Abstract
Pesticide use in agricultural areas requires the application of numerous chemicals to control target organisms, leaving non-target organisms at risk. The present study evaluates the hepatic metabolomic profile of one group of non-target organisms, amphibians, after exposure to a single pesticide and pesticide mixtures. Five common-use pesticide active ingredients were used in this study, three herbicides (atrazine, metolachlor and 2,4-d), one insecticide (malathion) and one fungicide (propiconazole). Juvenile green frogs (Lithobates clamitans) were reared for 60–90 days post-metamorphosis then exposed to a single pesticide or a combination of pesticides at the labeled application rate on soil. Amphibian livers were excised for metabolomic analysis and pesticides were quantified for whole body homogenates. Based on the current study, metabolomic profiling of livers support both individual and interactive effects where pesticide exposures altered biochemical processes, potentially indicating a different response between active ingredients in pesticide mixtures, among these non-target species. Amphibian metabolomic response is likely dependent on the pesticides present in each mixture and their ability to perturb biochemical networks, thereby confounding efforts with risk assessment.
Graphical abstract
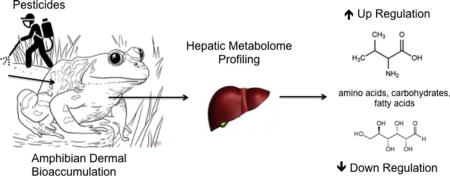
1. Introduction
Pesticide use has become commonplace in agricultural settings to control nuisance or unwanted species. This widespread use also has negative effects on non-target species inhabiting or moving throughout the same landscape (Mann et al., 2009; Pisa et al., 2015). From a cost-benefit perspective, multiple pesticides are often applied to crops as tank or prepared mixtures to minimize resources such as time, money and manpower (Cloyd, 2012). Post-application, the persistence of certain pesticides in the environment can leave non-target organisms at risk of exposure to numerous compounds, in addition to disruption of healthy ecosystem function, after application to crops has ended for the season (Goulson, 2013; Köhler and Triebskorn, 2013). Efforts with Cumulative Risk Assessment (CRA) are being developed to gain a foundational understanding of the risks associated with these multiple pesticide exposures (USEPA, 2007; Meek et al., 2011; Moretto et al., 2016). Pesticides that reside in the environment concurrently, post-application, frequently have different modes of action (MOA) and thus have been particularly challenging for risk assessors (Moretto et al., 2016). Moreover, pesticide effects in target organisms may be different from those of non-target organisms (e.g., Hayes et al., 2006). With their complex life history, amphibians in particular may experience repeated exposures to pesticides as larvae, juveniles and adults through aquatic and terrestrial habitats (reviewed in Mann et al., 2009; Brühl et al., 2011). Furthermore, dermal exposure and accumulation in amphibians may be the result of direct contact with pesticides during application events or indirect contact with residues on vegetation, soils or pond water (see Van Meter et al., 2015). While general pesticide risks to many non-target organisms are still poorly understood, amphibians add the challenge of risk across biphasic life stages (Johnson et al., 2017).
Despite the fact that amphibians are often exposed to pesticides and susceptible across multiple life stages, they are often underrepresented in risk assessment and registration process assessments (Brühl et al., 2011). Relative to organisms that are well represented in risk assessment, particularly mammals, frog dermis is vastly more permeable to pesticides (Quaranta et al., 2009). Dermal accumulation of pesticides from both aquatic (Storrs-Mendez et al., 2009; Reynaud et al., 2012) and terrestrial (Glinski et al., 2017a, b; Henson-Ramsey et al., 2008; Van Meter et al., 2014, 2015, 2016) habitats has been documented in the lab and field-based studies have verified that pesticide accumulation is occurring among amphibians in the environment (Smalling et al., 2013, 2015). While pesticides are known to cause adverse impacts to amphibians during larval stages, such as endocrine disruption, increased disease susceptibility, deformities and mortality (reviewed in Mann et al., 2009), the effects on post-metamorphic amphibians have not been well studied. Malathion decreased acetylcholinesterase activity in adult tiger salamanders exposed through contaminated soil (Henson-Ramsey et al., 2008), glyphosate altered hepatic metabolism in a leptodactylid frog species (Pérez-Iglesias et al., 2016) and terrestrial exposure to a variety of other common-use pesticides has resulted in increased mortality among other amphibians (Dinehart et al., 2009; Brühl et al., 2013; Cusaac et al., 2015). Adult amphibians are known to move large distances across terrestrial landscapes in search of breeding ponds and/or overwintering habitats (Fryday and Thompson, 2012; Lenhardt et al., 2014). In agriculturally intensive areas, this leaves terrestrial phase amphibians at risk of dermal pesticide exposure from soils and contaminated vegetation. In Argentina, amphibians sampled in agricultural landscapes had lowered body condition and increased stress responses, which may have been further exacerbated by extreme changes in climatic conditions (Brodeur et al., 2011, 2012).
Given rising global amphibian losses, field and lab-based studies of pesticide effects to juvenile and adult amphibians are urgently needed for this imperilled taxa (see Johnson et al., 2016). Environmental samples of both soils and water collected from amphibian habitats in urban and agricultural landscapes document the co-occurrence of multiple pesticides at any given time throughout the year (Smalling et al., 2013, 2015). The behavior of pesticides when present in mixtures may result in antagonistic, additive or synergistic effects in amphibians (Mann et al., 2009; Meek et al., 2011), and the type of interaction will depend on the pesticides present in the mixture. Therefore, it can be challenging to predict the consequence of exposure to multiple pesticides in these non-target organisms. Studies of pesticide mixtures on larval amphibians have reported both additive and synergistic effects including but not limited to reduced survival, smaller size at and longer time to metamorphosis, endocrine and immunosuppression, and increased disease susceptibility (Boone and James, 2003; Hayes et al., 2006; Kerby and Storfer, 2009). To our knowledge, individual, population and community level effects of mixed pesticide exposure in post-metamorphic juvenile amphibians have not been researched in the lab prior to this study. Co-exposure to numerous pesticides among amphibians in natural habitats is highly probable given the relatively lengthy half-lives as well as repeated and co-application of pesticides throughout agricultural landscapes.
Environmental metabolomics is a growing area of ecotoxicology that may help close the knowledge gaps and aid in an understanding of multiple pesticide stressors on amphibians. In general, metabolomics can provide an evaluation of the overall biological functioning of an individual at the molecular level after interaction with an environmental stressor. The goal of a metabolomics approach in ecotoxicology is to identify unique metabolite profiles or “fingerprints” in an organism after toxin exposure that may serve as a biomarker for future exposures to the same compound or mixture of compounds (e.g., Miller, 2007; Viant, 2008; Bundy et al., 2009). This approach has been used successfully in terrestrial ecotoxicology studies to evaluate effects of metal exposure in mammals and metal and pesticide exposures in earthworms (reviewed in Bundy et al., 2009), but the use of metabolomics for terrestrial amphibians or multiple pesticide exposures in any organism is deficient for comprehensive risk assessment. Among amphibians, biomarker identification through biochemical and molecular techniques has focused largely on embryonic and larval stages following contaminant exposure (reviewed in Venturino et al., 2003; Venturino and de D'Angelo, 2005). More recently, biomarkers of pesticide exposure have been evaluated in tadpoles through behavioral, biochemical and biospectral approaches (Denoël et al., 2012; Margido et al., 2013; Strong et al., 2016, respectively) and through in vitro studies with adult amphibian tissues (Attademo et al., 2014). Given the biphasic life history of amphibians and unique physiology associated with amphibian metamorphosis, effects of pesticide exposure are likely to vary and may require separate biomarker analyses for tadpole, juvenile and adult life stages (see Johnson et al., 2016).
Consideration of the short- and long-term stability of amphibian populations experiencing acute or chronic pesticide exposure during post-metamorphic life stages should be incorporated into future risk assessments. However, given that this field has received little research emphasis to date, there are very limited data for risk assessors to rely on (see Johnson et al., 2016). In an effort to expand our fundamental understanding of the effects of pesticides among amphibians in terrestrial habitats, this research was designed to explore mixed pesticide exposure from contaminated soils on an amphibian species widespread throughout the United States, the green frog (Lithobates clamitans). In particular, we exposed juvenile green frogs to five pesticides as single, double or triple pesticide mixtures. The herbicide treatments included atrazine (ATZ), 2,4-d (D) and/or metolachlor (ME) while the mixed pesticide treatment consisted of ATZ, malathion (MA) and/or propiconazole (PZ), an herbicide, insecticide and fungicide, respectively. These treatments are realistic representations of pesticides that may be encountered concurrently on vegetation and soils in corn, bean and cereal fields throughout the United States (USEPA, 2017). Following exposure, we profiled the amphibian metabolome for biochemical perturbations. We hypothesized that exposure to pesticide mixtures would result in observable, cumulative effects on the hepatic metabolomic profile in amphibians, relative to frogs exposed to single or no pesticides.
2. Methods
2.1. Chemicals
All chemicals and solvents were obtained from Fisher Scientific (Pittsburgh, PA). Experiments were conducted with pesticide active ingredients (purity ≥ 98%). Atrazine, 2,4-d, metolachlor, malathion and propiconazole were obtained from Chem Service (West Chester, PA).
2.2. Soil collection
Soil was collected from an experimental grassland restoration site at the Chester River Field Station of Washington College in Chestertown, MD in June 2015. The soil is classified as a Unicorn-Sassafras loam with low organic matter, typical of the agricultural sites on Maryland's Eastern Shore. The grasslands plots have not received widespread, direct application of pesticides in recent years (Dan Small, Washington College Center for Environment and Society, Chestertown, MD, personal communication). After collection, soils were stored in a walk-in cooler at 4 °C at Washington College.
