Abstract
Purpose
From 1990 to 1994, patients with newly diagnosed malignant gliomas were enrolled and randomized between hyperfractionated radiation (HFX) of 72.0 Gy in 60 fractions given twice daily and 60.0 Gy in 30 fractions given once daily. All patients received 80 mg/m2 of 1,3 bis (2 chloroethyl)-1 nitrosourea on days 1–3 q8 weeks for 1 year.
Methods
Patients were stratified by age, KPS, and histology. The primary endpoint was overall survival (OS), with secondary endpoints including progression-free survival (PFS) and toxicity.
Results
Out of the 712 patients accrued, 694 (97.5%) were analyzable cases (350 HFX, 344 standard arm). There was no significant difference between the arms on overall acute or late treatment-related toxicity. No statistically significant effect for HFX, as compared to standard therapy, was found on either OS, with a median survival time (MST) of 11.3 mo vs. 13.1 mo (p=0.20) or PFS, with a median PFS time of 5.7 mo vs. 6.9 mo (p=0.18). The treatment effect on OS remained insignificant based on the multivariate analysis (hazard ratio=1.16; p=0.0682). When OS was analyzed by histology subgroup there was also no significant difference between the two arms for patients with glioblastoma multiforme (MST: 10.3 mo vs. 11.2 mo; p=0.34), anaplastic astrocytoma (MST: 69.8 mo vs 50.0 mo; p=0.91) or anaplastic oligodendroglioma (MST: 92.1 mo vs. 66.5 mo; p=0.33).
Conclusion
Though this trial provided many invaluable secondary analyses, there was no trend or indication of a benefit to HFX radiation to 72.0 Gy in any subset of malignant glioma patients.
Keywords: GBM, Glioma, Hyperfractionated, Astrocytoma, Oligodendroglioma
INTRODUCTION
The diagnosis of malignant glioblastoma multiforme (GBM) or anaplastic astrocytoma (AA) remains just as devastating today as it has been in the past. In the continual and relentless fight against these tumors, there have been numerous treatment strategies that have been explored. Some of these strategies have provided direct, incremental success and improvement in outcome, while many others, though necessary to be thoroughly investigated and published, have not demonstrated primary endpoint benefit, but have provided valuable information via secondary analyses. The NRG Oncology Radiation Therapy Oncology Group (RTOG) trial 9006 is an example of the latter. NRG RTOG 9006, at the time of its conception, was the logical next step in a long series of trials exploring the use of radiation combined with chemotherapy in the treatment of malignant gliomas.
The use of radiation to treat patients with malignant gliomas after biopsy or surgical resection was initially supported by the Brain Tumor Study Group (BTSG) trials 69-01 and 72-01, which demonstrated improved survival for patients with malignant gliomas receiving whole brain radiation to 60 Gy in standard daily fractionations (1.8 Gy to 2.0 Gy), with or without chemotherapy, as compared to either chemotherapy alone or observation [1,2]. Additional evaluation on these trials as well as other data suggested that an overall survival (OS) dose response existed to at least 60 Gy [3].
The use of hyperfractionation (multiple daily radiation fractions in smaller than standard doses) theoretically offers the benefit of a larger total radiation dose without excessive late toxicity and has been investigated in many clinical trials involving a wide range of tumor types [4,5]. Late toxicity for central nervous system (CNS) tumors, in particular, is especially important due to the low estimated alpha/beta ratio of normal CNS tissue and thus sensitivity to fraction size [6–9].
The use of concurrent temozolomide chemotherapy with radiation is now the standard of care for GBM primarily due to the successful results of the European Organization for Research and Treatment of Cancer (EORTC) and National Cancer Institute of Canada (NCIC) intergroup trial published by Stupp et al. in 2005 [10]. This trial was built upon a foundation laid by multiple previous clinical trials demonstrating benefit of the addition of chemotherapy to radiation for gliomas. One of those trials, BTSG 69-01, demonstrated improved survival for patients receiving 1,3 bis (2 chloroethyl)-1 nitrosourea (ie. BCNU) compared to standard radiation alone [1].
NRG Oncology RTOG 8302 built upon the successful results of concurrent BCNU in BTSG 69-01 and combined them with the theoretical benefits of hyperfractionation in a phase I/II trial that randomized patients with supratentorial malignant gliomas to receive BCNU concurrent with either hyperfractionated (HFX) radiation (1.2 Gy twice daily to 64.8 Gy, 72 Gy, 76.8 Gy, or 81.6 Gy) or accelerated HFX radiation therapy (1.6 Gy twice daily to 48 Gy or 54.4 Gy) after biopsy or resection [11,12]. The patients with the best survival outcome were those treated with 72 Gy in 1.2 Gy fractions twice daily on the initial analysis and publication (median OS of 14 months for 72 Gy arm vs. 12.8 months for all other patients) [11]. The worse outcome of the patients treated with 81.6 Gy was attributed to excessive neurotoxicity [11]. Having established 72 Gy as the optimal HFX dose with concurrent BCNU, conducting a phase III trial to test the outcome of glioma patients treated with HFX radiation with BCNU vs. standard fraction radiation with BCNU was the logical next step.
