Abstract
Objective
Chronic Pain (CP) is a disabling illness, often comorbid with depression. We performed a randomized controlled pilot study on mindfulness-based cognitive therapy (MBCT) targeting depression in a CP population.
Methods
Participants with CP lasting ≥ 3 months, DSM-IV Major Depressive Disorder (MDD), Dysthymic Disorder, or Depressive disorder NOS, and a Quick Inventory of Depression scale (QIDS-C16) score ≥ 6 were randomized to MBCT (n = 26) or waitlist (n = 14). We adapted the original MBCT intervention for depression relapse prevention by modifying the psychoeducation and Cognitive Behavioral Therapy (CBT) elements to an actively depressed chronic pain population. We analyzed an intent-to treat (ITT) and a per protocol sample; the per protocol sample included participants in the MBCT group who completed at least 4 out of 8 sessions. The change in the QIDS-C16 and Hamilton Rating Sale for Depression (HRSD17) were the primary outcome measures. Pain, quality of life and anxiety were secondary outcome measures. Data collection took place between January 2012 and July 2013.
Results
Nineteen (73%) participants completed the MBCT program. No significant adverse events were reported in either treatment group. ITT analysis (n=40) revealed no significant differences. Repeated measures ANOVAs for the per protocol sample (n=33) revealed a significant treatment × time interaction (F (1, 31) = 4.67, p = 0.039, η2p = 0.13) for the QIDS-C16, driven by a significant decrease in the MBCT group (t (18) = 5.15, p < 0.001, d = 1.6), but not in the control group (t (13) = 2.01, p = 0.066). The HRSD17 scores did not differ significantly between groups. The study ended before the projected sample size was obtained, which might have prevented effect detection in some outcome measures.
Conclusions
MBCT shows potential as a treatment for depression in individuals with CP, but larger controlled trials are needed.
Trial registration
www.clinicaltrials.gov. Identifier: NCT01473615
INTRODUCTION
Chronic Pain (CP) is a highly prevalent, costly condition that poses a substantial burden on patients, their significant others, and society. In an internet-based survey, the prevalence of CP in the United States was calculated to be 30.7%, meaning that approximately 1 in 3 Americans lives with chronic pain (Johannes et al., 2010).
Chronic pain has high comorbidity with psychiatric disorders (Demyttenaere et al., 2007). Depression is the most common of these, with a mean prevalence rate of comorbid major depressive disorder (MDD) in patients with chronic pain ranging from 18 % in population-based settings up to 85 % in specialized pain clinics (Bair et al., 2003). Patients with chronic pain and comorbid depression experience greater pain intensity, greater interference due to pain, and more pain behaviors, specifically including Affective Distress and Facial/Audible Expressions as measured by the Pain Behavior Check List (Haythornthwaite et al., 1991; Kerns et al., 1991). In addition, depression is associated with poorer occupational (Sullivan et al., 1992) and social function (Holroyd et al., 2000), increased health care utilization (Engel et al., 1996), and increased risk of attempted and completed suicide (Tang & Crane, 2006). Treatment of depression in people with chronic pain is therefore a major public health imperative.
Recently there has been an increasing interest in mindfulness-based therapies, such as mindfulness-based stress reduction (MBSR) (Kabat-Zinn, 1982) and the adapted program for depression relapse prevention, mindfulness-based cognitive therapy (MBCT) (Segal et al., 2002). Mindfulness-based interventions teach participants meditation techniques that increase awareness of current moment experience and promote an accepting attitude towards oneself (Bishop et al., 2004). These techniques are believed to help participants disengage from dysfunctional automatic thinking patterns and create a more accepting stance towards physical and emotional pain (Kabat-Zinn, 1990). MBSR was originally developed for stress reduction in a chronic medically ill population and it does not specifically target depression. MBCT integrates aspects of cognitive behavioral therapy (CBT) and MBSR; it focuses more explicitly on ‘decentering’, which refers to the process of disengagement of negative automatic thoughts, and is associated with a significant reduction in depressive symptoms (Fresco et al., 2007). MBCT has been proved effective for depression relapse prevention (Geschwind et al., 2012; Godfrin & van Heeringen, 2010; W. Kuyken et al., 2008; Willem Kuyken et al., 2015; Ma & Teasdale, 2004; Teasdale et al., 2000) and there is increasing evidence that it also can be effective for active depression (Barnhofer et al., 2009; Eisendrath et al., 2008; van Aalderen et al., 2012). Research in mindfulness-based interventions for chronic pain show promising results, (Day et al., 2014) but there is a lack of well designed studies (Veehof et al., 2011). To our knowledge there are no studies that specifically address the effectiveness of a mindfulness-based intervention for depression in a CP population.
Given what the literature has shown, there is a good chance that integrating aspects of mindfulness and CBT might create synergistic effects in the treatment of depression in patients with chronic pain (Bohlmeijer et al., 2010; Veehof et al., 2011). We therefore developed an MBCT program that specifically targets active depression in individuals with CP and carried out a randomized controlled pilot trial of this intervention. We predicted that MBCT would be a feasible and effective intervention in our sample, with minimal side effects and a retention rate of 70 to 80%. We hypothesized that participants who completed a predetermined “minimum effective dose” of at least 4 of 8 MBCT sessions as proposed by Teasdale et al. (2000) would demonstrate a significant decrease in depressive symptoms as measured on the Quick Inventory of Depressive Symptomatology – Clinician rated (QIDS-C16) and the Hamilton Rating Scale for Depression (HRSD17), compared to the control condition.
METHODS
Patient Recruitment and Inclusion/Exclusion Criteria
The study was carried out at the Depression Clinical and Research Program (DCRP) of the Massachusetts General Hospital (MGH). Participants were recruited by clinician referral from outpatient clinics that manage chronic pain, and by web-based advertisements. At the screening visit participants signed an informed consent approved by our Institutional Review Board and were administered the Structured Clinical Interview for DSM-IV (SCID) (First, 1994).
Inclusion criteria included: a) Age ≥18 years; b) The presence of chronic pain, which has persisted for at least 3 months; c) Meeting criteria for Major Depressive Disorder, Dysthymic Disorder, or Depressive Disorder Not Otherwise Specified (NOS) as defined by DSM-IV criteria; d) A score ≥10 on the QIDS-C16 scale. After initiating the study, the cutoff was reduced to a QIDS-C16 score ≥6 (indicative of at least mild depressive symptoms) to allow more ample recruitment. Concurrent psychotherapy, psychopharmacotherapy and chronic pain treatments were allowed, though subjects were asked to make as few changes as possible in their psychotherapy treatment and stay on a stable dose of psychotropics/analgesics as much as possible for the duration of the study and for 8 weeks prior to the study.
Exclusion criteria included: a) A primary diagnosis other than Major Depressive Disorder, Dysthymic Disorder, Depressive Disorder NOS, or any history of psychosis or mania; b) Substance abuse or dependence within the last 3 months; c) Serious medical conditions (e.g. poorly controlled diabetes, severe congestive heart failure) that had not been stable for at least 3 months; d) Current active suicidal or self-injurious potential necessitating immediate treatment; e) General conditions that would impede participation in a group intervention, as assessed by the evaluating clinician (e.g., severe personality disorders, cognitive impairment, tendencies toward physical aggression); f) Significant current meditation practice, specifically more than three hours of insight/mindfulness/vipassana meditation per week.
