Abstract
The lysosome degrades and recycles macromolecules, signals to the master growth regulator mTORC1, and is associated with human disease. Here, we performed quantitative proteomic analyses of lysosomes rapidly isolated using the LysoIP method and find that nutrient levels and mTOR dynamically modulate the lysosomal proteome. We focus on NUFIP1, a protein that upon mTORC1 inhibition redistributes from the nucleus to autophagosomes and lysosomes. Upon these conditions, NUFIP1 interacts with ribosomes and delivers them to autophagosomes by directly binding to LC3B. The starvation-induced degradation of ribosomes via autophagy (ribophagy) depends on the capacity of NUFIP1 to bind LC3B and promotes cell survival. We propose that NUFIP1 is a receptor for the selective autophagy of ribosomes.
The capacity of lysosomes to degrade macromolecules is necessary for cells to clear damaged components and to recycle nutrients for maintaining homeostasis upon starvation. In the context of disease, lysosomes are best known for their dysfunction in the rare lysosomal storage diseases, but also play roles in neurodegeneration and cancer as well as the aging process (reviewed in (1–3)). Over the last decade it has become apparent that mTOR Complex 1 (mTORC1), the major nutrient-sensitive regulator of growth (mass accumulation), has an intimate relationship with lysosomes (reviewed in (4)). Most components of the nutrient sensing machinery upstream of mTORC1 localize to the lysosomal surface, and nutrients generated by lysosomes regulate mTORC1 by promoting its translocation there, a key step in its activation. In turn, mTORC1 regulates the flux of macromolecules destined for lysosomal degradation by controlling autophagosome formation as well as lysosomal biogenesis through the TFEB transcription factor (4).
Using our recently developed LysoIP method to rapidly isolate highly pure lysosomes (5) we profiled the dynamics of the lysosomal proteome under conditions that inhibit mTORC1 signaling. We identify NUFIP1, a protein not previously associated with lysosomes, as necessary for the starvation-induced degradation of ribosomes and show that it fulfills multiple criteria for being an autophagy receptor for ribosomes.
mTORC1 and nutrients regulate the lysosomal proteome
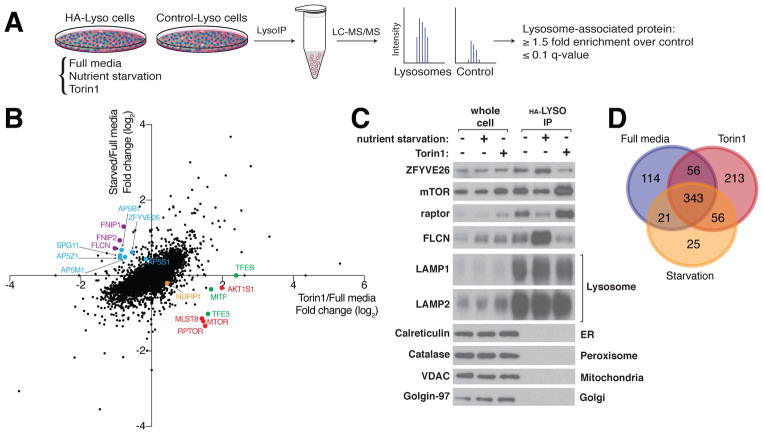
To define the mTORC1-regulated lysosomal proteome, we used high-resolution quantitative proteomics to analyze lysosomes isolated from HEK-293T cells cultured in nutrient-replete media (full media), starved of nutrients (amino acids and glucose), or treated with the mTOR inhibitor Torin1 for one hour (Fig. 1A and S1A). Nutrient starvation and Torin1 both inhibit mTORC1 signaling (Fig. S1A) but will have distinct effects because Torin1 inhibits mTORC1 more strongly than nutrient deprivation and there are mTORC1-independent mechanisms for sensing nutrients. Nutrient starvation and Torin1 did not impact the abundance of most proteins associated with the purified lysosomes, but even a cursory view of the datasets revealed many proteins affected by one or both treatments (Fig. 1B). Gratifyingly, these included proteins with established nutrient- and Torin1-sensitive associations with lysosomes (6–11), including components of mTORC1 (mTOR, Raptor, and mLST8) and the Folliculin complex (FLCN, FNIP1, and FNIP2) (Fig. 1B and C) as well as the TFEB, MITF, and TFE3 transcription factors (Fig. 1B). Proteins previously connected to lysosomes but not known to have regulated associations with them were also identified. For example, Torin1 decreased and amino acid starvation increased the lysosomal abundance of SPG11 and ZFYVE26 (Fig. 1B and 1C and Table S1), which are associated with Hereditary Spastic Paraplegia and interact with each other as well as the Adaptor-5 complex (12, 13), whose components (AP5B1, AP5M1, AP5S1 and AP5Z1) behaved similarly in the datasets (Table S1) (13).
Fig. 1. Regulation of the lysosomal proteome in response to nutrient starvation and mTOR inhibition.
(A) Schematic depicting the workflow for the LysoIP-proteomics method. HA-Lyso and Control-Lyso cells refer to cells stably expressing 3xHA-tagged TMEM192 or 2xFlag-tagged TMEM192, respectively.
(B) Nutrient starvation and mTOR inhibition regulate the lysosomal proteome. The scatter plot shows relative (fold) changes in protein abundances in lysosomes captured from the HA-Lyso cells starved for one hour of nutrients (amino acids and glucose) or treated for one hour with 250 nM Torin1 versus lysosomes from cells cultured in nutrient-replete media (full media). For each condition, three independent isolations were compared. Colors indicate proteins mentioned in the text. The dots denote the 5339 unique proteins detected amongst all the pre-filtered samples. The majority of these are in the immunoprecipitates from both the HA-Lyso and Control-Lyso cells. TFEB was detected only in the Torin1/Full media comparison and assigned an arbitrary log2 fold change of 0 in the Starved/Full media comparison.
(C) Validation of changes observed in the lysosomal abundances of the some of the proteins highlighted in Figure 1B. The immunoblot shows analyses for indicated proteins in whole cell lysates or lysosomes purified from HEK-293T cells subjected to the indicated treatments for 1 hour. ER, endoplasmic reticulum.
(D) Venn diagram representation of the number of proteins defined as lysosomal in each of the three conditions. A protein was deemed lysosomal if it had a significant (q ≤0.1) enrichment value of 1.5 fold (> 0.58, log2). Proteins not detected at all on the control beads were also classified as lysosomal.