METHODS
Patient characteristics
All patients were required to have supratentorial, histopathologically confirmed GBM, anaplastic oligodendroglioma or astrocytoma with foci of anaplasia (AAF). Patients must have had age ≥18, Karnofsky Performance Status (KPS) ≥60, and no prior radiation to the head or neck area, chemotherapy or any other radiosensitizer at registration. Normal hematologic, renal, pulmonary, and hepatic parameters were required. Additionally, patients could not have any prior malignancy within the past five years except for non-melanomatous skin cancer or carcinoma in-situ of the cervix. Eligible patients must have had an estimated survival of at least 8 weeks and a pre-surgical CT or MRI. Patients with multifocal disease, recurrent gliomas or acquired immune deficiency syndrome (AIDS) were excluded. Treatment was required to start within 4 weeks after surgery. Protocol approval was received from the Institutional Review Board at each study site and informed consent was obtained from each patient prior to participation.
Patients were stratified by age (<40 vs. ≥40 to <60 vs. ≥60), KPS (≥80 vs. <80), and histology (astrocytoma with foci of anaplasia vs. glioblastoma) and then randomized with a 1:1 ratio to either arm 1 (standard fraction radiation with BCNU) or arm 2 (HFX radiation with BCNU).
Radiation Therapy
The standard fractionated arm involved treating the contrast enhancing lesion and surrounding edema on a pre-operative CT or MRI scan with a 2 cm margin to 46 Gy in 23 daily fractions. The fields were then reduced to treat only the contrast enhancing lesion on the pre-operative CT or MRI with a 2.5 cm margin to an additional 14 Gy in 7 daily fractions for a total of 60 Gy in 30 daily fractions. The HFX radiation arm involved treating the contrast enhancing lesion and surrounding edema on the pre-operative CT or MRI with a 2 cm margin to 57.6 Gy in 48 twice-daily fractions (1.2 Gy per fraction separated by 4 to 8 hours). The fields were then reduced to treat only the contrast enhancing lesion on the pre-operative CT or MRI with a 2.5 cm margin to an additional 14.4 Gy in 12 twice-daily fractions (1.2 Gy per fraction separated by 4 to 8 hours).
The radiation delivery machines were either cobalt-60 based or linear accelerators with photon energies ranging from 1.25 MV to 10 MV with a source to axis distance of at least 80 cm. Treatment plans were required to use CT guidance and allowed opposed lateral fields, a wedge pair of fields, rotation, or multiple field techniques. Normal tissue dose constraints were 60 Gy for the chiasm, 50 Gy for the retina of at least one eye, and 60 Gy for the brainstem. The treatment prescription, calculation form, simulation films, and representative portal films were required to be sent to NRG Oncology RTOG headquarters prior to treatment start.
Chemotherapy
All patients on both arms received the same chemotherapy regimen, which included BCNU delivered intravenously to a dose of 80 mg/m2 on days 1, 2, and 3 of the first week of radiation and then every 8 weeks for a total of 6 cycles or 1 full year of therapy. Dose modifications were provided for those patients in excess of 125% of their ideal body weight or hematologic toxicity according to a pre-defined schema.
Pathology
Pathology for all patients was required to be centrally reviewed according to the RTOG criteria with tissue specimen submitted to NRG Oncology RTOG headquarters within 4 weeks prior to treatment initiation. A diagnosis of GBM required marked hypercellularity, foci of coagulation necrosis and vascular proliferation. The histologic variants of mixed glioblastoma and gliosarcoma were allowed. The diagnosis of AAF required that no foci of coagulation necrosis be present, but there must have been one or more of the following: increased cellularity, pleomorphic nuclei or cell bodies, mitotic figures, increase in blood vessels with mild endothelial proliferation, spongioblastic or incompletely differentiated glial cell [13]. Well-differentiated astrocytoma histologies were excluded from this study.
Follow-up
Patients received full neurologic examinations, CBC, serum chemistries, chest x-ray, and brain CT or MRI pre-operatively or post-operatively. During radiation, patients received weekly neurologic examinations, skin examinations within portal fields, a weekly CBC, and bi-weekly serum chemistries. Following the completion of radiation, patients received serum chemistries at least every 3 months for the first year. A brain CT or MRI was performed 4 months after the start of radiation and with each follow-up visit and at the time of neurologic deterioration. Follow-up visits were performed every month for first 3 months after treatment completion, then every 3 months for 9 months, then every 4 months for a year, then every 6 months for 3 years, and then annually. Treatment related normal tissue effects were evaluated and graded using RTOG toxicity criteria [12]. Acute and late toxicities were identified based on assessments made during the first 90 days after treatment start and after 90 days of treatment start, respectively.
