Abstract
Objectives
To assess initial and long-term outcome of children with persistent/chronic idiopathic thrombocytopenic purpura (ITP) treated with 4 infusions of rituximab and three 4-day cycles of dexamethasone (4R+3Dex) including cohorts with most benefit and/or treatment associated toxicity.
Study design
All pediatric patients with ITP at Weill-Cornell who received 4R+3Dex were included in this retrospective study. Duration was median time from first rituximab infusion to treatment failure. Patient cohort included 33 children ages 1-18 years with persistent/chronic ITP; 19 were female, 10 of whom were adolescents. Every patient had failed more than 1 and usually several ITP treatments.
Results
Children were treated with rituximab, 375 mg/m2 weekly for 4 weeks and three 4-day courses of dexamethasone 28 mg/m2 (40 mg max). Average age of nonresponders was 7.75 years, and initial responders averaged 12.7 years (P =.0073); 30% maintained continuing response at 60 months or last check-up. Eight of the 10 patients who underwent remission were female with ITP <24 months prior to initiating 4R+3Dex. All responding male patients except 2 relapsed.
Conclusions
Durable unmaintained ITP remission after 4R+3Dex was seen almost exclusively in female adolescents with <24 months duration of ITP. This provides a new therapeutic paradigm for a subpopulation with hard-to-treat chronic ITP. The pathophysiology of ITP underlying this distinction requires further elucidation.
Idiopathic thrombocytopenic purpura (ITP) is the most common cause of acquired, nonchemotherapy-induced thrombocytopenia and is autoimmune in origin.1 Autoimmunity in ITP is not only related to antiplatelet antibodies but also cytotoxic T cells.2–4 Antiplatelet antibodies and likely cytotoxic T cells not only accelerate platelet destruction but also impair platelet production.5,6
Immunomodulation has been the traditional approach to treatment of ITP. Frontline approaches include steroids, intravenous immunoglobulin, intravenous anti-D, and observation.7–9 However, even though ITP in most affected children will spontaneously improve, 13%-36% of patients will go on to develop chronic disease of greater than 1 year duration.10 For these patients and for a number of those with persistent disease (3-12 months duration) who are difficult to manage, additional treatments may be required or at least advantageous. The most commonly used second line treatments include therapeutics such as thrombopoietic agents, rituximab, azathioprine, mycophenolate mofetil, and splenectomy.11–15
Splenectomy may be curative but typically is avoided if at all possible.16 Rituximab alone has not been shown to provide any immediate benefit but may result in gradual long-term responses with increases in platelet counts over time.17 However, rituximab is generally considered the other treatment, with the possible exception of cyclophosphamide, with curative potential.18
Rituximab is a monoclonal antibody directed against CD20.19 As such it depletes circulating splenic and some nodal B cells but only a small fraction of plasma cells. Rituximab alone only achieves a 25% lasting response rate in children with ITP and long-term responses w ith dexamethasone alone are similarly disappointing.20–22 However, 2 studies in adults in which rituximab was combined with 1 4-day cycle of dexamethasone, and 2 reports from our group combining 3 instead of 1 cycle of dexamethasone with rituximab, strongly suggest that this combination results in a better outcome not only in terms of short-term response but also in regard to long-term outcome (“cure”).17,23–25 In view of the experience using rituximab combined with dexamethasone in children with ITP, this study explored treatment of 4 infusions of rituximab interspersed with three 4-day cycles of dexamethasone in 33 children up to 18 years of age with persistent or chronic ITP. Because studies have shown that cohorts of younger adult women with ITP tend to respond better to rituximab than other adult ITP populations,26 the goal of the study was to explore if the combination of rituximab with dexamethasone as a treatment protocol in pediatric patients is safe and tolerable, and what prognostic factors existed for long-term response.