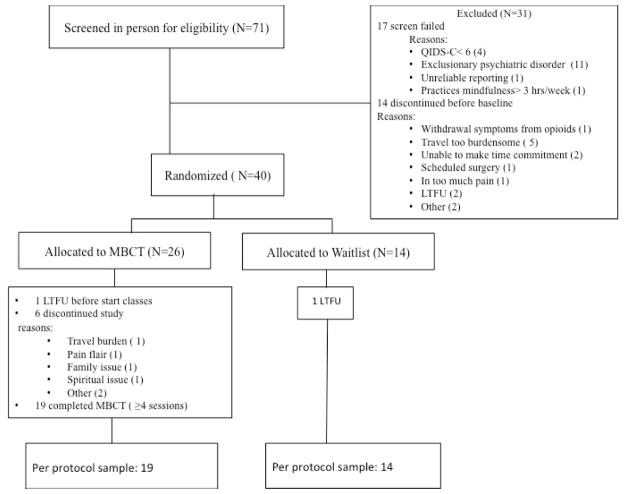
A total of 71 participants were screened, of whom 40 were ultimately randomized, as described in the Results section (Figure 1). Participants were reimbursed $ 40 for their participation. The MBCT program was provided free of charge. The study was approved by the Partners Human Research Committee, Massachusetts General Hospital (protocol 2011-P-001699/1).
Figure 1.
CONSORT Chart
Abbrevations: MBCT=Mindfulness-Based Cognitive Therapy, QIDS-C16= 16 item Quick Inventory of Depressive Symptoms-Clinician Rated.
Treatment Assignment
Participants who fulfilled the inclusion criteria were randomly allocated to MBCT in addition to their treatment as usual (TAU) or else to continue with their TAU, if any (waitlist control condition). The randomization ratio for waitlist control to MBCT was 1:2, which allowed us to fill the MBCT groups with participants more quickly. In order to assure equal gender distribution in both groups we stratified for gender.
Intervention
The intervention was based on the MBCT program developed by Segal et al (2013; 2002). The program combines elements of CBT with a ‘mindful’ approach to thoughts and feelings, characterized by non-judgmental awareness of internal experience, including a significant meditation component. MBCT comprises a manualized 8-week group skills program with sessions that each last 2 hours. The program includes daily homework exercises, which mainly consist of guided or unguided mindfulness techniques. We adapted the original program to our specific population by modifying the psychoeducation and CBT elements to a depressed CP population. This adaptation included psycho-education linking CP, negative thoughts, negative emotions, and depressive behaviors such as withdrawal; identifying automatic thoughts related to CP; and paying attention to behavioral elements such as pacing of activities. We also included meditations that specifically focused on cultivating mindfulness in relationship to CP.
The MBCT intervention was delivered at MGH in classes of 7 to 12 persons and was taught by an experienced licensed independent clinical social worker (LICSW) with advanced clinical training in therapeutic mindfulness and a fellow in psychology.
Waitlist
Participants who were randomly assigned to the waitlist control were offered the MBCT treatment at no costs after completion of the study.
Treatment as usual (TAU)
TAU for MBCT and control subjects included all regular visits with the pain physician, psychiatrist, psychotherapist and prescribed pain and/or antidepressant medications.
Efficacy, Safety, and Feasibility Measures
The change in the QIDS-C16 (Trivedi et al., 2004) and HRSD17 (Hamilton, 1960) scores were the primary outcome measures. Secondary outcome measures were based on the core outcome measures recommended by the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) (Dworkin et al., 2005), including pain, as measured by the Brief Pain Inventory short form (BPI-sf) (Cleeland & Ryan, 1994) and Visual Analogue Scale (VAS) ratings for average last week’s Pain Intensity; (Price et al., 1989) quality of life, as measured by the Short-Form Health Survey (SF-36 version 1.0 RAND)(Hays et al., 1993); and anxiety, as measured by the Beck Anxiety Inventory (BAI) (Beck et al., 1988). Within 2 weeks after the last treatment session, participants rated their subjective impression of improvement by the standardized Patient Global Impression of Change Questionnaire (PGIC) (Hurst & Bolton, 2004). All secondary outcome measures were collected using REDCap electronic data capture tools (Harris et al., 2009).
For safety reasons, all subjects completed a phone assessment every 2 weeks with a study clinician or a clinical fellow, at which time the QIDS-C16 and HRSD17 were administered. Documentation of any side effect or adverse event was completed at every assessment by having the study physician inquire about and record any adverse events experienced by the participants.
Feasibility was assessed descriptively by calculating overall retention rates based on number of classes completed.
Statistical analysis
Sample size calculation
Meta-analyses of mindfulness-based therapy in populations with mood disorders show relatively large effect sizes (Hedges’ g of 0.59) for improving depressive symptoms (Hofmann et al., 2010). Assuming an estimate of a medium effect size (Cohen’s d = 0.5), a total sample size of 46 subjects would provide 90 % power to detect a difference between treatments. Assuming an attrition rate of approximately 20 %, we originally sought to include 60 subjects.
Outcomes
Outcome measures were evaluated separately for an intent-to-treat (ITT) sample (n=40) and a per protocol sample (n= 33). The per protocol sample comprised all waitlist control participants and all MBCT participants who completed a predetermined “minimum effective dose” of at least 4 MBCT sessions as proposed by Teasdale et al. (2000). Because not all per protocol subjects would necessarily be “completers” (i.e. attend all study assessments conducted every 2 weeks), last-observation-carried forward (LOCF) was used for both per protocol and ITT analyses. Baseline characteristics were compared between groups by the independent samples t-test for continuous variables, and by the Chi-square test and Fisher’s exact test for categorical variables. The paired samples t-test was used to compare outcome variables from baseline to end. Repeated measures ANOVA was used to compare changes in depression, pain, anxiety and quality of life. The independent samples t-test was used to compare the PGIC ratings. For all analyses, significance was set as alpha = 0.05 (2-tailed). Statistical procedures were performed with SPSS software, version 22. Data collection took place between January 2012 and July 2013. The study is registered in clinicaltrials.gov under identifier: NCT01473615.
RESULTS
Of the 71 subjects screened, 17 failed to meet inclusion criteria and 14 withdrew before the baseline visit. Figure 1 shows the patient flow from screening to completion of the study, including reasons for screen fail and for early discontinuation. Demographic and clinical characteristics of the ITT sample are shown in Table 1.
Table 1.