Although these examples hinted at the utility of the proteomics data for discovery, we needed a way to a priori designate a protein as associated with lysosomes upon mTOR inhibition and/or nutrient deprivation as the set of such proteins has not been previously defined. To do so we generated a control dataset of proteins that bind non-specifically to magnetic beads coated with antibody to hemagglutinin (HA), used in the immune-isolation of the lysosomes. We defined as “lysosomal” any protein that was more abundant in purified lysosomes than on the control beads by a factor of at least 1.5 (at a significance value of q<0.1; Fig. 1A and 1D). We arrived at this value using a sliding-window method to identify a relative change that captured a protein set significantly enriched for those annotated as lysosomal in the UniProt database (Fig. S1B). This approach yielded a total of 828 unique proteins as associated with lysosomes in any of the three experimental conditions (Fig. 1D), with 343 proteins designated as lysosomal under all conditions (Fig. 1D and Table S2).
NUFIP1-ZNHIT3 accumulates on autophagosomes and lysosomes upon mTORC1 inhibition
Of the many proteins whose lysosomal abundance increased upon Torin1 treatment (Tables S1), Nuclear fragile X mental retardation-interacting protein 1 (NUFIP1) piqued our interest because previous work indicates that while NUFIP1 is largely a nuclear protein it has also been observed in the cytoplasm of some cell types (14). NUFIP1 forms a heterodimer with a smaller protein, Zinc finger HIT domain-containing protein 3 (ZNHIT3) (15, 16), whose lysosomal abundance also increased upon Torin1 treatment (Tables S1 and S2), albeit to a lesser extent. Similar to the behavior of many constitutively interacting proteins, CRISPR-Cas9-mediated loss of NUFIP1 caused the concomitant loss of ZNHIT3 (Fig. S2A).
Previous work implicates NUFIP1-ZNHIT3 in the assembly of the box C/D snoRNP (17–19). Consistent with such a role, NUFIP1 not only co-immunoprecipitated ZNHIT3 but also snoRNP core components such as Fibrillarin (FBL), NOP58, SNU13/15.5K, and NOP17/PIH1D1 (Fig. S2B). In addition, its loss caused modest reductions in the interaction of FBL with NOP58, and SNU13/15.5K as well as the expression of small nucleolar RNAs (snoRNAs) of the box C/D (U3 and U14), but not H/ACA (U19) or U4, class (Fig. S2, C and D). Although these results confirm a role for NUFIP1 in the function of the box C/D snoRNP, it was intriguing that none of its core components were in our proteomics data, suggesting that NUFIP1-ZNHIT3 might have a previously unappreciated role involving lysosomes.
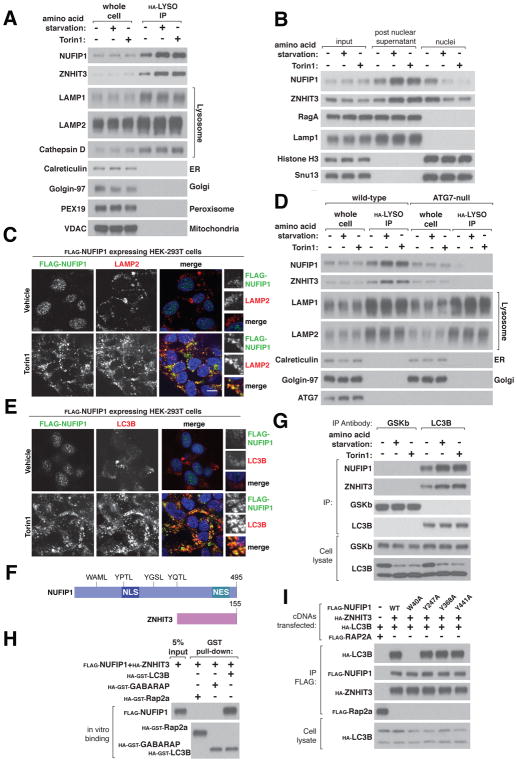
Acute inhibition of mTORC1 in HEK-293T cells, by either via Torin1 treatment or amino acid starvation, strongly increased the lysosomal abundance of NUFIP1 and ZNHIT3 (Fig. 2A). mTORC1 inhibition did not change the total cellular amount of NUFIP1 or ZNHIT3, so we reasoned that it must affect their subcellular localization. Indeed, upon Torin1 treatment and amino acid starvation NUFIP1-ZNHIT3, but not SNU13/15.5K, redistributed from the nuclear fraction to the post-nuclear supernatant (PNS) that contains lysosomes (Fig. 2B). Imaging studies using HEK-293T cells stably expressing FLAG-NUFIP1 confirmed that Torin1 caused NUFIP1 to translocate from the nucleus to LAMP2-positive lysosomes (Fig. 2C). Consistent with this shift in localization, Torin1 reduced the amount of NUFIP1-ZNHIT3 that co-immunuprecipitated with FBL, a C/D snoRNP component, without impacting the FBL-SNU13/15.5K interaction (Fig. S2E). Thus, mTOR inhibition promotes the lysosomal accumulation of NUFIP1-ZNHIT3 at the expense of its interaction with the nuclear C/D snoRNP.
Fig. 2. Upon starvation NUFIP1-ZNHIT3 accumulates at lysosomes in an autophagosome-dependent manner.
(A) NUFIP1-ZNHIT3 accumulates at lysosomes upon mTORC1 inhibition. Lysates and immunoprecipitates were prepared from HEK-293T cells cultured in full media, or deprived of amino acids or treated with 250 nM Torin1 for 1 hour as described in the supplementary materials.
(B) Upon mTORC1 inhibition NUFIP1-ZNHIT3 shifts from the nuclear fraction to the post-nuclear supernatant that contains lysosomes. HEK-293T cells were fractionated after being deprived of amino acids or treated with 250 nM Torin1 for 1 hour and the amounts of endogenous NUFIP1 and ZNHIT3 analyzed by immunoblotting. RagA and LAMP1 are lysosome-associated proteins; histone H3 and SNU13 are nuclear.
(C) mTOR inhibition shifts NUFIP1 from the nucleus to LAMP1-positive lysosomes. HEK-293T cells stably expressing FLAG-NUFIP1 were treated with 250 nM Torin1 for 1 hour and analyzed as described in the supplementary materials. Scale bar, 10 μm.
(D) Loss of ATG7 greatly decreases the amount of NUFIP1-ZNHIT3 on lysosomes. Wild-type and ATG7-null HEK-293T cells stably expressing the HA-Lyso tag were deprived of amino acids or treated with 250 nM Torin1 for 1 hour and the amounts of NUFIP1 and ZNHIT3 on lysosomes and in total cell lysates were analyzed as in (A).
(E) mTOR inhibition shifts NUFIP1 from the nucleus to LC3B-positive puncta. HEK-293T cells stably expressing FLAG-NUFIP1 were treated with 250 nM Torin1 for 1 hour and processed as in (C).
(F) Schematic depicting the localization of the four putative LC3B-binding regions (LIRs) in NUFIP1.
