Abstract
Visual (i.e., optical flow) perturbations can be used to study balance control and balance deficits. However, it remains unclear whether walking balance control adapts to such perturbations over time. Our purpose was to investigate the propensity for visuomotor adaptation in walking balance control using prolonged exposure to optical flow perturbations. Ten subjects (age: 25.4 ± 3.8 years) walked on a treadmill while watching a speed-matched virtual hallway with and without continuous mediolateral optical flow perturbations of three different amplitudes. Each of three perturbation trials consisted of 8 minutes of prolonged exposure followed by 1 min of unperturbed walking. Using 3D motion capture, we analyzed changes in foot placement kinematics and mediolateral sacrum motion. At their onset, perturbations elicited wider and shorter steps, alluding to a more cautious, general anticipatory balance control strategy. As perturbations continued, foot placement tended toward values seen during unperturbed walking while step width variability and mediolateral sacrum motion concurrently increased. Our findings suggest that subjects progressively shifted from a general anticipatory balance control strategy to a reactive, task-specific strategy using step-to-step adjustments. Prolonged exposure to optical flow perturbations may have clinical utility to reinforce reactive, task-specific balance control through training.
Keywords: Sensorimotor, Virtual Reality, Gait, Vision, Visual Feedback
1. Introduction
Walking balance control depends on integrating reliable sensory feedback and planning and executing appropriate motor responses (O’Connor & Kuo, 2009). Accordingly, sensory perturbations are increasingly used to study balance control mechanisms in walking. Visual (i.e., optical flow) perturbations in particular can elicit strong and acute motor responses to regulate balance from step to step (O’Connor & Kuo, 2009; Terry, Sinitski, Dingwell, & Wilken, 2012). Moreover, these acute motor responses are remarkably more intense in subjects with sensorimotor deficits, such as those due to advancing age (Franz, Francis, Allen, O’Connor, & Thelen, 2015). These results forecast the promising potential of optical flow perturbations applied during walking in the diagnosis of people at risk of falls. However, some evidence from the postural control of standing suggests that subjects may adapt to such perturbations, effectively adjusting their sensitivity to visual feedback over time (Jeka, Allison, & Kiemel, 2010). Although highly relevant to their translational potential, it remains unclear whether walking balance exhibits this time-dependent behavior, which we refer to as visuomotor adaptation.
Multisensory reweighting, the central process that determines the relative priority placed on somatosensory, visual, and vestibular feedback, is considered an essential component of balance control (Horak, Shupert, & Mirka, 1989; Jeka et al., 2010; Oie, Kiemel, & Jeka, 2002). However, to the best of our knowledge, this adaptive sensorimotor process has been exclusively studied in the context of postural sway during standing. For example, Jeka et al. (2010) used anterior-posterior (AP) optical flow perturbations to reveal that the relative priority placed on visual feedback in regulating standing balance is reduced when perturbation amplitudes are larger, and that this dynamic response is tuned over prolonged durations. Indeed, depending on environmental conditions, one would expect sensory feedback modalities deemed more reliable to be those prioritized in balance control. Although optical flow perturbations have been used in studies of walking, these studies have not been designed to investigate the propensity for visuomotor adaptation, with trial durations limited to between 30 s and 3 min and time-averaged outcome measures generally reported.
How would visuomotor adaptation manifest in the control of walking balance? Foremost, walking balance is governed via highly coordinated adjustments in whole-body center of mass (CoM) motion and foot placement from step to step. Adjustments in foot placement in response to optical flow perturbations can be well characterized via step-to-step variability (Brach, Studenski, Perera, VanSwearingen, & Newman, 2008; O’Connor & Kuo, 2009). Step width variability in particular has emerged as a robust metric of dynamic balance in walking and is uniquely compromised in the presence of perturbations (O’Connor & Kuo, 2009). Indeed, although AP balance in standing is highly susceptible to optical flow perturbations, mediolateral (ML) balance is more susceptible in walking and is governed in part by step-to-step adjustments in lateral foot placement (i.e., step width variability). (Bauby & Kuo, 2000; Collins & Kuo, 2013; Donelan, Shipman, Kram, & Kuo, 2004; O’Connor & Kuo, 2009; O’Connor, Xu, & Kuo, 2012). In addition, we and others have used the spectrum of ML CoM motion to provide insight into the relative priority placed on visual feedback for walking balance control (Franz et al., 2015; Latt, Menz, Fung, & Lord, 2008). Specifically, human walking exhibits naturally emerging entrainment to frequencies directly present in ML optical flow perturbations, and the strength of this entrainment can be interpreted to signify one’s sensitivity to visual stimuli (Franz et al., 2015). However, time-dependent changes in foot placement variability and ML CoM motion following exposure to perturbations in walking have yet to be investigated. A return of these metrics of walking balance control toward values seen during normal, unperturbed walking despite ongoing perturbations may allude to the occurrence of visuomotor adaptation.