Methods
Inclusion criteria were pediatric patients, 1-18 years of age with persistent/chronic ITP (defined as ITP >6-month duration). Patients were referred for primary ITP, although 3 patients were subsequently determined to have evolving Evan’s or common variable immune deficiency and one had acute disseminated encephalomyelitis, which declared during their prolonged course (prior to initiating 4 infusions of rituximab and three 4-day cycles of dexamethasone [4R+3Dex]). All patients had failed at least 1 previous therapy (usually 2 or 3) prior to initiation of 4R+3Dex. Patient demographics and clinical details are described in the Table. Antibody screening (ie, antinuclear antibody) was not routinely done if a patient had isolated thrombocytopenia. Although we did not determine Tanner stage for each patient upon enrollment, review of the medical charts indicated that on average, patient cohorts were of equal pubertal maturity. Patients were selected for this treatment protocol in discussion with families about the various therapies and trials available at that time and what would be most conducive to maintaining quality of life. The patients analyzed here received 4R+3Dex over a 5-year period (2010-2015). This retrospective chart review protocol was approved by the Weill Cornell Medicine Institutional Review Board but not listed on Clinicaltrials.gov because it was retrospective.
Table.
Patient demographics for children age 1-18 years old with ITP that participated in 4R+3Dex protocol
| Sex | Age at therapy (y) | Prior treatment | Number of previous therapies | Starting platelet count (+ = therapy) | Number of rituximab cycles | Number of dexamethasone cycles | Duration of ITP (mo) |
|---|---|---|---|---|---|---|---|
| F | 7 | Prednisone, IVIG, WinRho, solumedrol | 4 | 69+ | 4 | 3 | 5 |
| M | 12 | IVIG, Nplate, prednisone | 3 | 129+ | 4 | 3 | 27 |
| M | 4 | Prednisone, anti-D, IVIG | 3 | 45+ | 4 | 3 | 24 |
| M | 4 | Prednisone, anti-D, IVIG | 3 | 49+ | 4 | 3 | 81 |
| F | 17 | Steroid, IVIG, WinRho, eltrombopag | 3 | 78+ | 4 | 3 | 6 |
| F | 7 | IVIG, rituximab, prednisone | 3 | 17 | 4 | 4 | 36 |
| F | 17 | Prednisone | 1 | 16 | 4 | 2 | 84 |
| F | 12 | IVIG | 1 | 20 | 4 | 3 | 21 |
| F | 17 | IVIG, anti-D | 2 | 16 | 4 | 3 | 159 |
| F | 15 | Prednisone, IVIG | 2 | 12+ | 4 | 3 | 1 |
| M | 17 | IVIG, dexamethasone | 2 | 8 | 4 | 3 | 12 |
| F | 3 | Prednisone, IVIG | 2 | 126+ | 4 | 3 | 7 |
| F | 15 | Prednisone, IVIG | 2 | 11 | 4 | 3 | 1 |
| M | 10 | Prednisone, IVIG | 2 | 7 | 4 | 3 | 7 |
| F | 15 | Anti-D | 1 | 39 | 4 | 3 | 7 |
| F | 8 | Prednisone, IVIG | 2 | 42 | 4 | 3 | 86 |
| M | 4 | IVIG, anti-D, methylpred, Nplate | 4 | 177+ | 4 | 4 | 10 |
| F | 4 | IVIG, prednisone | 2 | 25 | 4 | 3 | 11 |
| F | 15 | IVIG, anti-D, prednisone | 3 | 98+ | 4 | 3 | 9 |
| M | 13 | Prednisone for other health reasons | 1 | 5 | 2 | 1 | 12 |
| F | 4 | IVIG | 1 | 14 | 4 | 3 | 14 |
| F | 3 | IVIG, anti-D | 2 | 18 | 4 | 3 | 9 |
| F | 8 | Prednisone, IVIG | 2 | 9 | 2 | 1 | 3 |
| M | 4 | Prednisone, Nplate, anti-D, IVIG | 4 | 7+ | 4 | 3* | 4 |
| M | 2 | Prednisone, IVIG, Nplate, vincristine, anti-D | 5 | 5+ | 4 | 3 | 7 |
| F | 12 | IVIG | 1 | 9 | 4 | 3 | 9 |
| M | 10 | IVIG, solumedrol, dexamethasone, anti-D | 4 | 25 | 4 | 3 | 12 |
| M | 11 | Prednisone, anti-D | 2 | 36 | 4 | 3 | 28 |
| M | 15 | Prednisone, IVIG | 2 | 19 | 4 | 3 | 5 |
| M | 17 | Prednisone, IVIG | 2 | 19 | 4 | 3 | 84 |
| F | 1 | IVIG, anti-D | 2 | 35 | 4 | 1 | 8 |
| M | 11 | Prednisone, anti-D, Amicar | 2 | 18 | 4 | 3 | 49 |
| F | 14 | Prednisone, IVIG | 2 | 56+ | 4 | 3* | 11 |
F, female; IVIG, intravenous immunoglobulin; M, male.
