Abstract
Alzheimer’s disease (AD) is a chronic neurodegenerative disorder that is mainly characterized by beta-amyloid (Aβ) plaque deposition, Tau pathology and dysfunction of the cholinergic system causing memory impairment. The aim of the present study was to examine (1) anxiety and cognition, (2) Aβ plaque deposition and (3) degeneration of cholinergic neurons in the nucleus basalis of Meynert (nbM) and cortical cholinergic innervation in an Alzheimer mouse model (APP_SweDI; overexpressing amyloid precursor protein (APP) with the Swedish K670N/M671L, Dutch E693Q, and Iowa D694N mutations). Our results show that 12-month-old APP_SweDI mice were more anxious and had more memory impairment. A large number of Aβ plaques were already visible at the age of 6 months and increased with age. A significant decrease in cholinergic neurons was seen in the transgenic mouse model in comparison to the wild-type mice, identified by immunohistochemistry against choline acetyltransferase (ChAT) and p75 neurotrophin receptor as well as by in situ hybridization. Moreover, a significant decrease in cortical cholinergic fiber density was found in the transgenic mice as compared to the wild-type. In the cerebral cortex of APP_SweDI mice, swollen cholinergic varicosities were seen in the vicinity of Aβ plaques. In conclusion, the present study shows that in an AD mouse model (APP_SweDI mice) a high Aβ plaque load in the cortex causes damage to cholinergic axons in the cortex, followed by subsequent retrograde-induced cell death of cholinergic neurons and some forms of compensatory processes. This degeneration was accompanied by enhanced anxiety and impaired cognition.
Keywords: Alzheimer, Plaques, Cholinergic neurodegeneration, Transgenic mice
1. Introduction
Alzheimer’s disease (AD) is a chronic neurodegenerative disorder that accounts for most cases of dementia in the elderly population. The neuropathological hallmarks of AD are beta-amyloid (Aβ) plaque deposits, Tau neurofibrillary tangles, neuronal and synaptic loss, inflammation and cerebrovascular damage. Senile Aβ plaques consist mainly of insoluble deposits of 40- or 42- amino acid long Aβ peptides that are produced by sequential cleavage of the transmembrane glycoprotein amyloid precursor protein (APP) (Selkoe, 2001). It has been shown that mutations in human APP are associated with early onset familial AD (Chartier-Harlin, Crawford, Houlden, et al., 1991; Goate et al., 1991; Murrell, Farlow, Ghetti, & Benson, 1991). The exact processes of genesis of Aβ plaques in AD is not entirely understood, but the Aβ cascade is the most prominent hypothesis for the development of AD (Hardy & Higgins, 1992; Marchesi, 2005; Reitz, 2012), suggesting that the excessive accumulation of insoluble Aβ peptides induces a series of events that leads to neuronal dysfunction and death.
Degeneration of the basal forebrain cholinergic system is an important pathophysiology of AD (Perry et al., 1978; Schliebs & Arendt, 2006). Cholinergic neurons provide the main source of acetylcholine (ACh) in the cortex and a decline in ACh directly correlates with cognitive impairment (Perry et al., 1978). The number of cholinergic neurons has consistently been found to be reduced in the basal forebrain of advanced AD (Davies & Maloney, 1976; Whitehouse, Price, Struble, & Clarke, 1982), accompanied by decreases in choline acetyltransferase (ChAT) activity (Lehéricy et al., 1993; Wilcock, Esiri, Bowen, & Smith, 1982). A lack of ACh in the cerebral cortex directly correlates with cognitive decline. These findings led to the cholinergic hypothesis of AD (Francis, Palmer, Snape, & Wilcock, 1999), proposing that the loss of basal forebrain cholinergic neurons and the associated decline in cholinergic innervation of cortical areas is causal for cognitive dysfunction in AD patients.
Murine transgenic mouse models are the state of the art for exploring mechanisms of plaque genesis in AD pathology. A variety of transgenic murine models for AD have been generated by overexpression of human mutated APP (Games et al., 1995). In the present study we used an AD mouse strain expressing human APP with the Swedish K670N/M671L, Dutch E693Q, and Iowa D694N mutations (APP_SweDI). These mice develop plaques within four to six months in all areas of the brain and also in vessels, called amyloid angiopathy (Davis et al., 2004). However, these mice lack any Tau pathology. Previous analysis of cholinergic neuropathology in another APP transgenic mouse strain (the PDAPP mice) showed an age-related significant reduction in the density of cholinergic nerve terminals in the cerebral cortex, but no decrease in basal forebrain cholinergic somata (German et al., 2003). However, so far, cholinergic neurodegeneration has not been investigated in the APP_SweDI AD mouse model.
The aim of the present study was to explore in this AD mouse model whether the deposition of plaques in the brain directly correlates with a decline in cholinergic neurons and innervation. As the neurotransmitter ACh is responsible for memory and learning, we want to study whether these mice are also impaired in learning and memory in the classical labyrinth. To support these findings the mice will also be tested for anxiety in the black/white box and the plus maze. These data should provide more insights into the complex interaction between plaques, cholinergic innervation and memory and anxiety.
2. Methods
2.1. Transgenic Alzheimer mice
Wildtype (WT, C57BL/6N) and transgenic APPSweDI (TG, expressing amyloid precursor protein (APP) harboring the Swedish K670N/M671L, Dutch E693Q, and Iowa D694N mutations; C57BL/6-Tg(Thy1-APPSwDutIowa) BWevn/Mmjax) mice were purchased from The Jackson Laboratory (MMRRC, Bar Harbor, ME, USA) and housed at the Medical University of Innsbruck animal facility providing open access to food and water under 12 h/12 h light/dark cycles. The mice were generated and extensively characterized by Davis et al. (2004). All animals were genotyped according to standardized methods. All animal experiments were approved by the Austrian Ministry of Science and Research (BMWF-66.011/0044-II/3b/2011 and BMWF-66.011/0059-II/3b/2011) and conformed to the Austrian guidelines on animal welfare and experimentation. All possible steps were taken to reduce suffering and the number of animals used during the experiment.
2.2. Black/white test box
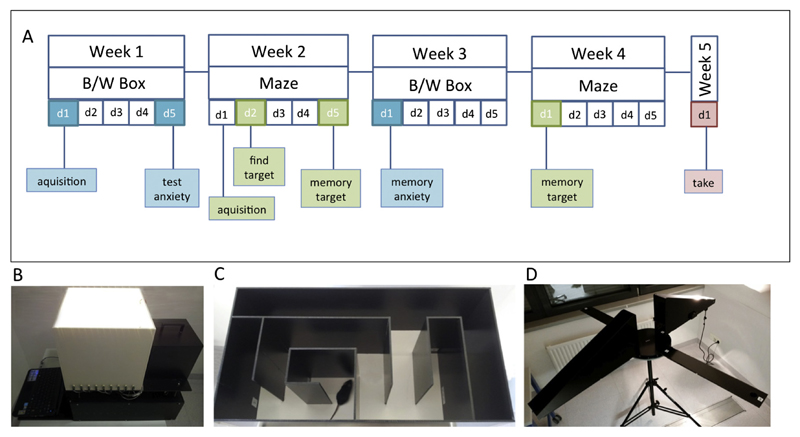
Anxiety was assessed using the black/white (B/W) box, also known as the light/dark exploration test (Fig. 1B). This model consists of two inter-connected compartments that vary in color (white/black) and illumination (light/dark) (Fig. 1B). In the first week (day 1) mice were adapted to the environment and the researcher; anxiety was recorded on day 5 (Fig. 1A). Memory of anxiety was tested in week 3 on day 1. (Fig. 1A). Mice were placed in the black, shaded area where they naturally feel safer than in the illuminated white area. To quantify anxiety the first move from the black to the white area and the time spent in the black area were recorded.