The purpose of this study was to investigate the propensity for visuomotor adaptation in walking balance control using prolonged exposure to optical flow perturbations of different amplitudes. We used a virtual reality environment to apply continuous ML optical flow perturbations during treadmill walking and recorded the time course of effects on measures of balance control. We first hypothesized that subjects would exhibit visuomotor adaptation, such that the effect of perturbations on walking balance would decrease with walking duration. We also hypothesized that this adaptation would scale with perturbation amplitude, with larger perturbations exhibiting more persistent effects on walking balance.
2. Methods
Ten healthy, young adult subjects (mean ± standard deviation; age: 25.4 ± 3.8 years, height: 1.75 ± 0.10 m, weight: 74.4 ± 16.7 kg, 4 female) participated in this study. Before testing, all subjects provided written informed consent according to the University of Wisconsin Health Sciences Institutional Review Board.
2.1 Experimental Procedures and Measurements
Subjects first walked down a 10 m walkway at their self-selected comfortable speed. We calculated subjects’ preferred overground walking speed as the average of two times taken to traverse the middle 4 m of the walkway (1.38 ± 0.13 m/s). Subjects then completed all treadmill walking trials on a force-sensing treadmill (Bertec, Inc., Columbus, OH) at their preferred overground speed while watching a custom, speed-matched virtual hallway (Fig. 1). The virtual hallway was rear-projected onto a semicircular curved screen that surrounded the treadmill and measured 2.75 m high and 2.25 m wide. Prior to completing the treadmill trials, we instructed subjects simply to “walk while looking down the hallway” so that subjects could naturally respond to the projected visual stimuli.
Figure 1.
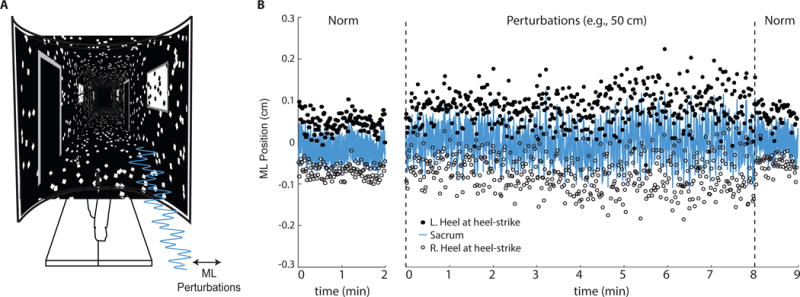
(A) Subjects walked on a treadmill while watching a speed-matched, immersive virtual hallway with and without continuous mediolateral optical flow perturbations of different amplitudes. (B) Mediolateral (ML) sacrum motion and lateral step placement during normal walking (“Norm”) and walking with the largest amplitude visual perturbation for a representative subject. Perturbation trials consisted of 8 min of prolonged exposure followed by 1 min of normal, unperturbed walking. L.heel and R.heel refer to the mediolateral locations of markers placed on the left and right heels at the instant of heel-strike, respectively.
Participants began by completing a 2 minute normal, unperturbed walking trial in which the virtual hallway moved posteriorly according to the prescribed walking speed. Subjects then completed three 9 minute walking trials that included optical flow perturbations (Fig. 1B). For the first 8 minutes, participants walked while subjected to continuous mediolateral (i.e., side to side) optical flow perturbations superimposed onto the moving hallway. Perturbations ceased for the final minute of each trial. Each trial consisted of a continuous mediolateral perturbation at one of three amplitudes (i.e., 20, 35, and 50 cm), prescribed in randomized order. To replicate perturbations used in earlier studies, those employed here consisted of a sum of three sinusoids (phase, φ = 0), such that the full amplitude was applied at 0.250 Hz and half that amplitude was applied at 0.125 Hz and 0.442 Hz (O’Connor et al., 2012). Subjects rested at least 5 minutes between trials. For the duration of each trial, a motion capture system (Motion Analysis Corp., Santa Rosa, CA) operating at 100 Hz recorded the three dimensional positions of markers placed on subjects’ right and left heels and sacrum, the latter used as a surrogate for their center of mass (Yang & Pai, 2014).