Statistics
The primary endpoint of this study was overall survival (OS). Secondary endpoints included progression-free survival (PFS) and severe (grade 4+) toxicity. OS and PFS were determined from the date of randomization. For OS, death due to any cause was considered as failure. For PFS, disease progression or death due to any cause, whichever came first, was considered as failure. The study was initially designed to have an 80% power to detect a difference in the median survival time (MST) of 5 months (16 vs. 21 months) in favor of the HFX arm, at a significance level of 0.05 (two-sided). The initial accrual goal was an overall of 564 patients (assuming a 5% rate of ineligibility) for these parameters, but was eventually increased to 708 patients. Based on the accrual at the early stage of this trial, the study design was adjusted to detect a difference in the MST of 4.3 months (16.0 vs. 20.3 months) with the same power and significance level, as recommended by the Data Monitoring Committee. Severe toxicity was compared between the two treatment arms using Pearson’s Chi-square test. OS and PFS were estimated using the Kaplan-Meier method [14]. Differences in survival distributions were tested using the Wilcoxon test [15]. Multivariate analysis was conducted using Cox proportional hazard model [16] with treatment assignment, KPS, age, histology and extent of surgery as covariates.
RESULTS
Patient Characteristics
This study was open from November 1990 to March 1994 with a total accrual of 712 patients. Fifteen cases were determined to be ineligible and another 3 had insufficient data, leaving a total of 694 analyzable cases (344 on the standard arm and 350 on the HFX arm). Reasons for ineligibility included ineligible histology (5 cases), excessive delay in starting radiation (3 cases), multifocal disease (2 cases), lab values out of range (3 cases) and other (2 cases).
Overall, 91% of the patients in each arm had received radiotherapy per protocol or with acceptable variations. There were 8% and 9% of the cases in the standard and HFX arm, respectively, who had minor radiation protocol deviations. Only 1% in each arm had major radiation protocol deviations. For both arms combined, 22 patients died prior to completing radiation, 7 progressed while on radiation, and another 15 refused radiation. For chemotherapy administration, 91% of the patients in both arms received chemotherapy per protocol or with acceptable variations. Acceptable variations occurred in 29% and 22% of the patients in the standard and HFX arm, respectively. Unacceptable chemotherapy deviations occurred in only 1% of the patients in each arm.
The distributions of patient pre-treatment characteristics for both the standard and HFX arm are listed in Table 1. As seen, the two arms were well balanced for all of the evaluated variables. The median ages for the standard and HFX arm were 52 and 53 years, respectively. Approximately 78 and 77% of the patients in respective arms had a KPS of 80 or higher. After central review, 70 and 72% of the patients in respective arms were confirmed to have GBM while 14 and 10% were found to have AAF. Though the glioma recursive partitioning analysis (RPA) was published after the initiation of this trial, the patient classifications are included in Table 1. The RPA classifications of this study have been previously discussed [17].
Table 1.
Pre-treatment patient characteristics
| Assigned Treatment | ||||
|---|---|---|---|---|
| Standard (n=344) |
HFX (n=350) |
|||
| Age | ||||
| Median | 52 | 53 | ||
| Range | 18 – 83 | 18 – 82 | ||
| n | % | n | % | |
| Age | ||||
| <40 | 76 | 22 | 75 | 21 |
| ≥40–<60 | 154 | 45 | 154 | 44 |
| ≥60 | 114 | 33 | 121 | 35 |
| Sex | ||||
| Male | 209 | 61 | 227 | 65 |
| Female | 135 | 39 | 123 | 35 |
| Race | ||||
| White | 311 | 90 | 308 | 88 |
| Hispanic | 16 | 5 | 17 | 5 |
| Black | 8 | 2 | 13 | 4 |
| Asian | 4 | 1 | 5 | 1 |
| Native American (Indian, Eskimo, etc) | 1 | 0 | 1 | 0 |
| Other | 4 | 1 | 6 | 2 |
| Marital Status | ||||
| Married or other live-in relationship | 218 | 63 | 224 | 64 |
| Single, divorced, separated, or widowed | 62 | 18 | 73 | 21 |
| Unknown | 64 | 19 | 53 | 15 |
| Number of Persons Living with Patient | ||||
| Lives alone | 23 | 7 | 33 | 9 |
| One other person | 128 | 37 | 118 | 34 |
| Two to four other people | 105 | 31 | 122 | 35 |
| Five or more other people | 24 | 7 | 21 | 6 |