Demographic and Clinical Characteristics of the Intent-to-Treat Sample
| Characteristica | All Evaluable Subjects (N=40) | Baseline MBCT (n=26) | Baseline Waitlist (n=14) | P Value (t/ χ2-test) |
|---|---|---|---|---|
| Sex | 0.28 | |||
| Male | 10 (25.0) | 5 (19.2) | 5 (35.7) | |
| Female | 30 (75.0) | 21 (80.8) | 9 (64.3) | |
| Race | 0.60 | |||
| White | 36 (90.0) | 24 (92.3) | 12(85.7) | |
| African-American | 4 (10.0) | 2 (7.7) | 2 (14.3) | |
| Ethnicity | 0.29 | |||
| Hispanic | 2 (5.0) | 1 (3.8) | 1 (7.1) | |
| Non-Hispanic | 34 (85.0) | 21 (80.8) | 13 (92.9) | |
| Not reported | 4 (10.0) | 4 (15.4) | 0 (0) | |
| Marital Status b | 0.91 | |||
| Married/live together | 15 (48.4) | 9 (50.0) | 6 (46.2) | |
| Separated/widowed/divorced | 6 (19.4) | 3 (16.7) | 3 (23.1) | |
| Never Married | 10 (32.3) | 6 (33.3) | 4 (30.8) | |
| Employment Status c | 0.75 | |||
| Employed | 11(30.6) | 8 (34.8) | 3 (23.1) | |
| Disabled | 13 (36.1) | 8 (34.8) | 5 (38.5) | |
| Other | 12 (33.3) | 7 (30.4) | 5 (38.5) | |
| Depression Diagnosis | 1.0 | |||
| Major depressive disorder | 34 (85.0) | 22 (84.6) | 12 (85.7) | |
| Not otherwise specified | 6 (15.0) | 4 (15.4) | 2 (14.3) | |
| Antidepressant medication use b | 18 (48.6) | 11 (44.0) | 7 (58.3) | 0.32 |
| Age, mean (SD), y | 50.7 (11.4) | 51.3 (11.9) | 49.9 (11.1) | 0.75 |
| Years of Education, mean (SD) b | 16.5 (2.5) | 16.4 (2.6) | 16.6 (2.5) | 0.82 |
Values shown as n (%) unless otherwise specified
based on N=37 due to 3 missing values.
based on N=36 due to 4 missing values.
Abbreviation: MBCT = Mindfulness-Based Cognitive Therapy
All 40 participants randomized (26 in the intervention arm and 14 in the control group) were evaluable by ITT for the primary outcome measures.
Of the 26 participants who started the MBCT classes, 19 (73 %) completed the “minimum effective dose” of 4 MBCT sessions (mean attendance was 7 of 8 classes). In total, 33 participants (including the aforementioned 19 subjects in the intervention arm and 14 in the control group) were evaluable by per protocol analysis for the primary outcome measures (6 of these had baseline QIDSC scores between 6 and 9 per the modified entry criteria). Due to both technical and compliance problems with the REDCap surveys, fewer participants were evaluable for the secondary outcome measures (Tables 2 and 3).
Table 2.
Baseline and Endpoint Data on Outcome Measures for the Intent-to-Treat Sample a
| Outcome Measure | All Evaluable Subjects (N = 40) | Baseline | Endpoint | Significance of Time-by Treatment Interaction, F (df) | P | ||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| MBCT (n = 26) | Waitlist (n = 14) | MBCT | Waitlist | ||||
| QIDS-C16 | 12.6 (3.5) | 13.1 (3.4) | 11.5 (3.7) | 9.4 (6.1) | 9.5 (3.7) | 1.31 (1,38) | 0.26 |
| HRSD17 | 18.6 (4.9) | 18.9 (4.6) | 18.1 (5.6) | 14.9 (8.1) | 15.6 (4.9) | 0.50 (1,38) | 0.48 |
| VAS Average Pain Intensity | 6.1 (1.5) | 6.1 (1.6) | 5.9 (1.4) | 5.6 (1.7) | 5.6 (2.3) | 0.09 (1,38) | 0.77 |
| BPI Pain Interference b | 6.7 (2.2) | 6.7 (2.1) | 6.9 (2.4) | 6.0 (2.0) | 6.4 (2.4) | 0.11 (1,32) | 0.74 |
| BAI c | 35.5 (10.1) | 36.8 (11.3) | 33.0 (7.1) | 33.2 (8.8) | 33.2 (6.6) | 2.32 (1,33) | 0.14 |
| SF-36 Physical Functioning c | 46.9 (24.3) | 48.0 (24.9) | 44.6 (24.1) | 52.4 (26.0) | 44.2 (24.6) | 1.36 (1,33) | 0.25 |
| SF-36 Role Limitations Physical c | 10.7 (24.5) | 14.1 (29.0) | 4.1 (9.7) | 17.4 (33.2) | 12.5 (29.2) | 0.24 (1,33) | 0.63 |
| SF-36 Role Limitations Emotional c | 21.0 (32.4) | 21.7 (29.5) | 19.4 (38.8) | 33.3 (41.4) | 22.2 (29.6) | 0.43 (1,33) | 0.52 |
| SF-36 Vitality c | 18.7 (17.5) | 13.7 (11.0) | 28.3 (23.6)† | 21.7 (20.3) | 22.1 (18.0) | 8.45 (1,33) | 0.01* |
| SF-36 Mental Health c | 41.4 (17.8) | 37.7 (18.1) | 48.3 (15.4) | 48.9 (21.0) | 47.7 (19.9) | 5.10 (1,33) | 0.03* |
| SF-36 Social Functioning c | 36.4 (22.9) | 33.6 (19.6) | 41.7 (28.4) | 42.7 (25.4) | 40.0 (25.7) | 1.80 (1,33) | 0.19 |
| SF-36 Pain c | 29.3 (16.7) | 29.1 (16.8) | 29.6 (17.2) | 31.0 (20.1) | 39.8 (24.4) | 4.34 (1,33) | 0.05 |
| SF-36 General Health c | 38.4 (18.1) | 36.1 (18.6) | 42.9 (16.8) | 40.0 (23.2) | 47.1 (18.9) | 0.00 (1,33) | 0.96 |
| PGICd | … | … | … | 2.7 (1.0) | 4.0 (0.9) | … | … |
Values shown as mean (SD).
based on N= 34 due to 6 missing values.
based on N=35 due to 5 missing values.
based on N= 27 due to 13 missing values.
Statistically significant time x treatment interaction.
P < .05 vs MBCT group at baseline.
Abbreviations: QIDS-C16 = 16 item Quick Inventory of Depressive Symptoms-Clinician Rated, HRSD17 = 17-item Hamilton Depression Rating Scale, VAS = Visual Analogue Scale., BPI = Brief Pain Inventory, BAI = Beck Anxiety Inventory, MBCT = Mindfulness-Based Cognitive Therapy, PGIC = Patient Global Impression of Change Questionnaire. SF-36 = 36-item Short-Form Health Survey.
Symbol: …= not applicable.
Table 3.