2.2 Data Analysis
Marker trajectories were filtered using a 4th order Butterworth filter with a 12 Hz low-pass cutoff frequency. Because we sought to investigate natural adaption to perturbations over time, we did not instruct participants to keep one foot on each treadmill belt, and subjects responded with significant changes in mediolateral foot placement. Accordingly, we focused on our analyses on kinematically-derived measures of walking balance control. We first used methods described by Zeni, Richards, and Higginson (2008) to identify the instants of right and left heel-strikes from the peak anterior heel positions relative to the sacral marker. We then calculated time series of step widths (SW) by averaging heel marker positions during midstance prior to heel rise (i.e., 12–25% of the gait cycle) and determining the mediolateral distance between consecutive steps (Perry & Burnfield, 2010). We computed step lengths (SL) using the relative anterior-posterior positions of successive heel markers at 20% of the gait cycle plus the treadmill belt translation during each step. To test for adaptation in SW and SL over the trial durations, rather than step to step variations, we used a moving average with a window of 30 steps to remove short term fluctuations (Bruijn, Impe, Duysens, & Swinnen, 2012; Fujiki et al., 2015; Malone & Bastian, 2010; Noel, Fortin, & Bouyer, 2009). Using the original time series, we calculated step width and length variabilities (SWV and SLV, respectively) as the standard deviation of SW and SL over steps occurring in 60 s bins.
Finally, we used spectral analysis to quantify each subject’s dynamic response to optical flow perturbations as the intensity of mediolateral sacrum motion (i.e., ML sway intensity) at each of the three frequencies comprising the perturbations (Loughlin & Redfern, 2001). Specifically, algorithms implemented in Matlab computed the fast Fourier transform (FFT) of the ML sacrum marker trajectories, partitioned into 60 s bins. From these 60 s bins, we extracted peaks values of sway intensity occurring at each perturbation frequency. This analysis provided three amplitudes of ML sway (i.e., one each occurring at 0.125, 0.250 and 0.442 Hz) that we quantified for each 60s bin comprising the protocol.
2.3 Statistical Analysis
We used the following seven dependent variables to statistically evaluate our hypotheses: step length, step width, step length and width variabilities, and mediolateral sacrum motion at each of the three perturbation frequencies. First, to provide initial context for any time-dependent changes evident in our outcome measures, a repeated measures analysis of variance (rmANOVA) tested for significant main effects of perturbation amplitude between normal, unperturbed walking and the first and last minutes of visually perturbed walking. Second, to assess the propensity for visuomotor adaptation, a two-way rmANOVA tested for significant main effects of and interactions between time (i.e., minutes 1–8; Hypothesis 1) and perturbation amplitude (i.e., 20, 35, and 50 cm; Hypothesis 2) on each outcome measure. When a significant main effect or interaction was found, we performed post-hoc pairwise comparisons using Tukey’s Honest Significant Difference for multiple comparisons. Finally, in an exploratory test for the presence of perturbation aftereffects, a one-way rmANOVA compared the final minute of unperturbed walking after removal of each perturbation to normal walking at the beginning of the session. We defined significance for all comparisons using an alpha level of 0.05.
3. Results
3.1. Initial response to perturbed optical flow
Compared to walking normally, the onset of perturbations elicited 39% and 62% increases in SWV and SLV, respectively (SWV: F (3, 27) = 6.50, p<0.01; SLV: F (3, 27) = 6.35, p<0.01), accompanied by significantly shorter steps (F (3, 27) = 7.14, p = 0.01) (Fig. 2). Subjects also tended to take 14% wider steps than normal in response to the onset of perturbations (F (3, 27) = 2.59, p = 0.07). ML CoM motion was also highly susceptible to perturbed optical flow. At the onset of perturbations, distinct peaks at the individual perturbation frequencies appeared in the spectrum of ML sacral motion (Fig. 3A). During their first minute of exposure, perturbations significantly increased the intensities of ML sacral motion by an average of 109% (0.125 Hz) and 333% (0.442 Hz) compared to walking normally (F (3, 27) = 4.17 and F (3, 27) = 5.29, respectively, p’s<0.02). (Fig. 3B).
Figure 2.
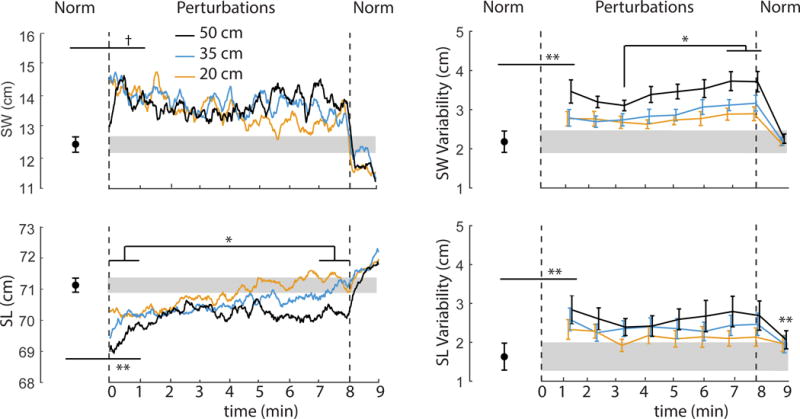
Mean (standard error) perturbation-induced effects on step width (SW), step length (SL), step width variability (SWV), and step length variability (SLV) for each perturbation amplitude. Gray shading represents plus and minus one standard error of each measure during normal walking (“Norm”). Vertical dashed lines represent cessation of optical flow perturbations and a return to normal, unperturbed walking. Double asterisks (**) indicate significantly different from normal walking for the onset of visual perturbations and/or following their cessation (p<0.05). Single asterisks (*) indicate significant post-hoc differences between the identified time points (p<0.05). We also note a statistical trend approaching significance (p = 0.07) for increased step width at the onset of perturbations (†).