(G) mTORC1 inhibition increases the interaction between endogenous LC3B and NUFIP1-ZNHIT3. Anti-LC3B immunoprecipitates were prepared from HEK-293T cells deprived of amino acids or treated with 250 nM Torin1 for 1 hour and lysates and immunoprecipitates were analyzed for the indicated proteins. Immunoprecipitates prepared with an antibody to GSKb were used as negative controls.
(H) NUFIP1-ZNHIT3 interacts with LC3B in vitro. Purified HA-GST-LC3B immobilized on a glutathione affinity resin was incubated with the purified FLAG-NUFIP1-HA-ZNHIT3 complex. HA-GST-Rap2a and HA-GST-GABARAP were used as negative controls. Proteins captured in the glutathione resin pull-down were analyzed by immunoblotting for the indicated proteins using anti-epitope tag antibodies. GST, glutathione S-transferase.
(I) Identification of a NUFIP1 mutant that does not bind LC3B. Wild-type (WT) FLAG-NUFIP1 or a series of point mutants in its putative LIR motifs were co-expressed with HA-ZNHIT3 and HA-LC3B. HA-Rap2A was used as a negative control. FLAG-immunoprecipitates and lysates were prepared and analyzed by immunoblotting.
Because mTORC1 inhibition strongly induces autophagy, we hypothesized that NUFIP1-ZNHIT3 may travel to lysosomes through an association with incipient autophagosomes. Indeed, NUFIP1 and ZNHIT3 were absent from lysosomes isolated from cells lacking the ATG7 protein (Fig. 2D), which is necessary for the formation of autophagosomes (20), and in Torin1 treated cells FLAG-NUFIP1 co-localized with LC3B-positive autophagosomes (Fig. 2E).
NUFIP1-ZNHIT3 interacts with LC3B
Sequence analyses predict that NUFIP1, but not ZNHIT3, has four potential LC3B-interacting regions (LIR) (Fig.2F). LIRs are found in autophagy receptors that physically link their cargo to the autophagosomal membrane through an LC3B/ATG8-binding domain that contains the Trp/Phe-X-X-Leu/Ile/Val sequence motif (reviewed in (21)). Consistent with the presence of LIRs in NUFIP1, endogenous LC3B co-immunoprecipitated endogenous NUFIP1 and ZNHIT3 from detergent lysates of HEK-293T cells cultured in full media and, to a much greater extent, in cells treated with Torin1 or starved of amino acids (Fig. 2G). Even when overexpressed, ZNHIT3 did not co-immunoprecipitate with LC3B in cells lacking NUFIP1 (Fig. S3A). In vitro, purified NUFIP1-ZNHIT3 bound to purified LC3B but not to GABARAP, another autophagosome-associated protein, or to the Rap2A control protein (Fig. 2H).
We sought a NUFIP1 mutant that dissociates its function in the nuclear C/D snoRNP from its capacity to bind LC3B. We generated NUFIP1 mutants with point mutations in each of the four potential LIRs and identified one, W40A NUFIP1, which no longer interacts with LC3B (Fig. 2I). When expressed in NUFIP1-null cells, neither NUFIP1 W40A nor ZNHIT3 associated with lysosomes, whether or not mTORC1 was inhibited (Fig. S3B). The W40A NUFIP1 mutant was indistinguishable from the wild-type protein in its capacity to co-immunoprecipitate FBL and reverse the modest reductions in U3 and U14 snoRNA expression caused by NUFIP1 loss (Fig. S3,C–D). Thus, NUFIP1 interacts with LC3B and the W40A mutant distinguishes between the role of NUFIP1 in C/D snoRNP function and its capacity to bind LC3B and localize to lysosomes.
NUFIP1-ZNHIT3 associates with ribosomes in a nutrient-dependent manner
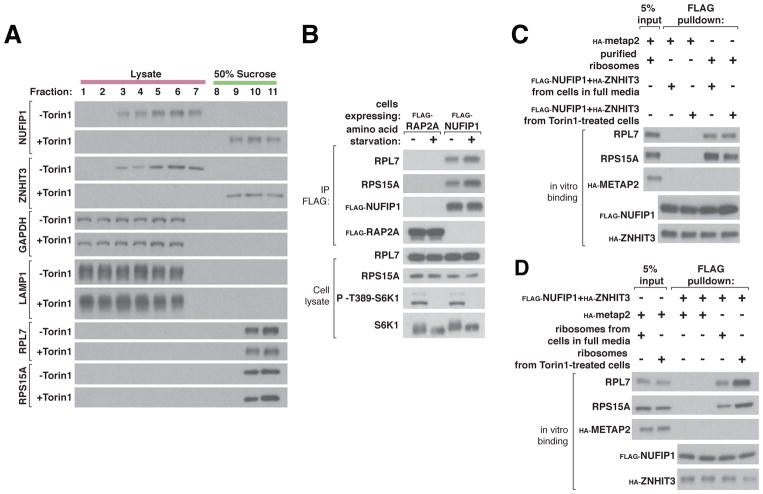
Because NUFIP1 binds to LC3B we hypothesized that it might serve as a selective autophagy receptor for an unknown cargo in the cytoplasm. Given its role in modifying ribosomal RNA and its reported co-localization with ribosomes in some cell types (14), we considered the possibility that NUFIP1-ZNHIT3 can associate with ribosomes. We began by fractionating cell lysates to determine the amount of NUFIP1-ZNHIT3 that co-migrates with ribosomes pelleted through a 50% sucrose cushion. In lysates from control cells, NUFIP1-ZNHIT3 did not enter the sucrose cushion, but in those from cells treated with Torin1 or deprived of amino acids both proteins shifted markedly to the ribosome-containing pellet (Fig. 3A and Fig. S4A). Consistent with this finding, amino acid starvation increased the amount of ribosomes, as monitored via small (40S) and large (60S) ribosomal subunit proteins, that co-immunoprecipitated with NUFIP1 (Fig. 3B). To probe which ribosomal subunit NUFIP1-ZNHIT3 might associate with, we took advantage of the capacity of EDTA to dissociate ribosomes into their 40S and 60S subunits. When lysates of amino acid starved cells were fractionated through a 10 to 45% sucrose gradient, endogenous NUFIP1 and ZNHIT3 co-migrated with monosomes (80S) and polysomes. The addition of EDTA to the same lysates increased the amount of NUFIP1-ZNHIT3 that migrated with the large ribosomal subunits (Fig. S4B). Collectively, these results suggest that upon mTORC1 inhibition NUFIP1 binds to LC3B and associates with the ribosome, likely through its large subunit.
Fig. 3. NUFIP1-ZNHIT3 interacts with ribosomes in an mTORC1-dependent fashion.
