Abstract
Objective
Determine the impact of cytolytic versus IL-2 receptor antibody (IL-2RA) induction on acute rejection, graft loss and death in African-American (AA) kidney transplant (KTX) recipients.
Background
AAs are underrepresented in clinical trials in transplantation; thus, there is controversy regarding the optimal choice of perioperative antibody induction in KTX to improve outcomes.
Methods
National cohort study using US transplant registry data from January 1, 2000 to December 31, 2009 in adult solitary AA KTX recipients, with at least 5 years of follow-up. Multivariable logistic and Cox regression were utilized to assess the outcomes of acute rejection, graft loss, and mortality, with interaction terms to assess effect modification.
Results
Twenty-five thousand eighty-four adult AAs receiving solitary KTX were included, 16,927 (67.5%) received cytolytic induction and 8157 (32.5%) received IL-2RA induction. After adjustment for recipient sociodemographics, donor, and transplant characteristics, the use of cytolytic induction therapy reduced the risk of acute rejection by 32% (OR 0.68, 0.62–0.75), graft loss by 9% (HR 0.91, 0.86–0.97), and death by 12% (HR 0.88, 0.83–0.94). There were a number of significant effect modifiers, including public insurance, panel reactive antibody, delayed graft function, and steroid withdrawal; in these groups, cytolytic induction substantially improved clinical outcomes.
Conclusions
These data demonstrate that cytolytic induction therapy, as compared with IL-2RA, reduces the risk of rejection, graft loss, and death in adult AA KTX recipients, particularly in those who are sensitized, receive public insurance, develop delayed graft function, or undergo steroid withdrawal.
Keywords: acute rejection, African American, graft loss, induction therapy, kidney transplant, mortality
The use of induction therapy within kidney transplantation has a long and well-studied history.1,2 Before the advent of antibody preparations, clinicians utilized lymph depletion, high-dose intravenous corticosteroids, and high doses of other immunosuppressants as induction therapies.3–5 From the 1990s onward, induction therapy in transplantation is typically thought of as using mono or polyclonal antibody preparations given perioperatively. Studies have demonstrated that induction therapies using antibody preparations clearly reduce the risk of acute rejection. However, their impact on long-term graft and patient survival is not as well defined. High immunologic risk patients may benefit from more intense induction therapy preparations, such as the cytolytic agents.1,2 The use of the non-T-cell depleting agents [IL-2 receptor antagonist (IL-2 RA) preparations] is still popular choices within kidney transplantation, but is generally recommended for use in lower immunologic risk patients.6–8
In transplantation, African Americans (AAs) are considered high-immunologic risk patients for a wide array of biologic and socioeconomic reasons.9–12 Because of this, there is considerable debate about which antibody induction therapies to utilize within AAs, with no current data demonstrating improved patient or graft survival with the use of cytolytic induction therapies within AAs; however, these studies are restricted by their size and short follow-up, limiting the power to detect differences even if they do exist.13–24 Because of these shortcomings and the limited numbers of AAs enrolled in randomized controlled trials (RCTs), the question of the effectiveness of cytolytic versus IL-2RA induction therapies specifically within the AA kidney transplant population is not well studied or understood. Therefore, the aim of this analysis was to utilize contemporary data available from the national transplant registry to conduct an adequately powered long-term analysis assessing the impact of cytolytic versus IL-2RA induction therapy on clinical outcomes within adult AA kidney transplant recipients.
METHODS
Study Design and Data Source
A nationwide population cohort study of kidney transplant recipients was conducted in a retrospective manner utilizing the United Network of Organ Sharing (UNOS) national registry database. UNOS is a nonprofit private organization that has held the US Government Organ Procurement and Transplantation Network contract to manage the nation’s organ transplant system, including capturing and tracking outcomes on all transplants that occur within the United States.25 After gaining local IRB exempt approval and signing a data use agreement with UNOS, the deidentified standard transplant analysis and research files were acquired, which contained all transplants performed from October 1, 1986 through March 31, 2016.
Patients
Patients were considered eligible for the inclusion in this study if they received a kidney transplant within the United States between January 1, 2000 and December 31, 2009. This starting time period was chosen as it represents a contemporary era of transplantation, such that modern induction therapy and maintenance immunosuppression were commonly utilized.26 The ending date was chosen so that patients had the opportunity to acquire at least 5 years of clinical follow-up to adequately assess long-term graft and patient survival. Pediatrics (<18 yrs of age), those receiving nonrenal transplants (either before, simultaneous, or after the kidney), non-AAs and those who did not receive any induction therapy were excluded from this study.