Deidentified information on the 33 pediatric patients with ITP enrolled in the trial. The first column indicates sex, the next indicates age. The third column enumerates prior ITP treatments received, and the next column lists the number of previous therapies. The fifth column provides the platelet count at the outset of the 4R+3Dex trial, and the next 2 columns list how many cycles of rituximab and dexamethasone the patient ultimately received. The last column provides duration of ITP diagnosis until start of 4R+3Dex therapy. The * next to dexamethasone cycles indicates transition from dexamethasone to methylprednisolone. The + next to the platelet count indicates a recent (less than 4 weeks) ITP related treatment that may have transiently increased platelet counts prior to 4R+3Dex initiation.
Thirty-three children were treated with 4R+3Dex at, or in close consultation with, the Platelet Disorders Center at Weill Cornell Medical College, and all such children in the specified time period of the study are included in this report. Children were treated with rituximab 375 mg/m2 weekly for 4 weeks (days 1, 8, 15, 22) and three 4-day courses of dexamethasone 28 mg/m2 (40 mg max) on days 1-4, 15-18, and 29-32. Some deviations occurred in several patients as clinically indicated, specifically when patients required tailoring of either their rituximab or dexamethasone (eg, when upon referral the patient had just received one or the other treatment or when approval for rituximab could not be immediately obtained in which case dexamethasone was initiated first). Treatment did not need to be discontinued for serious adverse drug events in any of our patients. In several cases (Table), IV methylprednisolone was substituted for dexamethasone because of poor tolerance of the first cycle of dexamethasone.
Statistical Analyses
Platelet responses were divided into 2 groups: partial response (PR) (50-100 × 109/L), or complete response (CR) (≥100 × 109/L); those with peak platelet counts <50 × 109/L were considered to be nonresponders. Initial response was measured at or around the 8-week mark from the initiation of therapy. This time point was chosen so as to minimize short-term temporizing effects of the dexamethasone treatment. Response was determined based on the highest platelet count achieved spontaneously without additional treatment. Long-term response duration was estimated by Kaplan- Meier analysis. Relapse was defined as either 2 consecutive platelet counts <50 × 109/L and/or need for additional therapy. The duration of response was calculated from date of first rituximab administration to relapse or latest follow-up as of May 31, 2015. IBM SPSS Statistics 22 (Armonk, NY) was used for statistical analysis including Kaplan-Meier graphs. Individual subgroups were compared using Student tests and the Fisher exact tests. Two-tailed values of <.05 were considered significant.
Results
The goal of this study was to provide both an estimate of initial response and of a lasting response with no additional therapy in children with ITP of ≥6 months duration. 4R+3Dex induces a prolonged remission for 10 out of 33 pediatric patients. Thirty-three children ages 1-18 years with chronic ITP received 4 R+3Dex. Forty-five percent (15/33) initially responded to treatment, defined as platelets greater than 50 × 109/L at a minimum of 8 weeks after initiation of 4R+3Dex. One additional patient had a delayed CR that was recorded initially as nonresponder. Of the patients that responded to treatment, 10 remain in remission without additional therapy for a median of 35.5 months (range 10-58 months).
The average age of patients who did not respond to treatment was 7.75 years (SD 4.73 years), and the average age of children who had an initial treatment response was 12.69 years of age (SD 4.98 years) (=.0073). Male and female patients were similar in these subsets (Figure 1). The average age of male initial responders was 13 years compared with 7 years for nonresponders. The average age of female initial responders was 12.67 years, compared with 8 years for nonresponders.
Figure 1.