Fig. 1.
Experimental design. Anxiety was assessed using the black/white (B/W) box, also known as the light/dark exploration test. This model consists of two inter-connected compartments that vary in color (white/black) and illumination (light/dark) (B). In the first week (day 1) mice were adapted to the environment and the researcher; anxiety was recorded on day 5. Memory of anxiety was tested in week 3 on day 1 (A). Spatial learning and memory were assessed in a well-established simple classical mouse labyrinth (C). On the first day of week 2 mice were adapted to a classical labyrinth maze and the environment. On day 2 (week 2) mice were conditioned to find a target (28 ± 2 mg chocolate, Ferrero© Nutella). The memory for finding this target was recorded on day 5 (week 2) as well as in week 4 on day 1 (A). Figure D shows the setup of the plus maze discriminative avoidance task. The training and the test session were performed on two consecutive days.
2.3. Classical mouse labyrinth
Spatial learning and memory were assessed in a well-established simple classical mouse labyrinth (Fig. 1C). On the first day of week 2 mice were adapted to the maze and the environment. On day 2 mice were trained to find a target. Mice were conditioned to 28 ± 2 mg chocolate (Ferrero© Nutella). The memory to find this target was recorded on day 5 (week 2) as well as in week 4 on day 1 (Fig. 1A). Quantification of cognitive performance (spatial learning and memory) was done by measuring the time taken by the mice to find the target and by calculating the number of working memory errors made by entering a wrong maze arm.
2.4. Plus maze discriminative avoidance task
To support the data obtained with the anxiety model and the classical maze we additionally performed the plus maze discriminative avoidance task as reported by Silva and Frussa-Filho (2000). This model permits learning/memory and anxiety to be measured simultaneously. On the first day, the training session was performed. Each mouse was placed in the centre of the apparatus (Fig. 1D), where they had 10 min time to explore the maze. The apparatus consists of two enclosed arms (one of them with aversive stimuli from a 100-W light bulb and an 80-dB noise every time a mouse entered the arm) and two open arms. The noise was induced by beating a metal stick against the wooden wall of the enclosed aversive arm and at the same time the light was turned on for as long as the animal stayed in this arm. Additional visual cues (stickers showing arrows, plus signs, hands, stop sign) that the mice could use to differentiate between the arms were placed on each side of the maze. Twenty-four hours after the training session the test session was performed. The mice were again placed in the middle of the maze, but had only 3 min time to explore the maze without being given any aversive stimuli. By measuring the time spent in each arm, memory related to the learned aversive stimuli was quantified. At the same time information about the anxiety level can also be given by measuring the time the mice spent in the open arms of the apparatus. To exclude olfactory cues, all of the apparatuses were cleaned with 70% EtOH after every trial.
2.5. Tissue collection
At the end of the experiment (one week after the last behavioral testing), animals were anesthetized by subcutaneous sodium thiopental (12.5 mg/ml, 1 ml) injection. A transcardial perfusion was performed with 20 ml of phosphate-buffered saline (PBS) containing EDTA and heparin and then subsequently with 50 ml of 4% paraformaldehyde (PFA) in PBS. The brain was removed, post-fixed in 4% PFA for 3 h at 4 °C, and then immersed in a 20% sucrose/PBS solution overnight. Brains were frozen in a CO2 stream and cryosectioned (40 μm).
2.6. Immunohistochemistry
Immunohistochemistry was performed as previously described under free-floating conditions (Ullrich, Pirchl, & Humpel, 2010). The sections were washed with PBS and incubated in PBS/0.1% Triton (T-PBS) for 30 min at 20 °C while shaking. To quench endogenous peroxidase, sections were treated with PBS/1%H2O2/5% methanol. After incubation, the sections were blocked in T-PBS/20% horse serum (GIBCO Invitrogen)/0.2% BSA (SERVA) for 30 min at 20 °C while shaking. Following blocking, brain sections were incubated with primary antibody (beta-amyloid, 1-16 (6E10) Covance SIG-39300; p75 neurotrophin receptor Promega G3231; choline acetyltransferase ChAT Millipore AB144P) in T-PBS/0.2% BSA for two days at 4 °C. The sections were then washed and incubated with the corresponding biotinylated secondary (βA-mouse; ChAT-goat; p75NT-rabbit) antibody (1:200, Vector Laboratories) in T-PBS/0.2% BSA for 1 h at 20 °C while shaking. Following secondary antibody incubation, sections were rinsed with PBS and incubated in avidin-biotin complex solution (Elite ABC kit, Vector Laboratories) for 1 h at 20 °C while shaking. Finally, the sections were washed with 50 mM Tris-buffered saline (TBS) and then incubated in 0.5 mg/ml 3,3′-diaminobenzidine (DAB, Sigma)/TBS/0.003% H2O2 at 20 °C in the dark until a signal was detected. Once DAB staining was visible, the reaction was stopped by adding TBS to the sections. The brain sections were rinsed with TBS, inserted into a 6-well plate, covered with Vectashield (Vector) and cover-slipped and then evaluated under an inverse microscope (Leica DM IRB). Some sections were stained with Thiazine Red (1.6 µg/ml overnight at 4 °C) to label plaques. Some sections were co-stained by fluorescence for ChAT (Alexa-488) and nuclear dye DAPI or ChAT (Alexa-488) and Aβ (Alexa-546).
2.7. Acetylcholinesterase histochemistry
Acetylcholinesterase (AChE) histochemistry was performed as described by us (Humpel & Weis, 2002). The sections were washed with 100 mM maleate buffer (pH 6.0) and then incubated in a fresh incubation solution of acetylthiocholine iodide, K3Fe(CN)6(III), CuSO4, sodium citrate, ethopropazine in maleate buffer for 1.5 h at room temperature. Then, sections were rinsed three times in PBS buffer (pH 7.4) and twice in Tris buffer (pH 7.6). Subsequently, sections were developed in a 0.04% DAB/0.3% nickel ammonium sulfate/0.003% H2O2/Tris pH 7.6 solution.
2.8. In situ hybridization
In situ hybridization was performed as described (Daschil et al., 2015). Mice (12-month-old WT and TG) were decapitated and the brains frozen under a CO2 stream, sectioned (14 μm) with a cryostat (Leica) and thawed onto slides (ProbeOn™ slides, Fisher Biotech, Austria). Oligonucleotides (5 pmol) specific for cholineacetyltransferase (5′-ggg-cac-ctg-gct-ggt-gga-gag-aat-aaa-ccg-gtt-gct-cat-cag-3′) were labeled at the 3′ end with [α-35S]dATP using terminal deoxyribonucleotidyl transferase (Roche, Austria) and purified on Qiagen columns. Brain sections were hybridized overnight at 42 °C in a humidified chamber with 100 µl per section of the hybridization solution (50% formamide, 4×SSC, 0.02% polyvinylpyrrolidone, 0.02% Ficoll, 0.02% bovine serum albumin, 10% dextrane sulfate, 0.5 mg/ml sheared salmon sperm DNA, 1% sarcosyl (N-lauroyl sarcosine), 0.02 M NaPO4 (pH 7.0), 50 mM dithiothreitol) containing 1 × 107 CPM/ml probe. Sections were subsequently rinsed, washed four times (15 min each) in 1×SSC (saline sodium citrate) at 54 °C, cooled to room temperature, dehydrated through 70%, 90% and 99.9% ethanol and subsequently air-dried. Sections were dipped in Kodak NTB photo emulsion, exposed for five weeks at −20 °C, developed, fixed and mounted with Mowiol. Sections were stored in the dark at 4 °C until analysis.