(A) mTOR inhibition increases the amount of NUFIP1-ZNHIT3 that co-migrates with ribosomes. HEK-293T cell lysates prepared from cells in full media or treated with 250 nM Torin1 were fractionated over a 50% sucrose cushion. Fractions were collected and the indicated proteins analyzed by immunoblotting.
(B) Amino acid deprivation increases the amount of ribosomes that co-immunoprecipitates with NUFIP1. HEK-293T cells stably expressing FLAG-NUFIP1 were deprived of amino acid for 1 hour. Lysates and FLAG immunoprecipitates were prepared and analyzed for the indicated proteins by immunoblotting. FLAG-Rap2A was used as a negative control.
(C) In vitro, purified NUFIP1-ZNHIT3 binds to ribosomes and the interaction is not affected by whether or not NUFIP1-ZNHIT3 was obtained from cells with inhibited mTOR. The FLAG-NUFIP1-HA-ZNHIT3 complex was purified from HEK-293T cells in full media or treated with 250 nM Torin1 for 1 hour and immobilized on a FLAG affinity resin. Equal amounts of ribosomes obtained from cells in full media were added to the immobilized FLAG-NUFIP1-HA-ZNHIT3 complex and the proteins captured analyzed by immunoblotting. Ribosomes were purified as described in the supplementary materials. Purified HA-METAP was used as a negative control.
(D) In vitro, ribosomes purified from cells with mTOR inhibition bind better to NUFIP1-ZNHIT3 than those from cells in full media. Ribosomes were purified from HEK-293T cells in full media conditions or treated with 250 nM Torin1 for 1 hour. The FLAG-NUFIP1-HA-ZNHIT3 complex was immobilized on FLAG affinity beads and equal amounts of ribosomes were added. Proteins captured by the FLAG affinity beads were analyzed by immunoblotting. HA-METAP2 served as a negative control.
Given that mTORC1 inhibition increases the interaction of NUFIP1-ZNHIT3 with ribosomes, we considered the possibility that an mTORC1-dependent modification of either NUFIP1-ZNHIT3 or ribosomes regulates the interaction. To test this, we purified ribosomes or NUFIP1-ZNHIT3 from cells cultured in full media or treated with Torin1 and examined their capacity to interact with each other in vitro. Interestingly, only ribosomes from Torin1-treated cells bound strongly to NUFIP1-ZNHIT3, while the source of NUFIP1-ZNHIT3 did not impact the strength of the interaction (Fig. 3,C and D). These results suggest that mTOR inhibition leads to a stable alteration of ribosomes that promotes their interaction with NUFIP1-ZNHIT3. In contrast, the in vitro interaction of NUFIP1-ZNHIT3 with LC3B was unaffected by the source of either (i.e., control or Torin1-treated cells) (Fig. S4C). Taken together, these data suggest that the loss of nuclear NUFIP1 and the increase in the LC3B-NUFIP1 interaction caused by mTORC1 inhibition results from the binding and trapping of NUFIP1 by modified ribosomes in the cytoplasm. We cannot exclude the possibility that an mTORC1-dependent modification of NUFIP1 also regulates its nuclear entry or exit, but we have failed to identify mTORC1-regulated phosphorylation sites on NUFIP1.
NUFIP1 is required for ribosomal degradation induced by nutrient starvation
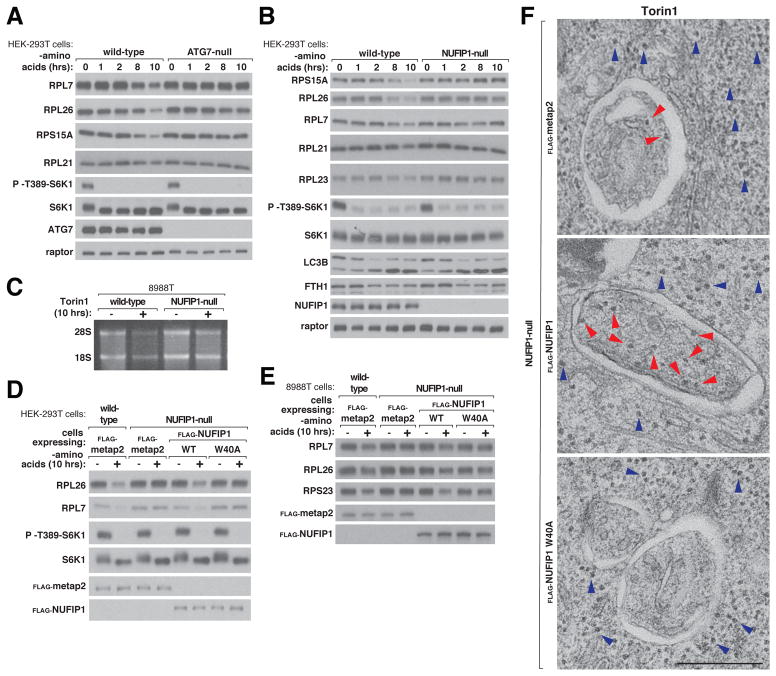
While it is known that the proteasome degrades ribosomal proteins that do not incorporate into ribosomal subunits, which we verified (Fig. S5, A to C), how intact ribosomes are degraded is less understood, particularly in mammalian cells (22–26). Upon amino acid deprivation or Torin1 treatment, ribosomal proteins decreased in a time-dependent manner in a fashion that depended on ATG7 and a low lysosomal pH (Fig. 4A and S6, A and B), in accord with previous work in yeast showing that intact ribosomes are degraded via autophagy (27). In GATOR1 mutant (DEPDC5 KO) cells that have nutrient-insensitive mTORC1 signaling, amino acid starvation did not reduce the abundance of ribosomal proteins while Torin1 still did (Fig. S6C). Thus, mTORC1 likely mediates the loss of ribosomal proteins caused by mTOR inhibition.
Fig. 4. NUFIP1 is required for ribophagy.
(A) ATG7 loss suppresses the degradation of ribosomes caused by amino acid starvation. Wild-type or ATG7-null HEK-293T cells were deprived of amino acids for the indicated time points. Cell lysates were analyzed by immunoblotting for the total levels and phosphorylation states of the indicated proteins.
(B) Loss of NUFIP1 inhibits the degradation of ribosomes caused by amino acid starvation. Wild-type or NUFIP1-null HEK-293T cells were deprived of amino acids for the indicated time points. Cell lysates were analyzed by immunoblotting for the total levels and phosphorylation states of the indicated proteins.
(C) Loss of NUFIP1 inhibits the loss of 28S and 18S rRNA caused by mTOR inhibition. Wild-type and NUFIP1-null 8988T cells were treated with 250 nM Torin1 for 10 hrs and total RNA was extracted and analyzed on a formaldehyde agarose gel. RNA from equal numbers of cells was loaded in each lane.