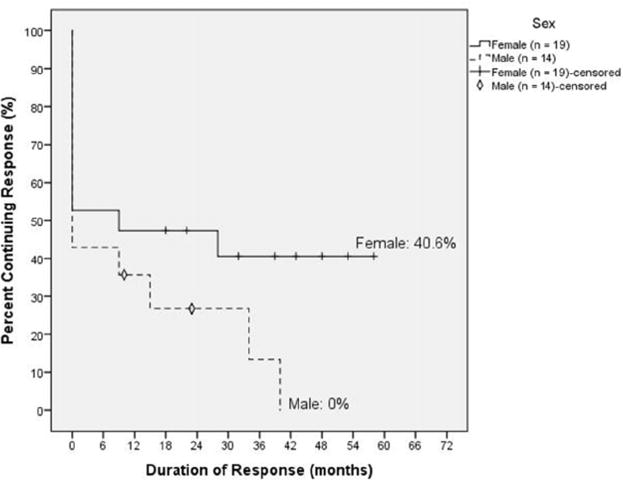
Kaplan-Meier plot of male and female patients with ITP treated with 4R+3Dex. Male and female patients had similar initial response rates, but over time all but 2 male patients relapsed, whereas 80% of female responders maintained a durable remission without additional treatment.
Forty percent of initial responders (6 of 15) or 6 of 16 total responders ultimately relapsed in the 60-month period following 4R+3D treatment. Two initial responders achieved a PR while the other 13 of 15 had an initial CR. One of 2 patients that had an initial PR progressed to a CR and did not relapse, while the second patient remained in PR and ultimately relapsed. Relapses occurred at 9, 9, 15, 28, 34, and 40 months post-treatment.
Female and male pateints had similar initial response rates after receiving 4R+3Dex (9 initial responders/19 total treated female patients compared with 6 initial responders/14 total treated male patients). At 60 months from initial rituximab infusion or at the last check-up, 80% (8/10) of all female responders (initial and delayed) continued to have unmaintained platelet levels in the CR range. Interestingly, the 2 female patients that relapsed were both 8 years of age. The remaining initial female responders who achieved unmaintained remission were each greater than 12 years old. The 1 patient who did not initially respond but ultimately had gradual response to a CR was less than 10 years of age, and this phenomenon may have represented a partial spontaneous response. In contrast only 2 of 6 male initial responders continued in remission, with 4 of 6 male responders relapsing within 36 months. Taken together with the average age of initial female r esponders (average age = 12.67 years), these data indicate that female patients at puberty or older who had an initial response to 4R+3Dex were substantially better able to continue in an unmaintained long-term remission than either younger girls or male patients of any age.
Female patients diagnosed <12 months prior to initial rituximab infusion had a 54% (7 of 13) initial response rate compared with 33% (2 of 6) for those diagnosed with ITP greater than 12 months previously. Among initial responders to 4R+3Dex, 86% (6 of 7) of the female patients with less than 12 months of chronic ITP maintained a long-term remission. There was only 1 female initial responder with ITP duration of >12 months but <24 months (21 months), and she maintained her initial response (Figure 2).
Figure 2.
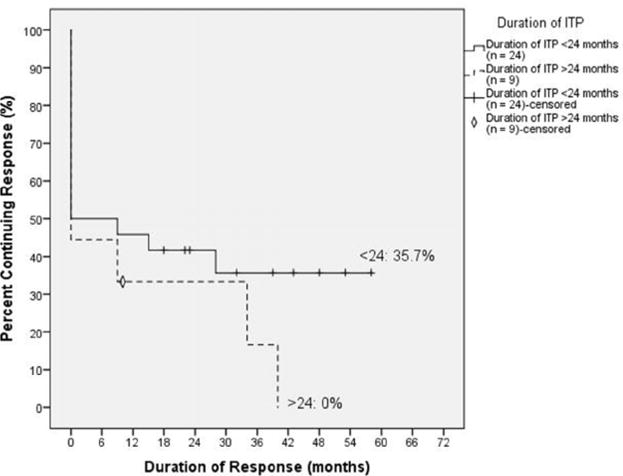
Kaplan-Meier curve of response rates in pediatric patients with ITP based on duration of diagnosis. Duration of ITP prior to initiation of 4R+3Dex did not predict initial response to therapy but did have a significant effect on the likelihood of maintaining remission.