2.9. Evaluation of plaques
Sections at the cortical level were photographed with the Leica inverse microscope at 10× magnification under a red filter. The exposure time was always 23 ms with the bright light set at the lowest level. The software Openlab was used on a Mac computer connected to the microscope. Pictures were saved as JPG files and the analysis was performed using Image J. The pictures were transformed to an 8-bit grayscale image. The calibration was set at 0.470 (distance in pixels), 1.00 (known distance), 1.0 (pixel aspect ratio) and μm (unit in length) and global was activated. The picture was transformed into a binary image and the threshold was adapted to 30–40. The number of particles was counted setting the size at 100–8000 μm2. The number of plaques was counted in a defined circle in a 2.5 mm2 area.
2.10. Quantitative analysis of neurons and nerve fibers
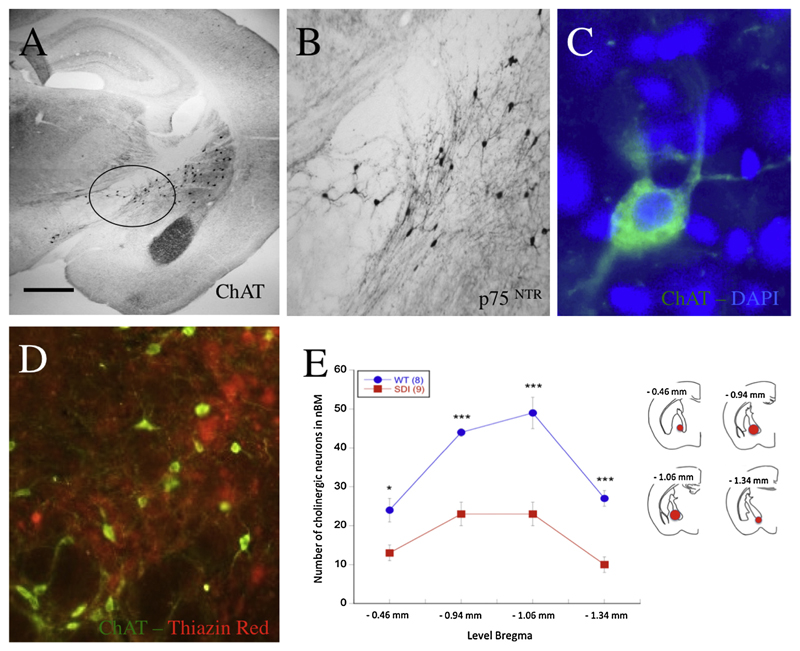
Brain sections of the nBM were analyzed under the microscope at 20× magnification. A simple scheme is given to show delineation of nucleus basalis of Meynert in Fig. 4E as well as the levels of Bregma we refer to. The neurons were identified by immunostainings having a free stained nucleus. We analyzed 267 sections of 40 μm thickness located between –0.46 mm and –1.34 mm from Bregma in coronary slices. Measurement of cholinergic nerve fiber density was performed as described previously (Humpel & Weis, 2002). Brain sections were photographed with the Leica inverse microscope at 40× magnification under a red filter. The procedure was the same as for the evaluation of plaques. The threshold was adapted to 50–60 and the number of all particles visible in the picture was counted setting the size to 50–500 μm2. The number of particles was deemed equivalent to the density of nerve fibers.
Fig. 4.
Cholinergic neurons in the wild-type (A–C) and APP_SweDI Alzheimer (D) mice. Brain sections were immunohistochemically stained with an antibody against choline acetyltransferase (ChAT) (A, C, D) or the p75 neurotrophin receptor (B). The circle in the overview marks the nucleus basalis of Meynert (nBM) region also used for quantification (A). Cholinergic neurons were stained either with the chromogenic DAB method (A and B) or using fluorescence (Alexa-488, green, C and D), counterstained with the blue nuclear dye DAPI (note the cytoplasmic staining of ChAT, C). In the nBM the number of beta-amyloid plaques (Alexa-546, red) was rare, as costained with Thiazine Red (D). The number of ChAT+ cholinergic neurons was counted at four different Bregma levels, namely from –0.46 to –1.34 mm (E). Schemes for the different chosen areas of the nBM (red circles) are shown in mouse brain map drafts. Values are given as mean ± SEM (n = 8 WT; n = 9 APP_SweDI). Statistical analysis was performed with one-way ANOVA with a Fisher LSD posthoc test (* p < 0.05; *** p < 0.001). Scale bar in A: 300 μm (A) or 115 μm (B) or 29 μm (C) or 58 μm (D). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
2.11. Semiquantitative analysis of ChAT mRNA
Using computer-assisted analysis the silver grains were counted in 10 neurons per field at 20× magnification using Image J. Background was subtracted.
2.12. Statistical analysis
Statistical analysis was performed with Student’s T test and one-way ANOVA with a subsequent Dunnett posthoc test, where p < 0.05 represents significance.
3. Results
3.1. Anxiety in the black-white box
There was a clear indication that APP_SweDI were less anxious at the beginning of the experiment, because these mice left the black (secure) compartment significantly faster than did the control mice (Fig. 2A; W1/d1). However, at the end of the experiment (week 3) the APP_SweDI were more anxious and spent more time in the secure black compartment (Fig. 2B; W3/d1). Also, the time until moving out of the secure black compartment for the first time increased during the experiment (Fig. 2A; W1/d5).
Fig. 2.
Analysis of anxiety (A & B) and spatial memory (C & D) in the 12-month-old control wild-type mice (WT blue) and APP_SweDI Alzheimer mice (TG red). Anxiety was tested in the black/white box by measuring the time until the mouse left the black compartment for the first time (A) and the total time spent in the black compartment (B). Memory was tested in the classical labyrinth; the time taken to find a target (28 ± 2 mg chocolate) as well as the number of errors made by entering a wrong arm were recorded. Anxiety was tested in week 1 and week 3 on day 1 (W1/d1-W1/d5-W3/d1). Memory was tested in week 2 on days 2 and 5 (W2/d2-W2/d5) and in week 4 on day 1 (W4/d1). Values are given as mean ± SEM. The number of tested animals is given in parenthesis. Statistical analysis was performed with Student’s T test (* p < 0.05; ** p < 0.01). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2. Memory in the classical labyrinth
There was a clear indication that APP_SweDI displayed markedly reduced learning and memory as shown by increased time taken to find the target (Fig. 2C) and an increased number of errors made (Fig. 2D), at least at the beginning of the experiment in week 2. Interestingly, the mice improved in learning and memory in week 4, because they differed neither in finding the target nor in making errors as compared to control mice (Fig. 2C and D).