2.3. Amphibian care
Green frog (Lithobates clamitans) eggs were collected from two egg masses in a permanent pond in Queen Anne's County, MD the morning after oviposition in June 2014. Eggs were transported to a laboratory at Washington College where they were kept in 10-gal aquariums through hatching (Gosner Stage 17; Gosner, 1960). Upon reaching Gosner Stage 25 (the free-feeding stage), tadpoles were fed Tetramin® Tropical Fish Flakes ad libitum. Two weeks after reaching Gosner Stage 25, tadpoles were transferred to 600 L polyethylene outdoor mesocosms that were filled with aged tap water. Tadpoles were housed outdoors through October 2014, then transferred to indoor mesocosms filled with aged tap water for overwintering. Tadpoles began to metamorphose in April 2015. All metamorphs were transferred to 600 L polyethylene outdoor mesocosms lined with moist sphagnum moss to simulate a terrestrial environment. Juvenile green frogs were fed crickets ad libitum for 60–90 days post-metamorphosis when experimentation began in July 2015.
2.4. Multiple pesticide exposure study
All pesticides were applied at the maximum-labeled application rate (Table 1) individually and in mixtures of two or three pesticides within an herbicide or a mixed pesticide group. The herbicide group consisted of atrazine (ATZ), 2,4-d (D) and metolachlor (ME). The mixed-pesticide group consisted of ATZ, malathion (MA) and propiconazole (PZ), an herbicide, insecticide and fungicide, respectively. In total, there were seven herbicide treatments (ATZ, ME, D, ATZME (atrazine + metolachlor), ATZD (atrazine + 2,4-d), MED (metolachlor + 2,4-d) and ATZMED (atrazine + metolachlor + 2,4-d; Table 1)) and seven mixed pesticide treatments (ATZ, MA, PZ, ATZMA (atrazine + malathion), ATZPZ (atrazine + propiconazole), MAPZ (malathion + propiconazole) and ATZMAPZ (atrazine + malathion + propiconazole; Table 1)) with the atrazine individual treatment group being used in both group comparisons to minimize the number of frogs receiving pesticide exposure. Six replicate frogs were used within a treatment group, in addition to nine control frogs that did not receive pesticide exposure, for a total sample size of 87 frogs (N = 87). Pesticide exposures occurred over a 2-day period, from June 30–July 1, 2015, due to the large number of samples and treatments involved in the experimental design. All frogs within the same treatment were exposed on the same day in a randomized block design, with the exception of the control and atrazine treatments where half of the frogs within those treatment groups were exposed on consecutive days.
Table 1.
Maximum labeled application rates (µg cm− 2) for each pesticide treatment.
| Treatment ID |
Application rate | |
|---|---|---|
| Herbicide treatments | ||
| Atrazine | ATZ | 23.6 |
| 2,4-D | D | 14.3 |
| Metolachlor | ME | 30.9 |
| Atrazine & 2,4-D | ATZD | 37.9 |
| Atrazine & metolachlor | ATZME | 54.5 |
| 2,4-d & metolachlor | MED | 45.2 |
| Atrazine, 2,4-D & metolachlor | ATZMED | 68.8 |
| Mixed pesticide treatments | ||
| Atrazine | ATZ | 23.6 |
| Malathion | MA | 25.9 |
| Propiconazole | PZ | 2.6 |
| Atrazine & malathion | ATZMA | 49.5 |
| Atrazine & propiconazole | ATZPZ | 26.2 |
| Malathion & propiconazole | MAPZ | 28.5 |
| Atrazine, malathion & propiconazole | ATZMAPZ | 52.1 |
This experimental design is intended to simulate a worst-case exposure where pesticides at maximum application rates are applied to bare soils. In addition, the night before exposure, all juvenile green frogs were brought into the laboratory and placed in clean, empty 10-gal aquariums for a 12-hour period of dehydration prior to experimentation. This dehydration period was intended to facilitate the movement of water, and associated pesticides, from the soil through the amphibian dermis during rehydration upon initiation of the exposure study. Experimental chambers were 0.94 L Pyrex glass bowls lined with 150 g of soil. All pesticide treatments were dissolved in 50 mL of 100% methanol (MeOH) and sprayed onto the soil surface using Preval Spray Gun Canisters® attached to clean, glass jars. The control treatment bowls received 50 mL of 100% MeOH. After pesticide/MeOH application, bowls were then transferred to the fume hood overnight to allow the MeOH to evaporate off the soil surfaces completely. The following morning, soils in each bowl were rehydrated with 50 mL spring water using a standard spray bottle. Immediately following soil rehydration, an individual frog was placed on the soil surface and the bowl was covered with window mesh and a rubber band to prevent frog escape. Pesticide exposures were 8-h in duration, after which frogs were rapidly euthanized by submersion in liquid nitrogen followed by storage in a − 80 °C freezer. Amphibians were subsequently thawed and dissected to remove a small fraction (~ 20 mg) of the liver for metabolomic profiling. The remaining frog tissues were extracted as whole body tissue homogenates as described below for body burden analysis. Soils samples from each bowl were also collected at the termination of the experiment and stored in a − 80 °C freezer until extraction and pesticide analysis.
2.5. Amphibian and soil extractions
Van Meter et al. (2014, 2015) detail the extraction methods for both amphibians and soils. In summary, all amphibian and soil samples were extracted two times with MeOH followed by evaporation under nitrogen gas. Following evaporation, final extraction of pesticides was achieved using milli-q water, methyl-tert-butyl ether (MTBE) and sodium sulfate. The MTBE layer was transferred off the top of the final sample, centrifuged, and 1 mL of the final extract was analyzed using GC/MS. Extracts were then analyzed using LC/MS after being evaporated under nitrogen and reconstituted with 30% methanol.
In addition to the five active pesticide ingredients, soil and frog tissue samples were scanned for primary metabolites. When metabolites were detected, their concentrations were summed with that of the associated parent compound as follows: desethyl-atrazine (DEA) and deisopropyl atrazine (DIA) with atrazine, metolachlor ethanesulfonic acid (MESA) and metolachlor oxanilic acid (MOXA) with metolachlor, and malaoxon with malathion. After analysis, bioconcentration factors (BCFs) were determined for each species and pesticide as:
where Cf is the frog whole-body tissue concentration and Cs is the average composite soil concentration within each treatment, both at the end of the 8-hour exposure. While BCFs typically refer to accumulation of contaminants from an aquatic medium at steady state, they also describe dietary and dermal accumulation in terrestrial environments, as presented in our study (Kenaga, 1980; Henson-Ramsey et al., 2008).
2.6. GC/MS (malaoxon)
The metabolite of malathion, malaoxon, was analyzed on an Agilent 6890 gas chromatograph (GC) coupled to a 5973 mass selective detector (MSD) controlled using ChemStation software. Injections (4 µL) were made in splitless mode and helium was the carrier gas maintained at a constant flow of 1.0 mL/min. Chromatographic separation was achieved on a DB5-MS column (30 m, 0.25 µm thickness, and 0.25 mm ID; Agilent, CA, USA). The inlet and transfer line were held constant at 280 °C, while the MS source and MS quad were 230 °C and 150 °C, respectively. The initial oven temperature was held at 80 °C for 2 min, ramped 10 °C/min to 300 °C and held for 6 min (total runtime 30 min). Malaoxon and tetraconazole (internal standard), were analyzed in selected ion monitoring (SIM) mode, monitoring malaoxon at 127, 109, and 99 m/z, and tetraconazole was monitored at 336 and 338 m/z ions. Standards and blanks were analyzed at the beginning, end and intermittently throughout the run sequence.
2.7. LC/MS/MS
Active ingredients and their corresponding metabolites were analyzed on a Varian Prostar HPLC interfaced to a Varian 1200L triple quadrupole mass spectrometer. Chromatographic separation was achieved on an Eclipse XDB-C18 column (3.5 µm particle size, 3.0 × 150 mm; Agilent Technologies, CA, USA). Briefly, initial mobile phase was 70% water with 0.1% formic acid (A) and 30% acetonitrile with 0.1% formic acid (B). Starting conditions were held for 2 min, ramped to 90% B over 16 min, and held for 4 min, before returning to initial conditions of 30% B and re-equilibrated for 5 min (total run time of 30 min). The flow rate was 300 µL/min and injection volume was 10 µL. The drying gas was set at 225 °C and the capillary voltage was at 60 V for all compounds analyzed. All compounds were analyzed in multiple reactions monitoring (MRM) mode; MOXA, MESA, and 2,4-d were the only analytes analyzed in negative mode, all other pesticides were detected in positive mode. The SRM transitions (m/z) ions were 216 to 174 for atrazine, 219 to 161 for 2,4-d, 284 to 252 for metolachlor, 331 to 127 for malathion, 342 to 159 for propiconazole and 372 to 159 for tetraconazole. For metabolites, m/z ions were 174 to 68 for DIA, 188 to 146 for DEA, 328 to 80 for MESA, and 278 to 206 for MOXA.
2.8. Amphibian metabolomics sample preparation
Metabolomic extraction methods follow those detailed in Viant (2007). Briefly, liver samples (~ 20 mg) were homogenized using a tissuelyser and extracted using MeOH and chloroform to separate the polar and nonpolar phases. Following phase separation, samples were placed in a Savant Speed Vac Plus evaporator overnight. The polar fraction of each sample was derivatized with 50 µL of methoxyamine hydrochloride at 20 mg/mL in pyridine and placed in a 60 °C oven for 2.5 h. During the incubation process, samples were vortexed at 30 min intervals. After cooling, 80 µL BSTFA (N,O-bistrifluoroacetamide) with 10% TCMS (methyltrichlorosilane) was added to each sample. The samples were then placed in the oven again for 1.5 h and vortexed every 30 min. These final derivatized samples were then cooled to room temperature and transferred to GC vials with inserts for GC/MS analysis as described below.