Overall, female adolescent patients with ITP <24 months duration had a very positive response r ate to 4R+3Dex. Although initial response rates were similar among all pediatric patients with ITP treated with 4R+3Dex, ability to remain in an unmaintained remission varied significantly. In female patients with persistent or chronic ITP diagnosed less than 24 months prior to 4R+3Dex initiation, 53% (8 of 15) had an initial response to therapy and 7 (47% of the total, or 87.5% of the responders) maintained a long-term remission without additional therapy compared with 27% of the entire group and 7% of male patients. In comparison only 1 of 4 female patients with a duration of ITP diagnosis of >24 months prior to 4R+3Dex therapy had an initial response; she ultimately relapsed (Figure 3).
Figure 3.
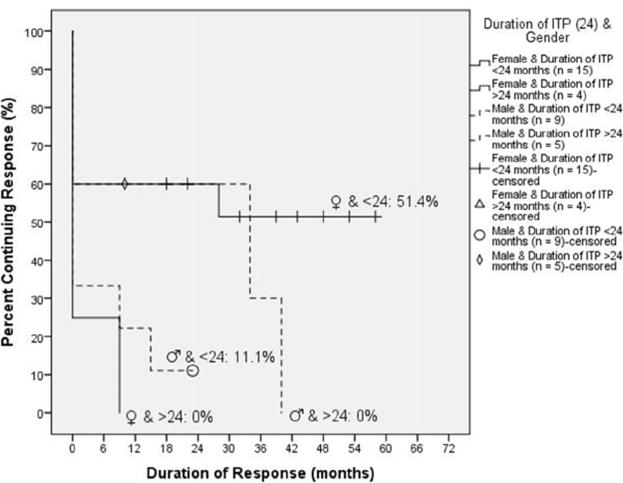
The effects of sex and duration of ITP on prognosis after 4R+3Dex therapy. In this graph, we stratify many of the study variables together. Female patients who were diagnosed less than 24 months before receiving 4R+3Dex therapy were most likely to achieve and maintain a response.
The 4R+3Dex therapy was relatively well tolerated in our pediatric population. Four of 33 patients had gastrointestinal issues related to dexamethasone and were, thus, switched to different steroid infusions (ie, intravenous methylprednisolone or other oral steroids). Prophylactic gastrointestinal treatment (eg, proton pump inhibitor), was recommended to all patients. Five patients (15%) developed substantial but transient hypogammaglobulinemia. This was treated with monthly intravenous immunoglobulin infusions for several months until resolution.
Discussion
Pediatric patients with persistent/chronic ITP would benefit from curative therapy.27 Splenectomy has traditionally filled that role but is limited by a success rate of only 60%-75%, along with perioperative complications (hemorrhage, thrombosis, and infection), long-term prevalence of thromboembolic events, and a life-long risk of overwhelming sepsis.28 This study, there-fore, explored whether 4 infusions of rituximab 375 mg/m2 with three 4-day cycles of dexamethasone 28 mg/m2/day (maximum 40 mg/day) would be curative in children w ith ITP of ≥6 months duration. Because the focus was on cure rather than on “response,” the data description emphasizes long-term outcomes.
In the 33 patient cohort of pediatric patients with ITP, we found that the ability to achieve long-term remission using 4R+3Dex was influenced by a combination of age, sex, and duration of ITP before the initiation of 4R+3Dex treatment. Male and female patients with ITP had similar rates of initial response to 4R+3Dex therapy, and initial response rates tended to be higher in the adolescent age group in both cohorts. Among these initial responders to 4R+3Dex therapy, 80% of female initial responders have remained in remission whereas approximately 70% of male responders have relapsed to date. Female patients who received 4R+3Dex less than 24 months from time of the initial diagnosis had a much higher probability of remaining in remission. Thus, despite the limited number of children included in this study, it is clear that the combination of female sex, adolescent age, initial response and duration of ITP diagnosis less than 24 months from initiation of 4R+3Dex therapy are all positive indicators that a patient will continue in an unmaintained remission. Younger children, males, and those with longer duration of ITP are much less likely to remain in remission.
The overall long-term unmaintained response rate was disappointing (27%) but comparable with our previous multicenter report, which identified 25%-26% as long-term responders.25 However, the finding of a very specific group of long-term responders echoes similar findings identified in older women with persistent/chronic ITP and suggests that there is a previously unidentified group of children uniquely suited to be cured by this therapy.