Study Variables and Outcomes
The primary variable of interest was induction therapy utilized at the time of transplant. For analytic purposes, this was categorized into 2 groups: cytolytic therapy versus IL-2RA therapy. Cytolytic therapy included receiving at least 1 dose of antithymocyte globulin [rabbit (rATG) or horse], muromonab (OKT3), or alemtuzumab. IL-2RA therapy included receiving at least 1 dose of either daclizumab or basiliximab. Those who received both cytolytic and IL-2RA therapy were placed in the cytolytic group, as this is the more potent therapy. Those who did not have the use of induction therapy documented in the registry were excluded (no induction). Covariates that were collected and included in this analysis were age, sex, body mass index, primary insurance at the time of transplant (categorized as Medicaid/Medicare or other), primary end-stage renal disease diagnosis (classified as hypertension, diabetes, or other), history of diabetes at the time of transplant, history of hypertension at the time of transplant, time on the waiting list, receiving dialysis at the time of transplant, donor age, donor sex, donor race (categorized as AA or other), donor type (living, deceased, extended criteria, or circulatory death), human leukocyte antigen (HLA) mismatches and type (A, B, and DR), panel reactive antibody (PRA) at the time of transplant (current) and peak, previous kidney transplant, delayed graft function (need for dialysis within 7 d after transplant) and baseline immunosuppression received at discharge from the index transplant hospitalization, including tacrolimus, cyclosporine, mycophenolate, azathioprine, mammalian target of rapamycin therapy (sirolimus or everolimus), and corticosteroids.
There were 3 primary outcomes that were assessed for this study, which included time to graft loss, time to death, and incidence of acute rejection. Graft loss was defined as the return to chronic dialysis or need for retransplantation, with death accounted for as a competing event. Death was defined by transplant center reporting with mapping to the Social Security Master Death File to validate these events. Acute rejection was reported by transplant centers to UNOS and included biopsy-proven and clinically suspected rejections that were treated with antirejection therapy.25,27 Incident acute rejection was assessed at 6-months posttransplant, 1-year posttransplant, and at the end of follow-up.
Statistical Analysis
Analyses included both univariate and multivariable components. For univariate analyses, continuous normally distributed data were summarized using means ± standard deviations, with non-normal data displayed as medians and interquartile ranges. The 2-sided independent t test was used to compare normally distributed continuous data and the Mann–Whitney U test was used for comparisons of non-normal data. Categorical data were summarized using percentages and compared between induction groups using the χ2square test. To assess the impact of induction therapy on the time to graft loss, Cox regression was utilized, with death accounted for as a competing risk event using the Fine and Gray method.28 Cox models were fully adjusted using all the baseline variables described above; fully adjusted cumulative incidence functions were estimated from these models, comparing induction therapy groups.28 Time to death was assessed using standard Cox models, also fully adjusted. Fully adjusted survival curves were estimated from this model, stratified by induction group. We utilized a marginal model to provide robust sandwich covariance matrix adjusted estimates by accounting for clustering by duplicate patients (repeat transplants), transplant center, and transplant year.29 Cox regression model results are reported as hazard ratios and adjusted hazard ratios (aHR). Binary logistic regression was utilized to assess the impact of induction therapy on acute rejection with results reported as odds ratios (OR) and adjusted odds ratios (aOR). These models were also fully adjusted using baseline variables and the probability of developing acute rejection based on induction therapy was estimated. Interaction terms were utilized in iterative models to determine if statistically significant effect modification was present for baseline variables listed above. Datasets were then stratified and models rerun for those variables where the interaction terms (induction therapy × variable) were statistically significant. Multiple imputation, using fully conditional specification methodology, was used to assess the impact of missing data on the inferred estimates, by comparing the estimates from the complete case analysis to the imputed analysis.30,31 A 2-sided P value of <0.05 was considered statistically significant. SAS version 9.4 (SAS Institute, Cary, NC) was utilized to conduct all analyses.
RESULTS
A total of 171,104 kidney recipients, transplanted between 2000 and 2009, were eligible for the inclusion in this cohort study. Of these, 8293 were excluded for age <18 years, 2123 were excluded for receiving a nonrenal transplant, 125,349 were excluded for not being AA, and 10,255 were excluded for not receiving either IL-2RA or cytolytic induction therapy; leaving 25,084 included in the final analysis; 8157 (32.5%) received IL-2RA induction therapy and 16,927 (67.5%) received cytolytic induction therapy (see Supplemental Figure 1 for the consort diagram, http://links.lww.com/SLA/B267). Baseline recipient and donor demographics, compared by induction type, are displayed in Table 1. Those who received cytolytic induction were more likely to be female, have a higher body mass index, have higher use of public insurance, spend longer on the waiting list, and were significantly less likely to receive a living donor, while being more likely to receive an ECD or DCD donor kidney. Table 2 displays the baseline recipient immunologic risk factors and maintenance immunosuppression, also stratified by induction type. Those who received cytolytic induction had more HLA mismatches, were more likely to be sensitized to HLA antigens (higher % PRA), had longer cold ischemic times, were more likely to be retransplants and develop delayed graft function. Baseline immunosuppression was more likely to be tacrolimus-based and steroid withdrawal was 3 times more common in those who received cytolytic induction. To summarize, there were significant differences between the induction cohorts, with the cytolytic induction group having more immunologic risk factors to develop acute rejection and graft loss, particular as it relates to donor type and the development of delayed graft function.
TABLE 1.