| Unknown | 64 | 19 | 56 | 16 |
| Education | ||||
| Grade 1–8 only | 11 | 3 | 19 | 5 |
| Some high school, did not graduate | 31 | 9 | 27 | 8 |
| High school graduate/GED | 105 | 31 | 89 | 25 |
| Attended college/technical school | 120 | 35 | 141 | 40 |
| Unknown | 77 | 22 | 74 | 21 |
| Employment Status Prior to Illness | ||||
| Outside home/full time | 142 | 41 | 163 | 47 |
| Outside home/part time | 15 | 4 | 25 | 7 |
| Homemaker/employed at home | 32 | 9 | 28 | 8 |
| Retired | 59 | 17 | 49 | 14 |
| Disabled | 6 | 2 | 1 | 0 |
| In school | 2 | 1 | 5 | 1 |
| Not working | 15 | 4 | 16 | 5 |
| Unknown | 73 | 21 | 63 | 18 |
| Neurologic function | ||||
| No symptoms | 53 | 15 | 52 | 15 |
| Minor symptoms | 178 | 52 | 172 | 49 |
| Moderate symptoms (fully active at home) | 55 | 16 | 73 | 21 |
| Moderate symptoms (less than fully active at home) | 54 | 16 | 40 | 11 |
| Severe symptoms | 4 | 1 | 13 | 4 |
| Prior surgery | ||||
| Biopsy only | 83 | 24 | 85 | 24 |
| Partial resection | 189 | 55 | 189 | 54 |
| Total resection | 72 | 21 | 75 | 21 |
| Shunt plus partial resection | 0 | 0 | 1 | 0 |
| Karnofsky performance status | ||||
| 60–70 | 75 | 22 | 79 | 23 |
| 80–100 | 269 | 78 | 271 | 77 |
| Lateralization of tumor | ||||
| Right | 187 | 54 | 172 | 49 |
| Left | 133 | 39 | 148 | 42 |
| Bilateral | 24 | 7 | 30 | 9 |
| Histology (central review) | ||||
| GBM | 240 | 70 | 253 | 72 |
| Glioma NOS | 16 | 5 | 16 | 5 |
| AAF | 48 | 14 | 35 | 10 |
| Oligodendroglioma | 16 | 5 | 12 | 3 |
| AA/Oligo mixed | 8 | 2 | 17 | 5 |
| Other | 8 | 2 | 7 | 2 |
| Unknown | 8 | 2 | 10 | 3 |
| RPA | ||||
| I | 34 | 10 | 34 | 10 |
| II | 15 | 4 | 8 | 2 |
| III | 55 | 16 | 48 | 14 |
| IV | 111 | 32 | 139 | 40 |
| V | 83 | 24 | 81 | 23 |
| VI | 14 | 4 | 10 | 3 |
| Cannot classify | 32 | 9 | 30 | 9 |
Toxicity
As might be expected, the hematologic acute toxicities were similar between the standard and HFX arm for grade 3 (28% vs. 30%), grade 4 (21% vs. 19%) and grade 5 (0% vs. <1%) levels. Likewise, the non-hematologic toxicity was also similar between the two arms (9% vs. 11% for grade 3; 3% vs. 1% for grade 4; <1% vs. 1% for grade 5). Specifically, there were 10 (3%) patients in the standard arm and 12 (3%) in the HFX arm with grade 3 or worse neurologic acute toxicity. The most common grade 5 acute toxicity was infection (5 patients). The only other patient death due to acute toxicity was hepatic in nature. Since only a few patients had grade 5 toxicities, patients with severe toxicities from each arm were categorized by having experienced the worst toxicity of grade 3 or grade 4+. There was no statistically significant difference in chemotherapy and acute radiotherapy toxicity between the two arms for worst hematologic toxicity grade (p=0.49), worst non-hematologic toxicity grade (p=0.45), or worst overall toxicity grade (p=0.40).
The most common late grade 3 or worse toxicities in the standard and HFX arm were hematologic (26% and 21%, respectively). Neurologic grade 3 or worse late toxicity was similar between the standard and HFX arm (2% and 3%, respectively). Overall, there were 8 patients who died due to late toxicity, most of which were due to pulmonary toxicity (6 out of 8). The other 2 patients died of bleeding and an “idiosyncratic” reaction to BCNU. Similarly, severe toxicities were compared between the two arms with respect to having experienced the worst toxicity of grade 3 or grade 4+. There was no statistically significant difference in late toxicity between the two experimental arms for worst hematologic late toxicity grade (p=0.94), worst non-hematologic late toxicity grade (p=0.37), or worst overall late toxicity grade (p=0.37).
Survival
Out of the 694 analyzable patients, 615 have died by the time of the analysis. The treatment effect was not shown to be statistically significant from the univariate analyses on either OS or PFS. The MST was 13.1 months for the standard arm and 11.3 months for the HFX arm, with a p=0.20. The median PFS time was 6.9 months vs. 5.7 months, with a p=0.18. Figures 1 and 2 display the Kaplan-Meier curves by treatment arm for OS and PFS, respectively. When OS was analyzed by histology, the MST was 10.8 months for GBM patients, 65.8 months for AAF patients, and 77.9 months for anaplastic oligodendroglioma patients, with the corresponding 2-year OS rates of 15%, 64%, and 79%.
Figure 1.