Female patients undergoing or having completed puberty were better long-term responders to 4R+3Dex than younger female patients and all male patients. This suggests that pubertal hormonal changes in women may alter the milieu of the autoimmune response and make it more amenable to treatment with 4R+3Dex.29 The hormonal changes and relatively immunosuppressed state in female patients during reproductive age seems to influence response to this therapy. These findings are consistent with similar findings of the impact on response in adults with ITP of the combination of female sex and shorter duration of ITP.25
We believe that female adolescents and women with a short duration of ITP may have a largely or almost entirely autoantibody-mediated mechanism of ITP compared with a T-cell driven pathophysiology in those with chronic disease. Female patients of this age group are at increased risk of other autoantibody-mediated diseases, and autoantibody-mediated persistent and early chronic ITP may be part of this spectrum.30 If so, the combination of rituximab (for B cells) and dexamethasone (for plasma cells) treatment might be expected to be more effective than either modality alone.
Prolonged duration of ITP (ie, greater than 24 months) in children with chronic ITP may be associated with the development of T-cell driven pathways of autoimmunity. This mirrors the phenomena of difficult to cure, long-standing disease that has been described in many different autoimmune diseases of childhood and “autoimmune expansion” may be but one of the underlying mechanisms.31 To the extent that our “T cell hypothesis” is correct, this would suggest that the failure of 4R+3Dex in these instances may be secondary to lack of anti-T cell agents in this particular combination. The addition of an agent such as mycophenolate mofetil to the 4R+3Dex regimen may contribute to curative therapy in a higher number and broader spectrum of patients.
Given that the addition of anti-T cell agents may be important for certain patient cohorts, it will be important to define biomarkers or indicators of which patients will most likely benefit from 4R+3Dex with or without a T cell agent prior to beginning the therapy. The inability to accurately and quantitatively measure antiplatelet antibodies makes direct testing of the “autoantibody” hypothesis difficult.32 T and B cell subsets from a very limited number of patients did not reveal significant differences between responders and nonresponders. We are currently using sophisticated immune cell profiles such as T cell receptor V-beta (TCR-Vbeta) repertoires to analyze patients and preliminary data in adults treated with 4R+3Dex showed that among 10 patients in unmaintained complete responses, 9 had nor mal distribution of their TCR-Vbeta repertoire. In contrast, 13 of 26 nonresponders (8 patients) or relapses (18 patients) had oligoclonal or monoclonal patterns suggesting that persistent abnormal T cell responses are linked to a poorer response. Thus, further refinement of this technique may yield a potential biomarker based assay to stratify patients to 4R+3Dex or 4R+3Dex with an anti-T cell agent.
Acknowledgments
Funded by the Children’s Cancer and Blood Foundation (J.B.B.). J.B.B. receives research support from Amgen, Boehringer Ingelheim, GlaxoSmithKline, Novartis Pharmaceuticals Corporation, Prophylix Pharma, Protalex, and Rigel Pharmaceuticals. J.B.B. has participated on Advisory Boards for Amgen, Momenta Pharmaceuticals, Novartis Pharmaceuticals, Prophylix Pharma, Protalex, and Rigel Pharmaceuticals. J.B.B. has participated in Speaker’s Bureaus for Novartis Pharmaceuticals and Physicians Education Resource. J.B.B. receives honoraria from UpToDate, Inc. The other authors declare no conflicts of interest.