2.9. GC/MS (metabolomics)
Metabolomic samples were analyzed on an Agilent 6890 gas chromatograph (Agilent Technologies, CA, USA) linked to a Waters magnetic sector mass spectrometer (Waters, Milford, MA) and all data were collected and then processed with MassLynx®. Metabolite derivatives were separated on an Rxi-5Sil MS (30 m, 0.25 µm thickness, and 0.25 mm ID; Restek, PA, USA). All injections (2 µL) were made in the splitless mode. The injector temperature was 250 °C, the transfer line temperature was held constant at 280 °C, the source temperature was 200 °C, and the trap and detector were set for 350 µA and 300 V, respectively. The carrier gas was helium and maintained at a constant pressure of 65.20 kPa. The initial oven temperature was held for 2 min (60 °C) and then ramped at 6 °C/min to 280 °C with a hold time of 3 min. Blanks were run at the beginning and intermittently throughout the run to verify no carry over. Mass spectra were acquired over a mass range from 50 to 650 m/z. Chromatograms were then exported as netcdf files and imported into MetAlign 041012 for data preprocessing and alignment. Distributor recommended parameters for fast scan analysis was used (Lommen, 2009). Following alignment, Excel was used to filter and truncate the data as described in Niu et al. (2014). Excel was used to generate t-test filtered chromatograms and Metaboanalyst® 3.0 used for ANOVA and metabolite pathway analysis (Xia and Wishart, 2016). Statistical analysis of herbicide and mixed pesticide studies was independent for metabolomic profiling. t-Test filtered chromatograms are generated by comparing the control and treated m/z abundances at each retention time (p ≤ 0.05), and if statistically different, subtracting the average spectral response of the control frogs from that of the pesticide treatment. Therefore, peaks that are above the axis can be assumed higher in treated samples through ‘up’ regulation while negative peaks are ‘down’ regulated by pesticide exposure.
3. Results
3.1. Tissue & soil concentrations
In both the herbicide and mixed pesticide studies, atrazine was the most abundant pesticide accumulated in amphibian whole body tissue homogenates despite similar or greater concentrations of both metolachlor and malathion as measured in soils (Table 2). Within the herbicide study, the combination of atrazine and the additional herbicides tested reduced the atrazine body burden among frogs tested. The atrazine treatment tissue concentration was 89%, 63% and 9.5% greater than the atrazine concentration among frogs in the double pesticide ATZD and ATZME and the triple pesticide ATZMED treatment groups, respectively (16.8 ± 6.2(SE), 19.3 ± 13.2 and 29.1 ± 9.5 ppm atrazine, respectively). Atrazine body burdens among amphibians in the mixed pesticide study were, on average, 113%, 244% and 185% greater among frogs in the ATZ treatment relative to those in the double pesticide ATZME and ATZPZ and the triple pesticide ATZMAPZ treatment groups (14.9 ± 3.5, 9.3 ± 2.6 and 11.2 ± 3.0 ppm, respectively).
Table 2.
Amphibian whole body tissue (average ppm ± SE) and soil concentrations (average ppm ± SE) summed across all pesticides in a given herbicide or mixed pesticide treatment.
| Tissue concentration |
Soil concentration | |
|---|---|---|
| Herbicide treatments | ||
| ATZ | 31.89(± 9.23) | 15.83(± 1.27) |
| D | 0.07(± 0.03) | 0.45(± 0.09) |
| ME | 4.32(± 1.31) | 36.89(± 8.17) |
| ATZD | 16.96(± 6.19) | 26.15(± 2.30) |
| ATZME | 29.63(± 8.67) | 58.61(± 2.61) |
| MED | 1.72(± 0.70) | 25.04(± 1.32) |
| ATZMED | 38.71(± 7.56) | 63.12(± 5.93) |
| Mixed pesticide treatments | ||
| ATZ | 31.89(± 9.23) | 15.83(± 1.27) |
| MA | 0.68(± 0.34) | 13.06(± 2.32) |
| PZ | 0.24(± 0.06) | 1.65(± 0.24) |
| ATZMA | 16.68(± 4.30) | 28.02(± 1.09) |
| ATZPZ | 10.00(± 2.80) | 13.76(± 0.82) |
| MAPZ | 1.25(± 0.36) | 12.30(± 1.64) |
| ATZMAPZ | 15.93(± 2.64) | 27.82(± 5.43) |
For the remaining herbicides tested, both metolachlor and 2,4-d shared similar patterns in amphibian accumulation (Table 2). Relative to their average individual tissue concentrations, both metolachlor and 2,4-d concentrations were increased by 133% to 10.1 ± 3.5 ppm and 63% to 0.12 ± 0.04 ppm, respectively, when exposed simultaneously with atrazine. Similarly, tissue concentrations of metolachlor and 2,4-d also increased when present in the triple herbicide mixture ATZMED by 117% to 9.4 ± 2.1 ppm and 159% to 0.19 ± 0.05 ppm, respectively. However, the concentration of both of these herbicides was reduced in the presence of one another; 61% to 1.7 ± 0.7 ppm for metolachlor and 20% to 0.06 ± 0.01 ppm for 2,4-d.
Within the mixed pesticide study, malathion (MA) and propiconazole (PZ) also resulted in a similar pattern where the combined application of any additional pesticide increased tissue concentrations relative to individual exposures (Table 2). The addition of atrazine increased malathion body burdens by 159% to 1.76 ± 0.90 ppm and 2,4-d body burdens by 206% to 0.73 ± 0.25 ppm. When malathion and propiconazole were applied in tandem, tissue concentrations increased by 22% to 0.83 ± 0.28 ppm for malathion and 43% to 0.42 ± 0.08 ppm for propiconazole. Among the triple pesticide mixture ATZMAPZ, malathion tissue concentrations were also increased by 80% to 3.51 ± 2.05 ppm and propiconazole by 81% to 1.26 ± 0.31 ppm.
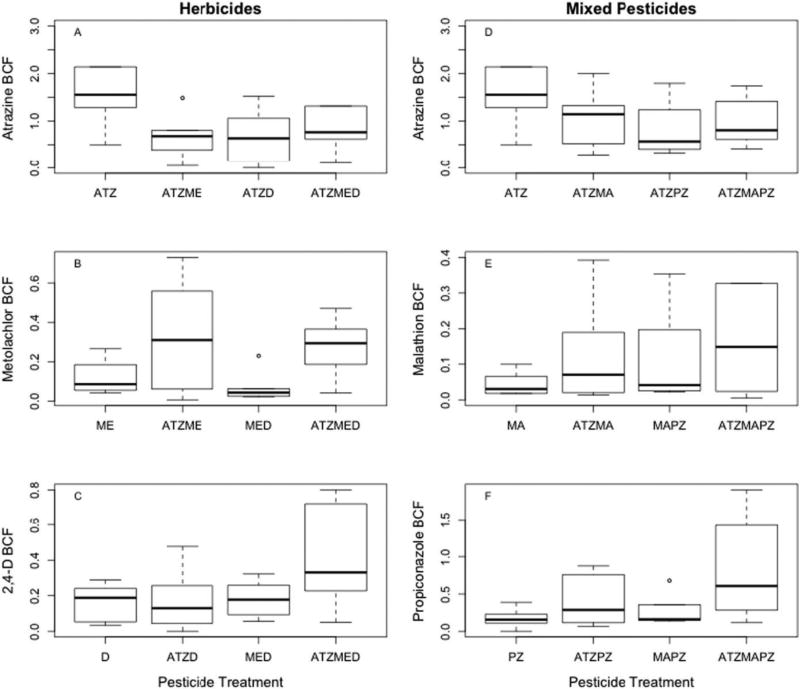
3.2. Bioconcentration factors (BCFs)
Amphibian BCFs were the highest within the atrazine only treatment for both the herbicide (Fig. 1A) and mixed pesticide studies (Fig. 1D). Metolachlor (Fig. 1B), 2,4-d (Fig. 1C), malathion (Fig. 1E), and propiconazole (Fig. 1F) bioconcentration factors reached their maximum, on average, in the triple herbicide and mixed pesticide treatments (ATZMED and ATZMAPZ, respectively). The addition of herbicides in the double pesticide treatments decreased atrazine bioconcentration roughly 3-fold (Fig. 1A) whereas metolachlor BCF was 2.75 times greater in the double pesticide ATZME treatment group (Fig. 1B). Within the mixed pesticide study, the double pesticide ATZMA treatment decreased atrazine bioconcentration nearly two times while increasing malathion BCF 2.5 times relative to single treatments (Fig. 1E). Similarly, the ATZPZ treatment resulted in 2.5-fold reduction in atrazine bioconcentration but a 2.3-fold increase in propiconazole bioconcentration (Fig. 1F).
Fig. 1.
Bioconcentration factors for green frogs (Lithobates clamitans) across individual, double and triple pesticide treatments within the herbicide and mixed pesticide studies.
In the triple pesticide treatment for both the herbicide and mixed pesticide studies, atrazine BCF was nearly half of the bioconcentration factor of the atrazine treatment alone (Fig. 1A & D, respectively). Among 2,4-d treatment groups, bioconcentration was nearly 2.5 times greater in the triple herbicide ATZMED treatment than all other 2,4-d treatments (Fig. 1C). In the mixed pesticide study, malathion bioconcentration was 9.8 times greater and propiconazole 4.8 times greater in the triple pesticide treatment relative to the individual respective treatments (Fig. 1E & F).