Baseline Recipient and Donor Demographics Stratified and Compared by Induction Therapy
| Characteristic | IL-2RA Induction (n = 8157) | Cytolytic Induction (n = 16,927) | P Value |
|---|---|---|---|
| Mean age (yrs ±SD) | 47.8 ± 13.1 | 48.1 ± 12.7 | 0.077 |
| Female sex | 39.6% | 42.0% | <0.001 |
| Mean BMI (kg/m2 ± SD) | 27.7 ± 5.5 | 28.2 ± 5.6 | <0.001 |
| Primary insurance— Medicare/Medicaid | 70.2% | 74.5% | <0.001 |
| Primary diagnosis for ESRD | |||
| Hypertension | 39.5% | 40.7% | 0.119 |
| Diabetes | 22.2% | 21.3% | |
| Other | 38.3% | 38.1% | |
| History of diabetes | 30.7% | 31.2% | 0.409 |
| History of hypertension | 90.0% | 92.3% | <0.001 |
| Mean time on waiting list (yrs ± SD) | 2.2 ± 1.9 | 2.5 ± 2.1 | <0.001 |
| Dialysis prior to transplant | 91.8% | 92.1% | 0.485 |
| Donor mean age (yrs ± SD) | 37.2 ± 14.8 | 38.7 ± 5.6 | <0.001 |
| Donor female sex | 44.7% | 44.1% | 0.349 |
| Donor African-American | 39.4% | 33.6% | <0.001 |
| Living donor | 29.1% | 20.7% | <0.001 |
| Extended criteria donor | 11.1% | 14.8% | <0.001 |
| Circulatory death donor | 3.9% | 8.2% | <0.001 |
BMI indicates body mass index; ESRD, end-stage renal disease; IL-2RA, IL-2 receptor antibody; SD, standard deviation.
TABLE 2.
Baseline Recipient Immunologic Risk and Immunosuppression Stratified and Compared by Induction Therapy
| Characteristic | IL-2RA Induction (n = 8157) | Cytolytic Induction (n = 16,927) | P Value |
|---|---|---|---|
| Median HLA mismatches (IQR) | 4 (3, 5) | 4 (3, 5) | |
| 0 Mismatches | 5.2% | 4.0% | |
| 1 Mismatch | 2.8% | 2.0% | |
| 2 Mismatches | 8.0% | 5.9% | <0.001 |
| 3 Mismatches | 18.3% | 15.0% | |
| 4 Mismatches | 21.2% | 23.9% | |
| 5 Mismatches | 28.1% | 31.9% | |
| 6 Mismatches | 16.4% | 17.2% | |
| Median type A mismatches (IQR) | 1 (1, 2) | 1 (1, 2) | <0.001 |
| Median type B mismatches (IQR) | 2 (1, 2) | 2 (1, 2) | <0.001 |
| Median type DR mismatches (IQR) | 1 (1, 2) | 1 (1, 2) | <0.001 |
| Median current PRA (%, IQR) | 0 (0, 3) | 0 (0, 15) | <0.001 |
| Current PRA >20% | 11.4% | 22.6% | <0.001 |
| Median peak PRA (%, IQR) | 0 (0, 13) | 3 (0, 35) | <0.001 |
| Peak PRA >20% | 19.5% | 30.7% | <0.001 |
| Mean cold ischemic time (h ± SD) | 14.6 ± 10.2 | 16.4 ± 11.3 | <0.001 |
| Previous transplant | 6.0% | 11.6% | <0.001 |
| Delayed graft function | 20.6% | 26.7% | <0.001 |
| Baseline immunosuppression | |||
| Tacrolimus | 65.9% | 83.9% | <0.001 |
| Cyclosporine | 28.5% | 10.7% | <0.001 |
| Mycophenolate | 87.8% | 88.0% | 0.601 |
| Azathioprine | 1.8% | 1.1% | <0.001 |
| mTOR inhibitor | 10.9% | 9.0% | <0.001 |
| Steroid withdrawal | 8.0% | 29.2% | <0.001 |
IL-2RA indicates IL-2 receptor antibody; IQR, interquartile ranges; PRA, panel reactive antibody; SD, standard deviation.
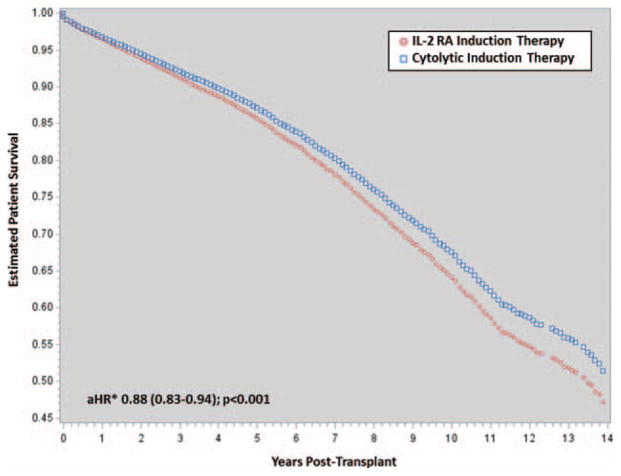
In AA kidney transplant recipients, the use of cytolytic induction therapy reduced the crude and adjusted odds of developing acute rejection by 32% (OR 0.68, 95% CI 0.63–0.73; P < 0.001, aOR 0.68, 95% CI 0.62–0.75, P < 0.001). Figure 1 displays the crude and adjusted incident rate of acute rejection across posttransplant time periods; cytolytic induction therapy reduced the relative risk of acute rejection by 20% to 28% across all time periods (P < 0.001). Cytolytic induction therapy also reduced the risk of graft loss by 9% (aHR 0.91, 95% CI 0.86–0.97, P = 0.003) and the risk of death by 12% (aHR 0.88, 95% CI 0.83–0.94, P < 0.001) in AAs. The fully adjusted cumulative incidence estimates for graft loss are displayed in Figure 2 and the adjusted patient survival estimates are displayed in Figure 3.