Baseline and End Point Data on Outcome Measures for the Per- Protocol Samplea
| Outcome Measure | All Evaluable Subjects (N = 33) | Baseline | Endpoint | Significance of Time-by Treatment Interaction, F (df) | P | ||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| MBCT (n = 19) | Waitlist (n = 14) | MBCT | Waitlist | ||||
| QIDS-C16 | 11.9 (3.2) | 12.3 (2.9) | 11.5 (3.7) | 7.2 (3.5) | 9.5 (3.7) | 4.67 (1,31) | 0.04* |
| HRSD17 | 18.0 (5.1) | 18.0(4.8) | 18.1 (5.6) | 12.8 (6.7) | 15.6 (4.5) | 1.53 (1,31) | 0.23 |
| VAS Average Pain Intensity | 5.9 (1.6) | 6.0 (1.7) | 5.9 (1.4) | 5.5 (1.7) | 5.6 (2.3) | 0.05 (1,31) | 0.83 |
| BPI Pain Interference b | 6.7 (2.2) | 6.6 (2.2) | 6.9 (2.4) | 5.7 (2.0) | 6.3 (2.4) | 0,55 (1,27) | 0.47 |
| BAI c | 34.4 (9.5) | 35.4 (10.9) | 33.0 (7.1) | 30.7 (5.9) | 33.2 (6.6) | 3.03 (1,28) | 0.10 |
| SF-36 Physical Functioning c | 46.2 (24.4) | 47.2 (25.2) | 44.6 (24.1) | 52.8 (26.6) | 44.1 (24.6) | 1.71 (1,28) | 0.20 |
| SF-36 Role Limitations Physical c | 12.5 (26.1) | 18.1 (31.9) | 4.1 (9.7) | 22.2 (36.3) | 12.5 (29.2) | 0.12 (1,28) | 0.73 |
| SF-36 Role Limitations Emotional c | 22.2 (34.3) | 24.1 (32.0) | 19.4 (38.8) | 38.9 (44.6) | 22.2 (29.6) | 0.64 (1,28) | 0.43 |
| SF-36 Vitality c | 21.7 (17.2) | 17.2 (9.7) | 28.3 (23.6)† | 27.5 (19.3) | 22.1 (18.0) | 9.37 (1,28) | 0.01* |
| SF-36 Mental Health c | 44.3 (16.6) | 41.5 (17.3) | 48.3 (15.4) | 55.8 (16.8) | 47.7 (19.9) | 7.09 (1,28) | 0.01* |
| SF-36 Social Functioning c | 38.9 (23.1) | 37.1 (19.5) | 41.7 (28.4) | 48.8 (24.5) | 40.0 (25.7) | 2.20 (1,28) | 0.15 |
| SF-36 Pain c | 29.0 (16.7) | 28.6 (16.7) | 29.6 (17.2) | 30.1 (21.1) | 39.8 (24.4) | 3.00 (1,28) | 0.10 |
| SF-36 General Health c | 39.3 (17.5) | 36.9 (18.0) | 42.9 (16.8) | 41.9 (23.5) | 47.1 (18.9) | 0.02 (1,28) | 0.89 |
| PGICd | … | … | … | 2.7 (1.0) | 4.0 (0.9) | … | … |
Values shown as mean (SD).
based on N= 29 due to 4 missing values.
based on N=30 due to 3 missing values.
based on N= 27 due to 6 missing values.
Statistically significant time x treatment interaction.
Abbreviations. QIDS-C16 = 16 item Quick Inventory of Depressive Symptoms-Clinician Rated, HRSD17 = 17 –item Hamilton Depression Rating Scale, VAS = Visual Analogue Scale, BPI = Brief Pain Inventory, BAI = Beck Anxiety Inventory, PGIC = Patient Global Impression of Change Questionnaire, SF-36 = 36-item Short-Form Health Survey, MBCT = Mindfulness-Based Cognitive Therapy.
Symbol: … = not applicable.
There were no significant adverse events (AEs) in either treatment arm, except for 1 participant in the intervention group who discontinued because she had spiritual issues, possibly related to the treatment. Other AEs reported included bronchitis, nausea and urinary burning, which were not related to the intervention.
Independent samples t –test, Chi-square and Fischer’s exact test yielded no statistically significant differences in baseline demographic and clinical characteristics between the groups (Table 1). The SF-36 subscales, except for ‘vitality’ (lower in the intervention group), showed no statistically significant differences between the groups (Table 2). The study population suffered from a wide variety of pain conditions, including chronic back pain, migraines, neuropathic pain, osteoarthritis and fibromyalgia. Eighty-five percent of the participants suffered from MDD, and 15 % from Minor Depressive Disorder (within the broader category of Depressive Disorder NOS). None of the participants suffered from Dysthymic Disorder. Forty-nine percent of the participants were taking antidepressant medication, including monotherapy with serotonin-norepinephrine reuptake inhibitors (SNRI’s) (18.9%), tricyclic antidepressants (5.4%), selective serotonin reuptake inhibitors (SSRI’s) (2.7 %) and other antidepressants including bupropion and trazodone (8.1%), or combined pharmacotherapy (13.5%).
Primary outcome measures for intent-to-treat sample
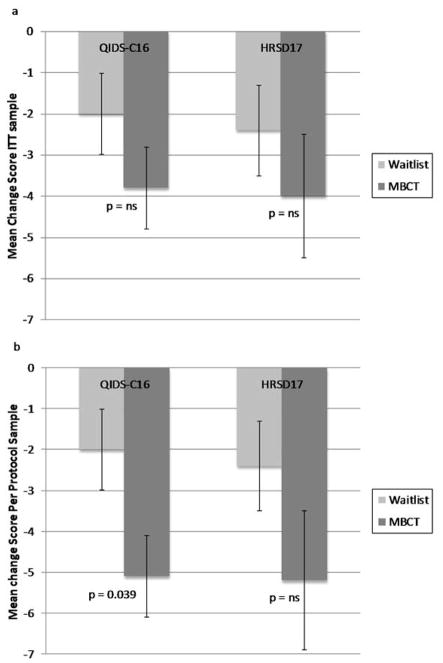
Clinical improvement measured by both the QIDS-C16 and the HRSD17 was greater for the MBCT group than for the control group, but the difference between groups did not reach statistical significance (Figure 2a).
Figure 2.
Depression rating scale score changes. Intent to treat sample (a) and per protocol sample (b).
Error bars are ± 1 SEM ; p - values refer to group by time interaction effects as revealed by repeated -measures ANOVAs.
Abbreviations: HDRS 17 = 17-item Hamilton Depression Rating Scale, ITT = intent to treat, MBCT = mindfulness-based cognitive therapy, NS = nonsignificant,, QIDS-C16 = 16-item Quick Inventory of Depressive Symptoms-Clinician Rated.
Primary outcome measures for per protocol sample
In the per protocol sample, repeated measures ANOVA with time (baseline and endpoint) as the repeated measure, treatment arm as the between-subjects factor, and the QIDS-C16 score as the dependent variable, revealed a significant time × treatment interaction (F (1, 31) = 4.67, p = 0.039, η2p = 0. 13) for the QIDS-C16, driven by a significant decrease in the MBCT (t (18) = 5.15, p < 0.001, d=1.6), but not in the control group (t (13) = 2.01, p = 0.066) (Figure 2b). The Cohen’s d effect size of the improvement on the QIDSC over time for MBCT relative to waiting list control was 0.77. Clinical improvement measured by the HRSD17 was also greater for the MBCT group than for the control group, but the difference between groups did not reach statistical significance (Figure 2b).