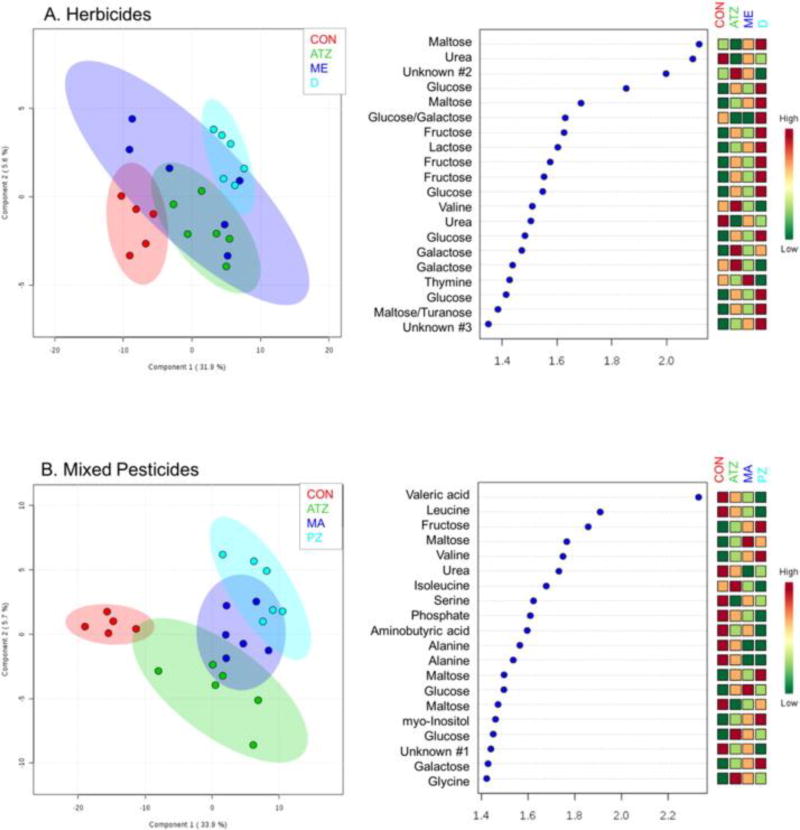
3.3. Metabolomic profiling
Partial least squares discriminant analysis (PLS-DA) models were constructed for the individual pesticides used in the herbicide and mixed pesticide studies to aid in visualization of potential differences in biological response (Fig. 2). Based on this analysis, there appears to be three distinct biochemical profiles in the mixed pesticide group, evidenced by class separation along principal component (PC) 2 (up and down). The greatest separation in both models (along PC1) appears to be between control and treated amphibians. In the herbicide model, there was greater overlap between each treatment class, potentially suggesting that these compounds elicit similar responses in the hepatic metabolome (Fig. 2A). When identifying peaks in the variable importance in projection plots for PC2, metabolites that were important in the mixed pesticide exposure group include leucine, urea, serine and alanine (all higher in control samples) (Fig. 2B). In the mixed pesticide group, generally, sugars and energetic molecules appear to be higher in treated animals. While, a decrease in urea and mixed flux in sugars (i.e. disaccharides) appear to explain the greatest variation in the herbicide exposures. Interestingly, fewer amino acids or other biomolecules appear to be significant and > 50% of the top 25 metabolites response for PC2 separation are sugar derivatives.
Fig. 2.
PLS-DA scores plot (left) and VIP scores from principal component 2 of the PLS-DA (right) for the herbicide exposure model (A) and mixed pesticide exposure model (B) identifying the top 25 GC/MS features responsible for separation between treatment classes (x-axis is the correlation score generated by Metaboanalyst®).
Based on t-test filtering of the chromatograms, a total of 38 putatively identified metabolites were significantly affected by at least one herbicide treatment relative to control livers (ANOVA p ≤ 0.05). Among single herbicides, 2,4-d resulted in the greatest number of metabolites that were up or down regulated and had the largest number of significant metabolomic spectral features (or m/z values) identified relative to the control group (Table 3 & Fig. 3A; t-test p < 0.05). When paired with atrazine, the metabolite profile and number of spectral features of the ATZD treatment was intermediate to the single pesticide treatments, with the exception of the down regulation of hydroxybutyric acid (Table 3; t-test p < 0.05). Similarly, when paired with metolachlor, the MED treatment produced an intermediate number of m/z values and metabolomic profile relative to single treatments, however, both butyric and lactic acid were up regulated in the double herbicide treatment (Table 3; t-test p < 0.05). Urea was upregulated by the ME and D single herbicide treatments and their associated double treatments that were paired with atrazine (ATZME and ATZD). Similarly, leucine was up regulated by all double and triple herbicide mixtures (ATZME, ATZD, MED and ATZMED) as well as the individual 2,4-d treatment (D).
Table 3.
Metabolites significantly up (↑) or down (↓) regulated by an herbicide treatment relative to the control group (t-test p < 0.05) following exposure in amphibians.
| Herbicide treatment | |||||||
|---|---|---|---|---|---|---|---|
| Metabolite | ATZ | ME | D | ATZME | ATZD | MED | ATZMED |
| Alanine | ↓ | ↓ | ↓ | ||||
| Aspartic acid | ↓ | ↓ | ↓ | ||||
| Butyric acid | ↑ | ↑ | |||||
| Disaccharide | ↓, ↑ | ↑ | ↓ | ||||
| Galactose | ↑ | ↑ | |||||
| Glucose/galactose | ↑ | ↑ | ↑ | ↑ | |||
| Glucuronic acid | ↑ | ↑ | ↑ | ↑ | ↑ | ||
| Guanosine | ↑ | ↑ | |||||
| Hydroxybutyric acid | ↓ | ↓ | |||||
| Hypoxanthine | ↓ | ↑ | |||||
| Lactic acid | ↑ | ↑ | |||||
| Leucine | ↑ | ↑ | ↑ | ↑ | ↑ | ||
| Maltose | ↑ | ↑ | ↑ | ||||
| Maltose/turanose | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | |
| Myo-inositol | ↑ | ↑ | ↑ | ||||
| Oxalic acid | ↓ | ↓ | ↓ | ↓ | |||
| phosphorylethanolamine | ↑ | ↑ | ↑ | ↑ | ↑ | ||
| Ribitol | ↑ | ↑ | ↑ | ↑ | |||
| Ribose/fructose | ↓ | ↑ | |||||
| Sugar phosphate | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ |
| Tyrosine | ↑ | ↑ | ↑ | ||||
| Urea | ↑ | ↑ | ↑ | ↑ | |||
| Total up regulated | 7 | 8 | 14 | 12 | 7 | 7 | 6 |
| Total down regulated | 2 | 0 | 4 | 5 | 5 | 0 | 0 |
| Total up or down regulated | 9 | 8 | 18 | 17 | 12 | 7 | 6 |
CON = control, ATZ = atrazine, ME = metolachlor, D = 2,4-d.
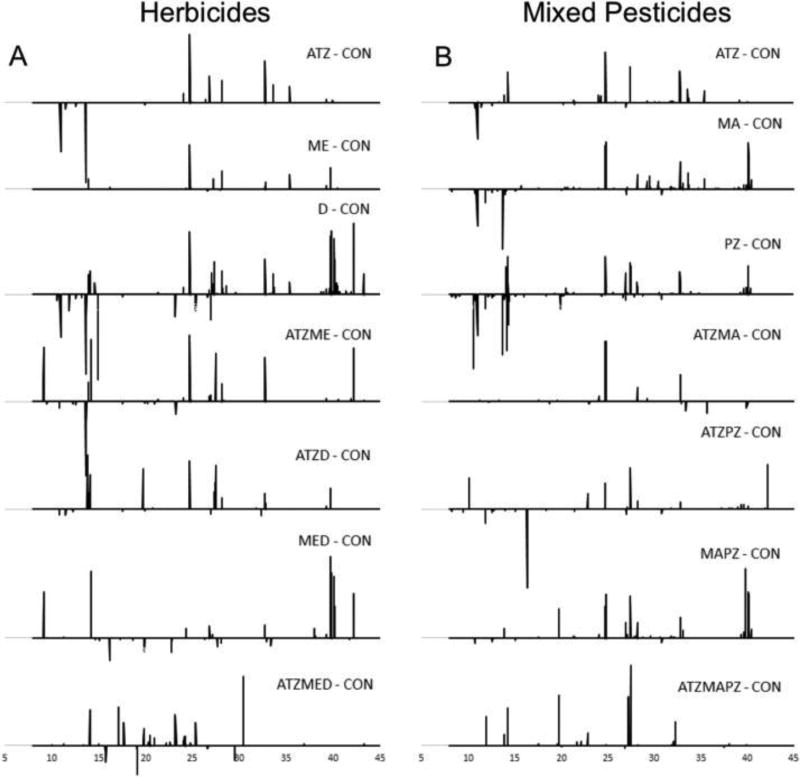
Fig. 3.
t-Test filtered chromatograms for single, double and triple herbicide (A) and mixed pesticide (B) exposures relative to the control. Each peak represents a metabolite that was up or down regulated when compared to control samples.
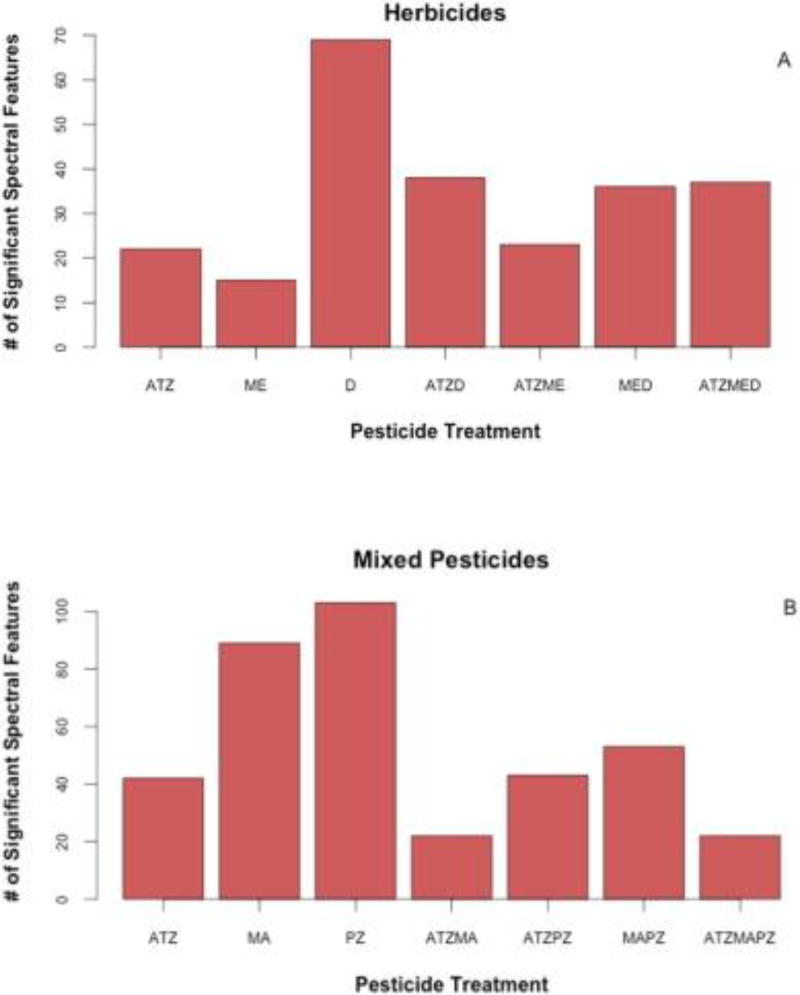
While the single atrazine (ATZ) and metolachlor (ME) treatments resulted in fewer up and down regulated metabolites relative to 2,4-d (D), when combined together in the double herbicide treatment, ATZME, 17 metabolites were up or down regulated (Table 3; t-test p < 0.05). Of these 17 metabolites, 8 were novel metabolites that were not affected by the single ATZ or ME treatments. Among the combined herbicide treatments, this double herbicide ATZME treatment resulted in the largest number of significant spectral features in the amphibians we tested, although the ATZMED triple herbicide and MED double herbicide results were very similar in number of spectral features (Fig. 4A). Interestingly, the ATZMED treatment resulted in up regulation of both hypoxanthine and monosaccharides, whereas these metabolites were largely unaffected by other herbicide treatments and even down regulated in double MED and ATZME treatments, respectively (Table 3; t-test p < 0.05).