FIGURE 1.
Crude and adjusted estimated rates of acute rejection compared between cytolytic and IL-2RA induction groups.
FIGURE 2.
Estimate of adjusted cumulative incidence of graft loss, accounting for death as a competing risk, stratified and compared by induction therapy. *Adjusted for age, sex, BMI, insurance, waiting time, dialysis at the time of transplant, previous transplant, primary diagnosis, history of diabetes, history of hypertension, current PRA, peak PRA, HLA mismatches, cold ischemic time, living donor, expanded criteria donor, circulatory death donor, donor sex, donor race, baseline immunosuppression, steroid withdrawal, and delayed graft function. BMI indicates body mass index.
FIGURE 3.
Estimate of adjusted patient survival, stratified and compared by induction therapy. *Adjusted for age, sex, BMI, insurance, waiting time, dialysis at the time of transplant, previous transplant, primary diagnosis, history of diabetes, history of hypertension, current PRA, peak PRA, HLA mismatches, cold ischemic time, living donor, expanded criteria donor, circulatory death donor, donor sex, donor race, baseline immunosuppression, steroid withdrawal and delayed graft function. BMI indicates body mass index.
A number of variables significantly modified the effect of cytolytic induction therapy on the outcomes of acute rejection, graft loss, and death. For acute rejection, those receiving public insurance, those with PRA>20%, and those with delayed graft function had a substantially larger reduction in risk of acute rejection when receiving cytolytic induction (top of Fig. 4). For the outcome of graft loss, cold ischemic time, AA donor race, and steroid withdrawal were all significant effect modifiers (middle of Fig. 4), while for the outcome of death, public health insurance was a significant effect modifier of cytolytic induction therapy (bottom of Fig. 4). There were no measured variables that led to effect modification such that cytolytic induction therapy induced harm by significantly increasing event rates. This included characteristics that would traditionally be considered low immunologic risk factors or factors that may increase the incidence or severity of opportunistic infections, such as diabetes, living donor, and advanced recipient age.
FIGURE 4.
Impact of cytolytic induction therapy on acute rejection, graft loss and death, stratified by variables that had a significant impact on these outcomes.
Sensitivity analyses were conducted to assess the degree and impact of missing data on the estimates. Supplemental Table 1, http://links.lww.com/SLA/B267 displays the level of missingness across all covariates. Of the 25,084 transplant events included in this analysis, 7354 (29.4%) were missing at least 1 variable and 17,730 (70.6%) were complete cases. Supplemental Table 1, http://links.lww.com/SLA/B267 displays all of the covariates included in analyses and the level of missingness, which was highest for history of hypertension (11.8%), cold ischemic time (14.1%), and time on the waiting list (5.7%). All other variables had very low rates of missing data (≤2%). Analysis using multiple imputation demonstrated that missingness was likely missing at random, as it had no appreciable impact on the estimates for the effect of induction therapy on the outcomes of acute rejection (0% difference in estimates), graft loss (1% difference in estimates), and death (0% difference in estimates), see Supplemental Table 2, http://links.lww.com/SLA/B267.
DISCUSSION
The results of this analysis of all adult AA recipients of solitary kidney transplants between 2000 and 2009 demonstrate that cytolytic induction therapy, as compared with IL-2RA therapy, substantially reduces the risk of acute rejection, and significantly prolongs graft and patient survival. This is the first large-scale analysis we are aware of demonstrating improved graft and patient survival through induction therapy in AA kidney transplant recipients. Further, the results demonstrated that all subgroups of AAs benefited from cytolytic induction therapy, particularly those who were sensitized, developed delayed graft function and underwent steroid withdrawal. No AA subgroups, including those of low immunologic risk or high infectious risk, demonstrated deleterious clinical outcomes from cytolytic induction therapy. Taken in their entirety, these results provide considerable evidence to suggest that most, if not all, contemporary adult AA solitary kidney transplant should receive cytolytic induction therapy as part of their immunosuppressant regimen.