(D) For amino acid starvation to cause ribosomal degradation, NUFIP1 must be able to interact with LC3B. Wild-type or NUFIP1-null HEK-293T cells stably expressing the indicated proteins were deprived of amino acids for 10 hours and analyzed for the total levels and phosphorylation states of the indicated proteins.
(E) For amino acid starvation to cause ribosomal degradation, NUFIP1 must interact with LC3B. Wild-type or NUFIP1-null 8988T cells stably expressing the indicated proteins were treated as in (B).
(F) Autophagosomes from HEK-293T cells lacking NUFIP1 or expressing the LC3B-binding W40A mutant contain fewer ribosomes than those from control cells. NUFIP1-null HEK-293T cells expressing the control protein metap2, NUFIP1, or NUFIP1 W40A were treated with Torin1 and ConcanamycinA for 4 hours and analyzed by electron microscopy. Autophagosomes were identified by the presence of a double membrane. Red arrows indicate ribosomes inside an autophagosome. Blue arrows indicate ribosomes present in the cytoplasm. Scale bar, 500 nm
Given that NUFIP1 binds LC3B and also makes an mTORC1-regulated association with ribosomes, we hypothesized that NUFIP1 is required for the degradation of ribosomes via autophagy, a process that has been termed ribophagy in yeast (27). Indeed, in multiple cell types, loss of NUFIP1 prevented the depletion of ribosomal proteins caused by nutrient deprivation or Torin1 treatment (Fig. 4B and S6, D to G).
Loss of NUFIP1 had no impact the induction of autophagy, as assessed by LC3B lipidation, nor on the degradation of ferritin, another selective autophagy substrate, whether it was induced by nutrient starvation or iron chelation (Fig. 4B and S6H). NUFIP1 loss also blocked the Torin1-induced depletion of ribosomal RNA (rRNA) (Fig. 4C). Re-expression at levels near that of the endogenous protein of wild-type NUFIP1, but not of the W40A mutant deficient in LC3B binding, restored the capacity of NUFIP1-null HEK-293T and 8988T cells to degrade ribosomes upon nutrient depletion (Fig. 4, D and E and S6I). As assessed by electron microscopy, autophagosomes in HEK-293T cells lacking NUFIP1 or just its LC3B-binding capacity contained many fewer ribosomes than those from control cells (Fig. 4F and S6, J and K). Loss of NUFIP1 did not affect the morphology of the ER, mitochondria, or Golgi (Fig. S6L).
To test the role of the subcellular localization of NUFIP1 in the induction of ribosome degradation, we generated NUFIP1 mutants lacking either a nuclear localization (NLS) or export (NES) signal and expressed them in NUFIP1-null cells. The NLS mutant localized to the cytoplasm even in cells in full media, but this did not cause ribosome loss. The NLS mutant colocalized to a greater extent with LAMP2 upon Torin1 treatment and restored the capacity of the null cells to degrade ribosomes upon amino acid starvation (Fig. S7, A and B). The NES mutant was constitutively nuclear and did not support ribosome degradation even upon nutrient starvation (Fig. S7, B and C). Loss of ATG7 or LC3B did not affect the nuclear exit of wild-type NUFIP1 (Fig. S7, D and E). Thus, in cells in full media, the presence of NUFIP1 in the cytoplasm is not sufficient to induce ribosome degradation, presumably because mTORC1 inhibition is still needed to promote the interaction of NUFIP1 with ribosomes.
NUFIP1 is important for cells to survive starvation
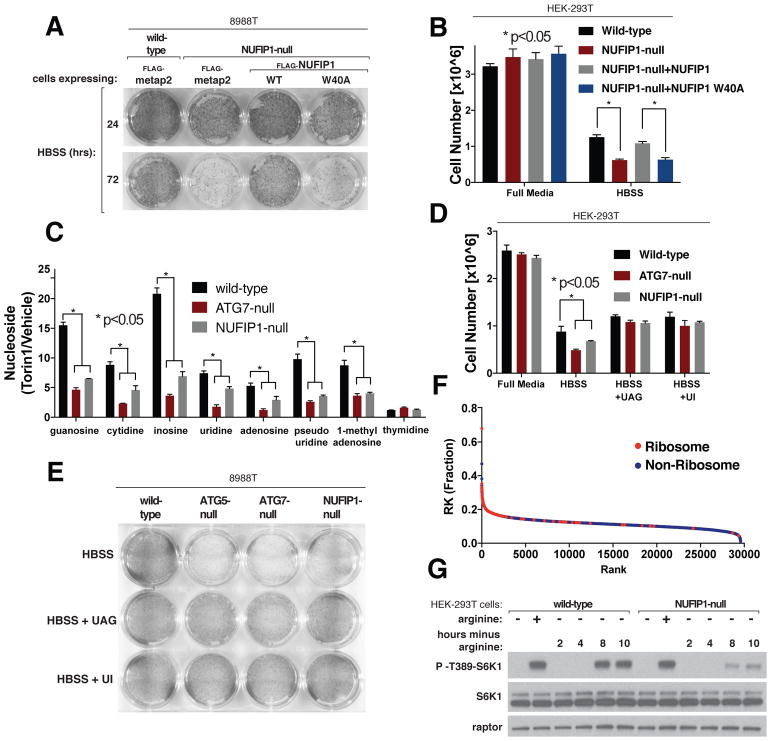
Because NUFIP1 is required for starvation-induced ribophagy and ribosomes constitute a major fraction of the total cell mass (28–30), we asked whether NUFIP1 is important for the cellular response to nutrient deprivation. Indeed, loss of NUFIP1 or just its LC3B-binding ability reduced the capacity of multiple cell types to survive nutrient starvation, as measured in clonogenic survival assays or via direct cell counting (Fig. 5, A and B and Fig. S8, A to C). These results suggest that the NUFIP1-mediated degradation of ribosomes supplies metabolites needed for survival during starvation. Consistent with this possibility, loss of either ATG7 or NUFIP1 suppressed the large increase in nucleoside levels (including inosine, which is generated by the deamination of adenosine in lysosomes (31)), caused by mTOR inhibition that we recently described (5), which suggests that most of this increase results from the lysosomal degradation of rRNA (Fig. 5C) (32). The addition of nucleosides to the starvation media rescued the survival defect of the NUFIP1-null cells, and, as previously shown (33), of cells lacking the canonical autophagy pathway (Fig. 5D and E and S8D).
Fig. 5. NUFIP1 is important for cells to survive starvation.