Fig. 4.
Number of statistically significant spectral features (i.e. m/z values) identified by GC/MS analysis of amphibian livers exposed to herbicides (A) or mixed pesticides (B). Statistical analysis of herbicide and mixed pesticide studies was independent for metabolomic profiling.
Among the mixed pesticide treatments, 50 putatively identified metabolites were significantly altered by at least one of the mixed pesticide treatments (ANOVA p < 0.05). The individual propiconazole (PZ) and malathion (MA) treatments resulted in significant up or down regulation of approximately 23 and 25 putatively identified metabolites relative to the control, respectively (Table 4 & Fig. 3B; t-test p < 0.05), and also resulted in the largest number of significant m/z values among treatments in the mixed pesticide study (Fig. 4B). When paired with each other in the double pesticide MAPZ treatment, the number of significant m/z values was greatly diminished relative to the individual treatments (Fig. 4B). Despite this decrease in number of spectral features, 20 metabolites were up or down regulated relative to the controls (Table 4). Among these 20 metabolites, cytosine was up regulated in the double MAPZ treatment while down regulated in the single PZ treatment. Methionine was also up regulated in the double MAPZ treatment as well as the triple ATZMAPZ treatment, although neither up nor down regulated in any of the single or remaining double pesticide treatments (Table 4).
Table 4.
Metabolites significantly up (↑) or down (↓) regulated by a mixed pesticide treatment relative to the control group (t-test p < 0.05) following exposure in amphibians.
| Mixed pesticide treatment | |||||||
|---|---|---|---|---|---|---|---|
| Metabolite | ATZ | MA | PZ | ATZMA | ATZPZ | MAPZ | ATZMAPZ |
| Adenine | ↑ | ↑ | ↓ | ||||
| Alanine | ↑ | ↑ | ↑ | ↑ | |||
| Altronic acid | ↑ | ↑ | ↑ | ↑ | |||
| Arabitol | ↑ | ↑ | |||||
| Asparagine | ↑ | ↑ | |||||
| Creatinine | ↓ | ↓ | ↓ | ||||
| Cytosine | ↓ | ↓ | ↑ | ||||
| Fructose phosphate | ↓ | ↓ | ↓ | ↓ | |||
| Galactose/glucose | ↑ | ↑ | ↑ | ↑ | ↑ | ||
| Glucose phosphate | ↑ | ↑ | |||||
| Glucuronic acid | ↑ | ↑ | ↑ | ↑ | |||
| Glycine | ↓ | ↓ | ↓ | ↓ | |||
| Hypoxanthine | ↑ | ↑ | |||||
| Lactic acid | ↓ | ↓ | ↓ | ↓ | |||
| Malic acid | ↑ | ↑ | |||||
| Malonic acid | ↑ | ↑ | ↑ | ||||
| Maltose/turanose | ↑ | ↑ | ↑ | ↓ | ↑ | ↑ | ↑ |
| Melibiose | ↑ | ↑ | |||||
| Methionine | ↑ | ↑ | |||||
| Myo-inositol | ↑ | ↑ | |||||
| Ornithine | ↑ | ↑ | |||||
| Phenylethanolamine | ↓ | ↓ | ↓ | ↓ | |||
| Phosphate | ↑ | ↑ | ↑ | ||||
| Putrescine | ↑ | ↑ | ↑ | ↑ | ↑ | ||
| Pyrrolidine carboxylic acid | ↓ | ↑ | ↑ | ↓ | ↑ | ||
| Ribitol | ↑ | ↑ | |||||
| Serine | ↓ | ↓ | |||||
| Spermine | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | |
| Sugar alcohol | ↑ | ↑ | |||||
| Sugar phosphate | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ |
| Threitol | ↑ | ↑ | |||||
| Urea | ↓ | ↓ | |||||
| Valine | ↓ | ↓ | ↓ | ↓ | |||
| Xanthine | ↑ | ↑ | ↑ | ||||
| Sum up regulated | 8 | 17 | 15 | 5 | 10 | 15 | 9 |
| Sum down regulated | 3 | 8 | 8 | 1 | 5 | 5 | 2 |
| Total up or down regulated | 11 | 25 | 23 | 6 | 15 | 20 | 11 |
CON = control, ATZ = atrazine, MA = malathion, PZ = propiconazole.
Among the ATZPZ and ATZMA double pesticide treatments, the total number of significant spectral features also decreased suggesting an observable effect of atrazine on both the action of malathion and propiconazole or a different mode of action when the pesticides where combined (Fig. 4B). Several disaccharides were down regulated in the ATZMA treatment, although they were up regulated relative to control amphibians in all other pesticide treatments (Table 4). The triple mixed pesticide treatment ATZMAPZ had a similarly low number of significant m/z values for the frogs we tested (Figs. 3B & 4B). Adenine was down regulated in the triple ATZMAPZ treatment while up regulated by the PZ and MAPZ treatments, also suggesting an altered effect of atrazine on amphibians when paired with other pesticides.
In looking at the overall chromatographic spectra for the amphibian metabolome, the magnitude of the changes in up or down regulation of metabolites was the lowest in the triple mixed pesticide and triple herbicide treatment relative to the control (Fig. 4). Furthermore, metabolomic profiling supports tissue concentrations and BCFs whereby a possible cascading failure or disruption of typical biological function may have been induced by pesticide overload in the triple herbicide treatment. A potential inhibition of uptake mechanisms for pesticide mixtures warrants further investigation.
Pathways analysis on the metabolites of significance highlighted eight biological pathways that may be impaired by the herbicide treatments and twelve by the mixed pesticide treatments (Table 5). Among these pathways, five were identified as significant biological pathways impaired in both studies. This overlap in affected pathways suggests that atrazine may play a dominant role in altering typical biological function in exposed amphibians or that co-exposure to a variety of pesticides may cause some similar effects due to generalized modes of action and resulting toxicity when pesticides are present in mixtures.
Table 5.
Biological pathways significantly affected by pesticide treatments in frogs.
| Pathway | Hits | Total | p-Value |
|---|---|---|---|
| Herbicide treatments | |||
| Galactose metabolisma | 5 | 26 | 0.0004 |
| Starch and sucrose metabolisma | 4 | 19 | 0.001 |
| Aminoacyl-tRNA biosynthesisa | 7 | 69 | 0.0013 |
| Alanine, aspartate and glutamate metabolisma | 4 | 24 | 0.0026 |
| Cysteine and methionine metabolisma | 4 | 27 | 0.0041 |
| Purine metabolism | 5 | 68 | 0.026 |
| Inositol phosphate metabolism | 3 | 28 | 0.0319 |
| Glycine, serine and threonine metabolism | 3 | 31 | 0.0416 |
| Mixed pesticide treatments | |||
| Aminoacyl-tRNA biosynthesisa | 13 | 67 | 0 |
| Galactose metabolisma | 7 | 26 | 0 |
| Alanine, aspartate and glutamate metabolisma | 6 | 24 | 0.0001 |
| Arginine and proline metabolism | 7 | 43 | 0.0006 |
| beta-Alanine metabolism | 4 | 16 | 0.0019 |
| Glutathione metabolism | 4 | 26 | 0.0122 |
| Pyrimidine metabolism | 5 | 41 | 0.0136 |
| Pantothenate and CoA biosynthesis | 3 | 15 | 0.0145 |
| Cysteine and methionine metabolisma | 4 | 29 | 0.0179 |
| Sulfur metabolism | 2 | 9 | 0.0386 |
| Starch and sucrose metabolisma | 3 | 22 | 0.0413 |
| Pyruvate metabolism | 3 | 22 | 0.0413 |
Indicates a pathway shared between both herbicide & mixed pesticide studies.
4. Discussion
The hepatic metabolomic profiling of juvenile amphibians presented here is the first study, to the best of our knowledge, to evaluate comprehensive toxic effects of pesticide mixtures on post-metamorphic juvenile amphibians at the biochemical level and only the second to report on the metabolome of juvenile frogs in general (see Ichu et al., 2014). At the onset of this study, it was predicted that cumulative effects on amphibian metabolomic profiles would emerge when multiple pesticides were combined. However, across all pesticides and mixtures tested in this study, the propiconazole and 2,4-d treatments resulted in the greatest number of significant spectral features in juvenile frogs and the individual application of atrazine consistently produced the highest BCFs.
In total between both the herbicide and mixed pesticide exposure studies, the production of 44 different metabolites was affected by at least two pesticide treatments in the amphibians tested. Of these 44 metabolites, 12 were metabolites impacted in both studies and may represent a generalized response to pesticide exposure and/or an effect of atrazine exposure, since atrazine was common to both studies. Among non-target vertebrates, atrazine is an endocrine disruptor, malathion is a known acetylcholinesterase inhibitor, while the remaining pesticides tested are considered possible endocrine disruptors, carcinogens and/or teratogens (reviewed in Mniff et al., 2011; Sparks and Nauen, 2015). However, non-target MOA is not clearly known and impacts on the metabolome in juvenile amphibians has not been investigated previously. Further exploration of the data generated in this study may help elucidate whether atrazine is responsible for many of the effects, or if mixed modes of action between pesticides is the causative agent for the shifts in metabolomics seen here.