Figure 3.
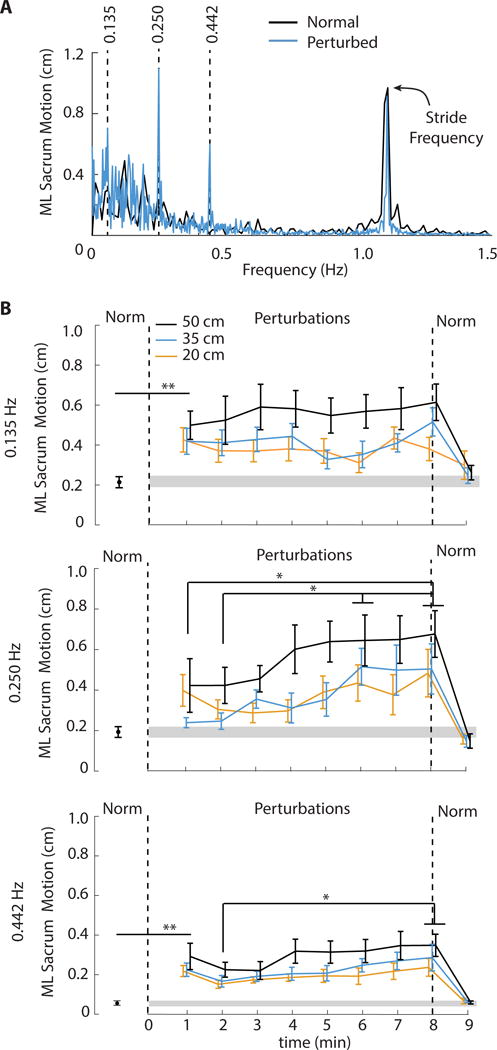
(A) Group-average (standard error) spectrum of ML sacrum motion reveals distinct peaks of signal intensity at, and thus naturally emerging entrainment to, each of the three perturbation frequencies (0.135, 0.250 and 0.442 Hz). (B) Mean (standard error) peak mediolateral (ML) sacrum motion at each perturbation frequency as a functional of perturbation amplitude and time compared to normal, unperturbed walking (“Norm”). Gray shading represents plus and minus one standard error of each measure during normal walking. Vertical dashed lines represent cessation of optical flow perturbations and a return to normal, unperturbed walking. Double asterisks (**) indicate significantly different from normal walking for the onset of visual perturbations and/or following their cessation (p<0.05). Single asterisks (*) indicate significant post-hoc differences between the identified time points (p<0.05).
3.2. Propensity for visuomotor adaptation
As perturbations continued, SL exhibited a significant time dependence (Table 1), tending toward values seen during normal, unperturbed walking (Fig. 2). Post-hoc comparisons during the final minute revealed that the largest amplitude perturbation (i.e., 50 cm) elicited persistent, though small, reductions in step length compared to 20 cm (p = 0.01) and 35 cm (p = 0.05). SW did not exhibit a significant main effect of time (p = 0.15) (Table 1). However, SW values at the end of the perturbation trials did not differ significantly from unperturbed walking (p = 0.10). Neither SWV, SLV, nor the intensity of ML sacral motion at the individual perturbation frequencies decreased from perturbation beginning to end. Rather, SWV exhibited a more complex timedependent response (p<0.01) (Table 1), with significant increases beyond the first three minutes as perturbations continued (Fig. 2). We also observed a concurrent and significant timedependent increase in the intensity of ML sacrum motion at the two fastest perturbation frequencies (0.250 Hz: p<0.01; 0.442 Hz: p = 0.01) (Table 1). Pairwise comparisons revealed that this effect was driven by further increases in ML sacral motion after the third minute of walking with perturbations (Fig. 3B).
Table 1.
