. 2015;74:140–145. doi: 10.1016/j.bone.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller E.L., Rosen C.J. What's the matter with MAT? Marrow adipose tissue, metabolism, and skeletal health. Ann. N. Y. Acad. Sci. 2014;1311:14–30. doi: 10.1111/nyas.12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellinger D., Lin C.S., Hatipoglu H.G., Fertikh D. Potential value of vertebral proton MR spectroscopy in determining bone weakness. AJNR Am. J. Neuroradiol. 2001;22(8):1620–1627. [PMC free article] [PubMed] [Google Scholar]
- Schollenberger A.E., Heinze J.M., Meile T., Peter A., Königsrainer A., Bischoff S.C. Markers of bone metabolism in obese individuals undergoing laparoscopic sleeve gastrectomy. Obes. Surg. 2015;25(8):1439–1445. doi: 10.1007/s11695-014-1509-2. [DOI] [PubMed] [Google Scholar]
- Shanbhogue V.V., Støving R.K., Frederiksen K.H., Hanson S., Brixen K., Gram J. Bone structural changes after gastric bypass surgery evaluated by HR-pQCT: a two-year longitudinal study. Eur. J. Endocrinol. 2017;176(6):685–693. doi: 10.1530/EJE-17-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signori C., Zalesin K.C., Franklin B., Miller W.L., McCullough P.A. Effect of gastric bypass on vitamin D and secondary hyperparathyroidism. Obes. Surg. 2010;20(7):949–952. doi: 10.1007/s11695-010-0178-z. [DOI] [PubMed] [Google Scholar]
- Sinha N., Shieh A., Stein E.M., Strain G., Schulman A., Pomp A. Increased PTH and 1.25(OH)(2)D levels associated with increased markers of bone turnover following bariatric surgery. Obesity (Silver Spring) 2011;19(12):2388–2393. doi: 10.1038/oby.2011.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Søvik T.T., Aasheim E.T., Taha O., Engström M., Fagerland M.W., Björkman S. Weight loss, cardiovascular risk factors, and quality of life after gastric bypass and duodenal switch: a randomized trial. Ann. Intern. Med. 2011;155(5):281–291. doi: 10.7326/0003-4819-155-5-201109060-00005. [DOI] [PubMed] [Google Scholar]
- Stein E.M., Carrelli A., Young P., Bucovsky M., Zhang C., Schrope B. Bariatric surgery results in cortical bone loss. J. Clin. Endocrinol. Metab. 2013;98(2):541–549. doi: 10.1210/jc.2012-2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemmer K., Bielohuby M., Grayson B.E., Begg D.P., Chambers A.P., Neff C. Roux-en-Y gastric bypass surgery but not vertical sleeve gastrectomy decreases bone mass in male rats. Endocrinology. 2013;154(6):2015–2024. doi: 10.1210/en.2012-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szulc P., Naylor K., Hoyle N.R., Eastell R., Leary E.T., Project N.B.H.A.B.T.M. Use of CTX-I and PINP as bone turnover markers: National Bone Health Alliance recommendations to standardize sample handling and patient preparation to reduce pre-analytical variability. Osteoporos. Int. 2017;28(9):2541–2556. doi: 10.1007/s00198-017-4082-4. [DOI] [PubMed] [Google Scholar]
- Tsuji K., Maeda T., Kawane T., Matsunuma A., Horiuchi N. Leptin stimulates fibroblast growth factor 23 expression in bone and suppresses renal 1alpha,25-dihydroxyvitamin D3 synthesis in leptin-deficient mice. J. Bone Miner. Res. 2010;25(8):1711–1723. doi: 10.1002/jbmr.65. [DOI] [PubMed] [Google Scholar]