Secondary outcome measures for intent-to-treat sample
The secondary outcome measures revealed no significant findings on time × treatment interaction for pain (VAS ratings Pain Intensity and BPI pain interference) and anxiety (BAI). For quality of life, as measured by the SF-36, two health dimensions showed a significant time x group interaction; mental health (F (1, 33) = 5.10, p = 0.031, η2p = 0.13) driven by a significant increase in the MBCT group (t (22) = 3.40, p = 0.003, d = 0.57), but not in the control group (t (11) = −0.19, p = 0.854); and vitality (F (1, 33) = 8.45, p = 0.006, η2p = 0.20), driven by a significant increase in the MBCT group (t (22) = 2.59, p = 0.017, d = 0.50), but not in the control group (t (11 ) = −1.92, p = 0.082); however, because the MBCT group had a significantly lower vitality score at baseline, this positive interaction might be due to regression to the mean. All other measured health dimensions revealed no significant differences between groups. Participants who had received MBCT treatment reported a significantly higher subjective impression of clinical improvement (mean = 2.7 or “minimally improved”-“much improved”) compared to control subjects (mean = 4.0 or “no change”) (t (25) = 3.47, p = 0.002).
Secondary outcome measures for per protocol sample
The secondary outcome measures revealed no significant findings on time × treatment interaction for pain (VAS ratings Pain Intensity and BPI pain interference) and anxiety (BAI). For quality of life, as measured by the SF-36, two health dimensions showed a significant time x group interaction; mental health (F (1, 28) = 7.09, p = 0.013, η2p = 0. 20), driven by a significant increase in the MBCT group (t (17) = −3.650, p = 0.002, d = 0.83), but not in the control group (t (11), p = 0.854); and vitality (F (1, 28) = 9.37, p = 0.005, η2p = 0.25), driven by a significant increase in the MBCT group (t (17) = −2.69, p = 0.016, d = 0.68), but not in the control group (t (11) = 1.92, p = 0.082). All other measured health dimensions revealed no significant differences between groups. Participants who had received MBCT treatment reported a significantly higher subjective impression of clinical improvement (mean=2. 5 or “minimally improved”-“much improved”) compared to controls (mean =4.0 or “no change”) (t (23) = 3.696, p = 0.001).
DISCUSSION
This pilot study shows positive outcomes for MBCT for depression (in the per protocol sample) and mental health (both in the ITT and the per protocol sample), but not for pain intensity. This finding is in line with the treatment goal of our MBCT program and psychotherapeutic interventions of CP in general, which is not to remove or reduce the pain itself, but rather to find ways of managing the pain and the adverse consequences on mental health and quality of life. Interestingly, a recent randomized controlled trial from van Ravesteijn et al. (2013) focusing on MBCT for patients with medically unexplained symptoms, including CP, also found significant improvement on mental health functioning, in particular with regard to vitality, but no significant findings on physical health and bodily pain.
While pain has both physical and psychological components, MBCT may address primarily the psychological components, by minimizing reactions to physical pain, such as worrying, catastrophizing, avoidance, and self-blaming. That this study shows no effects at all on pain intensity was somewhat unexpected, given that meta-analyses of psychological therapies typically do show small to moderate effects on pain intensity (Veehof et al., 2011; Williams et al., 2012). However, the most recent Cochrane meta-analysis on psychological therapies for CP found the strongest effects on mood, and the weakest effects on pain (Williams et al., 2012).
Because this pilot study had only a small sample size, we might have had just enough power to detect effects on mood in the per protocol sample, but may have been underpowered to detect the smaller effects on pain intensity. Larger studies should help to answer this question.
The measurement of depression in a chronic pain population is particularly challenging because chronic pain can cause many symptoms that are also common in depression (Wilson et al., 2001). In this study, the decrease of depression severity in the MBCT completers was only significant only in the per-protocol sample as measured by the QIDS-C16. Interestingly, HRSD17 scores did not decrease significantly. This finding might be explained by the fact that the HRSD17, as opposed to the QIDS-C16, has a larger emphasis on somatic symptoms such as insomnia, which are less likely to change in a chronic pain population. Despite the limited research on the application of depression rating scales in chronic pain populations, there is some evidence that a substantial number of HRSD17 items do not seem to track changes in depression in CP populations (Moran & Mohr, 2005). To our knowledge, no study has been carried out to validate the QIDS-C16 in a medically ill population. Because the QIDS-C16 has less emphasis on somatic symptoms, it might be less prone to confounding by nonspecific symptoms in a CP population. As a post hoc analysis, we examined a sub portion of the HRSD17 including only psychological symptoms (mood, guilt, suicide, anxiety psychic and insight). Improvement in these selective symptoms was greater for the intervention group than for the control group, but did not reach significance (data not shown). With respect to anxiety, a similar explanation might have played a role in the non-significant findings, because the BAI also emphasizes somatic items (O Donnchadha et al., 2013). Hoge et al. (2013) reported similar concerns regarding the Hamilton Anxiety Scale.
As expected, tolerability and acceptability were good, with almost no adverse effects and a retention rate of 73%, which can be considered high in a population suffering from chronic pain (Turk & Rudy, 1990).
This pilot study had several limitations. Due to various challenges in recruitment, we were unable to obtain the full complement of participants that we hoped for, resulting in underpowering. Also, this study was initially powered according to a 1:1 randomization ratio, but due to recruitment challenges we switched to a randomization ratio of 2 MBCT subjects for every control subject, which resulted in a greater loss of statistical power. In addition, design sensitivity was reduced by lowering the symptom threshold to a level of mild depressive symptoms (QIDS-C16 score ≥6). However, despite the fact that there might be less room for improvement (floor effect), the inclusion of less severely depressed individuals adds to the generalizability of our results to chronic pain populations in general with varying levels of depression severity. Moreover, the lower symptom threshold is more consistent with our inclusion diagnoses (MDD and depressive disorder NOS (including minor depression). These methodological shortcomings might have prevented detection of relevant effects on some clinical outcome measures. For example, the SF-36 yielded mixed results, with benefits primarily in psychological measures, such as mental health and vitality, but less impact on physical domains such as pain and physical functioning. Nonetheless, this result is consistent with the rest of the findings, which favor impact on depression rather than pain.
The second major limitation is the heterogeneity of the population regarding chronic pain conditions and depressive disorders, as well as treatments and prescribed medications, which may confound the results. In addition, some participants did change their medications and other concurrent treatments during the course of the study, and this too may have impacted the findings. Because of our small sample size and variability in treatment changes, we did not attempt to correct for concomitant medications and treatments. Future larger investigations should incorporate more rigorous detailing and controlling for concomitant medications and other therapies. Because data were missing on some of the secondary outcome measures, this too may have prevented more robust findings of effect.
Finally, because of the pilot nature of this study, we did not include an active comparison group but used a waitlist control group, which does not adequately control for “placebo effects” induced by non-specific factors such as attention in patient-provider interactions (Goyal et al., 2014). We did try to control for attention as much as possible by keeping all office and phone visits with the clinician equal in both the MBCT group and the control group.
It is nonetheless promising that despite these limitations, we found a significant decrease of depressive symptoms as noted with the QIDS-C16 in the per protocol analysis, with a large effect size. Also, patients in the MBCT group reported a significantly higher subjective impression of clinical improvement compared to controls, indicating a subjective improvement in all components of their health experience. These findings support our hypothesis that integrating CBT and mindfulness might create a synergistic effect and result in improved outcomes, at least with regard to depression.