Repeated measures ANOVA results (Perturbation trial, Minutes 1-8)
| Amplitude | Time | Amplitude × Time | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| F statistic | p-value | partial η2 | F statistic | p-value | partial η2 | F statistic | p-value | partial η2 | |
|
|
|||||||||
| Step Width | F(2,18)=0.126 | 0.883 | 0.014 | F(1,9)=4.30 | 0.153 | 0.213 | F(2,18)=0.684 | 0.517 | 0.071 |
| Step Length | F(2,18)=5.240 | 0.016 | 0.368 | F(1,9)=9.555 | 0.013 | 0.515 | F(2,18)=0.274 | 0.764 | 0.030 |
| SWV | F(2,18)=8.353 | 0.003 | 0.481 | F(7,63)=3.375 | 0.004 | 0.273 | F(14,126)=0.679 | 0.791 | 0.070 |
| SLV | F(2,18)=7.432 | 0.004 | 0.452 | F(7,63)=1.100 | 0.374 | 0.109 | F(14,126)=0.763 | 0.706 | 0.078 |
| Sway (0.125 Hz) | F(2,18)=6.325 | 0.008 | 0.413 | F(7,63)=0.667 | 0.699 | 0.069 | F(14,126)=0.446 | 0.956 | 0.047 |
| Sway (0.250 Hz) | F(2,18)=5.953 | 0.010 | 0.398 | F(7,63)=3.989 | 0.001 | 0.307 | F(14,126)=1.425 | 0.151 | 0.137 |
| Sway (0.442 Hz) | F(2,18)=4.553 | 0.025 | 0.336 | F(7,63)=2.881 | 0.011 | 0.243 | F(14,126)=0.658 | 0.810 | 0.068 |
SWV: step width variability; SLV: step length variability
3.3. Presence of perturbation aftereffects
Within the temporal resolution of our analyses, following cessation of optical flow perturbations, all but one outcome measure immediately returned to values seen during normal walking; only SLV remained significantly higher than values seen during normal walking (F (3, 27) = 4.16, p = 0.02) (Fig. 2).
4. Discussion
We investigated the prevalence of visuomotor adaption in walking balance control using the time-dependent response of gait kinematics to visual (i.e., optical flow) perturbations of different amplitudes. Consistent with prior studies, we found that optical flow perturbations elicited an immediate increase in mediolateral sacrum motion and shorter, wider, and more variable steps compared to normal walking - effects that generally scaled with perturbation amplitude (Franz et al., 2015; O’Connor et al., 2012; Terry et al., 2012). We first hypothesized that walking balance control would adapt to prolonged optical flow perturbations, evidenced by a return of these outcome measures to values seen during normal, unperturbed walking. In partial support of this hypothesis, step length and width exhibited a time-dependent return toward unperturbed values. However, step width variability and step length variability did not decrease from perturbation beginning to end, nor did mediolateral sacrum motion. Rather, these outcome measures exhibited a more complex response to prolonged perturbations, with significant increases as perturbations continued beyond the third minute. Thus, our findings demonstrate that while walking balance control exhibits persistent susceptibility to perturbed optical flow, time-dependent changes in gait kinematics do emerge with prolonged exposure, but not in the manner hypothesized. As we elaborate more below, these time-dependent changes may provide insight into strategies used to accommodate perturbations during walking which may also be highly relevant to their translational potential.
In response to perturbation onset, subjects immediately adopted wider and shorter steps. This response, prominent when walking in challenging and unpredictable environment conditions, may be interpreted as the adoption of a more cautious or general anticipatory balance control strategy (Maki, 1997; Wu, Matsubara, & Gordon, 2015). Walking with wider steps increases lateral margins of stability and shorter steps both better position the body’s CoM within those margins and are more resistant to unexpected slips or trips (Espy, Yang, & Pai, 2010). Thus, this general anticipatory balance control strategy adopted at the onset of perturbations was a logical response from subjects experiencing a novel stimuli designed to elicit a perceived and unpredictable loss of balance. However, as the perturbations progressed, step width and step length tended toward values seen during normal, unperturbed walking. We interpret these changes to suggest that as subjects grew accustomed to the perturbations, they progressively abandoned, or at least deprioritized, the general anticipatory balance control strategy.