3.3. Plus maze discriminative avoidance task
In the training session the APP_SweDI mice spent significantly more time in the open arms and in the middle platform than did the wild-type mice (Table 1). In the test session performed twenty-four hours later, no significant differences between the groups were obvious (Table 1).
Table 1.
APP_SweDI Alzheimer mice compared to wildtype (WT) controls tested in the plus maze discriminative avoidance task.
| WT | APP_SweDI | p | |
|---|---|---|---|
| Training (600 s total) | |||
| Time in aversive closed | 151 ± 80 | 16 ± 6 | 0.09 |
| Time in non-aversive closed | 407 ± 85 | 364 ± 36 | 0.6 |
| Time in open arms | 0 ± 0 | 58 ± 20 | 0.02* |
| Time in middle platform | 42 ± 9 | 162 ± 30 | 0.004** |
| Test (180 s total) | |||
| Time in aversive closed | 106 ± 34 | 64 ± 24 | 0.3 |
| Time in non-aversive closed | 64 ± 36 | 73 ± 26 | 0.9 |
| Time in open arms | 0 ± 0 | 27 ± 22 | 0.3 |
| Time in middle platform | 10 ± 3 | 16 ± 4 | 0.2 |
On the first day (i.e. training) wild-type (WT, n = 6) and APP_SweDI (n = 7) mice were conditioned to the aversive stimuli (100 W light and 80 dB noise) while exploring the maze for 10 min. Twenty-four hours later (i.e. test) the mice were tested again for 3 min, this time without aversive stimuli. The time they spent in every single arm and also on the middle platform was measured. Results are shown as mean ± SEM. Statistical analysis was performed with Student’s T test (* p < 0.05; ** p < 0.01).
3.4. Beta-amyloid plaques
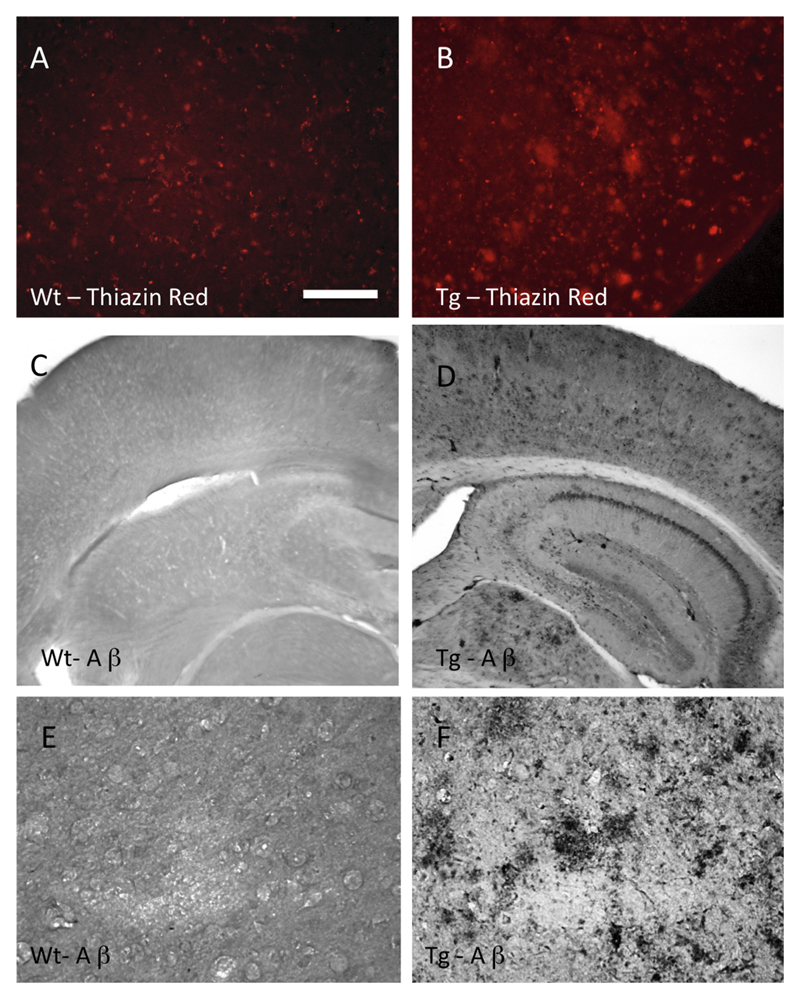
Diffuse Thiazine Red plaques (Fig. 3B) and Aβ plaques (Fig. 3D and F) were found throughout the brains already in 6-month-old APP_SweDI mice and markedly increased in 12-month-old transgenic mice (Table 2). There was no staining in sections from control wild-type mice (Fig. 3A, C and E). Omitting the primary antibody did not yield staining in any background (data not shown).
Fig. 3.
Beta-amyloid plaques in the APP_SweDI Alzheimer mouse model. Brain sections from wild-type (A, C, E) and 12-month-old transgenic (B, D, F) mice were immunohistochemically stained for Thiazine Red (A and B) or with antibodies against beta-amyloid (C–F). Scale bar in A: 29 μm (A, B, E) or 115 μm (C, D, E). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Table 2.
Quantification of plaques and nBM neurons and fibers.
| WT 6 mo | APP_SweDI 6 mo | WT 12 mo | APP_SweDI 12 mo | |
|---|---|---|---|---|
| Aβ plaques cortex | 2.5 ± 2.3 (6) | 34 ± 10 (6)* | 1.1 ± 0.4 (5) | 75 ± 8 (6)*** |
| ChAT+ nBM neurons | 40 ± 3 (6) | 41 ± 1 (6) ns | 43 ± 4 (5) | 23 ± 3 (8)** |
| p75+ nBM neurons | 45 ± 2 (5) | 43 ± 1 (3) ns | 41 ± 3 (5) | 17 ± 1 (6)*** |
| ChAT+ fibers cortex | 547 ± 73 (6) | 367 ± 38 (6)* | 665 ± 87 (5) | 223 ± 46 (6)*** |
| AChE+ fibers cortex | 307 ± 28 (6) | 223 ± 24 (6)* | 357 ± 42 (5) | 185 ± 10 (6)** |
Brain sections from 6- or 12-month-old wild-type (WT) and APP_SweDI transgenic mice were stained by immunohistochemistry with a beta-amyloid antibody (Aβ plaques, PLQ) or with choline-acetyltransferase (ChAT, cholinergic neurons and fibers) or with p75 neurotrophin receptor (p75, cholinergic neurons) or by acetylcholinesterase (AChE) histochemistry. The plaque number in the cortex was analyzed by computer-assisted imaging. Values are given as mean ± SEM (n = number of animals). The number of plaques (PLQ) is given as PLQ per 1.16 mm2 and the number of cholinergic nucleus basalis of Meynert (nBM) as neurons per section (average of six sections). The density of cholinergic nerve fibers in the cortex was determined with computer-assisted imaging in pixels and is given as density for a field size of 55,488 µm2. Statistical analysis was performed by one-way ANOVA with a subsequent Fisher LSD posthoc test (* p < 0.05; ** p < 0.01; *** p < 0.001; ns not significant).