Among the 12 metabolites commonly altered in both the herbicide and mixed pesticide studies, many are amino acids, nucleic acids (and analogs) and carbohydrates that are critical for protein synthesis, DNA structure and replication as well as stress response and energy production in amphibians. In particular, glucose and lactic acid are both molecules important to glycolysis and glucogenesis as part of the starch and sucrose metabolism pathway (Gray et al., 2014). In our amphibians, glucose was up regulated in both the herbicide and mixed pesticide studies relative to the control group possibly indicating an increase in energetics necessary for detoxification following pesticide exposure. While lactic acid was also up regulated in the ATZME and MED double herbicide treatments, it was down regulated in ATZ, MA, PZ and MAPZ treatments within the mixed pesticide study. In Xenopus laevis tadpoles, atrazine exposure caused down regulation of genes associated with glycolysis/glucogenesis (Zaya et al., 2011). This down regulation may have been a stress response to the atrazine exposure, where initial exposure induced a spike in glucose in the bloodstream, followed by a decrease in the conversion of glucose to energy stores which accompanied general shifts in nutritional status, energetics and homeostasis (Zaya et al., 2011). Similarly, bullfrog tadpoles exposed to one of three herbicides, including atrazine, showed a significant decrease in glycogen levels in all tissues measured, likely as a stress response to maintain homeostasis, but that ultimately depleted energy stores (Dornelles and Oliveira, 2016). Given that these tadpole studies report down regulation of metabolites critical to sucrose and starch pathways following pesticide exposure, it is interesting to note the up regulation we saw in these same metabolites in many instances in our juvenile frogs. This may indicate variations in biological response to pesticide exposure across life stages or a stress response in juvenile amphibians as they shift toward anaerobic respiration. Additional studies exploring these metabolomic pathways in post-metamorphic juvenile amphibians are needed to gain a comprehensive understanding of the biological effects of pesticides during this transitional and critical life stage.
Patterns of altered or decreased glycolysis/glucogenesis have also been seen in daphnia following atrazine exposure (Wagner et al., 2016), malathion exposure in mice (Wang et al., 2014), as well as endosulfan and carbofuran exposure in earthworms (Yuk et al., 2011; Mudian et al., 2013, respectively). Energetics were also altered in goldfish exposed to the pesticide butachlor, where glucose was converted to lactate through the intermediary pyruvate, which was believed to be a stress response and a shift from aerobic to anaerobic respiration (Xu et al., 2015). In eukaryotes, proper pyruvate metabolism is critical for disease control and alterations in this metabolic pathway can lead to the formation of cancer, heart failure and neurodegeneration (Gray et al., 2014). Given the widespread changes in glycolysis/glucogenesis and the adverse effects on energetics across many taxa and pesticides, these metabolomic changes may represent a generalized adverse response to pesticide exposure also seen in the amphibians presently studied.
The amino acid alanine was also altered in the frogs we studied in both the herbicide and mixed pesticide studies, though interestingly it was up regulated by several treatments in the herbicide study while down regulated by several treatments in the mixed pesticide study. The alanine cycle is critical as it syncs liver and muscle metabolic processes (Gray et al., 2014). Alanine may be considered a biomarker of stress given its role in glycolysis. In earthworms exposed to the pesticide cypermethrin, alanine and valine metabolism were among those processes that were impaired (Ch et al., 2015). Similarly, after atrazine exposure in Hyalella, valine and alanine were altered and suggested a shift in energetics, possibly due to amino acid catabolism (Ralston-Hooper et al., 2010). While valine was not significantly altered in our herbicide study, it was down regulated in several treatments within the mixed pesticide study. Decreases in valine along with increases in glucose and lactate were reported in Daphnia after malathion exposure and indicated a stress response (Nagato et al., 2016). Also common to both the herbicide and mixed pesticide treatments was the alteration in urea metabolism. Urea was down regulated by MA and PZ in the mixed pesticide treatments but up regulated by ME and D as well as their paired treatments with atrazine (ATZME and ATZD) in the herbicide study. Urea and the urea cycle are intimately linked with the arginine and purine/pyrimidine metabolic pathways (Ichu et al., 2014). In tadpoles exposed to atrazine, the urea cycle was up regulated and suggested an increase in protein breakdown that was associated with increased energetic demands (Zaya et al., 2011). Likewise, an increase in urea following atrazine exposure in Hyalella was believed to support proteolytic breakdown to supply energetic needs in response to the herbicide stressor (Ralston-Hooper et al., 2010).
Within the herbicide study, 10 unique metabolites were affected by exposure to atrazine, metolachlor, 2,4-d or any combination of these pesticides. Among these was the amino acid tyrosine that was up regulated in the D, ATZME and MED treatments. After atrazine exposure in mice, tyrosine metabolism was negatively affected. Tyrosine is important for normal brain function and is a precursor to the production of the neurotransmitter dopamine (Lin et al., 2014). Given the up regulation of tyrosine among frogs in several of our herbicide treatments, this suggests a possible increase in neurotransmitter function which may result in overstimulation of the brain and associated neural signalling.
In the mixed pesticide study, 22 unique metabolites were significantly affected by exposure to atrazine, malathion, propiconazole or a combination of these pesticides. The amino acids methionine and serine play an essential role in aminoacyl-tRNA biosynthesis, which is essential for DNA replication (O'Donoghue and Luthey-Schulten, 2003). Methionine was up regulated in our double MAPZ and triple ATZMAPZ treatments and serine was down regulated in the MA and PZ treatments. In addition to this highly conserved and fundamental role, methionine and serine are part of the cysteine methionine metabolism pathway. In amphibians, cysteine is a precursor to glutathione (GSH), a well-documented biomarker of oxidative stress (Ichu et al., 2014). Among tadpoles, exposure to a variety of pesticides is known to reduce GSH activity and result in significant mortality (reviewed in Venturino et al., 2003; Venturino and de D'Angelo, 2005). After atrazine exposure, glutathione metabolism was up regulated in Xenopus laevis tadpoles and may have facilitated an increase in the excretion of atrazine and its metabolites, but also increased the presence of reactive oxygen species (ROS) scavengers that indicate stress and may lead to cellular damage (Zaya et al., 2011). Similarly, rats receiving dietary intake of atrazine by gavage experienced a significant decrease in GSH, possibly due to oxidation reactions that reduce the toxicity of the atrazine (Singh et al., 2011). Methionine also plays a substantial role as part of the antioxidant defense system (Ichu et al., 2014) and may be a useful bioindicator of oxidative stress (Wagner et al., 2016). Methylation is another important role in methionine metabolism, but significant increases in DNA methylation can lead to cancer proliferation in the body (e.g., Suva et al., 2013). Pesticide dysregulation of amino acids in juvenile amphibians may have profound impacts on survival, disease resistance and immune function.
Additional metabolites that were uniquely affected by the mixed pesticide treatments were ornithine and creatinine. Ornithine was up regulated by the MA and PZ treatments whereas creatinine was down regulated by these same treatments in addition to the triple pesticide ATZMAPZ treatment. Ornithine plays an important role in the urea, arginine, and purine/pyrimidine metabolism pathways in amphibians (Ichu et al., 2014). Creatinine is produced by the breakdown of creatine in muscles and is related to ATP production and use during times of high energy demand in frogs (Ichu et al., 2014). In cockroaches exposed to an organophosphate pesticide, fenithrothion, creatine was significantly increased and also signalled a change in energetic needs (Southam et al., 2011). After butachlor exposure in goldfish, creatinine levels in blood serum increased and may have been suggestive of renal failure (Xu et al., 2015). Given that creatinine was down regulated in several of our treatments, this suggests a limited energetics response and possibly a decreased capacity for detoxification among amphibians after pesticide exposure. Pesticide induced changes to basic, yet essential metabolites in these biological pathways, as seen in this study, have the potential to significantly alter amphibian brain function and energetics. A targeted analytic approach focusing on the metabolites identified here would offer greater sensitivity and further our efforts in identifying biomarkers of multiple pesticide exposure in amphibians.
Bioaccumulation of pesticides, whether present individually or in mixtures, did not necessarily corroborate with associated impacts to the amphibian metabolome in this study. Across all pesticides tested, 2,4-d, malathion and propiconazole resulted in much lower BCFs than atrazine, but had equal or substantially larger impacts to individual metabolite production in amphibians. Similarly, several double pesticide treatments, such as ATZMA, ATZPZ and ATZD, along with the triple pesticide treatments, ATZMED and ATZMAPZ, altered amphibian BCF but not in a linear way that coincided with changes in the metabolome. While at first glance it may appear that atrazine has an inhibitory effect when paired with other pesticides, the impact on the metabolome induced by these pesticide combinations may be quite significant when looking at specific metabolite regulation or affected biological pathways.
4.1. Conclusions
The metabolites and pathways of significance often differed between the pesticide treatments we studied in amphibians, indicating that pesticide modes of action can and do change depending on specific chemical interactions with other pesticides present in mixtures. Pesticide impacts to the amphibian metabolome and overall biological functioning are likely to differ depending on the chemicals present, as evidenced by our data, and identifying those changes in modes of action will be essential in evaluating pesticide impacts to this imperilled taxa. Additionally, some pesticides and pesticide combinations had a large impact on the amphibian metabolome but were not among the treatments that resulted in the greatest bioaccumulation in our study.
To adequately inform Cumulative Risk Assessment for amphibians, much more data is needed. In the study presented here, we have explored the effects of five pesticides on one amphibian species. Given that there are hundreds of pesticides successfully registered in the United States, we encourage researchers to expand on mixed pesticide research by including juvenile and adult stage amphibians across different species. While metabolomic profiling is very time intensive and may not be feasible in all research labs, novel approaches, such as this, will help elucidate pesticide impacts in these non-target animals. Understanding pesticide modes of action in post-metamorphic juvenile amphibians, individually and when present in mixtures, will provide greatly needed insight into both short- and long-term effects that have the potential to propagate across all amphibian life stages.