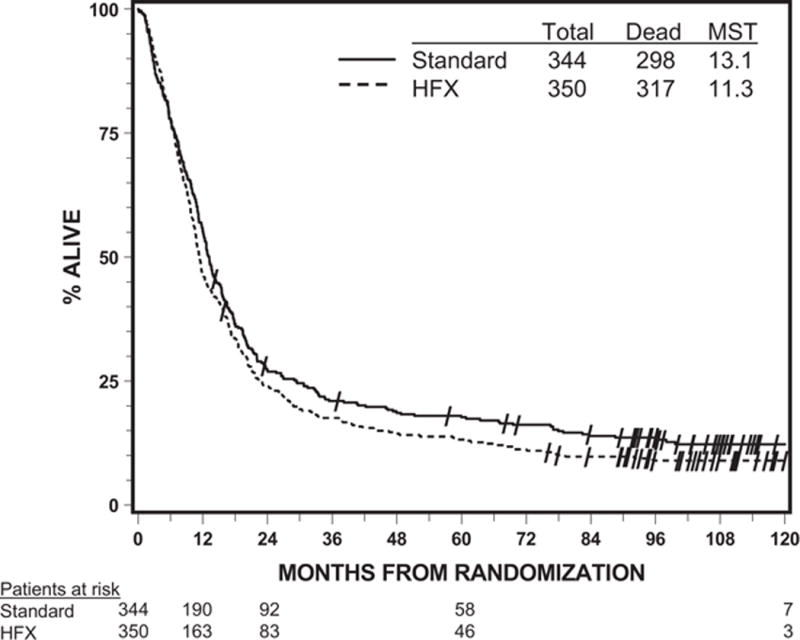
Overall survival by assigned treatment (all analyzable patients)
Figure 2.
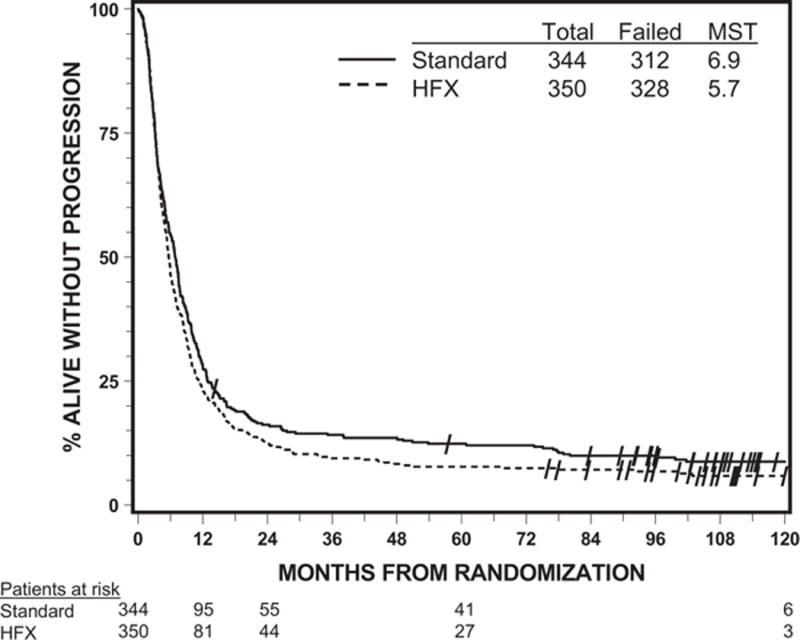
Progression-free survival by assigned treatment (all analyzable patients)
When OS was compared between the treatment arms within each histologic subtype (Table 2), there was no significant difference observed for patients with GBM (p=0.34), AAF (p=0.91) or anaplastic oligodendroglioma (p=0.33). When analyzed by age subgroup of 18 to <40 years, ≥40 to <60 years, and ≥60 years, there was again no significant difference observed on OS between the two arms (p=0.21, 0.23, and 0.41, respectively). For the analysis on patients with GBM/Glioma NOS, there was no significant difference in OS between the standard and HFX arm for patients with ages 18 to <40 years (MST: 18.9 mo vs. 14.6 mo; p=0.15), ≥40 to <60 years (MST: 12.3 mo vs. 11.5 mo; p=0.33), or ≥60 years (MST: 6.2 mo vs. 8.5 mo; p=0.34).
Table 2.