In summary, we have obtained preliminary evidence that MBCT may be a feasible and potentially effective intervention for treating depression in patients with CP, based on positive findings in 1 of 2 primary outcomes in the per protocol analysis, though not in the ITT analysis. While these findings should be interpreted with caution, they support follow-up investigation, particularly because depression is a highly debilitating and difficult to treat condition that, when present in a chronic pain population, complicates the treatment and outcome of CP. Adding MBCT to the available treatments may widen the therapeutic scope of potential interventions. Finally, because MBCT is delivered in a group setting, it may also represent a potentially cost-efficient treatment modality. Future larger randomized controlled trials comparing MBCT to attention control and comparative effectiveness studies of MBCT and CBT in this population are warranted.
Acknowledgments
We would like to thank Kiran K. Hug, B.A. 1, for helping with data entry, Lee Baer, Ph.D. 2 and Alisabet J. Clain, M.S. 2 for assistance with data base conversion and general statistical questions and Egilius Spierings, M.D., Ph.D. 3 for assistance with patient recruitment.
Footnotes
CONFLICTS OF INTEREST AND SOURCE OF FUNDING
Potential Conflicts of Interest
Dr Peeters received financial compensation as an independent symposium speaker from Astra-Zeneca, Eli Lilly, GlaxoSmithKline, Janssen-Cilag, Lundbeck, SCEM, Benecke, Servier. Dr Alpert has received research support from Abbott Laboratories, Alkermes, Lichtwer Pharma GmbH, Lorex Pharmaceuticals; Aspect Medical Systems, Astra-Zeneca, Bristol-Myers Squibb Company, Cephalon, Cyberonics, Eli Lilly & Company, Forest Pharmaceuticals Inc., GlaxoSmithkline, J & J Pharmaceuticals, National Institutes of Health, NARSAD, Novartis, Organon Inc., PamLab, LLC, Pfizer Inc, Pharmavite, Roche, Sanofi/Synthelabo, Solvay Pharmaceuticals, Inc., and Wyeth-Ayerst Laboratories; has participated on advisory boards for or consulted to Eli Lilly & Company, Luye Pharmaceuticals, Pamlab LLC, and Pharmavite LLC.Dr; has received speakers’ honoraria from: Eli Lilly & Company, Xian-Janssen, Organon, Psicofarma, MGH Academy,Reed Medical Education and Primedia, Nevada Psychiatric Association, American Society of Clinical Psychopharmacology, and the American Psychiatric Association and has received editorial fees from Belvoir Publishing. Dr Fava has received research support from Abbot Laboratories; Alkermes, Inc.; American Cyanamid;Aspect Medical Systems; AstraZeneca; Avanir Pharmaceuticals; BioResearch; BrainCells Inc.; Bristol-Myers Squibb; CeNeRx BioPharma; Cephalon; Cerecor; Clintara, LLC; Covance; Covidien; Eli Lilly and Company;EnVivo Pharmaceuticals, Inc.; Euthymics Bioscience, Inc.; Forest Pharmaceuticals, Inc.; FORUM Pharmaceuticals; Ganeden Biotech, Inc.; GlaxoSmithKline; Harvard Clinical Research Institute; Hoffman-LaRoche; Icon Clinical Research; i3 Innovus/Ingenix; Janssen R&D, LLC; Jed Foundation; Johnson & Johnson Pharmaceutical Research & Development; Lichtwer Pharma GmbH; Lorex Pharmaceuticals; Lundbeck Inc.; MedAvante; Methylation Sciences Inc; National Alliance for Research on Schizophrenia & Depression (NARSAD); National Center for Complementary and Alternative Medicine (NCCAM);National Coordinating Center for Integrated Medicine (NiiCM); National Institute of Drug Abuse (NIDA); National Institute of Mental Health (NIMH); Neuralstem, Inc.; Novartis AG; Organon Pharmaceuticals; PamLab, LLC.; Pfizer Inc.; Pharmacia-Upjohn; Pharmaceutical Research Associates., Inc.; Pharmavite® LLC;PharmoRx Therapeutics; Photothera; Reckitt Benckiser; Roche Pharmaceuticals; RCT Logic, LLC (formerly Clinical Trials Solutions, LLC); Sanofi-Aventis US LLC; Shire; Solvay Pharmaceuticals, Inc.; Stanley Medical Research Institute (SMRI); Synthelabo; Takeda Pharmaceuticals;Tal Medical; Wyeth-Ayerst Laboratories; has been an advisor/consultant for Abbott Laboratories; Acadia; Affectis Pharmaceuticals AG; Alkermes, Inc.; Amarin Pharma Inc.; Aspect Medical Systems; AstraZeneca; Auspex Pharmaceuticals; Avanir Pharmaceuticals; AXSOME Therapeutics; Bayer AG; Best Practice Project Management, Inc.; Biogen; BioMarin Pharmaceuticals, Inc.; Biovail Corporation; BrainCells Inc; Bristol-Myers Squibb; CeNeRx BioPharma; Cephalon, Inc.; Cerecor; CNS Response, Inc.; Compellis Pharmaceuticals; Cypress Pharmaceutical, Inc.; DiagnoSearch Life Sciences (P) Ltd.; Dinippon Sumitomo Pharma Co. Inc.; Dov Pharmaceuticals, Inc.; Edgemont Pharmaceuticals, Inc.; Eisai Inc.; Eli Lilly and Company; EnVivo Pharmaceuticals, Inc.; ePharmaSolutions; EPIX Pharmaceuticals, Inc.; Euthymics Bioscience, Inc.; Fabre-Kramer Pharmaceuticals, Inc.; Forest Pharmaceuticals, Inc.; Forum Pharmaceuticals; GenOmind, LLC; GlaxoSmithKline; Grunenthal GmbH; i3 Innovus/Ingenis; Intracellular; Janssen Pharmaceutica; Jazz Pharmaceuticals, Inc.; Johnson & Johnson Pharmaceutical Research & Development, LLC; Knoll Pharmaceuticals Corp.; Labopharm Inc.; Lorex Pharmaceuticals; Lundbeck Inc.; MedAvante, Inc.; Merck & Co., Inc.; MSI Methylation Sciences, Inc.; Naurex, Inc.; Nestle Health Sciences; Neuralstem, Inc.; Neuronetics, Inc.; NextWave Pharmaceuticals; Novartis AG;Nutrition 21; Orexigen Therapeutics, Inc.; Organon Pharmaceuticals; Osmotica; Otsuka Pharmaceuticals; Pamlab, LLC.; Pfizer Inc.; PharmaStar; Pharmavite® LLC.; PharmoRx Therapeutics; Precision Human Biolaboratory; Prexa Pharmaceuticals, Inc.; PPD; Puretech Ventures; PsychoGenics; Psylin Neurosciences, Inc.; RCT Logic, LLC ( formerly Clinical Trials Solutions, LLC); Rexahn Pharmaceuticals, Inc.; Ridge Diagnostics, Inc.; Roche; Sanofi-Aventis US LLC.; Sepracor Inc.; Servier Laboratories; Schering-Plough Corporation; Solvay Pharmaceuticals, Inc.; Somaxon Pharmaceuticals, Inc.; Somerset Pharmaceuticals, Inc.; Sunovion Pharmaceuticals; Supernus Pharmaceuticals, Inc.; Synthelabo; Taisho Pharmaceutical; Takeda Pharmaceutical Company Limited; Tal Medical, Inc.; Tetragenex Pharmaceuticals, Inc.; TransForm Pharmaceuticals, Inc.; Transcept Pharmaceuticals, Inc.; Vanda Pharmaceuticals, Inc.; VistaGen; has received speaking/publishing fees from Adamed, Co; Advanced Meeting Partners; American Psychiatric Association; American Society of Clinical Psychopharmacology; AstraZeneca; Belvoir Media Group; Boehringer Ingelheim GmbH; Bristol-Myers Squibb; Cephalon, Inc.; CME Institute/Physicians Postgraduate Press, Inc.; Eli Lilly and