The short-term comparative efficacy of different antibody induction therapies in the general kidney transplant population is fairly well studied, with a number of prospective RCTs. In 2006, Brennan et al22 published a multicenter RCT comparing rATG versus basiliximab induction in 278 patients at high risk of acute rejection or DGF. All patients received maintenance immunosuppression with cyclosporine, mycophenolate, and prednisone. At 1 year posttransplant, the incidence of acute rejection (16% vs. 26%, P = 0.02) was lower in the rATG group. DGF, graft loss, and death were similar between groups. The number of AAs in this study was relatively low at 28.8% (n = 80); additionally, the study was not powered and did not have enough follow-up to assess long-term outcomes, such as graft loss and death, particularly within the AA patients.22 Pilch et al23 published a single-center prospective RCT comparing rATG to IL2-RA induction in 200 moderate to high-risk kidney transplant recipients. All received tacrolimus, mycophenolate, and prednisone maintenance immunosuppression. The 1-year acute rejection rate was similar between groups (10% in basiliximab vs. 6% in rATG patients, P = 0.30), but was significantly lower in the AA patients, which represented 50% of patients (n = 99). Graft and patient survival rates were similar between induction groups, but this study was not powered and did not have enough follow-up to assess the long-term impact of induction therapy.23 Thus, in the short-term, these RCTs suggest AAs may have improved efficacy from cytolytic induction therapy as compared with Caucasians and other racial/ethnic groups.16,21,32 Our analysis utilized national registry data to allow for a study with sufficient power and follow-up to assess the impact of cytolytic induction on long-term patient and graft outcomes, while also confirming the substantial effect cytolytic induction has on short-term acute rejection rates in AAs. There are additional clinical trials published that demonstrate significant reductions in acute rejection rates with cytolytic induction therapy use, as compared with IL2-RA in immunologically high-risk patients. However, these studies were deficient in AAs, which represents the largest high-risk transplant subpopulation within the United States.33,34
In 2007, Patlolla et al35 published a study using 5 years of data from Scientific Registry of Transplant Recipients to conduct a large-scale retrospective cohort study comparing rATG to IL2-RA with no induction therapy. The analysis, using multivariable models to adjust for confounders, demonstrated that cytolytic induction therapy reduced acute rejection rates, but had similar rates of graft survival, as compared with IL-2RA. The reduction in acute rejection rates (aOR 0.90, 95% CI 0.83 – 0.99) in the rATG compared with IL-2RA was less impressive, as compared with the impact demonstrated within our study of only AA recipients (aOR 0.68, 95% CI 0.63–0.73). Our study also included a 10-year contemporary cohort with substantially longer follow-up, thus allowing for improved power to assess the impact of cytolytic induction therapy on long-term graft survival.35 Jindal et al14 analyzed transplant registry data in a 2009 publication, demonstrating that rATG use, as compared with IL-2RA induction therapy, was associated with a nonstatically significant reduction in the risk of graft loss in AAs (aHR 0.95, 95% CI 0.87–1.04). The population of AAs in the Jindal study was 58% smaller (n = 10,568) and had substantially shorter follow-up, as compared with our analysis, which is likely why they were unable to demonstrate a significant difference in long-term patient and graft outcomes.14 We improved on these limitations and because of a large sample-size with long-term follow-up, were able to utilize interaction terms in models to assess for clinically significant effect modification by induction type specifically within AAs.
Our results also demonstrate a number of important effect modifiers that varied the impact of cytolytic induction on outcomes within AA kidney transplant recipients. For acute rejection, those with a peak PRA >20% and those who developed DGF all had a substantially larger impact from cytolytic induction therapy. As DGF and PRA are known to be strong immunologic risk factors, it is logical that cytolytic induction therapy improves outcomes to a greater degree within these subgroups.36–39 The issue of public insurance (Medicare/Medicaid) as a significant effect modifier of cytolytic induction therapy for the outcomes of both acute rejection and patient mortality is intriguing. Previous analysis of the transplant registry demonstrates that public insurance is a significant risk factor for graft loss, independent of race.40 This risk may be mediated through medication adherence, as those with Medicare and Medicaid may have limited means to afford prescription copays and thus be at higher risk of medication nonadherence, a known risk factor for acute rejection and graft loss.40–42 For graft loss, steroid withdrawal was an important effect modifier. It is clear from these data and previous studies that cytolytic induction therapy should be used in transplant cases that are planned to undergo steroid withdrawal; this is particularly important within AA recipients.43–45
It is also important to note the factors that had no effect modification, in that they did not influence the impact of cytolytic induction therapy effectiveness. This includes variables that would traditionally place a patient at low risk for acute rejection and/or high risk for infection. These include advanced age, diabetes, and living donor status.46–49 Typically, in our experience and based on guidelines, clinicians would not consider cytolytic induction therapy as beneficial in these low-risk recipients;7 yet data from this study demonstrate that in AAs, these subgroups of patients had a significant benefit from cytolytic induction therapy, with lower rates of acute rejection, graft loss, and death. Thus, within the AA renal transplant population, there were no subgroups of patients that appear to have increased events rates with cytolytic induction therapy.