3.5. Cholinergic neurons in the nBM
ChAT-positive cholinergic neurons were found in the nucleus basalis of Meynert (nbM) of wild-type mice (Fig. 4A). Typical cytoplasmic staining was observed at a high magnification (Fig. 4C). These nBM neurons also expressed the p75 neurotrophin receptor (Fig. 4B). The number of ChAT+ and p75+ neurons in the nBM did not differ between 6- and 12-month-old WT mice. However, their number significantly decreased in 12-month-old but not in 6-month-old transgenic mice (Table 1; Fig. 4E). In the nBM not many Aβ plaques were observed close to the cholinergic neurons (Fig. 4D).
3.6. Cholinergic nerve fibers in the cortex
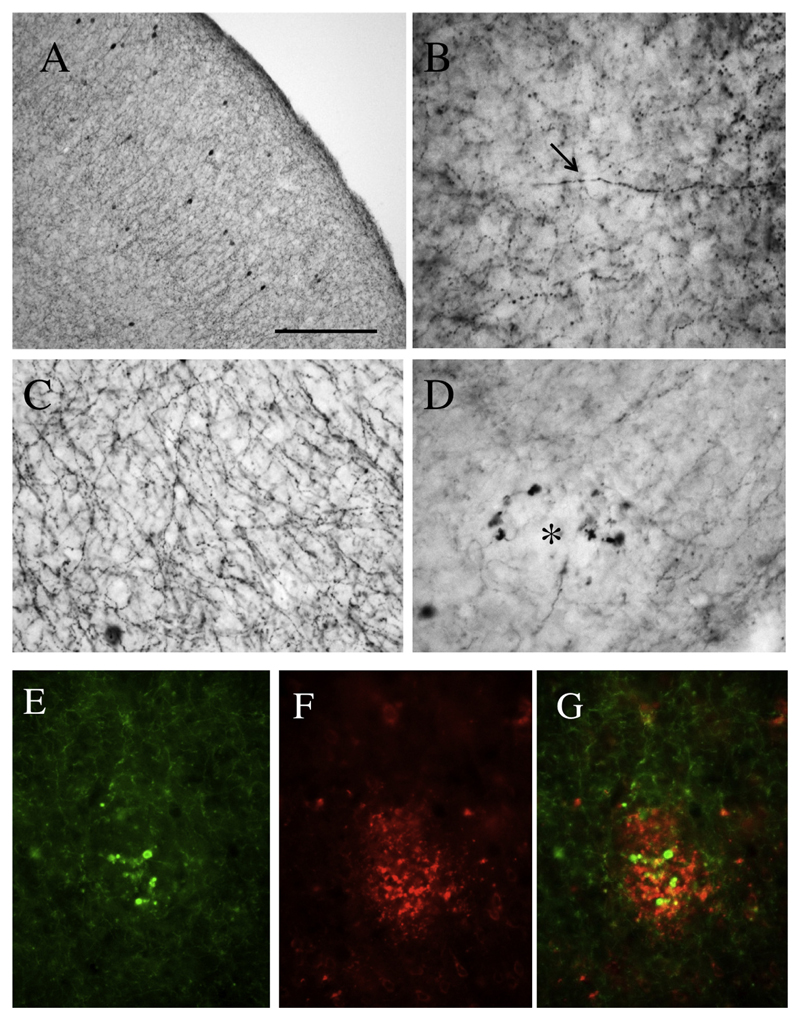
Cholinergic nerve fibers in the cortex were visualized by ChAT+ immunohistochemistry (Fig. 5A and B) or acetylcholinesterase histochemistry (Fig. 5C). The number of ChAT+ or AChE+ nerve fibers did not differ between 6- and 12-month-old WT mice (Table 1). However, in the APP_SweDI mice several degenerated ChAT+ nerve fibers were seen and also marked enhanced swollen axonal varicosities (Fig. 5D) in 6- as well as in 12-month-old mice (Table 1). In parallel, also the density of AChE+ fibers decreased in the APP_SweDI mouse model at both time points (Table 1). Co-localization experiments show that these strongly swollen axonal varicosities concentrate around the Aβ plaques (Fig. 5E–G).
Fig. 5.
Cholinergic nerve fibers in the cerebral cortex of wild-type (A, B, C) and 12-month-old APP_SweDI transgenic (D–G) mice. (A, B, D). Brain sections were stained by immunohistochemistry with an antibody against choline acetyltransferase (ChAT; A, B, D, E) or with Koelle-Friedenwald acetylcholinesterase histochemistry (C). Intact cholinergic ChAT+ nerve fibers are shown in wild-type mice (B, arrow) or swollen cholinergic varicosities in transgenic mice (D, asterisk). Co-staining of ChAT+ nerve fibers (Alexa-488, E) with beta-amyloid plaques (Alexa-546, F) shows that the swollen cholinergic varicosities are located close to a plaque in the cortex (G). Scale bar in A: 200 μm (A) or 50 μm (B–G).
3.7. ChAT mRNA expression
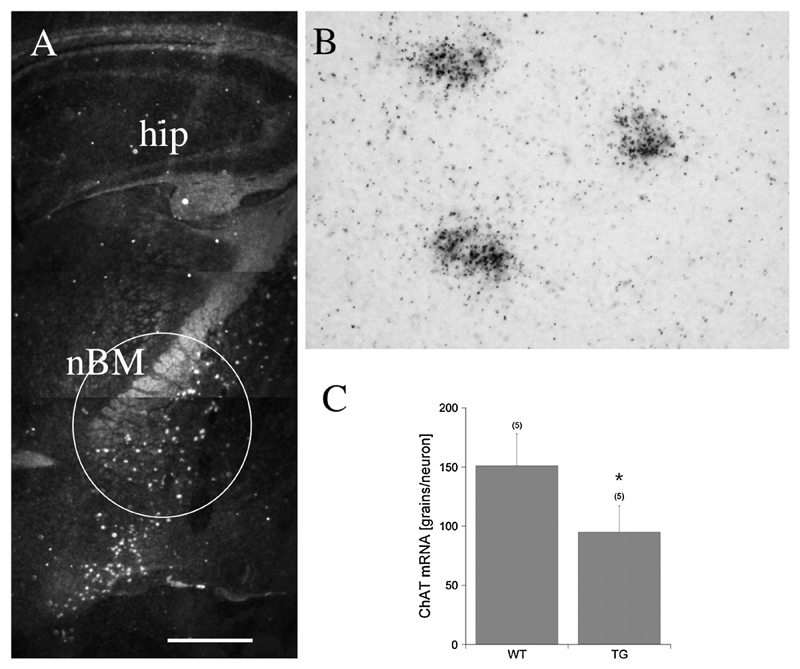
In situ hybridization for ChAT mRNA showed a specific expression in the nBM in dark field microscopy (Fig. 6A). In bright field microscopy black silver grains were located directly over the cells (Fig. 6B). Semi-quantitative analysis showed that the number of neurons expressing ChAT mRNA significantly decreased (Fig. 6C).
Fig. 6.
In situ hybridization detects mRNA expression of choline acetyltransferase (ChAT) in the basal nucleus of Meynert. Dark field microscopy shows several cholinergic neurons as white spots in wild-type control mice (A, circle; nBM, basal nucleus of Meynert; hip, hippocampus). In bright field microscopy black silver grains are located directly over three neurons (B). Semiquantitative analysis shows that the number of neurons expressing ChAT mRNA significantly declined (C). Values are given as mean ± SEM. Statistical analysis was performed using Student’s T test (* p < 0.05) comparing wild-type (WT) and transgenic APP_SweDI (TG) mice. Scale bar in A: 400 μm (A) or 77 μm (B).