Company; Forest Pharmaceuticals, Inc.; GlaxoSmithKline; Imedex, LLC; MGH Psychiatry Academy/Primedia; MGH Psychiatry Academy/Reed Elsevier; Novartis AG; Organon Pharmaceuticals; Pfizer Inc.; PharmaStar; United BioSource,Corp. and Wyeth-Ayerst Laboratories; has equity holdings in Compellis and PsyBrain, Inc; holds a patent for Sequential Parallel Comparison Design (SPCD), licensed by MGH to Pharmaceutical Product Development, LLC (PPD); and a patent application for a combination of Ketamine plus Scopolamine in Major Depressive Disorder (MDD), licensed by MGH to Biohaven; holds copyrights for the MGH Cognitive & Physical Functioning Questionnaire (CPFQ), Sexual Functioning Inventory (SFI), Antidepressant Treatment Response Questionnaire (ATRQ), Discontinuation-Emergent Signs & Symptoms (DESS), Symptoms of Depression Questionnaire (SDQ), and SAFER; and receives royalties from Lippincott, Williams & Wilkins; Wolkers Kluwer; and World Scientific Publishing Co. Pte.Ltd. Dr Mischoulon has received research support from the Bowman Family Foundation, FisherWallace, Nordic Naturals, Methylation Sciences, Inc. (MSI), and PharmoRx Therapeutics; has received honoraria for consulting, speaking, and writing from Pamlab, and the Massachusetts General Hospital Psychiatry Academy; has received royalties from Lippincott Williams & Wilkins for published book “Natural Medications for Psychiatric Disorders: Considering the Alternatives.” Dr Spierings has received research support from Amgen, Alder BioPharmaceuticals, Teva, Pfizer, Allergan, Daiichi Sankyo, Seres Therapeutics en Dr. Reddy’s Laboratories. Drs de Jong, Gard, Ashih, Kulich, Kueppenbender, Hoge, Britton, Lazar and Baer and Mr Doorley, Mss Walker, Rhoades, Hug, and Clain have no personal affiliations or financial relationships with any commercial interest to disclose relative to the article.
Source of Funding
This study was partially funded by an anonymous bequest and partially funded by the Mind and Life Francisco J. Varela Grant 2010-01-010 and the Brach Family Charitable Foundation. The Sponsors had no further role in the study design, collection, analysis and interpretation of data, writing of the report, and the decision to submit the paper for publication.
References
- Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163(20):2433–2445. doi: 10.1001/archinte.163.20.2433. [DOI] [PubMed] [Google Scholar]
- Barnhofer T, Crane C, Hargus E, Amarasinghe M, Winder R, Williams JM. Mindfulness-based cognitive therapy as a treatment for chronic depression: A preliminary study. Behav Res Ther. 2009;47(5):366–373. doi: 10.1016/j.brat.2009.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56(6):893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Bishop SR, Lau M, Shapiro S, Carlson L, Anderson ND, Carmody J, … Velting D. Mindfulness: A proposed operational definition. Clinical psychology: Science and practice. 2004;11(3):230–241. [Google Scholar]
- Bohlmeijer E, Prenger R, Taal E, Cuijpers P. The effects of mindfulness-based stress reduction therapy on mental health of adults with a chronic medical disease: A meta-analysis. Journal of psychosomatic research. 2010;68(6):539–544. doi: 10.1016/j.jpsychores.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23(2):129–138. [PubMed] [Google Scholar]
- Day MA, Thorn BE, Ward LC, Rubin N, Hickman SD, Scogin F, Kilgo GR. Mindfulness-based cognitive therapy for the treatment of headache pain: a pilot study. Clin J Pain. 2014;30(2):152–161. doi: 10.1097/AJP.0b013e318287a1dc. [DOI] [PubMed] [Google Scholar]
- Demyttenaere K, Bruffaerts R, Lee S, Posada-Villa J, Kovess V, Angermeyer MC, … Von Korff M. Mental disorders among persons with chronic back or neck pain: results from the World Mental Health Surveys. Pain. 2007;129(3):332–342. doi: 10.1016/j.pain.2007.01.022. [DOI] [PubMed] [Google Scholar]
- Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP … Immpact. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113(1–2):9–19. doi: 10.1016/j.pain.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Eisendrath SJ, Delucchi K, Bitner R, Fenimore P, Smit M, McLane M. Mindfulness-based cognitive therapy for treatment-resistant depression: a pilot study. Psychother Psychosom. 2008;77(5):319–320. doi: 10.1159/000142525. [DOI] [PubMed] [Google Scholar]
- Engel CC, von Korff M, Katon WJ. Back pain in primary care: predictors of high health-care costs. Pain. 1996;65(2–3):197–204. doi: 10.1016/0304-3959(95)00164-6. [DOI] [PubMed] [Google Scholar]
- First MS, Gibbon M, Williams J. Structured Clinical Interview for Axis I DSM-IV Disorders - Patient version (SCID-1/P version 2.0) New York: 1994. [Google Scholar]
- Fresco DM, Segal ZV, Buis T, Kennedy S. Relationship of posttreatment decentering and cognitive reactivity to relapse in major depression. Journal of Consulting and clinical Psychology. 2007;75(3):447. doi: 10.1037/0022-006X.75.3.447. [DOI] [PubMed] [Google Scholar]
- Geschwind N, Peeters F, Huibers M, van Os J, Wichers M. Efficacy of mindfulness-based cognitive therapy in relation to prior history of depression: randomised controlled trial. Br J Psychiatry. 2012;201(4):320–325. doi: 10.1192/bjp.bp.111.104851. [DOI] [PubMed] [Google Scholar]
- Godfrin KA, van Heeringen C. The effects of mindfulness-based cognitive therapy on recurrence of depressive episodes, mental health and quality of life: A randomized controlled study. Behav Res Ther. 2010;48(8):738–746. doi: 10.1016/j.brat.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Goyal M, Singh S, Sibinga EM, Gould NF, Rowland-Seymour A, Sharma R, … Haythornthwaite JA. Meditation programs for psychological stress and well-being: a systematic review and meta-analysis. JAMA Intern Med. 2014;174(3):357–368. doi: 10.1001/jamainternmed.2013.13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of neurology, neurosurgery, and psychiatry. 1960;23(1):56. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays RD, Sherbourne CD, Mazel RM. The RAND 36-Item Health Survey 1.0. Health Econ. 1993;2(3):217–227. doi: 10.1002/hec.4730020305. [DOI] [PubMed] [Google Scholar]