Overall Survival by Assigned Treatment within Central Review of Histology
| Months from Randomization | GBM/Glioma NOS patients | AAF patients | Anaplastic Oligodendroglioma patients | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Assigned Treatment | Assigned Treatment | Assigned Treatment | ||||||||||
| Standard | HFX | Standard | HFX | Standard | HFX | |||||||
| % Alive | # at Risk | % Alive | # at Risk | % Alive | # at Risk | % Alive | # at Risk | % Alive | # at Risk | % Alive | # at Risk | |
| 0 | 100 | 256 | 100 | 269 | 100 | 48 | 100 | 35 | 100 | 16 | 100 | 12 |
| 12 | 47 | 120 | 38 | 103 | 83 | 40 | 80 | 28 | 88 | 14 | 92 | 11 |
| 24 | 16 | 39 | 14 | 37 | 63 | 30 | 66 | 23 | 75 | 12 | 83 | 10 |
| 36 | 10 | 24 | 7 | 20 | 54 | 26 | 66 | 23 | 75 | 12 | 75 | 9 |
| 48 | 8 | 18 | 5 | 13 | 52 | 25 | 54 | 19 | 63 | 10 | 75 | 9 |
| 60 | 8 | 18 | 4 | 10 | 48 | 23 | 54 | 19 | 63 | 10 | 67 | 8 |
|
| ||||||||||||
| Dead/Total | 243/256 | 266/269 | 27/48 | 20/35 | 11/16 | 6/12 | ||||||
| Median | 11.2 | 10.3 | 50.0 | 69.8 | 66.5 | 92.1 | ||||||
| p-value | 0.34 | 0.91 | 0.33 | |||||||||
From the multivariate analysis (Table 3), the only covariates which were shown to have significant effects on OS were KPS (p<0.0001), age (p<0.0001), histology (p<0.0001 for AAF vs. GBM/Glioma NOS), and extent of resection (p=0.0017 for biopsy vs. partial resection; p<0.0001 for biopsy vs. total resection). The effect of the assigned treatment (standard fractionation vs. HFX) was not statistically significant with a trend towards improved survival for patients in the standard arm (hazard ratio=1.16; p=0.0682).
Table 3.
Cox Model for Survival
| Covariate (bolded group has the favorable response) | Parameter Estimate | Standard Error | P-value | Hazard Ratio (95% Hazard Ratio Confidence Limits) |
|---|---|---|---|---|
| RX | 0.14857 | 0.08147 | 0.0682 | 1.16 (0.99, 1.36) |
| Standard vs. HFX | ||||
| KPS | 0.42925 | 0.08293 | <0.0001 | 1.54 (1.31, 1.81) |
| 90–100 vs. 60–80 | ||||
| Age | 0.62833 | 0.12491 | <0.0001 | 1.87 (1.47, 2.39) |
| 18-<40 vs. ≥40-<60 | ||||
| Age | 1.19512 | 0.13316 | <0.0001 | 3.30 (2.55, 4.29) |
| 18-<40 vs. ≥60 | ||||
| Histology | 1.17017 | 0.16331 | <0.0001 | 3.22 (2.34, 4.44) |
| AAF vs. GBM/Glioma NOS | ||||
| Histology | 0.29584 | 0.19655 | 0.1323 | 1.34 (0.91, 1.98) |
| AAF vs. non GBM/Glioma NOS and non AAF | ||||
| Surgery | 0.31302 | 0.09985 | 0.0017 | 1.37 (1.12, 1.66) |
| partial resection vs. biopsy | ||||
| Surgery | 0.57497 | 0.12368 | <0.0001 | 1.78 (1.40, 2.27) |
| total resection vs. biopsy |
DISCUSSION
NRG Oncology RTOG 9006 was a phase III comparison of BCNU chemotherapy with either standard fraction radiation to 60 Gy in 30 once-daily fractions or HFX radiation to 72 Gy in 60 twice-daily fractions for the treatment of primary brain gliomas. This trial was the next logical step in a series of successful NRG Oncology RTOG trials demonstrating benefit of radiation therapy for gliomas [1–3]. A previous phase I/II trial, NRG Oncology RTOG 8302, evaluated several HFX and accelerated HFX radiation dose schemes in combination with BCNU and found that the best MST with the least toxicity was 72 Gy in 60 fractions, delivered twice daily, particularly for patients with AAF histology [12]. Accordingly, this fractionation scheme was then advanced as the experimental arm in this phase III trial.
Unfortunately, this study did not demonstrate any OS or PFS advantage of the HFX arm as compared with standard fractionation. Additional analyses on OS by histology and age subgroup did not demonstrate any benefit to the HFX treatment either. A multivariate analysis, adjusting for key predictors of OS including KPS, age, histology and extent of resection, did not show a significant treatment effect.
Though NRG Oncology RTOG 9006 was a negative study for the planned primary endpoint, there was significant benefit obtained from this trial through numerous secondary and combined database analyses [18–27]. Perhaps the most significant utility of this trial was its role as the motivation for the development of the RTOG RPA, which is still regarded as one of the key factors in the OS predictive model for gliomas today [17]. The negative survival results of the HFX treatment arm and the stark differences observed in OS between patients based on KPS, age, and histology indicated that these pre-treatment prognostic factors were likely more influential than minor modifications in therapy [17, 19]. The RPA model, that was developed based on previous NRG Oncology RTOG glioma trials, was successfully validated using the data from this study [17, 19]. Although there have been many incremental advances since NRG Oncology RTOG 9006 and the current standard of care for gliomas is different than during the time of this study, the influence of this trial, and its valuable secondary analyses, is still tightly woven into the fabric of modern glioma treatment and understanding.