Glossary
- 4R+3Dex
4 infusions of rituximab and three 4-day cycles of dexamethasone
- CR
Complete response
- ITP
Idiopathic thrombocytopenic purpura
- PR
Partial response
References
- 1.Cines DB, Bussel JB, Liebman HA, Luning Prak ET. The ITP syndrome: pathogenic and clinical diversity. Blood. 2009;113:6511–21. doi: 10.1182/blood-2009-01-129155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olson B, Andersson PO, Jernas M, Jacobsson S, Carlsson B, Carlsson LM, et al. T-cell mediated cytotoxicity toward platelets in chronic idiopathic thrombocytopenic purpura. Nat Med. 2003;9:1123–4. doi: 10.1038/nm921. [DOI] [PubMed] [Google Scholar]
- 3.McKenzie CG, Guo L, Freedman J, Semple JW. Cellular immune dysfunction in immune thrombocytopenia (ITP) Br J Haematol. 2013;163:10–23. doi: 10.1111/bjh.12480. [DOI] [PubMed] [Google Scholar]
- 4.Yazdanbakhsh K, Zhong H, Bao W. Immune dysregulation in immune thrombocytopenia. Semin Hematol. 2013;50:S63–7. doi: 10.1053/j.seminhematol.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang M, Nakagawa PA, Williams SA, Schwartz MR, Imfeld KL, Buzby JS, et al. Immune thrombocytopenic purpura (ITP) plasma and purified ITP monoclonal autoantibodies inhibit megakaryocytopoiesis in vitro. Blood. 2003;102:887–95. doi: 10.1182/blood-2002-05-1475. [DOI] [PubMed] [Google Scholar]
- 6.Barsam SJ, Psaila B, Forestier M, Page LK, Sloane PA, Geyer JT, et al. Platelet production and platelet destruction: assessing mechanisms of treatment effect in immune thrombocytopenia. Blood. 2011;117:5723–32. doi: 10.1182/blood-2010-11-321398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Izak M, Bussel JB. Contemporary treatment of immune thrombocytopenia. Expert Rev Hematol. 2013;6:697–712. doi: 10.1586/17474086.2013.841076. [DOI] [PubMed] [Google Scholar]
- 8.Eghbali A, Azadmanesh P, Bagheri B, Taherahmadi H, Sadeghi Sedeh B. Comparison between IV immune globulin (IVIG) and anti-D globulin for treatment immune thrombocytopenia: a randomized open-label study. Fundam Clin Pharmacol. 2016;30:385–9. doi: 10.1111/fcp.12198. [DOI] [PubMed] [Google Scholar]
- 9.Cheng Y, Wong RS, Soo YO, Chui CH, Lau FY, Chan P, et al. Initial treatment of immune thrombocytopenic purpura with high-dose dexamethasone. N Engl J Med. 2003;349:831–6. doi: 10.1056/NEJMoa030254. [DOI] [PubMed] [Google Scholar]
- 10.Psaila B, Bussel JB. Refractory immune thrombocytopenic purpura: current strategies for investigation and management. Br J Haematol. 2008;143:16–26. doi: 10.1111/j.1365-2141.2008.07275.x. [DOI] [PubMed] [Google Scholar]
- 11.Ramaswamy K, Hsie L, Leven E, Thompson MV, Nugent D, Bussel JB. Thrombopoietic agents for the treatment of persistent and chronic immune thrombocytopenia in children. J Pediatr. 2014;165:600–5. doi: 10.1016/j.jpeds.2014.03.060. [DOI] [PubMed] [Google Scholar]
- 12.Garzon AM, Mitchell WB. Use of thrombopoietin receptor agonists in childhood immune thrombocytopenia. Front Pediatr. 2015;3:70. doi: 10.3389/fped.2015.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Figueroa M, Gehlsen J, Hammond D, Ondreyco S, Piro L, Pomeroy T, et al. Combination chemotherapy in refractory immune thrombocytopenic purpura. N Engl J Med. 1993;328:1226–9. doi: 10.1056/NEJM199304293281703. [DOI] [PubMed] [Google Scholar]
- 14.Ghanima W, Godeau B, Cines DB, Bussel JB. How I treat immune thrombocytopenia: the choice between splenectomy or a medical therapy as a second-line treatment. Blood. 2012;120:960–9. doi: 10.1182/blood-2011-12-309153. [DOI] [PubMed] [Google Scholar]
- 15.Vianelli N, Palandri F, Polverelli N, Rodeghiero F, Joelsson J, Johansson E, et al. Splenectomy as a curative treatment for immune thrombocytopenia: a retrospective analysis of 233 patients with a minimum follow up of 10 years. Haematologica. 2013;98:875–80. doi: 10.3324/haematol.2012.075648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Provan D, Newland AC. Current management of primary immune thrombocytopenia. Adv Ther. 2015;32:875–87. doi: 10.1007/s12325-015-0251-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooper N, Bussel JB. The long-term impact of rituximab for childhood immune thrombocytopenia. Curr Rheumatol Rep. 2010;12:94–100. doi: 10.1007/s11926-010-0090-5. [DOI] [PubMed] [Google Scholar]