- Haythornthwaite JA, Sieber WJ, Kerns RD. Depression and the chronic pain experience. Pain. 1991;46(2):177–184. doi: 10.1016/0304-3959(91)90073-7. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Sawyer AT, Witt AA, Oh D. The effect of mindfulness-based therapy on anxiety and depression: A meta-analytic review. J Consult Clin Psychol. 2010;78(2):169–183. doi: 10.1037/a0018555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoge EA, Bui E, Marques L, Metcalf CA, Morris LK, Robinaugh DJ, … Simon NM. Randomized controlled trial of mindfulness meditation for generalized anxiety disorder: effects on anxiety and stress reactivity. J Clin Psychiatry. 2013;74(8):786–792. doi: 10.4088/JCP.12m08083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd KA, Stensland M, Lipchik GL, Hill KR, O’Donnell FS, Cordingley G. Psychosocial correlates and impact of chronic tension-type headaches. Headache. 2000;40(1):3–16. doi: 10.1046/j.1526-4610.2000.00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst H, Bolton J. Assessing the clinical significance of change scores recorded on subjective outcome measures. J Manipulative Physiol Ther. 2004;27(1):26–35. doi: 10.1016/j.jmpt.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Johannes CB, Le TK, Zhou X, Johnston JA, Dworkin RH. The prevalence of chronic pain in United States adults: results of an Internet-based survey. J Pain. 2010;11(11):1230–1239. doi: 10.1016/j.jpain.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J. An outpatient program in behavioral medicine for chronic pain patients based on the practice of mindfulness meditation: theoretical considerations and preliminary results. Gen Hosp Psychiatry. 1982;4(1):33–47. doi: 10.1016/0163-8343(82)90026-3. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J. Full catastrophe living: Using the wisdom of your body and mind to face stress, pain, and illness. Delta: 1990. [Google Scholar]
- Kerns RD, Haythornthwaite J, Rosenberg R, Southwick S, Giller EL, Jacob MC. The Pain Behavior Check List (PBCL): factor structure and psychometric properties. J Behav Med. 1991;14(2):155–167. doi: 10.1007/BF00846177. [DOI] [PubMed] [Google Scholar]
- Kuyken W, Byford S, Taylor RS, Watkins E, Holden E, White K, … Teasdale JD. Mindfulness-based cognitive therapy to prevent relapse in recurrent depression. J Consult Clin Psychol. 2008;76(6):966–978. doi: 10.1037/a0013786. [DOI] [PubMed] [Google Scholar]
- Kuyken Willem, Hayes Rachel, Barrett Barbara, Byng Richard, Dalgleish Tim, Kessler David, … Byford Sarah. Effectiveness and cost-effectiveness of mindfulness-based cognitive therapy compared with maintenance antidepressant treatment in the prevention of depressive relapse or recurrence (PREVENT): a randomised controlled trial. The Lancet. 2015 doi: 10.1016/s0140-6736(14)62222-4. [DOI] [PubMed] [Google Scholar]
- Ma SH, Teasdale JD. Mindfulness-based cognitive therapy for depression: replication and exploration of differential relapse prevention effects. J Consult Clin Psychol. 2004;72(1):31–40. doi: 10.1037/0022-006X.72.1.31. [DOI] [PubMed] [Google Scholar]
- Moran PJ, Mohr DC. The validity of Beck Depression Inventory and Hamilton Rating Scale for Depression items in the assessment of depression among patients with multiple sclerosis. J Behav Med. 2005;28(1):35–41. doi: 10.1007/s10865-005-2561-0. [DOI] [PubMed] [Google Scholar]
- Donnchadha OS, Burke T, Bramham J, O’Brien MC, Whelan R, Reilly R, … Tubridy N. Symptom overlap in anxiety and multiple sclerosis. Mult Scler. 2013;19(10):1349–1354. doi: 10.1177/1352458513476742. [DOI] [PubMed] [Google Scholar]
- Price DD, McHaffie JG, Larson MA. Spatial summation of heat-induced pain: influence of stimulus area and spatial separation of stimuli on perceived pain sensation intensity and unpleasantness. Journal of neurophysiology. 1989;62(6):1270. doi: 10.1152/jn.1989.62.6.1270. [DOI] [PubMed] [Google Scholar]
- Segal ZV, Williams JMG, Teasdale JD. Mindfulness-Based Cognitive Therapy for Depression. New York, NY: The Guilford Press; 2013. [Google Scholar]
- Segal ZV, Williams JMG, Teasdale JD. Mindfulness-based cognitive therapy for depression: A new approach to preventing relapse. The Guilford Press; 2002. [Google Scholar]
- Sullivan MJ, Reesor K, Mikail S, Fisher R. The treatment of depression in chronic low back pain: review and recommendations. Pain. 1992;50(1):5–13. doi: 10.1016/0304-3959(92)90107-M. [DOI] [PubMed] [Google Scholar]
- Tang NK, Crane C. Suicidality in chronic pain: a review of the prevalence, risk factors and psychological links. Psychol Med. 2006;36(5):575–586. doi: 10.1017/S0033291705006859. [DOI] [PubMed] [Google Scholar]
- Teasdale JD, Segal ZV, Williams JM, Ridgeway VA, Soulsby JM, Lau MA. Prevention of relapse/recurrence in major depression by mindfulness-based cognitive therapy. J Consult Clin Psychol. 2000;68(4):615–623. doi: 10.1037//0022-006x.68.4.615. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Ibrahim HM, Carmody TJ, Biggs MM, Suppes T, … Kashner TM. The Inventory of Depressive Symptomatology, Clinician Rating (IDS-C) and Self-Report (IDS-SR), and the Quick Inventory of Depressive Symptomatology, Clinician Rating (QIDS-C) and Self-Report (QIDS-SR) in public sector patients with mood disorders: a psychometric evaluation. Psychol Med. 2004;34(1):73–82. doi: 10.1017/s0033291703001107. [DOI] [PubMed] [Google Scholar]
- Turk DC, Rudy TE. Neglected factors in chronic pain treatment outcome studies--referral patterns, failure to enter treatment, and attrition. Pain. 1990;43(1):7–25. doi: 10.1016/0304-3959(90)90046-G. [DOI] [PubMed] [Google Scholar]
- van Aalderen JR, Donders AR, Giommi F, Spinhoven P, Barendregt HP, Speckens AE. The efficacy of mindfulness-based cognitive therapy in recurrent depressed patients with and without a current depressive episode: a randomized controlled trial. Psychol Med. 2012;42(5):989–1001. doi: 10.1017/S0033291711002054. [DOI] [PubMed] [Google Scholar]
- van Ravesteijn H, Lucassen P, Bor H, van Weel C, Speckens A. Mindfulness-based cognitive therapy for patients with medically unexplained symptoms: a randomized controlled trial. Psychother Psychosom. 2013;82(5):299–310. doi: 10.1159/000348588. [DOI] [PubMed] [Google Scholar]
- Veehof MM, Oskam MJ, Schreurs KM, Bohlmeijer ET. Acceptance-based interventions for the treatment of chronic pain: a systematic review and meta-analysis. Pain. 2011;152(3):533–542. doi: 10.1016/j.pain.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Williams AC, Eccleston C, Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst Rev. 2012;11:CD007407. doi: 10.1002/14651858.CD007407.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson KG, Mikail SF, D’Eon JL, Minns JE. Alternative diagnostic criteria for major depressive disorder in patients with chronic pain. Pain. 2001;91(3):227–234. doi: 10.1016/S0304-3959(00)00440-1. [DOI] [PubMed] [Google Scholar]