Acknowledgments
We thank Katie Washart and Heather Plocinik, both undergraduate research assistants at Washington College, for all of their time and effort in helping with the live amphibian care and general laboratory assistance. Our IACUC protocol (SU 14-001B) received approval from the Washington College Institutional Animal Care and Use Committee. This paper has been reviewed in accordance with Environmental Protection Agency policy and approved for publication. The views expressed in this article are those of the authors and do not necessarily represent the views or policies of the USEPA.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Data accessibility
The R code and data that implements the analyses and generates manuscript figures are available from a GitHub repository
References
- 1.Attademo AM, Peltzer PM, Lajmanovich RC, Basso A, Junges C. Tissue-specific variations of esterase activities in the tadpoles and adults of Pseudis paradoxa (Anura:Hylidae) Water Air Soil Pollut. 2014;225:1903. [Google Scholar]
- 2.Boone MD, James SM. Interactions of an insecticide, herbicide and natural stressors in amphibian community mesocosms. Ecol. Appl. 2003;13(3):829–841. [Google Scholar]
- 3.Brodeur JC, Suarez RP, Natale GS, Ronco AE, Zaccagnini ME. Reduced body condition and enzymatic alterations in frogs inhabiting intensive crop production areas. Ecotoxicol. Environ. Saf. 2011;74:1370–1380. doi: 10.1016/j.ecoenv.2011.04.024. [DOI] [PubMed] [Google Scholar]
- 4.Brodeur JC, Vera Candioti J, Soloneski S, Larramendy ML, Ronco AE. Evidence of reduced feeding and oxidative stress in common tree frogs (Hypsiboas pulchellus) from an agroecosystem experiencing severe drought. J. Herpetol. 2012;46:72–78. [Google Scholar]
- 5.Brühl CA, Pieper S, Weber B. Amphibians at risk? Susceptibility of terrestrial amphibian life stages to pesticides. Environ. Toxicol. Chem. 2011;30:2465–2472. doi: 10.1002/etc.650. [DOI] [PubMed] [Google Scholar]
- 6.Brühl CA, Schmidt T, Pieper S, Alscher A. Terrestrial pesticide exposure of amphibians: an underestimated cause of global decline? Sci. Report. 2013;3:1135. doi: 10.1038/srep01135. https://doi.org/10.1038/srep01135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bundy JG, Davey MP, Viant MR. Environmental metabolomics: a critical review and future perspectives. Metabolomics. 2009;5:3–21. [Google Scholar]
- 8.Ch R, Singh AK, Pandey P, Saxena PN, Mudiam MKR. Identifying the metabolic perturbations in earthworm induced by cypermethrin using gas chromatographymass spectrometry based metabolomics. Sci. Report. 2015;5:15674. doi: 10.1038/srep15674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cloyd RA. Pesticide Mixtures: Understanding Their Use in Horticultural Production Systems. Kansas State University Agricultural Experiment Station and Cooperative Extension Service (MF-3045); 2012. [Google Scholar]
- 10.Cusaac JPW, Mimbs WH, IV, Belden JB, Smith LM, McMurry ST. Terrestrial exposure and effects of headline AMP fungicide on amphibians. Ecotoxicology. 2015;24:1341–1351. doi: 10.1007/s10646-015-1509-6. [DOI] [PubMed] [Google Scholar]
- 11.Denoël M, D'Hooghe B, Ficetola GF, Brasseur C, De Pauw E, Thomé JP, Kestemont P. Using sets of behavioural biomarkers to assess short-term effects of pesticide: a study case with endosulfan on frog tadpoles. Ecotoxicology. 2012;21:1240–1250. doi: 10.1007/s10646-012-0878-3. [DOI] [PubMed] [Google Scholar]
- 12.Dinehart SK, Smith LM, McMurry ST, Anderson TA, Smith PN, Haukos DA. Toxicity of a glufosinate- and several glyphosate-based herbicides from Southern High Plains, USA. Sci. Total Environ. 2009;407:1065–1071. doi: 10.1016/j.scitotenv.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 13.Dornelles MF, Oliveira GT. Toxicity of atrazine, glyphosate, and quinclorac in bullfrog tadpoles exposed to concentrations below legal limits. Environ. Sci. Pollut. Res. 2016;23:1610–1620. doi: 10.1007/s11356-015-5388-4. [DOI] [PubMed] [Google Scholar]
- 14.Fryday S, Thompson H. Toxicity of Pesticides to Aquatic and Terrestrial Life Stages of Amphibians and Occurrence, Habitat Use and Exposure of Amphibian Species in Agricultural Environments. 2012 (Supporting Publications 2012:EN-343, [ www.efsa.europa.eu/publications])
- 15.Glinski DA, Henderson WM, Van Meter RJ, Purucker ST. Effect of hydration status on pesticide uptake in anurans following exposure to contaminated soils. Environ. Sci. Pollut. Res. 2017a doi: 10.1007/s11356-018-1830-8. (In review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glinski DA, Henderson WM, Van Meter RJ, Purucker ST. Using in vitro derived metabolic rate constants to inform pesticide body burdens in amphibians. Toxicol. Lett. 2017b doi: 10.1016/j.toxlet.2018.02.016. (In review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gosner KL. A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica. 1960;16:183–190. [Google Scholar]
- 18.Goulson D. An overview of the environmental risks posed by neonicotinoid insecticides. J. Appl. Ecol. 2013;50(997–987) [Google Scholar]
- Gray LR, Tompkins SC, Taylor EB. Regulation of pyruvate metabolism and human disease. Cell. Mol. Life Sci. 2014;71:2577–2604. doi: 10.1007/s00018-013-1539-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayes TB, Case P, Chui S, Chung D, Haefele C, Haston K, Lee M, Mai VP, Marjuoa Y, Parker J, Tsui M. Pesticide mixtures, endocrine disruption, and amphibian declines: are we underestimating the impact? Environ. Health Perspect. 2006 doi: 10.1289/ehp.8051. https://doi.org/10.1289/ehp.8051. [DOI] [PMC free article] [PubMed]
- 20.Henson-Ramsey H, Kennedy-Stoskopf S, Levine JF, Taylor SK, Shea D, Stoskopf MK. Acute toxicity and tissue distributions of malathion in Ambystoma tigrinum. Arch. Environ. Contam. Toxicol. 2008;55:481–487. doi: 10.1007/s00244-007-9091-4. [DOI] [PubMed] [Google Scholar]
- 21.Johnson MS, Aubee C, Salice CJ, Leigh SKB, Liu E, Pott U, Pillard D. A review of ecological risk assessment methods for amphibians: Comparative assessment of testing methodologies and available data. Integr. Environ. Assess. Manag. 2017;13:601–613. doi: 10.1002/ieam.1881. [DOI] [PubMed] [Google Scholar]
- 22.Ichu T, Han J, Borchers C, Lesperance M, Helbing CC. Metabolomic insights into system-wide coordination of vertebrate metamorphosis. BMC Dev. Biol. 2014;14:5. doi: 10.1186/1471-213X-14-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kenaga EE. Correlation of bioconcentration factors of chemicals in aquatic and terrestrial organisms with their physical and chemical properties. Environ. Sci. Technol. 1980;14:553–556. [Google Scholar]
- 24.Kerby JL, Storfer A. Combined effects of atrazine and chlorpyrifos on susceptibility of tiger salamander to Ambystoma tigrinum virus. EcoHealth. 2009;6:91–98. doi: 10.1007/s10393-009-0234-0. [DOI] [PubMed] [Google Scholar]
- 25.Köhler HR, Triebskorn R. Wildlife ecotoxicology of pesticides: can we track effects to the population level and beyond? Science. 2013;341:759. doi: 10.1126/science.1237591. [DOI] [PubMed] [Google Scholar]
- 26.Lenhardt PP, Brühl CA, Berger G. Temporal coincidence of amphibian migration and pesticide applications on arable fields in spring. Basic Appl. Ecol. 2014;16:54–63. [Google Scholar]
- 27.Lin Z, Roede JR, He C, Jones DP. Short-term oral atrazine exposure alters the plasma metabolome of male C57BL/6 mice and disrupts α-lineolate, tryptophan, tyrosine and other major metabolic pathways. Toxicology. 2014;326:130–141. doi: 10.1016/j.tox.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 28.Lommen A. MetAlign: an interface-driven, versatile metabolomics tool for hyphenated full-scan MS data pre-processing. Anal. Chem. 2009;81:3079–3086. doi: 10.1021/ac900036d. [DOI] [PubMed] [Google Scholar]
- 29.Mann RM, Hyne RV, Choung CB, Wilson SP. Amphibians and agricultural chemicals: review of the risks in a complex environment. Environ. Pollut. 2009;157:2903–2927. doi: 10.1016/j.envpol.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 30.Margido TCS, Felício AA, Rossa-Feres DdC, de Almeida EA. Biochemical biomarkers in Scinax fuscovarius tadpoles exposed to a commercial formulation of the pesticide fipronil. Mar. Environ. Res. 2013;91:61–67. doi: 10.1016/j.marenvres.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 31.Meek ME(Bette), Boobis AR, Crofton KM, Heinemeyer G, Raaij MW, Vickers C. Risk assessment of combined exposure to multiple chemicals: a WHO/IPCS framework. Regul. Toxicol. Pharmacol. 2011;60(2) doi: 10.1016/j.yrtph.2011.03.010. https://doi.org/10.1016/j.yrtph.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 32.Miller MG. Environmental metabolomics: a SWOT analysis (strengths, weaknesses, opportunities and threats) J. Proteome Res. 2007;6:540–545. doi: 10.1021/pr060623x. [DOI] [PubMed] [Google Scholar]