In addition to adopting shorter and wider steps at the onset of the perturbation, subjects simultaneously and significantly increased their step width and step length variabilities and mediolateral sacral motion, indicating larger adjustments in foot placement from one step to the next. Step to the step adjustments in foot placement, particularly those in the mediolateral direction, are a critical component of walking balance control and represent a second, more reactive and task-specific strategy to correct balance disturbances elicited by optical flow perturbations (Bauby & Kuo, 2000; Collins & Kuo, 2013; O’Connor & Kuo, 2009; O’Connor et al., 2012). Interestingly, the time course of changes in SL and SW coincided with notable increases in step width variability and mediolateral sacrum motion as perturbations progressed. Thus, we posit that during each trial, subjects underwent a progressive shift in balance control, from a general anticipatory balance control strategy at perturbation onset to a reactive, task-specific strategy of step to step adjustments as perturbations continued. While not surprising that subjects would adopt a strategy of balance corrections, the time-dependent nature of these particular balance control strategies was more complex than anticipated. Wu et al. (2015) noted similar changes in balance control in subjects responding to anticipated or unanticipated lateral changes in movement direction while walking. When the prompted change in movement direction was unanticipated, subjects adopted wider steps and increased their margins of stability, described by the authors as general anticipatory adjustments. Finally, we also hypothesized that adaptation to perturbations would scale with perturbation amplitude, with larger perturbations exhibiting more persistent effects on walking balance. Step length exhibited the hypothesized return to values seen during normal walking as perturbations progressed. Here, in partial support of our second hypothesis, we found that the largest amplitude perturbation elicited persistent reductions in step length. Compared to subjects’ response to smaller amplitude perturbations, this suggests that our most challenging condition may have prompted a more persistent dependence on the general anticipatory strategy.
The neuromechanical mechanism(s) governing the modest but apparent change in balance control strategy following prolonged exposure to perturbations are difficult to ascertain. Compared to those preferred when walking normally, wider and shorter steps, like those adopted at the onset of perturbations, are widely recognized to be more metabolically expensive (Bertram & Ruina, 2001; Donelan et al., 2004; Kuo, 2001; Wezenberg, de Haan, van Bennekom, & Houdijk, 2011). The increased metabolic cost of walking with wider, shorter steps arises at least in part from the increased mechanical work performed during step-to-step transitions and the increased rate of producing cyclic muscle force during leg swing, respectively. Although walking with increased step-to-step variability does independently increase the metabolic cost of walking (O’Connor et al., 2012), those increases (i.e., ∼6%) are generally smaller than those attributed to consistently adopting wider, shorter steps (Donelan et al., 2004). Thus, it may be more metabolically economical to execute reactive, step-to-step adjustments than to rely on a general anticipatory balance control strategy. Indeed, humans do have the capacity to continuously shape their movement patterns to minimize metabolic energy expenditure during walking (Selinger, O’Connor, Wong, & Donelan, 2015)
There are alternative, or perhaps complementary explanations for the time-dependent changes in subjects’ response to optical flow perturbations. In studying the dynamics of visual reweighting during standing, Jeka et al. (2010) noticed that some subjects’ response to larger amplitude anterior-posterior optical flow perturbations actually increased over time. These complex dynamics are similar to the time-dependent increases in CoM motion evident at the end of our walking trials. Jeka et al. interpreted their findings to suggest that subjects’ initial downweighting of visual feedback at perturbation onset went too far, resulting in small increases in the relative priority placed on vision as perturbations continued. It is possible that a similar process of multisensory fine-tuning could have governed our subjects’ time-dependent response to optical flow perturbations during walking and/or governed the modest aftereffects in step length variability that persisted after perturbation cessation.
There are several important limitations to this study. First, we used moving averages (e.g., step width and length) or analyzed bins of time-averaged data (i.e., step width and length variabilities) and were thus by design limited in our temporal resolution to identify changes that may have occurred on a step by step basis. As a strength of our approach, we used moving averages to quantify time-dependent changes in step width and step length over the course of each perturbation trial separately from time-dependent changes in the magnitude of their step-to-step fluctuations (i.e., step width variability and step length variability), a common practice in studies of locomotor adaptation (Bruijn et al., 2012; Fujiki et al., 2015; Malone & Bastian, 2010; Noel et al., 2009). Indeed, our primary objective was to investigate adaptation to optical flow perturbations that may occur over the course of several minutes of walking. However, we acknowledge that alternative approaches exist (e.g., time-dependent filtering). Second, although unlikely, we cannot exclude the possibility that subjects learned to anticipate the continuous perturbations used in this study. Although the perturbations were continuous, they prescribed a complex combination of three sinusoids of different frequencies that would be challenging for subjects to predict. Nevertheless, time-dependent adaptation to these perturbations, even if driven in part by prediction, are important to consider; similar combinations of sinusoidal perturbations are frequently used to study walking balance control (Dingwell & Cusumano, 2010; Franz et al., 2015; O’Connor et al., 2012). Finally, we used a series of kinematic outcome measures derived from a reduced lower extremity marker set that may not fully describe the dynamics of walking balance control. Other highly relevant metrics of walking balance control include margins of stability, whole-body angular momentum, and dynamic stability, and the time-dependent nature of these metrics during perturbed walking has yet to be carefully examined (Dingwell & Cusumano, 2010; Neptune & Mcgowan, 2016). In addition, to investigate the naturally-emergent response to perturbations, we did not instruct subjects to keep one foot on each treadmill belt. Thus, we could not resolve the individual leg ground reaction forces necessary to compute metrics of balance control based on kinetic measurements.