There are a number of limitations with this study that are worthy of discussion. As this was a retrospective cohort analysis, choice of induction therapy was not random or controlled and there were significant baseline differences between those who received cytolytic versus IL-2RA induction therapies; the cytolytic group had substantially more risk factors for acute rejection and graft loss, when compared with the IL-2RA group.47 We attempted to account for this through adjustment in multivariable models, but there is likely residual confounding and unmeasured risk that was not accounted for in these models.50 However, these would likely lead to bias toward the null and thus the estimates from this analysis are conservative. Missing data were also a limitation. Models contained a large number of covariates and thus the complete case analysis comprised of only 71% of the study population. We assessed the impact of this using contemporary missing data sensitivity analyses and concluded that missing data were at random and not likely to bias or skew the results.30,31 It should also be noted that variables and outcomes utilized from UNOS are not fully validated, and there may be under-reporting of graft loss rates.27 However, previous analyses have demonstrated that the quality of the UNOS registry data is quite good, and under-reporting of graft loss would not impact the aHR estimates unless it differed by induction therapy type.25 Finally, because of limitations of data reporting within the UNOS registry, we did not assess the impact of induction therapy on infectious or cancer risk. Thus, future studies, using different data sources, are needed to fully understand the risk-benefit of cytolytic induction therapy in AAs with regard to these potential hazards.
In conclusion, these results demonstrate that AA kidney transplant recipients receiving cytolytic induction therapy have substantially reduced risk of acute rejection and significantly prolonged graft and overall survival, as compared with those receiving IL2-RA induction therapy. Based on these results, coupled with previous RCTs, we recommend clinicians consider using cytolytic induction therapy in most to all adult AA transplant recipients; particularly those with known risk factors for reduced post-transplant outcomes (public insurance, elevated PRA, DGF, and steroid withdrawal).
Acknowledgments
Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Number K23DK099440. This work was supported in part by Health Resources and Services Administration contract 234-2005-37011C. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Footnotes
The authors report no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.annalsofsurgery.com).
References
- 1.Hill P, Cross NB, Barnett AN, et al. Polyclonal and monoclonal antibodies for induction therapy in kidney transplant recipients. Cochrane Database Syst Rev. 2017;1:CD004759. doi: 10.1002/14651858.CD004759.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones-Hughes T, Snowsill T, Haasova M, et al. Immunosuppressive therapy for kidney transplantation in adults: a systematic review and economic model. Health Technol Assess. 2016;20:1–594. doi: 10.3310/hta20620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franksson C, Lundgren G, Magnusson G, et al. Drainage of thoracic duct lymph in renal transplant patients. Transplantation. 1976;21:133–140. doi: 10.1097/00007890-197602000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Merion RM, White DJ, Thiru S, et al. Cyclosporine: five years’ experience in cadaveric renal transplantation. N Engl J Med. 1984;310:148–154. doi: 10.1056/NEJM198401193100303. [DOI] [PubMed] [Google Scholar]
- 5.Starzl TE, Weil R, 3rd, Iwatsuki S, et al. The use of cyclosporin A and prednisone in cadaver kidney transplantation. Surg Gynecol Obstet. 1980;151:17–26. [PMC free article] [PubMed] [Google Scholar]
- 6.Bakr MA, Nagib AM, Donia AF. Induction immunosuppressive therapy in kidney transplantation. Exp Clin Transpl. 2014;12:60–69. doi: 10.6002/ect.25liver.l58. [DOI] [PubMed] [Google Scholar]
- 7.Kasiske BL, Zeier MG, Chapman JR, et al. KDIGO clinical practice guideline for the care of kidney transplant recipients: a summary. Kidney Int. 2010;77:299–311. doi: 10.1038/ki.2009.377. [DOI] [PubMed] [Google Scholar]
- 8.Ponticelli C. Basiliximab: efficacy and safety evaluation in kidney transplantation. Expert Opinion Drug Safety. 2014;13:373–381. doi: 10.1517/14740338.2014.861816. [DOI] [PubMed] [Google Scholar]
- 9.Padiyar A, Hricik DE. Immune factors influencing ethnic disparities in kidney transplantation outcomes. Expert Rev Clin Immunol. 2011;7:769–778. doi: 10.1586/eci.11.32. [DOI] [PubMed] [Google Scholar]