- Valderas J.P., Velasco S., Solari S., Liberona Y., Viviani P., Maiz A. Increase of bone resorption and the parathyroid hormone in postmenopausal women in the long-term after Roux-en-Y gastric bypass. Obes. Surg. 2009;19(8):1132–1138. doi: 10.1007/s11695-009-9890-y. [DOI] [PubMed] [Google Scholar]
- Van der Schueren B., Green N., Gorroochurn P., Teixeira J., Altiner S., Laferrère B. Low vitamin D in candidates for bariatric surgery: are the Americans better off than the French? Obes. Surg. 2011;21(7):948–949. doi: 10.1007/s11695-011-0439-5. (author reply 50) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viapiana O., Fracassi E., Troplini S., Idolazzi L., Rossini M., Adami S. Sclerostin and DKK1 in primary hyperparathyroidism. Calcif. Tissue Int. 2013;92(4):324–329. doi: 10.1007/s00223-012-9665-7. [DOI] [PubMed] [Google Scholar]
- Vilarrasa N., de Gordejuela A.G., Gómez-Vaquero C., Pujol J., Elio I., San José P. Effect of bariatric surgery on bone mineral density: comparison of gastric bypass and sleeve gastrectomy. Obes. Surg. 2013;23(12):2086–2091. doi: 10.1007/s11695-013-1016-x. [DOI] [PubMed] [Google Scholar]
- Wagner C.A., Imenez Silva P.H., Rubio-Aliaga I. And the fat lady sings about phosphate and calcium. Kidney Int. 2017;91(2):270–272. doi: 10.1016/j.kint.2016.11.009. [DOI] [PubMed] [Google Scholar]
- World Health Organization Obesity and Overweight Factsheet No. 311. 2017.
- Wucher H., Ciangura C., Poitou C., Czernichow S. Effects of weight loss on bone status after bariatric surgery: association between adipokines and bone markers. Obes. Surg. 2008;18(1):58–65. doi: 10.1007/s11695-007-9258-0. [DOI] [PubMed] [Google Scholar]
- Ybarra J., Sánchez-Hernández J., Gich I., De Leiva A., Rius X., Rodríguez-Espinosa J. Unchanged hypovitaminosis D and secondary hyperparathyroidism in morbid obesity after bariatric surgery. Obes. Surg. 2005;15(3):330–335. doi: 10.1381/0960892053576758. [DOI] [PubMed] [Google Scholar]
- Youssef Y., Richards W.O., Sekhar N., Kaiser J., Spagnoli A., Abumrad N. Risk of secondary hyperparathyroidism after laparoscopic gastric bypass surgery in obese women. Surg. Endosc. 2007;21(8):1393–1396. doi: 10.1007/s00464-007-9228-6. [DOI] [PubMed] [Google Scholar]
- Yu E.W. Bone metabolism after bariatric surgery. J. Bone Miner. Res. 2014;29(7):1507–1518. doi: 10.1002/jbmr.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu E.W., Bouxsein M.L., Roy A.E., Baldwin C., Cange A., Neer R.M. Bone loss after bariatric surgery: discordant results between DXA and QCT bone density. J. Bone Miner. Res. 2014;29(3):542–550. doi: 10.1002/jbmr.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu E.W., Bouxsein M.L., Putman M.S., Monis E.L., Roy A.E., Pratt J.S. Two-year changes in bone density after Roux-en-Y gastric bypass surgery. J. Clin. Endocrinol. Metab. 2015;100(4):1452–1459. doi: 10.1210/jc.2014-4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu E.W., Carmody J.S., Brooks D.J., LaJoie S., Kaplan L.M., Bouxsein M.L. Cortical and trabecular deterioration in mouse models of Roux-en-Y gastric bypass. Bone. 2016;85:23–28. doi: 10.1016/j.bone.2016.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu E.W., Wewalka M., Ding S.A., Simonson D.C., Foster K., Holst J.J. Effects of gastric bypass and gastric banding on bone remodeling in obese patients with type 2 diabetes. J. Clin. Endocrinol. Metab. 2016;101(2):714–722. doi: 10.1210/jc.2015-3437. [DOI] [PMC free article] [PubMed] [Google Scholar]