5. Conclusions
Our findings suggest that gait kinematics do adapt to prolonged exposure to optical flow perturbations and allude to shifts in the underlying strategies used to regulate walking control. At their onset, perturbations elicited shorter, wider steps indicative of a more cautious, general anticipatory balance control strategy that scaled with perturbation amplitude. As perturbations continued, subjects abandoned, or at least deprioritized, this anticipatory strategy in favor of using a more reactive, task-specific strategy of step to step adjustments. These responses were also amplitude dependent; only the largest, and presumably most challenging, perturbation elicited persistent effects on step length lasting the entire trial. Future studies should consider the effects of prolonged exposure to optical flow perturbations in people at risk of falls due to aging or disease. Prolonged exposure to perturbations may have clinical utility to reinforce a reactive, task-specific strategy of step to step adjustments for balance control through training.
Do Gait Kinematics Adapt to Perturbed Optical Flow?
Jessica D. Thompson and Jason R. Franz
Joint Department of Biomedical Engineering, University of North Carolina at Chapel Hill and North Carolina State University, Chapel Hill, NC, USA
Research Highlights.
A virtual reality environment perturbed optical flow during treadmill walking
Investigated propensity for visuomotor adaptation in walking balance control
Gait kinematics and CoM motion exhibited time-dependent effects of perturbations
At their onset, perturbations elicit a general anticipatory balance control
Subjects shift to reactive, task-specific balance control as perturbations continue
Acknowledgments
We gratefully thank Dr. Shawn O’Connor for help with the VR environment used in this study and Dr. Darryl Thelen for his helpful insights. This study was supported in part by grants from the National Center for Advancing Translational Sciences (UL1TR001111) and the National Institute on Aging (F32AG044904).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bauby CE, Kuo AD. Active control of lateral balance in human walking. Journal of Biomechanics. 2000;33:1433–1440. doi: 10.1016/s0021-9290(00)00101-9. [DOI] [PubMed] [Google Scholar]
- Bertram JE, Ruina A. Multiple walking speed-frequency relations are predicted by constrained optimization. Journal of Theoretical Biology. 2001;209(4):445–453. doi: 10.1006/jtbi.2001.2279. [DOI] [PubMed] [Google Scholar]
- Bruijn SM, Impe AV, Duysens J, Swinnen SP. Split-belt walking: adaptation differences between young and older adults. Journal of Neurophysiology. 2012;108(4):1149–1157. doi: 10.1152/jn.00018.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins SH, Kuo AD. Two independent contributions to step variability during overground human walking. PLoS One. 2013;8(8) doi: 10.1371/journal.pone.0073597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingwell JB, Cusumano JP. Re-Interpreting Detrended Fluctuation Analyses of Stride-To-Stride Variability in Human Walking. Gait and Posture. 2010;32(3):348–353. doi: 10.1016/j.gaitpost.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donelan JM, Shipman DW, Kram R, Kuo AD. Mechanical and metabolic requirements for active lateral stabilization in human walking. Journal of Biomechanics. 2004;37:827–835. doi: 10.1016/j.jbiomech.2003.06.002. [DOI] [PubMed] [Google Scholar]
- Espy DD, Yang F, Pai YC. Control of center of mas motion state through cuing and decoupling of spontaneous gait parameters in level walking. Journal of Biomechanics. 2010;43(13):2548–2553. doi: 10.1016/j.jbiomech.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz JR, Francis CA, Allen MS, O’Connor SM, Thelen DG. Advanced age brings a greater reliance on visual feedback to maintain balance during walking. Human Movement Science. 2015;40:381–392. doi: 10.1016/j.humov.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz JR, Francis CA, Allen MS, Thelen DG. Visuomotor entrainment and the frequency-dependent response of walking balance to perturbations. IEEE Trans Neural Syst Rehabil Eng. doi: 10.1109/TNSRE.2016.2603340. In revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki S, Aoi S, Funato T, Tomita N, Senda K, Tsuchiya K. Adaptation mechanism of interlimb coordination in human split-belt treadmill walking through learning of foot contact timing: a robotics study. Journal of the Royal Society Interface. 2015;12(110):20150542. doi: 10.1098/rsif.2015.0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horak FB, Shupert C, Mirka A. Components of postural dyscontrol in the elderly: A review. Neurobiology of Aging. 1989;10:727–738. doi: 10.1016/0197-4580(89)90010-9. [DOI] [PubMed] [Google Scholar]