The current standard of care treatment for GBM is driven, in part, by the results of the EORTC/NCIC intergroup trial which demonstrated an overall survival benefit with the addition of concurrent and adjuvant temozolomide to 60 Gy of standard fractionated radiation [10]. NRG Oncology RTOG 9006 and other negative clinical trials which evaluated radiation dose escalation and altered fractionation included chemotherapies other than temozolomide. Thus, the question of radiation dose escalation benefit for GBM patients in the era of concurrent and adjuvant temozolomide still remains and is the topic of active clinical trials.
Acknowledgments
Funding
This study was supported by grants U10CA21661 (RTOG-Ops-Stat), U10CA180868 (NRG Oncology Operations), U10CA180822 (NRG Oncology SDMC), U10CA37422 (CCOP), from the National Cancer Institute (NCI).
Footnotes
Conflicts of interest
Dr. Movsas reports research grants from Varian, Inc. and Philips, Inc. Drs. Chen and Penas-Prado report grants from NCI. Dr. Jones reports receiving speaker fees from Lilly. Dr. Yung reports research grant to his institution from RTOG and fees for serving on scientific advisory board of DNAtrix.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- 1.Walker MD, Alexander E, Jr, Hunt WE, MacCarty CS, Mahaley MS, Jr, Mealey J, Jr, Norrell HA, Owens G, Ransohoff J, Wilson CB, Gehan EA, Strike TA. Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas. J Neurosurg. 1978;49(3):333–343. doi: 10.3171/jns.1978.49.3.0333. [DOI] [PubMed] [Google Scholar]
- 2.Walker MD, Green SB, Byar DP, Alexander E, Jr, Batzdorf U, Brooks WH, Hunt WE, MacCarty CS, Mahaley MS, Jr, Mealey J, Jr, Owens G, Ransohoff J, 2nd, Robertson JT, Shapiro WR, Smith KR, Jr, Wilson CB, Strike TA. Randomized Comparisons of Radiotherapy and Nitrosoureas for the Treatment of Malignant Glioma after Surgery. N Engl J Med. 1980;303(23):1323–1329. doi: 10.1056/NEJM198012043032303. [DOI] [PubMed] [Google Scholar]
- 3.Walker MD, Strike TA, Sheline GE. An analysis of dose-effect relationship in the radiotherapy of malignant gliomas. Int J Radiat Oncol Biol Phys. 1979;5(10):1725–1731. doi: 10.1016/0360-3016(79)90553-4. [DOI] [PubMed] [Google Scholar]
- 4.Withers HR. Biologic basis for altered fractionation schemes. Cancer. 1985;55(S9):2086–2095. doi: 10.1002/1097-0142(19850501)55:9+<2086::aid-cncr2820551409>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 5.Thames HD, Jr, Peters LJ, Withers HR, Fletcher GH. Accelerated fractionation vs hyperfractionation: rationales for several treatments per day. Int J Radiat Oncol Biol Phys. 1983;9(2):127–138. doi: 10.1016/0360-3016(83)90089-5. [DOI] [PubMed] [Google Scholar]
- 6.Sheline GE, Wara WM, Smith V. Therapeutic irradiation and brain injury. Int J Radiat Oncol Biol Phys. 1980;6(9):215–1228. doi: 10.1016/0360-3016(80)90175-3. [DOI] [PubMed] [Google Scholar]
- 7.Schultheiss TE, Kun LE, Ang KK, Stephens LC. Radiation response of the central nervous system. Int J Radiat Oncol Biol Phys. 1995;31(5):1093–1112. doi: 10.1016/0360-3016(94)00655-5. [DOI] [PubMed] [Google Scholar]
- 8.Fowler JF. The linear-quadratic formula and progress in fractionated radiotherapy. Br J Radiol. 1989;62(740):679–694. doi: 10.1259/0007-1285-62-740-679. [DOI] [PubMed] [Google Scholar]
- 9.Lawrence YR, Li XA, el Naqa I, Hahn CA, Marks LB, Merchant TE, Dicker AP. Radiation dose–volume effects in the brain. Int J Radiat Oncol Biol Phys. 2010;76(3):S20–S27. doi: 10.1016/j.ijrobp.2009.02.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stupp R, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 11.Nelson DF, et al. Hyperfractionated radiation therapy and bis-chlorethyl nitrosourea in the treatment of malignant glioma—possible advantage observed at 72.0 Gy in 1.2 Gy BID fractions: report of the Radiation Therapy Oncology Group Protocol 8302. Int J Radiat Oncol Biol Phys. 1993;25(2):193–207. doi: 10.1016/0360-3016(93)90340-2. [DOI] [PubMed] [Google Scholar]
- 12.Werner-Wasik M, Scott CB, Nelson DF, Gaspar LE, Murray KJ, Fischbach JA, Nelson JS, Weinstein AS, Curran WJ., Jr Final report of a phase I/II trial of hyperfractionated and accelerated hyperfractionated radiation therapy with carmustine for adults with supratentorial malignant gliomas: Radiation therapy oncology group study 83-02. Cancer. 1996;77(8):1535–1543. doi: 10.1002/(SICI)1097-0142(19960415)77:8<1535::AID-CNCR17>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 13.Curran W, et al. No survival benefit of hyperfractionated radiotherapy (RT) to 72.0 Gy and carmustine versus standard RT and carmustine for malignant glioma patients: preliminary results of RTOG 90-06. J Clin Oncol. 1996;15(Suppl):154. [Google Scholar]