(A) Loss of NUFIP1 or just its capacity to interact with LC3B impairs cell survival upon nutrient starvation. Wild-type or NUFIP1-null 8988T cells stably expressing the indicated proteins were deprived of nutrients by culturing them in Hank’s Balanced Salt Solution (HBSS); after the indicated times the surviving cells were stained and imaged.
(B) Loss of NUFIP1 or just its capacity to interact with LC3B impairs cell survival upon nutrient starvation. Wild-type or NUFIP1-null HEK-293T cells stably expressing the indicated proteins were deprived of nutrients by HBSS, and after 48 hours the number of surviving cells was quantified using cell counting. Values are normalized relative to cell numbers at the start of the starvation period and are mean +/− SD (*P<0.05; n=3).
(C) Loss of NUFIP1 or ATG7 inhibits the increase in nucleosides caused by mTOR inhibition. Data represent relative change in whole-cell concentrations of nucleosides in wild-type, ATG7-null, and NUFIP1-null HEK-293T cells treated with 250 nM Torin1 for 1 hour. Values are mean −/+ SEM (*P<0.05; n=3)
(D) Nucleoside supplementation rescues the survival defects of ATG7-null and NUFIP1-null HEK-293T cells. Indicated cells were deprived of nutrients by culturing in HBSS with or without the indicated nucleosides (2 mM each). After 48 hours, the number of surviving cells was quantified. Values were normalized relative to cell numbers at the start of the starvation period and are mean +/− SD (*P<0.05; n=3).
(E) Nucleoside supplementation rescues survival defect of ATG5-null, ATG7-null, and NUFIP1-null 8988T cells. Wild-type, ATG5-null, ATG7-null, or NUFIP1-null cells were deprived of nutrients by culturing in HBSS with or without the indicated nucleosides (2 mM each). After 48 hours the surviving cells were stained and imaged.
(F) Ribosomes are highly enriched for arginine and lysine. Protein sequences in the UniProt database (including isoforms) were ranked based on their fraction content of arginine and lysine. Ribosomal proteins are shown in red; all other proteins are shown in blue. Mitochondrial ribosomal proteins were not designated as ribosomal in this analysis.
(G) Loss of NUFIP1 suppresses the reactivation of mTORC1 that occurs after long-term arginine deprivation. Wild-type or NUFIP1 HEK-293T cells were deprived of arginine for 50 mins (first lanes of each set) or the indicated times and, where indicated, re-stimulated with arginine for 10 mins. Cell lysates were analyzed by immunoblotting for the levels and phosphorylation states of the indicated proteins.
Starvation of single amino acids acutely inhibits mTORC1 signaling, but over time it reactivates because of the release of endogenous amino acids by the autophagic degradation of proteins. Analysis of the human proteome annotated in the UniProt database (which includes isoforms) revealed that ribosomal proteins are amongst the most highly enriched for arginine and lysine (Fig. 5F). Because mTORC1 senses lysosomal arginine (and likely lysine) through SLC38A9 (34), ribophagy might be important for generating the amino acids necessary for mTORC1 re-activation. Indeed, loss of NUFIP1 severely diminished the reactivation of mTORC1 normally observed after long-term arginine deprivation (Fig. 5G). Thus, NUFIP1-mediated ribophagy contributes significantly to the cellular response to nutrient starvation.
Conclusions
NUFIP1 has several properties suggesting it functions as an autophagy receptor for ribosomes during starvation-induced ribophagy: (i) it is required for nutrient deprivation to degrade ribosomes, (ii) it binds LC3B and ribosomes, and (iii) a NUFIP1 mutant that does not bind LC3B cannot support ribosomal degradation upon autophagy induction. We propose that NUFIP1 cycles in and out of the nucleus (14) (Fig. 2C and S7, A and C), and, upon nutrient starvation, accumulates in the cytoplasm because it binds to ribosomes that acquire an mTORC1-regulated alteration. In the cytoplasm, NUFIP1 transports its ribosome cargo to autophagic vesicles by directly binding LC3B in a fashion that is likely not directly regulated by nutrients and mTORC1. Our NUFIP1 findings suggest that our dataset of lysosomal proteins can serve as a resource for future discoveries.
Many questions remain. Our in vitro data indicate that the ribosome is likely altered upon mTORC1 inhibition in a fashion that strengthens its interaction with NUFIP1-ZNHIT3, but the nature of this alteration—whether a post-translational modification, such as the addition of ubiquitin, or the binding of a protein to the ribosome—is unknown. As NUFIP1-ZNHIT3 most likely interacts with the 60S ribosomal subunit and the atomic structure of the human ribosome is available (35), it should be possible to determine where NUFIP1-ZNHIT3 interacts. Note that that NUFIP1-ZNHIT3 may not bind directly to an established ribosomal protein or the rRNA; other currently unknown proteins may be involved in mediating the interaction.
Our identification of NUFIP1 as an autophagy receptor for ribosomes in mammalian cells adds to the growing list of selective autophagy receptors, including for ferritin, mitochondria, peroxisomes, endoplasmic reticulum, and bacteria (36–43). We find that upon ribophagy is an important source of nutrients (particularly nucleosides) upon starvation, and that loss of NUFIP1 decreases the survival of cells under low nutrient conditions. As RNA-binding proteins, most ribosomal proteins have a high content of basic amino acids, and we find that NUFIP1 is required for the reactivation of mTORC1 that occurs after prolonged arginine starvation. This result suggests that a key role for SLC38A9, which senses lysosomal basic amino acids upstream of mTORC1 (34), is to signal the successful degradation of ribosomes in lysosomes to mTORC1.
In the human and mouse cells we have examined, loss of NUFIP1 prevents starvation-induced ribosome degradation but it is possible that other ribosome receptors or mechanisms for ribosome degradation also exist. For example, recent work suggests that ribosomes can be degraded via bulk autophagy at longer starvation times (24 hours) than those we have examined (44). In yeast, it has been proposed that the ubiquitin protease Ubp3p/Bre5p is required for the selective degradation of ribosomes, but it is unclear if its homologues play such a role in animals (27). As it is estimated that in growing cells ribosomes account for ~50% and ~80% of total cellular protein and RNA (28–30), respectively. Our work identifies a key link between starvation and one of the most abundant nutrient sources in cells.
Supplementary Material
Acknowledgments
We thank all members of the Sabatini Laboratory for helpful insights, particularly R.L. Wolfson, and the FLI proteomics core facility, in particular J. Kirkpatrick.