- 10.Goldfarb-Rumyantzev AS, Sandhu GS, Baird B, et al. Effect of education on racial disparities in access to kidney transplantation. Clin Transplant. 2012;26:74–81. doi: 10.1111/j.1399-0012.2010.01390.x. [DOI] [PubMed] [Google Scholar]
- 11.Taber DJ, Hamedi M, Rodrigue JR, et al. Quantifying the race stratified impact of socioeconomics on graft outcomes in kidney transplant recipients. Transplantation. 2015;100:1550–1557. doi: 10.1097/TP.0000000000000931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taber DJ, Douglass K, Srinivas T, et al. Significant racial differences in the key factors associated with early graft loss in kidney transplant recipients. Am J Nephrol. 2014;40:19–28. doi: 10.1159/000363393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oliver JD, III, Neff RT, Leeser DB, et al. Influence of race on kidney transplantation in the Department of Defense healthcare system. Am J Nephrol. 2009;29:327–333. doi: 10.1159/000163558. [DOI] [PubMed] [Google Scholar]
- 14.Jindal RM, Das NP, Neff RT, et al. Outcomes in African-Americans vs. Caucasians using thymoglobulin or interleukin-2 receptor inhibitor induction: analysis of USRDS database. Am J Nephrol. 2008;29:501–508. doi: 10.1159/000182816. [DOI] [PubMed] [Google Scholar]
- 15.Haririan A, Morawski K, Sillix DH, et al. Induction therapy with basiliximab versus Thymoglobulin in African-American kidney transplant recipients. Transplantation. 2005;79:716–721. doi: 10.1097/01.tp.0000153506.07816.f0. [DOI] [PubMed] [Google Scholar]
- 16.Hardinger KL, Brennan DC, Klein CL. Selection of induction therapy in kidney transplantation. Transplant Int. 2013;26:662–672. doi: 10.1111/tri.12043. [DOI] [PubMed] [Google Scholar]
- 17.Gralla J, Le CN, Cooper JE, et al. The risk of acute rejection and the influence of induction agents in lower-risk African American kidney transplant recipients receiving modern immunosuppression. Clin Transplant. 2014;28:292–298. doi: 10.1111/ctr.12311. [DOI] [PubMed] [Google Scholar]
- 18.Fleming JN, Taber DJ, Weimert NA, et al. Comparison of efficacy of induction therapy in low immunologic risk African-American kidney transplant recipients. Transplant Int. 2010;23:500–505. doi: 10.1111/j.1432-2277.2009.01004.x. [DOI] [PubMed] [Google Scholar]
- 19.Patel SJ, Suki WN, Loucks-DeVos J, et al. Disparate rates of acute rejection and donor-specific antibodies among high-immunologic risk renal transplant subgroups receiving antithymocyte globulin induction. Transplant Int. 2016;29:897–908. doi: 10.1111/tri.12791. [DOI] [PubMed] [Google Scholar]
- 20.Hammond EB, Taber DJ, Weimert NA, et al. Efficacy of induction therapy on acute rejection and graft outcomes in African American kidney transplantation. Clin Transplant. 2010;24:40–47. doi: 10.1111/j.1399-0012.2009.00974.x. [DOI] [PubMed] [Google Scholar]
- 21.Malat GE, Culkin C, Palya A, et al. African American kidney transplantation survival. Drugs. 2009;69:2045–2062. doi: 10.2165/11318570-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 22.Brennan DC, Daller JA, Lake KD, et al. Rabbit antithymocyte globulin versus basiliximab in renal transplantation. N Engl J Med. 2006;355:1967–1977. doi: 10.1056/NEJMoa060068. [DOI] [PubMed] [Google Scholar]
- 23.Pilch NA, Taber DJ, Moussa O, et al. Prospective randomized controlled trial of rabbit antithymocyte globulin compared with IL-2 receptor antagonist induction therapy in kidney transplantation. Ann Surg. 2014;259:888–893. doi: 10.1097/SLA.0000000000000496. [DOI] [PubMed] [Google Scholar]
- 24.Sollinger H, Kaplan B, Pescovitz MD, et al. Basiliximab versus antithymocyte globulin for prevention of acute renal allograft rejection1, 2. Transplantation. 2001;72:1915–1919. doi: 10.1097/00007890-200112270-00008. [DOI] [PubMed] [Google Scholar]
- 25.Hanto DW. Quality control of the OPTN/UNOS transplant registry. Transplantation. 2004;77:1309–1310. doi: 10.1097/01.tp.0000120943.94789.e4. [DOI] [PubMed] [Google Scholar]
- 26.Organ Procurement and Transplantation Network (OPTN) and Scientific Registry of Transplant Recipients (SRTR) OPTN /SRTR 2010 Annual Data Report. Rockville, MD: Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation; 2011. [Accessed February 3, 2017]. Available at: http://edmgr.ovid.com/annsurg/accounts/ifauth.htm. [Google Scholar]
- 27.Massie AB, Kuricka L, Segev DL. Big data in organ transplantation: registries and administrative claims. Am J Transplant. 2014;14:1723–1730. doi: 10.1111/ajt.12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 29.King G, Roberts ME. How robust standard errors expose methodological problems they do not fix, and what to do about it. [Accessed February 3, 2017];Political Analysis. 2014 :1–21. Available at: https://dash.harvard.edu/bitstream/handle/1/13572089/32225/robust.pdf?sequence=1.