4. Discussion
In the present study we show that 12-month-old APP_SweDI mice contain a very high load of Aβ plaques, which are accompanied by an extensive cholinergic neurodegeneration of the nBM and cortical innervation. APP_SweDI mice are also more anxious and exhibit severe cognitive impairment, at least at the beginning of the behavioral tests.
4.1. Anxiety, learning and memory in the APP_SweDI mice
Our behavioral studies showed increased anxiety in the 12-month-old APP_SweDI AD mice, which is in line with another study (Beauquis et al., 2014). In contrast, as compared to the control group, the AD mice showed less anxiety at the beginning of the test phase, which might be caused by an impaired fear memory of the animals. While the healthy control animals showed a normal learning curve by remembering that the test area does not pose any danger, the APP_SweDI AD mice did not build up confidence and thus exhibited a less exploratory behaviour. It seems likely that the enhanced fear also caused reduced motor activity and exploration. This declined exploration may mask an impairment in memory in our behavioral tests. With regard to learning and memory processes, our results show impaired general learning in the APP_SweDI mice in the second week. The mice needed significantly more time to find (and therefore to remember) the target, and furthermore the number of errors made by visiting wrong arms was larger than in the control group. However, when we tested memory in relation to the target in week 4, we could not see a difference in memory between AD and control mice. These data point to different mechanisms in the short- and long-term memory of the AD mice or rapid compensatory mechanisms.
As already shown in the black/white box, the APP_SweDI mice exhibited less anxiety at the beginning of the tests, which was also observed in the plus maze test. The AD mice were also more inclined to exploration, because they entered the open arms and the middle platform more often. This could be due to an impaired learning mechanism, so that the animals were not able to recognize the negative situation in the aversive enclosed arm. However, the wildtype mice immediately understood the dangerous situation in the aversive enclosed arm and thus hid mostly in the non-aversive enclosed arm and refrained from exploring the open arms. In the test session performed on the next day, the APP_SweDI mice showed no differences to the WT mice. Therefore, our data support the finding that the anxiety level did not influence performance of memory-related measures in the classical labyrinth.
4.2. Plaque pathology in the APP_SweDI mice
Senile plaques are one of the key pathological hallmarks of AD, and the amyloid cascade hypothesis proposes that Aβ plaque deposition is the central event in the onset and progression of AD (Hardy & Higgins, 1992). Regardless of some controversies surrounding the Aβ cascade hypothesis, it is generally agreed that Aβ plays a very important role in the progression of AD, and most animal models of AD focus on the overexpression of APP or on mutations that enhance Aβ aggregation. Indeed, transgenic AD mouse models overexpressing mutated APP have been widely used in AD research to study the development of plaques. Our present study clearly verifies plaques in the APP_SweDI model using highly specific antibodies. Aβ plaque quantification shows a highly significant increase in Aβ plaque density in the cortex of 6-month-old APP_SweDI mice as compared to the wild-type mice, and a marked increase in the 12-month-old APP_SweDI mice. These results are in line with findings in other AD mouse models, such as the PDAPP mouse (Reilly et al., 2003) or the APPSWE/PSEN1dE9 mouse (Darvesh et al., 2012). However, it needs to be pointed out that all AD mouse models are only a compromise because they do not fully reflect the whole AD pathology (such as e.g. the lack of Tau pathology) or the sporadic form of AD (>95% of all AD cases).
4.3. Cholinergic neurodegeneration in the APP_SweDI mice
The basal forebrain cholinergic system consists of the nucleus basalis of Meynert (nbM), the diagonal bands of Broca and the medial septal nucleus. The nbM sends its projections to all cortical regions (Woolf, Eckenstein, & Butcher, 1984) and was of particular interest in our present study. Several studies revealed substantial impairments of the cholinergic system in AD, including neocortical deficits in ChAT (Bowen, Smith, White, & Davison, 1976; Perry, Gibson, Blessed, Perry, & Tomlinson, 1977), reduced choline uptake (Rylett, Ball, & Colhoun, 1983) and ACh release (Nilsson, Nordberg, Hardy, Wester, & Winblad, 1986), and degeneration of cholinergic somata in the nbM (Whitehouse et al., 1982). Several studies confirmed a decline in cholinergic neurons in the postmortem brains of AD patients (Bird, Stranahan, & Sumi, 1983; Etienne et al., 1986), and reduced ACh synthesis has been found in biopsy samples from AD patients (Bartus, Dean, & Beer, 1982). Thus, the cholinergic hypothesis of AD proposes that the degeneration of cholinergic neurons in the basal forebrain and the associated loss of cholinergic transmission in the cerebral cortex are the key factors for the cognitive decline and memory loss (Bartus et al., 1982; Mesulam, Mufson, Levey, & Wainer, 1983). Cholinergic neurons were identified by highly selective immunostaining using anti-ChAT antibodies. It is well established that a decline in ChAT + neurons directly correlates with neurodegeneration. However, to exclude only down-regulation of the enzyme, cholinergic neurons were also stained using antibodies against the p75 neurotrophin receptors and, in fact, it is well established that cholinergic neurons express this receptor. Our present data show a significant decline in the number of cholinergic somata in the nbM of 12-month-old transgenic mice as compared with the 6-month-old transgenic or wild-type mice, with both ChAT and p75 neurotrophin receptor immunohistochemistry, which clearly points to the neurodegeneration of cholinergic neurons in the APP_SweDI AD mouse model. This decline in cholinergic neurons was also verified by in situ hybridization at the mRNA level. Cholinergic nerve fibers in the cortex were positively stained by immunostaining against ChAT, because these nBM neurons anterogradely transport the enzyme to the synapse. In addition, the cholinergic nerve fibers were also stained with a well-established AChE histochemistry. Using both methods, we observed a marked decline in cholinergic innervation in the cortex of the brains of 6- and 12-month-old APP_SweDI transgenic mice. This is consistent with findings in the PDAPP (German et al., 2003) and the APP23 (Boncristiano et al., 2002) mice. However, our data also show a very strong up-regulation of ChAT+ immunoreactivity in a few specific nerve fibers. Dual fluorescence immunostaining of Aβ with ChAT revealed that these swollen axonal cholinergic varicosities were mainly located in the vicinity of Aβ plaques in the cortex. Similar morphological abnormalities have been found in other AD mouse models (Bronfman, Moechars, & Van Leuven, 2000; German et al., 2003; Kelley, Perez, & Overk, 2011; Perez, Dar, & Ikonomovic, 2007). This clearly indicates that swollen terminals represent a compensatory mechanism for the reduction of cholinergic nerve fiber density in the cortex.