- 18.Liang Y, Zhang L, Gao J, Hu D, Ai Y. Rituximab for children with immune thrombocytopenia. PLoS ONE. 2012;7:e36698. doi: 10.1371/journal.pone.0036698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reff ME, Carner K, Chambers KS, Chinn PC, Leonard JE, Raab R, et al. Depletion of B-cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood. 1994;83:435–45. [PubMed] [Google Scholar]
- 20.Hasan A, Michel M, Patel V, Stasi R, Cunningham-Rundles S, Leonard JP, et al. Repeated courses of rituximab in chronic ITP: three different regimens. Am J Hematol. 2009;84:661–5. doi: 10.1002/ajh.21512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel VL, Mahevas M, Lee SY, Stasi R, Cunningham-Rundles S, Godeau B, et al. Outcomes 5 years after response to rituximab therapy in children and adults with immune thrombocytopenia. Blood. 2012;119:5989–95. doi: 10.1182/blood-2011-11-393975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zaja F, Baccarani M, Mazza P, Bocchia M, Gugliotta L, Zaccaria A, et al. Dexamethasone plus rituximab yields higher sustained response rates than dexamethasone monotherapy in adults with primary immune thrombocytopenia. Blood. 2010;115:2755–62. doi: 10.1182/blood-2009-07-229815. [DOI] [PubMed] [Google Scholar]
- 23.Bussel JB, Lee CS, Seery C, Imahiyerobo AA, Thompson MV, Catellier D, et al. Rituximab and three dexamethasone cycles provide responses similar to splenectomy in women and those with immune thrombocytopenia of less than two years duration. Haematologica. 2014;99:1264–71. doi: 10.3324/haematol.2013.103291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cooper N, Stasi R, Cunningam-Rundles S, Cesarman E, McFarland JG, Bussel JB. Platelet associated antibodies, cellular immunity and FCGR3a genotype influence the response to rituximab in immune thrombocytopenia. Br J Haematol. 2012;158:539–47. doi: 10.1111/j.1365-2141.2012.09184.x. [DOI] [PubMed] [Google Scholar]
- 25.Chapin J, Lee CS, Zhang H, Zehnder JL, Bussel JB. Gender and duration of disease differentiate responses to rituximab-dexamethasone therapy in adults with immune thrombocytopenia. Am J Hematol. 2016;91:907–11. doi: 10.1002/ajh.24434. [DOI] [PubMed] [Google Scholar]
- 26.Marangon M, Vianelli N, Padandri F, Mazzzucconi MG, Santoro C, Barcellini W, et al. Rituximab in immune thrombocytopenia: gender, age and response as predictors of long-term response. Eur J Haematol. 2016;98:371–7. doi: 10.1111/ejh.12839.. [DOI] [PubMed] [Google Scholar]
- 27.Yacobovich J, Revel-Vilk S, Tamary H. Childhood immune thrombocytopenia — who will spontaneously recover? Semin Hematol. 2013;50(Suppl 1):S71–4. doi: 10.1053/j.seminhematol.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 28.Schifferli A, Kuhne T. Chronic immune thrombocytopenia in children: who needs splenectomy? Semin Hematol. 2013;50(Suppl 1):S58–62. doi: 10.1053/j.seminhematol.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 29.Coronel-Restrepo N, Posso-Osorio I, Naranjo-Escobar J, Tobon GJ. Autoimmune diseases and their relationship with immunological, neurological and endocrinological axes. Autoimmun Rev. 2017;6:684–92. doi: 10.1016/j.autrev.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 30.Ngo ST, Steyn FJ, McCombe PA. Gender differences in autoimmune disease. Front Neuroendocrinol. 2014;35:347–69. doi: 10.1016/j.yfrne.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 31.Mercadante ER, Lorenz UM. Breaking free of control: how conventional T cells overcome regulatory T cell suppression. Front Immunol. 2016;7:193. doi: 10.3389/fimmu.2016.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Labarque V, VanGeet C. Clinical practice: immune thrombocytopenia in paediatrics. Eur J Pediatr. 2014;173:163–72. doi: 10.1007/s00431-013-2254-6. [DOI] [PubMed] [Google Scholar]