- 30.Van Buuren S, Brand JP, Groothuis-Oudshoorn C, et al. Fully conditional specification in multivariate imputation. J Stat Comp Sim. 2006;76:1049–1064. [Google Scholar]
- 31.Van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16:219–242. doi: 10.1177/0962280206074463. [DOI] [PubMed] [Google Scholar]
- 32.Gralla J, Le CN, Cooper JE, et al. The risk of acute rejection and the influence of induction agents in lower-risk African American kidney transplant recipients receiving modern immunosuppression. Clin Transplant. 2014;28:292–298. doi: 10.1111/ctr.12311. [DOI] [PubMed] [Google Scholar]
- 33.Gaber AO, Knight RJ, Patel S, et al. A review of the evidence for use of thymoglobulin induction in renal transplantation. Transplant Proc. 2010;42:1395–1400. doi: 10.1016/j.transproceed.2010.04.019. [DOI] [PubMed] [Google Scholar]
- 34.Nol C, Abramowicz D, Durand D, et al. Daclizumab versus antithymocyte globulin in high-immunological-risk renal transplant recipients. J Am Soc Nephrol. 2009;20:1385–1392. doi: 10.1681/ASN.2008101037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patlolla V, Zhong X, Reed G, et al. Efficacy of anti-IL-2 receptor antibodies compared to no induction and to antilymphocyte antibodies in renal transplantation. Am J Transplant. 2007;7:1832–1842. doi: 10.1111/j.1600-6143.2007.01860.x. [DOI] [PubMed] [Google Scholar]
- 36.Gebel HM, Bray RA, Nickerson P. Pre-transplant assessment of donor-reactive, HLA-specific antibodies in renal transplantation: contraindication vs. risk. Am J Transplant. 2003;3:1488–1500. doi: 10.1046/j.1600-6135.2003.00273.x. [DOI] [PubMed] [Google Scholar]
- 37.Lefaucheur C, Loupy A, Hill GS, et al. Preexisting donor-specific HLA antibodies predict outcome in kidney transplantation. J Am Soc Nephrol. 2010;21:1398–1406. doi: 10.1681/ASN.2009101065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siedlecki A, Irish W, Brennan DC. Delayed graft function in the kidney transplant. Am J Transplant. 2011;11:2279–2296. doi: 10.1111/j.1600-6143.2011.03754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yarlagadda SG, Coca SG, Formica RN, Jr, et al. Association between delayed graft function and allograft and patient survival: a systematic review and meta-analysis. Nephrol Dial Transplant. 2009;24:1039–1047. doi: 10.1093/ndt/gfn667. [DOI] [PubMed] [Google Scholar]
- 40.Goldfarb-Rumyantzev AS, Koford JK, Baird BC, et al. Role of socioeconomic status in kidney transplant outcome. Clin J Am Soc Nephrol. 2006;1:313–322. doi: 10.2215/CJN.00630805. [DOI] [PubMed] [Google Scholar]
- 41.Sellares J, De Freitas D, Mengel M, et al. Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant. 2012;12:388–399. doi: 10.1111/j.1600-6143.2011.03840.x. [DOI] [PubMed] [Google Scholar]
- 42.Takemoto SK, Pinsky BW, Schnitzler MA, et al. A retrospective analysis of immunosuppression compliance, dose reduction and discontinuation in kidney transplant recipients. Am J Transplant. 2007;7:2704–2711. doi: 10.1111/j.1600-6143.2007.01966.x. [DOI] [PubMed] [Google Scholar]
- 43.Hanaway MJ, Woodle ES, Mulgaonkar S, et al. Alemtuzumab induction in renal transplantation. N Engl J Med. 2011;364:1909–1919. doi: 10.1056/NEJMoa1009546. [DOI] [PubMed] [Google Scholar]
- 44.Taber DJ, Hunt KJ, Gebregziabher M, et al. A comparative effectiveness analysis of early steroid withdrawal in black kidney transplant recipients. Clin J Am Soc Nephrol. 2017;12:131–139. doi: 10.2215/CJN.04880516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Woodle ES, First MR, Pirsch J, et al. A prospective, randomized, double-blind, placebo-controlled multicenter trial comparing early (7 day) corticosteroid cessation versus long-term, low-dose corticosteroid therapy. Ann Surg. 2008;248:564–577. doi: 10.1097/SLA.0b013e318187d1da. [DOI] [PubMed] [Google Scholar]
- 46.Kuo HT, Sampaio MS, Vincenti F, et al. Associations of pretransplant diabetes mellitus, new-onset diabetes after transplant, and acute rejection with transplant outcomes: an analysis of the Organ Procurement and Transplant Network/United Network for Organ Sharing (OPTN/UNOS) database. Am J Kidney Dis. 2010;56:1127–1139. doi: 10.1053/j.ajkd.2010.06.027. [DOI] [PubMed] [Google Scholar]
- 47.Lebranchu Y, Baan C, Biancone L, et al. Pretransplant identification of acute rejection risk following kidney transplantation. Transplant Int. 2014;27:129–138. doi: 10.1111/tri.12205. [DOI] [PubMed] [Google Scholar]
- 48.Schold JD, Srinivas TR, Braun WE, et al. The relative risk of overall graft loss and acute rejection among African American renal transplant recipients is attenuated with advancing age. Clin Transplant. 2011;25:721–730. doi: 10.1111/j.1399-0012.2010.01343.x. [DOI] [PubMed] [Google Scholar]
- 49.Taber DJ, Meadows HB, Pilch NA, et al. Pre-existing diabetes significantly increases the risk of graft failure and mortality following renal transplantation. Clin Transplant. 2013;27:274–282. doi: 10.1111/ctr.12080. [DOI] [PubMed] [Google Scholar]
- 50.Fewell Z, Davey Smith G, Sterne JA. The impact of residual and unmeasured confounding in epidemiologic studies: a simulation study. Am J Epidemiol. 2007;166:646–655. doi: 10.1093/aje/kwm165. [DOI] [PubMed] [Google Scholar]