4.4. Do plaques induce cholinergic neurodegeneration?
The central question arises: do Aβ plaques directly induce damage of synaptic nerve terminals and subsequent retrograde nerve fiber degeneration, or, alternatively, is the cholinergic neurodegeneration an event independent of the Aβ plaques? Interaction between Aβ and the cholinergic system has been extensively studied. Aβ injection into the brain evokes cholinergic hypofunction (Harkany et al., 1995) and evokes inflammatory responses such as reactive astrocytosis and microgliosis (Scali et al., 1999). Very low (pM – nM) concentrations of Aβ inhibit ACh release from cultured hippocampal and cortical slices (Kar et al., 1998) and inhibit nicotinic currents (Pettit, Shao, & Yakel, 2001). In addition, Aβ also interferes with ACh synthesis and inhibits the internalization of extracellular choline into basal forebrain cholinergic neurons (Kar et al., 1998). Aβ causes apoptosis in primary neuronal cultures (Kihara et al., 2005; Yao, Nguyen, & Pike, 2005) and, in particular, cholinergic neurons in the basal forebrain are selectively vulnerable to Aβ neurotoxicity (Harkany et al., 1995). Our present data suggest that the high plaque load in the cortex of 6-month-old mice directly causes damage and decline of cholinergic axons, while the number of cholinergic neurons remains the same. However, in older 12-month-old mice, the plaque load in the cortex markedly increased and in parallel the nerve fiber density further decreased. However, at this stage also the cholinergic neurons degenerated in the nBM, which points to retrograde-induced cell death of cholinergic neurons. In addition, the damage to cholinergic axons also induced compensatory events, seen as the swollen ChAT+ varicosities around the plaques.
Taken together, the present study shows that in an AD mouse model (APP_SweDI mice) a high Aβ plaque load in the cortex causes damage to cholinergic axons in the cortex, followed by subsequent retrograde-induced cell death of cholinergic neurons and some forms of compensatory processes. This degeneration was accompanied by enhanced anxiety and impaired cognition. In conclusion, our data provide evidence that the plaque load in AD brains may directly result in cholinergic decline accompanied by memory impairment. Therapeutic options in AD thus aim to enhance the cholinergic transmission (e.g. acetylcholinesterase inhibitors) or protect cholinergic neurons from cell death (e.g. nerve growth factor), which must be initiated before plaque deposition starts.
Acknowledgements
This study was supported by the Austrian Science Fund – Austria (P24734-B24). We thank Ursula Kirzenberger-Winkler, Marita Luchner and Karin Albrecht for excellent technical assistance.
References
- Bartus RT, Dean R, Beer B. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217(4558):408–414. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- Beauquis J, Vinuesa A, Pomilio C, Pavía P, Galván V, Saravia F. Neuronal and glial alterations, increased anxiety, and cognitive impairment before hippocampal amyloid deposition in PDAPP mice, model of Alzheimer’s disease. Hippocampus. 2014;24(3):257–269. doi: 10.1002/hipo.22219. [DOI] [PubMed] [Google Scholar]
- Bird T, Stranahan S, Sumi S. Alzheimer’s disease: Choline acetyltransferase activity in brain tissue from clinical and pathological subgroups. Annals of Neurology. 1983;14(3):284–293. doi: 10.1002/ana.410140306. [DOI] [PubMed] [Google Scholar]
- Boncristiano S, Calhoun ME, Kelly PH, Pfeifer M, Bondolfi L, Stalder M, et al. Jucker M. Cholinergic changes in the APP23 transgenic mouse model of cerebral amyloidosis. Journal of Neuroscience. 2002;22(8):3234–3243. doi: 10.1523/JNEUROSCI.22-08-03234.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen DM, Smith CB, White P, Davison AN. Neurotransmitter-related enzymes and indices of hypoxia in senile dementia and other abiotrophies. Brain. 1976;99(3):459–496. doi: 10.1093/brain/99.3.459. [DOI] [PubMed] [Google Scholar]
- Bronfman FC, Moechars D, Van Leuven F. Acetylcholinesterase-positive fiber deafferentation and cell shrinkage in the septohippocampal pathway of aged amyloid precursor protein london mutant transgenic mice. Neurobiology of Diseases. 2000;7(3):152–168. doi: 10.1006/nbdi.2000.0283. [DOI] [PubMed] [Google Scholar]
- Chartier-Harlin MC, Crawford F, Houlden H, et al. Early-onset Alzheimer’s disease caused by mutations at codon 717 of the beta-amyloid precursor protein gene. Nature. 1991;353(6347):844–846. doi: 10.1038/353844a0. [DOI] [PubMed] [Google Scholar]
- Darvesh S, Cash MK, Reid GA, Martin E, Mitnitski A, Geula C. Butyrylcholinesterase is associated with β-amyloid plaques in the transgenic APPSWE/PSEN1dE9 mouse model of Alzheimer disease. Journal of Neuropathology and Experimental Neurology. 2012;71(1):2–14. doi: 10.1097/NEN.0b013e31823cc7a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daschil N, Kniewallner KM, Obermair GJ, Hutter-Paier B, Windisch M, Marksteiner J, Humpel C. L-Type calcium channel blockers and substance P induce angiogenesis of cortical vessels associated with beta-amyloid plaques in an Alzheimer mouse model. Neurobiology of Aging. 2015;36:1333–1341. doi: 10.1016/j.neurobiolaging.2014.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P, Maloney AJ. Selective loss of central cholinergic neurons in Alzheimer’s disease. Lancet. 1976;2:1403. doi: 10.1016/s0140-6736(76)91936-x. [DOI] [PubMed] [Google Scholar]
- Davis J, Xu F, Deane R, Romanov G, Previti ML, Zeigler K, et al. Van Nostrand WE. Early-onset and robust cerebral microvascular accumulation of beta-amyloid-protein in transgenic mice expressing low levels of a vasculotropic Dutch/Iowa mutant form of beta-amyloid-protein precursor. Journal of Biological Chemistry. 2004;279(19):20296–20306. doi: 10.1074/jbc.M312946200. [DOI] [PubMed] [Google Scholar]
- Etienne P, Robitaille Y, Wood P, Gauthier S, Nair NP, Quirion R. Nucleus basalis neuronal loss, neuritic plaques and choline acetyltransferase activity in advanced Alzheimer’s disease. Neuroscience. 1986;19(4):1279–1291. doi: 10.1016/0306-4522(86)90142-9. [DOI] [PubMed] [Google Scholar]
- Francis PT, Palmer AM, Snape M, Wilcock GK. The cholinergic hypothesis of Alzheimer’s disease: A review of progress. Journal of Neurology, Neurosurgery and Psychiatry. 1999;66(2):137–147. doi: 10.1136/jnnp.66.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Games D, Adams D, Alessandrini R, Barbour R, Berthelette P, Blackwell C, et al. Alzheimer-type neuropathology in transgenic mice overexpressing V717F beta-amyloid precursor protein. Nature. 1995;373(6514):523–527. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- German DC, Yazdani U, Speciale SG, Pasbakhsh P, Games D, Liang CL. Cholinergic neuropathology in a mouse model of Alzheimer’s disease. Journal of Comparative Neurology. 2003;462(4):371–381. doi: 10.1002/cne.10737. [DOI] [PubMed] [Google Scholar]