Funding: This work was supported by grants from the NIH (R01 CA103866, R01 CA129105, and R37 AI47389) and Department of Defense (W81XWH-15-1-0230) and the Lustgarten Foundation to D.M.S., from the Department of Defense (W81XWH-15-1-0337) to E.F., as well an EMBO Long-Term Fellowship to M.A.-R, Saudi Aramco Ibn Khaldun Fellowship for Saudi Women to N.N.L., and an MIT School of Science Fellowship in Cancer Research to G.A.W.. A.O. and I.H. acknowledge support from the FLI proteomics core facility. The FLI is a member of the Leibniz Association and is financially supported by the Federal Government of Germany and the State of Thuringia. D.M.S. is an investigator of the Howard Hughes Medical Institute.
Footnotes
Author Contributions: G.A.W., M.A-R., and D.M.S. initiated the project and designed the research plan. G.A.W and M.A-R. performed the experiments and analyzed the data with help from E.M.F, N.L., and V.D.. A.O. designed the proteomic runs and analyzed the lysosomal proteomic data. C.A.L. and S.Z.H preformed LC/MS runs and quantified metabolites. G.A.W and M.A-R. wrote the manuscript and D.M.S. edited it. All the authors approved and edited the manuscript.
Competing Interests: D.M.S. is a founding member of the scientific advisory board, a paid consultant, and a shareholder of Navitor Pharmaceuticals, which is targeting for therapeutic benefit the amino acid sensing pathway upstream of mTORC1.
Data and Materials availability: The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org) (50) via the PRIDE partner repository (51) with the dataset identifier PXD009084.
References
- 1.Ballabio A, Gieselmann V. Lysosomal disorders: from storage to cellular damage. Biochimica et biophysica acta. 2009;1793:684–696. doi: 10.1016/j.bbamcr.2008.12.001. published online EpubApr. [DOI] [PubMed] [Google Scholar]
- 2.Platt FM, Boland B, van der Spoel AC. The cell biology of disease: lysosomal storage disorders: the cellular impact of lysosomal dysfunction. The Journal of cell biology. 2012;199:723–734. doi: 10.1083/jcb.201208152. published online EpubNov 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Groth-Pedersen L, Jaattela M. Combating apoptosis and multidrug resistant cancers by targeting lysosomes. Cancer letters. 2013;332:265–274. doi: 10.1016/j.canlet.2010.05.021. published online EpubMay 28. [DOI] [PubMed] [Google Scholar]
- 4.Saxton RA, Sabatini DM. mTOR Signaling in Growth, Metabolism, and Disease. Cell. 2017;168:960–976. doi: 10.1016/j.cell.2017.02.004. published online EpubMar 09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abu-Remaileh M, Wyant GA, Kim C, Laqtom NN, Abbasi M, Chan SH, Freinkman E, Sabatini DM. Lysosomal metabolomics reveals V-ATPase- and mTOR-dependent regulation of amino acid efflux from lysosomes. Science. 2017;358:807–813. doi: 10.1126/science.aan6298. published online EpubNov 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, Sabatini DM. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science. 2008;320:1496–1501. doi: 10.1126/science.1157535. published online EpubJun 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Settembre C, Zoncu R, Medina DL, Vetrini F, Erdin S, Erdin S, Huynh T, Ferron M, Karsenty G, Vellard MC, Facchinetti V, Sabatini DM, Ballabio A. A lysosome-to-nucleus signalling mechanism senses and regulates the lysosome via mTOR and TFEB. The EMBO journal. 2012;31:1095–1108. doi: 10.1038/emboj.2012.32. published online EpubMar 07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roczniak-Ferguson A, Petit CS, Froehlich F, Qian S, Ky J, Angarola B, Walther TC, Ferguson SM. The transcription factor TFEB links mTORC1 signaling to transcriptional control of lysosome homeostasis. Science signaling. 2012;5:ra42. doi: 10.1126/scisignal.2002790. published online EpubJun 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petit CS, Roczniak-Ferguson A, Ferguson SM. Recruitment of folliculin to lysosomes supports the amino acid-dependent activation of Rag GTPases. The Journal of cell biology. 2013;202:1107–1122. doi: 10.1083/jcb.201307084. published online EpubSep 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martina JA, Diab HI, Lishu L, Jeong AL, Patange S, Raben N, Puertollano R. The nutrient-responsive transcription factor TFE3 promotes autophagy, lysosomal biogenesis, and clearance of cellular debris. Science signaling. 2014;7:ra9. doi: 10.1126/scisignal.2004754. published online EpubJan 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsun ZY, Bar-Peled L, Chantranupong L, Zoncu R, Wang T, Kim C, Spooner E, Sabatini DM. The folliculin tumor suppressor is a GAP for the RagC/D GTPases that signal amino acid levels to mTORC1. Molecular cell. 2013;52:495–505. doi: 10.1016/j.molcel.2013.09.016. published online EpubNov 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murmu RP, Martin E, Rastetter A, Esteves T, Muriel MP, El Hachimi KH, Denora PS, Dauphin A, Fernandez JC, Duyckaerts C, Brice A, Darios F, Stevanin G. Cellular distribution and subcellular localization of spatacsin and spastizin, two proteins involved in hereditary spastic paraplegia. Molecular and cellular neurosciences. 2011;47:191–202. doi: 10.1016/j.mcn.2011.04.004. published online EpubJul. [DOI] [PubMed] [Google Scholar]
- 13.Hirst J, Borner GH, Edgar J, Hein MY, Mann M, Buchholz F, Antrobus R, Robinson MS. Interaction between AP-5 and the hereditary spastic paraplegia proteins SPG11 and SPG15. Molecular biology of the cell. 2013;24:2558–2569. doi: 10.1091/mbc.E13-03-0170. published online EpubAug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bardoni B, Willemsen R, Weiler IJ, Schenck A, Severijnen LA, Hindelang C, Lalli E, Mandel JL. NUFIP1 (nuclear FMRP interacting protein 1) is a nucleocytoplasmic shuttling protein associated with active synaptoneurosomes. Experimental cell research. 2003;289:95–107. doi: 10.1016/s0014-4827(03)00222-2. published online EpubSep 10 ( [DOI] [PubMed] [Google Scholar]
- 15.Quinternet M, Chagot ME, Rothe B, Tiotiu D, Charpentier B, Manival X. Structural Features of the Box C/D snoRNP Pre-assembly Process Are Conserved through Species. Structure. 2016;24:1693–1706. doi: 10.1016/j.str.2016.07.016. published online EpubOct 04. [DOI] [PubMed] [Google Scholar]