- 33.Mniff W, Hassine AIH, Bouaziz A, Bartegi A, Thomas O, Roig B. Effects of endocrine disruptor pesticides: a review. Int. J. Environ. Res. Public Health. 2011;8(6):2265–2303. doi: 10.3390/ijerph8062265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretto A, Bachman A, Boobis A, Solomon KR, Pastoor TP, Wilks MF, Embry MR. A framework for cumulative risk assessment in the 21st century. Crit. Rev. Toxicol. 2016 doi: 10.1080/10408444.2016.1211618. https://doi.org/10.1080/10408444.2016.1211618. [DOI] [PubMed]
- 34.Mudian MKR, Ch R, Saxena PN. Gas chromatography-mass spectrometry based metabolomic approach for optimization and toxicity evaluation of earthworm sub-lethal responses to carbofuran. PLoS ONE. 2013;8:e81077. doi: 10.1371/journal.pone.0081077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nagato ED, Simpson AJ, Simpson MJ. Metabolomics reveals energetic impairments in Daphnia magna exposed to diazinon, malathion and bisphenol-A. Aquat. Toxicol. 2016;170:175–186. doi: 10.1016/j.aquatox.2015.11.023. [DOI] [PubMed] [Google Scholar]
- 36.Niu W, Knight E, Xia Q, McGarvey BD. Comparative evaluation of eight software programs for alignment of gas chromatography-mass spectrometry chromatograms in metabolomics experiment. J. Chromatogr. A. 2014;1374:199–206. doi: 10.1016/j.chroma.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 37.O'Donoghue P, Luthey-Schulten Z. On the evolution of structure in aminoacyltRNA synthetases. Micro molecular. Biol. Rev. 2003;67:550–573. doi: 10.1128/MMBR.67.4.550-573.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pérez-Iglesias JM, Franco-Belussi L, Morenom L, Tripole S, Oliveira C, Natale GS. Effects of glyphosate on hepatic tissue evaluating melanomacrophages and erythrocytes responses in neotropical anuran Leptodactylus latinasus. Environ. Sci. Pollut. Res. 2016;23:9852–9861. doi: 10.1007/s11356-016-6153-z. [DOI] [PubMed] [Google Scholar]
- 39.Pisa LW, Amaral-Rogers V, Belzunces LP, Bonmatin JM, Downs CA, Goulson D, Kreutzweiser DP, Krupke C, Liess M, McField M, Morrissey CA, Noone DA, Settele J, Simon-Delso N, Stark JD, Van der Sluijs JP, Van Dyck J, Wiemers M. Effects of neonicotinoids and fipronil on non-target invertebrates. Environ. Sci. Pollut. Res. 2015;22(2015):68. doi: 10.1007/s11356-014-3471-x. https://doi.org/10.1007/s11356-014-3471-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quaranta A, Bellantuono V, Cassano G, Lippe C. Why amphibians are more sensitive than mammals to xenobiotics. PLoS One. 2009;4:e7699. doi: 10.1371/journal.pone.0007699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ralston-Hooper KJ, Adamec J, Jannash A, Mollenhaure R, Ochoa-Acuúúa H, Sepúlveda MS. Use of GC x GC/TOF-MS and LC/TOF-MS for metabolomic analyses of Hyalella azteca chronically exposed to atrazine and its primary metabolite, desethylatrazine. J. Appl. Toxicol. 2010;31:399–410. doi: 10.1002/jat.1587. [DOI] [PubMed] [Google Scholar]
- 42.Reynaud S, Worms IAM, Veyrenc S, Portier J, Maitre A, Miaud C, Raveton M. Toxicokinetic of benzo[a]pyrene and fipronil in female green frogs (Pelphylax kl. esculentus) Environ. Pollut. 2012;161:206–214. doi: 10.1016/j.envpol.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 43.Singh M, Sandhir R, Kiran R. Effects of antioxidant status of liver following atrazine exposure and its attenuation by vitamin E. Exp. Toxicol. Pathol. 2011;63:269–276. doi: 10.1016/j.etp.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 44.Smalling KL, Fellers GM, Kleeman PM, Kuivila KM. Accumulation of pesticide in Pacific chorus frogs (Pseudacris regilla) from California's Sierra Nevada Mountains, USA. Environ. Toxicol. Chem. 2013;32(9):2026–2034. doi: 10.1002/etc.2308. [DOI] [PubMed] [Google Scholar]
- 45.Smalling KL, Reeves R, Muths E, Andover M, Battaglin WA, Helladic ML, Pierre CL. Pesticide concentrations in frog tissue and wetland habitats in a landscape dominated by agriculture. Sci. Total Environ. 2015;502:80–90. doi: 10.1016/j.scitotenv.2014.08.114. [DOI] [PubMed] [Google Scholar]
- 46.Southam AD, Lange A, Hines A, Hill EM, Katsu Y, Iguchi T, Tyler CR, Viant MR. Metabolomics reveals target and off-target toxicities of a model organophosphate pesticide to roach (Rutilus rutilus): implications for biomonitoring. Environ. Sci. Technol. 2011;45:3759–3767. doi: 10.1021/es103814d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sparks TC, Nauen R. IRAC: mode of action classification and insecticide resistance management. Pestic. Biochem. Physiol. 2015;121:122–128. doi: 10.1016/j.pestbp.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 48.Storrs-Mendez SI, Tillitt DE, Rittenhouse TAG, Semlitsch RD. Behavioral response and kinetics of terrestrial atrazine exposure in American toads (Bufo americanus) Arch. Environ. Contam. Toxicol. 2009;57:590–597. doi: 10.1007/s00244-009-9292-0. [DOI] [PubMed] [Google Scholar]
- 49.Strong RJ, Halsall CJ, Ferenčík M, Jones KC, Shore RF, Martin FL. Biospectroscopy reveals the effect of varying water quality on tadpole tissues of the common frog (Rana temporaria) Environ. Pollut. 2016;213:3220337. doi: 10.1016/j.envpol.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 50.Suva ML, Riggi N, Bernstein BE. Epigenetic reprogramming in cancer. Science. 2013;339:1567–1570. doi: 10.1126/science.1230184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.USEPA. Concepts, methods and data sources for cumulative health risk assessment of multiple chemicals, exposures and effects: a resource document. U.S. Environmental Protection Agency, Office of Research and Development, National Center for Environmental Assessment; Cincinnati (OH): 2007. (Report EPA/600/R-06/014F) [Google Scholar]
- 52.USEPA. [Accessed date: 1 July 2017];Pesticide product and label system. 2017 https://iaspub.epa.gov/apex/pesticides/f?p=PPLS:1.
- 53.Van Meter RJ, Glinski DA, Hong T, Cyterski M, Henderson WM, Purucker ST. Estimating terrestrial amphibian pesticide body burden through dermal exposure. Environ. Pollut. 2014;193:262–268. doi: 10.1016/j.envpol.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 54.Van Meter RJ, Glinski DA, Henderson WM, Purucker ST. Pesticide uptake across the amphibian dermis through soil and overspray exposures. Arch. Environ. Contam. Toxicol. 2015;69:545–556. doi: 10.1007/s00244-015-0183-2. [DOI] [PubMed] [Google Scholar]
- 55.Van Meter RJ, Glinski DA, Henderson WM, Purucker ST. Soil organic matter content effects of dermal pesticide bioconcentration in American toads (Bufo americanus) Environ. Toxicol. Chem. 2016;11:2734–2741. doi: 10.1002/etc.3439. [DOI] [PubMed] [Google Scholar]
- 56.Van Meter RJ, Glinski DA, Henderson WM, Purucker ST. R Wrapper Code for This Paper. Zenodo. 2017 https://doi.org/10.5281/zenodo.1049111.
- 57.Venturino A, de D'Angelo AMP. Biochemical targets of xenobiotics: biomarkers in amphibian ecotoxicology. Appl. Herpetol. 2005;2:335–353. [Google Scholar]
- 58.Venturino A, Rosenbaum E, de Castro AC, Anguiano OL, Gauna L, de Shroeder TF, de D'Angelo AMP. Biomarkers of effect in toads and frogs. Biomarkers. 2003;8:167–186. doi: 10.1080/1354700031000120116. [DOI] [PubMed] [Google Scholar]
- 59.Viant MR. Revealing the metabolome of animal tissues using 1 H nuclear magnetic resonance spectroscopy. Methods Mol. Biol. 2007:229–246. doi: 10.1007/978-1-59745-244-1_13. [DOI] [PubMed] [Google Scholar]
- 60.Viant MR. Environmental metabolomics using 1 H-NMR spectroscopy. Methods Mol. Biol. 2008;410:137–150. doi: 10.1007/978-1-59745-548-0_9. [DOI] [PubMed] [Google Scholar]
- Wagner ND, Simpson AJ, Simpson MJ. Metabolomic responses to sublethal contaminant exposure in neonate and adult Daphnia magna. Environ. Toxicol. Chem. 2016 doi: 10.1002/etc.3604. https://doi.org/10.1002/etc.3604. [DOI] [PubMed]
- 61.Wang P, Wang H, Xu M, Lian Y, Sun Y, Yan L, Li L, Li W, Wu Y. Combined subchronic toxicity of dichlorvos with malathion or pirimicarb in mice liver and serum: a metabonomic study. Food Chem. Toxicol. 2014;70:222–230. doi: 10.1016/j.fct.2014.05.027. [DOI] [PubMed] [Google Scholar]
- 62.Xia J, Wishart DS. Using MetaboAnalyst 3.0 for comprehensive metabolomics data analysis. Curr. Protoc. Bioinformatics. 2016;55:1–14. doi: 10.1002/cpbi.11. [DOI] [PubMed] [Google Scholar]
- 63.Xu H, Wang J, Li M, Liu Y, Chen T, Jia A. H NMR based metabolomics approach to study the toxic effects of herbicide butachlor on goldfish (Carassius auratus) Aquat. Toxicol. 2015;159:69–80. doi: 10.1016/j.aquatox.2014.11.020. [DOI] [PubMed] [Google Scholar]
- 64.Yuk J, Simpson MJ, Simpson AJ. 1-D and 2-D NMR metabolomics of earthworm responses to sub-lethal trifluralin and endosulfan exposure. Environ. Chem. 2011;8:281–294. [Google Scholar]
- 65.Zaya RM, Amini Z, Whitaker AS, Ide CF. Exposure to atrazine affects the expression of key genes in metabolic pathways integral to energy homeostasis in Xenopus laevis tadpoles. Aquat. Toxicol. 2011;104:254–262. doi: 10.1016/j.aquatox.2011.04.022. [DOI] [PubMed] [Google Scholar]