- Goate A, Chartier-Harlin MC, Mullan M, Brown J, Crawford F, Fidani L, et al. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature. 1991;349(6311):704–706. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- Hardy J, Higgins G. Alzheimer’s disease: The amyloid cascade hypothesis. Science. 1992;256:185–5. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- Harkany T, Lengyel Z, Soos K, Penke B, Luiten PG, Gulya K. Cholinotoxic effects of beta-amyloid (1–42) peptide on cortical projections of the rat nucleus basalis magnocellularis. Brain Research. 1995;695(1):71–75. doi: 10.1016/0006-8993(95)00823-9. [DOI] [PubMed] [Google Scholar]
- Humpel C, Weis C. Nerve growth factor and cholinergic CNS neurons studied in organotypic brain slices. Implication in Alzheimer’s disease? Journal of Neural Transmission (Supplement) 2002;62:253–263. doi: 10.1007/978-3-7091-6139-5_23. [DOI] [PubMed] [Google Scholar]
- Kar S, Issa AM, Seto D, Auld DS, Collier B, Quirion R. Amyloid beta-peptide inhibits high-affinity choline uptake and acetylcholine release in rat hippocampal slices. Journal of Neurochemistry. 1998;70(5):2179–2187. doi: 10.1046/j.1471-4159.1998.70052179.x. [DOI] [PubMed] [Google Scholar]
- Kelley C, Perez S, Overk C. Effect of neocortical and hippocampal amyloid deposition upon galaninergic and cholinergic neurites in AβPPswe/PS1ΔE9 Mice. Journal of Alzheimer’s Disease. 2011;25(3):491–504. doi: 10.3233/JAD-2011-102097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihara T, Shimohama S, Sawada H, Kimura J, Kume T, Kochiyama H, et al. Akaike A. Nicotinic receptor stimulation protects neurons against beta-amyloid toxicity. Annals of Neurology. 2005;42(2):159–163. doi: 10.1002/ana.410420205. [DOI] [PubMed] [Google Scholar]
- Lehéricy S, Hirsch EC, Cervera-Piérot P, Hersh LB, Bakchine S, Piette F, et al. Agid Y. Heterogeneity and selectivity of the degeneration of cholinergic neurons in the basal forebrain of patients with Alzheimer’s disease. Journal of Comparative Neurology. 1993;330(1):15–31. doi: 10.1002/cne.903300103. [DOI] [PubMed] [Google Scholar]
- Marchesi VT. An alternative interpretation of the amyloid Aβ hypothesis with regard to the pathogenesis of Alzheimer’s disease. Proceedings of the National academy of Sciences of the United States of America. 2005;102(26):9093–9098. doi: 10.1073/pnas.0503181102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Levey AI, Wainer BH. Cholinergic innervation of cortex by the basal forebrain: Cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. Journal of Comparative Neurology. 1983;214(2):170–197. doi: 10.1002/cne.902140206. [DOI] [PubMed] [Google Scholar]
- Murrell J, Farlow M, Ghetti B, Benson MD. A mutation in the amyloid precursor protein associated with hereditary Alzheimer’s disease. Science. 1991;254(5028):97–99. doi: 10.1126/science.1925564. [DOI] [PubMed] [Google Scholar]
- Nilsson L, Nordberg A, Hardy J, Wester P, Winblad B. Physostigmine restores 3H-acetylcholine efflux from Alzheimer brain slices to normal level. Journal of Neural Transmission. 1986;67(3–4):275–285. doi: 10.1007/BF01243353. [DOI] [PubMed] [Google Scholar]
- Perez S, Dar S, Ikonomovic M. Cholinergic forebrain degeneration in the APPswe/PS1dE9 transgenic mouse. Neurobiology of Diseases. 2007;28(1):3–15. doi: 10.1016/j.nbd.2007.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry EK, Gibson PH, Blessed G, Perry RH, Tomlinson BE. Neurotransmitter enzyme abnormalities in senile dementia. Choline acetyltransferase and glutamic acid decarboxylase activities in necropsy brain tissue. Journal of the Neurological Sciences. 1977;34(2):247–265. doi: 10.1016/0022-510x(77)90073-9. [DOI] [PubMed] [Google Scholar]
- Perry EK, Tomlinson BE, Blessed G, Bergmann K, Gibson PH, Perry RH. Correlation of cholinergic abnormalities with senile plaques and mental test scores in senile dementia. British Medical Journal. 1978;2(6150):1457–1459. doi: 10.1136/bmj.2.6150.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettit DL, Shao Z, Yakel JL. Beta-Amyloid(1-42) peptide directly modulates nicotinic receptors in the rat hippocampal slice. Journal of Neuroscience. 2001;21:RC120. doi: 10.1523/JNEUROSCI.21-01-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly JF, Games D, Rydel RE, Freedman S, Schenk D, Young WG, et al. Bloom FE. Amyloid deposition in the hippocampus and entorhinal cortex: Quantitative analysis of a transgenic mouse model. Proceedings of the National academy of Sciences of the United States of America. 2003;100(8):4837–4842. doi: 10.1073/pnas.0330745100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitz C. Alzheimer’s disease and the amyloid cascade hypothesis: A critical review. International Journal of Alzheimer’s Disease. 2012;2012:e369808. doi: 10.1155/2012/369808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rylett RJ, Ball MJ, Colhoun EH. Evidence for high affinity choline transport in synaptosomes prepared from hippocampus and neocortex of patients with Alzheimer’s disease. Brain Research. 1983;289(1–2):169–175. doi: 10.1016/0006-8993(83)90017-3. [DOI] [PubMed] [Google Scholar]
- Scali C, Prosperi C, Giovannelli L, Bianchi L, Pepeu G, Casamenti F. Beta(1–40) amyloid peptide injection into the nucleus basalis of rats induces microglia reaction and enhances cortical gamma-aminobutyric acid release in vivo. Brain Research. 1999;831:319–321. doi: 10.1016/s0006-8993(99)01492-4. [DOI] [PubMed] [Google Scholar]
- Schliebs R, Arendt T. The significance of the cholinergic system in the brain during aging and in Alzheimer’s disease. Journal of Neural Transmission. 2006;113(11):1625–1644. doi: 10.1007/s00702-006-0579-2. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer’s disease: Genes, proteins, and therapy. Physiological Reviews. 2001;81(2):741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- Silva RH, Frussa-Filho R. The plus-maze discriminative avoidance task: A new model to study memory – anxiety interactions. Effects of chlordiazepoxide and caffeine. Journal of Neuroscience Methods. 2000;102:117–125. doi: 10.1016/s0165-0270(00)00289-2. [DOI] [PubMed] [Google Scholar]
- Ullrich C, Pirchl M, Humpel C. Hypercholesterolemia in rats impairs the cholinergic system and leads to memory deficits. Molecular and Cellular Neuroscience. 2010;45(4):408–417. doi: 10.1016/j.mcn.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse P, Price D, Struble R, Clarke A. Alzheimer’s disease in senile dementia: Loss of neurones in the basal forebrain. Science. 1982;215:1237–1239. doi: 10.1126/science.7058341. [DOI] [PubMed] [Google Scholar]
- Wilcock GK, Esiri MM, Bowen DM, Smith CC. Alzheimer’s disease. Correlation of cortical choline acetyltransferase activity with the severity of dementia and histological abnormalities. Journal of the Neurological Sciences. 1982;57(2–3):407–417. doi: 10.1016/0022-510x(82)90045-4. [DOI] [PubMed] [Google Scholar]
- Woolf NJ, Eckenstein F, Butcher LL. Cholinergic systems in the rat brain: I. Projections to the limbic telencephalon. Brain Research Bulletin. 1984;13(6):751–784. doi: 10.1016/0361-9230(84)90236-3. [DOI] [PubMed] [Google Scholar]
- Yao M, Nguyen TV, Pike CJ. Beta-amyloid-induced neuronal apoptosis involves c-Jun N-terminal kinase-dependent downregulation of Bcl-w. Journal of Neuroscience. 2005;25(5):1149–1158. doi: 10.1523/JNEUROSCI.4736-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]