- 14.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457–481. [Google Scholar]
- 15.Kalbfleisch JD, Prentice RL. Statistical Analysis of Failure Time Data. New York: Wiley; 1980. [Google Scholar]
- 16.Cox DR. Regression models and life-tables. J R Stat Soc Ser B. 1972;34:187–202. [Google Scholar]
- 17.Curran WJ, Jr, et al. Recursive partitioning analysis of prognostic factors in three radiation therapy oncology group malignant glioma trials. J Natl Cancer Inst. 1993;85(9):704–710. doi: 10.1093/jnci/85.9.704. [DOI] [PubMed] [Google Scholar]
- 18.Scott CB, Curran W, Yung W, Scarantino C, Urtasun R, Movsas B, Jones C, Simpson J, Fischbach A, Petito C, Nelson J. Long term results of RTOG 9006: a randomized trial of hyperfractioanted radiotherapy (RT) to 72.0 Gy and carmustine vs. standard RT and carmustine for malignant glioma patients with emphasis on anaplastic astrocytoma patients (Abstract) InProc Am Soc Clin Oncol. 1998;17:401. [Google Scholar]
- 19.Scott CB, Scarantino C, Urtasun R, Movsas B, Jones CU, Simpson JR, Fischbach AJ, Curran WJ., Jr Validation and predictive power of radiation therapy oncology group (RTOG) recursive partitioning analysis classes for malignant glioma patients: a report using RTOG 90-06. Int J Radiat Oncol Biol Phys. 1998;40(1):51–55. doi: 10.1016/s0360-3016(97)00485-9. [DOI] [PubMed] [Google Scholar]
- 20.Lawrence YR, Wang M, Dicker AP, Andrews D, Curran WJ, Jr, Michalski JM, Souhami L, Yung WK, Mehta M. Early toxicity predicts long-term survival in high-grade glioma. Br J Cancer. 2011;104(9):1365–1371. doi: 10.1038/bjc.2011.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choucair A, Moughan J, Schultz C, Schulsinger A, Mehta M, Curran W. Prognostic value of H-MLH1 after adjusting for RPA class in GBM patients. Front Biosci. 2011;3:1182–1191. doi: 10.2741/e321. [DOI] [PubMed] [Google Scholar]
- 22.Li J, Wang M, Won M, Shaw EG, Coughlin C, Curran WJ, Jr, Mehta MP. Validation and simplification of the radiation therapy oncology group recursive partitioning analysis classification for glioblastoma. Int J Radiat Oncol Biol Phys. 2011;81(3):623–630. doi: 10.1016/j.ijrobp.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blumenthal DT, Won M, Mehta MP, Curran WJ, Souhami L, Michalski JM, Rogers CL, Corn BW. Short delay in initiation of radiotherapy may not affect outcome of patients with glioblastoma: a secondary analysis from the radiation therapy oncology group database. J Clin Oncol. 2009;27(5):733–739. doi: 10.1200/JCO.2008.18.9035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chakravarti A, Seiferheld W, Tu X, Wang H, Zhang HZ, Ang KK, Hammond E, Curran W, Jr, Mehta M. Immunohistochemically determined total epidermal growth factor receptor levels not of prognostic value in newly diagnosed glioblastoma multiforme: report from the radiation therapy oncology group. Int J Radiat Oncol Biol Phys. 2005;62(2):318–327. doi: 10.1016/j.ijrobp.2004.10.037. [DOI] [PubMed] [Google Scholar]
- 25.Chinnaiyan P, Wang M, Rojiani AM, Tofilon PJ, Chakravarti A, Ang KK, Zhang HZ, Hammond E, Curran W, Jr, Mehta MP. The prognostic value of nestin expression in newly diagnosed glioblastoma: report from the radiation therapy oncology group. Radiat Oncol. 2008;3:32. doi: 10.1186/1748-717X-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lustig RA, Scott CB, Curran WJ. Does stereotactic eligibility for the treatment of glioblastoma cause selection bias in randomized studies? Am J Clin Oncol. 2004;27(5):516–521. doi: 10.1097/01.coc.0000135641.82026.c4. [DOI] [PubMed] [Google Scholar]
- 27.Prados MD, Scott C, Curran WJ, Jr, Nelson DF, Leibel S, Kramer S. Procarbazine, lomustine, and vincristine (PCV) chemotherapy for anaplastic astrocytoma: a retrospective review of radiation therapy oncology group protocols comparing survival with carmustine or PCV adjuvant chemotherapy. J Clin Oncol. 1999;17(11):3389–3395. doi: 10.1200/JCO.1999.17.11.3389. [DOI] [PubMed] [Google Scholar]