- Jeka JJ, Allison LK, Kiemel T. The dynamics of visual reweighting in healthy and fall-prone older adults. Journal of Motor Behavior. 2010;42(4):197–208. doi: 10.1080/00222895.2010.481693. [DOI] [PubMed] [Google Scholar]
- Kuo AD. A simple model of bipedal walking predicts the preferred speed-step length relationship. Journal of Biomechanical Engineering. 2001;123:364–269. doi: 10.1115/1.1372322. [DOI] [PubMed] [Google Scholar]
- Latt MD, Menz HB, Fung VS, Lord SR. Acceleration Patterns of the Head and Pelvis During Gait in Older People With Parkinson’s Disease: A Comparison of Fallers and Nonfallers. The Journals of Gerontology: Medical Sciences. 2008;64A(6):700–706. doi: 10.1093/gerona/glp009. [DOI] [PubMed] [Google Scholar]
- Loughlin PJ, Redfern MS. Spectral characteristics of visually induced posutral sway in healthy elderly and healthy young subjects. IEEE Trans Neural Syst Rehabil Eng. 2001;9(1):24–30. doi: 10.1109/7333.918273. [DOI] [PubMed] [Google Scholar]
- Maki BE. Gait changes in older adults: Predictors of falls or indicators of fear. Journal of the American Geriatrics Society. 1997;45(3):313–320. doi: 10.1111/j.1532-5415.1997.tb00946.x. [DOI] [PubMed] [Google Scholar]
- Malone LA, Bastian AJ. Thinking About Walking: Effects of Conscious Correction Versus Distraction on Locomotor Adaptation. Journal of Neurophysiology. 2010;103(4):1954–1962. doi: 10.1152/jn.00832.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neptune RR, Mcgowan CP. Muscle contribution of frontal p l ane angular momentum during walking. Journal of Biomechanics. 2016;49(13):2975–2981. doi: 10.1016/j.jbiomech.2016.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel M, Fortin K, Bouyer LJ. Using an electrohydraulic ankle foot orthosis to study modifications in feedforward control during locomotor adaptation to force fields applied in stance. Journal of NeuroEngineering and Rehabilitation. 2009;6(1):16. doi: 10.1186/1743-0003-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor SM, Kuo AD. Direction-Dependent Control of Balance During Walking and Standing. Journal of Neurophysiology. 2009;102(3):1411–1419. doi: 10.1152/jn.00131.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor SM, Xu HZ, Kuo AD. Energetic cost of walking with increased step variability. Gait and Posture. 2012;36:102–107. doi: 10.1016/j.gaitpost.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oie KS, Kiemel T, Jeka JJ. Multi-sensory fusion: Simultaneous re-weighting of vision and touch for the control of human posture. Cognitive Brain Research. 2002;14:164–176. doi: 10.1016/s0926-6410(02)00071-x. [DOI] [PubMed] [Google Scholar]
- Perry J, Burnfield JM. Gait Analysis: Normal and Pathological Function. 2. Thorofar, NJ: Slack Incorporated; 2010. [Google Scholar]
- Selinger JC, O’Connor SM, Wong JD, Donelan JM. Humans can continuously optimize energetic cost during walking. Current Biology. 2015;25(18):2452–2456. doi: 10.1016/j.cub.2015.08.016. [DOI] [PubMed] [Google Scholar]
- Terry K, Sinitski EH, Dingwell JB, Wilken JM. Amplitude effects of medio-lateral mechanical and visual perturbations on gait. Journal of Biomechanics. 2012;45:1979–1986. doi: 10.1016/j.jbiomech.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wezenberg D, de Haan A, van Bennekom CAM, Houdijk H. Mind your step: Metabolic energy cost while walking an enforced gait pattern. Gait and Posture. 2011;33(4):544–549. doi: 10.1016/j.gaitpost.2011.01.007. [DOI] [PubMed] [Google Scholar]
- Wu M, Matsubara JH, Gordon KE. General and Specific Strategies Used to Facilitate Locomotor Maneuvers. PLoS One. 2015;10(7) doi: 10.1371/journal.pone.0132707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Pai YC. Can Sacral Marker Approximate Center of Mass During Gait and Slip-Fall Recovery Among Community-Dwelling Older Adults? Journal of Biomechanics. 2014;47(16):3807–3812. doi: 10.1016/j.jbiomech.2014.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeni J, Richards J, Higginson J. Two simple methods for determining gait events during treadmill and overground walking using kinematic data. Gait and Posture. 2008;27(4):710–714. doi: 10.1016/j.gaitpost.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]