- 16.Rothe B, Saliou JM, Quinternet M, Back R, Tiotiu D, Jacquemin C, Loegler C, Schlotter F, Pena V, Eckert K, Morera S, Dorsselaer AV, Branlant C, Massenet S, Sanglier-Cianferani S, Manival X, Charpentier B. Protein Hit1, a novel box C/D snoRNP assembly factor, controls cellular concentration of the scaffolding protein Rsa1 by direct interaction. Nucleic acids research. 2014;42:10731–10747. doi: 10.1093/nar/gku612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boulon S, Marmier-Gourrier N, Pradet-Balade B, Wurth L, Verheggen C, Jady BE, Rothe B, Pescia C, Robert MC, Kiss T, Bardoni B, Krol A, Branlant C, Allmang C, Bertrand E, Charpentier B. The Hsp90 chaperone controls the biogenesis of L7Ae RNPs through conserved machinery. The Journal of cell biology. 2008;180:579–595. doi: 10.1083/jcb.200708110. published online EpubFeb 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKeegan KS, Debieux CM, Boulon S, Bertrand E, Watkins NJ. A dynamic scaffold of pre-snoRNP factors facilitates human box C/D snoRNP assembly. Molecular and cellular biology. 2007;27:6782–6793. doi: 10.1128/MCB.01097-07. published online EpubOct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quinternet M, Rothe B, Barbier M, Bobo C, Saliou JM, Jacquemin C, Back R, Chagot ME, Cianferani S, Meyer P, Branlant C, Charpentier B, Manival X. Structure/Function Analysis of Protein-Protein Interactions Developed by the Yeast Pih1 Platform Protein and Its Partners in Box C/D snoRNP Assembly. Journal of molecular biology. 2015;427:2816–2839. doi: 10.1016/j.jmb.2015.07.012. published online EpubAug 28. [DOI] [PubMed] [Google Scholar]
- 20.Komatsu M, Waguri S, Ueno T, Iwata J, Murata S, Tanida I, Ezaki J, Mizushima N, Ohsumi Y, Uchiyama Y, Kominami E, Tanaka K, Chiba T. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. The Journal of cell biology. 2005;169:425–434. doi: 10.1083/jcb.200412022. published online EpubMay 09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Birgisdottir AB, Lamark T, Johansen T. The LIR motif - crucial for selective autophagy. Journal of cell science. 2013;126:3237–3247. doi: 10.1242/jcs.126128. published online EpubAug 01. [DOI] [PubMed] [Google Scholar]
- 22.Sung MK, Porras-Yakushi TR, Reitsma JM, Huber FM, Sweredoski MJ, Hoelz A, Hess S, Deshaies RJ. A conserved quality-control pathway that mediates degradation of unassembled ribosomal proteins. eLife. 2016;5 doi: 10.7554/eLife.19105. published online EpubAug 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sung MK, Reitsma JM, Sweredoski MJ, Hess S, Deshaies RJ. Ribosomal proteins produced in excess are degraded by the ubiquitin-proteasome system. Molecular biology of the cell. 2016;27:2642–2652. doi: 10.1091/mbc.E16-05-0290. published online EpubSep 01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Warner JR. In the absence of ribosomal RNA synthesis, the ribosomal proteins of HeLa cells are synthesized normally and degraded rapidly. Journal of molecular biology. 1977;115:315–333. doi: 10.1016/0022-2836(77)90157-7. published online EpubSep 25 ( [DOI] [PubMed] [Google Scholar]
- 25.Mathis AD, Naylor BC, Carson RH, Evans E, Harwell J, Knecht J, Hexem E, Peelor FF, 3rd, Miller BF, Hamilton KL, Transtrum MK, Bikman BT, Price JC. Mechanisms of In Vivo Ribosome Maintenance Change in Response to Nutrient Signals. Molecular & cellular proteomics: MCP. 2017;16:243–254. doi: 10.1074/mcp.M116.063255. published online EpubFeb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kristensen AR, Schandorff S, Hoyer-Hansen M, Nielsen MO, Jaattela M, Dengjel J, Andersen JS. Ordered organelle degradation during starvation-induced autophagy. Molecular & cellular proteomics: MCP. 2008;7:2419–2428. doi: 10.1074/mcp.M800184-MCP200. published online EpubDec. [DOI] [PubMed] [Google Scholar]
- 27.Kraft C, Deplazes A, Sohrmann M, Peter M. Mature ribosomes are selectively degraded upon starvation by an autophagy pathway requiring the Ubp3p/Bre5p ubiquitin protease. Nature cell biology. 2008;10:602–610. doi: 10.1038/ncb1723. published online EpubMay. [DOI] [PubMed] [Google Scholar]
- 28.Warner JR. The economics of ribosome biosynthesis in yeast. Trends in biochemical sciences. 1999;24:437–440. doi: 10.1016/s0968-0004(99)01460-7. published online EpubNov ( [DOI] [PubMed] [Google Scholar]
- 29.Weinberg DE, Shah P, Eichhorn SW, Hussmann JA, Plotkin JB, Bartel DP. Improved Ribosome-Footprint and mRNA Measurements Provide Insights into Dynamics and Regulation of Yeast Translation. Cell reports. 2016;14:1787–1799. doi: 10.1016/j.celrep.2016.01.043. published online EpubFeb 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Darnell JE., Jr Ribonucleic acids from animal cells. Bacteriological reviews. 1968;32:262–290. doi: 10.1128/br.32.3.262-290.1968. published online EpubSep ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lindley ER, Pisoni RL. Demonstration of adenosine deaminase activity in human fibroblast lysosomes. The Biochemical journal. 1993;290(Pt 2):457–462. doi: 10.1042/bj2900457. published online EpubMar 1 ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang H, Kawamata T, Horie T, Tsugawa H, Nakayama Y, Ohsumi Y, Fukusaki E. Bulk RNA degradation by nitrogen starvation-induced autophagy in yeast. The EMBO journal. 2015;34:154–168. doi: 10.15252/embj.201489083. published online EpubJan 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo JY, Teng X, Laddha SV, Ma S, Van Nostrand SC, Yang Y, Khor S, Chan CS, Rabinowitz JD, White E. Autophagy provides metabolic substrates to maintain energy charge and nucleotide pools in Ras-driven lung cancer cells. Genes & development. 2016;30:1704–1717. doi: 10.1101/gad.283416.116. published online EpubAug 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wyant GA, Abu-Remaileh M, Wolfson RL, Chen WW, Freinkman E, Danai LV, Vander Heiden MG, Sabatini DM. mTORC1 Activator SLC38A9 Is Required to Efflux Essential Amino Acids from Lysosomes and Use Protein as a Nutrient. Cell. 2017;171:642–654. e612. doi: 10.1016/j.cell.2017.09.046. published online EpubOct 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klinge S, Voigts-Hoffmann F, Leibundgut M, Ban N. Atomic structures of the eukaryotic ribosome. Trends in biochemical sciences. 2012;37:189–198. doi: 10.1016/j.tibs.2012.02.007. published online EpubMay. [DOI] [PubMed] [Google Scholar]
- 36.Khaminets A, Heinrich T, Mari M, Grumati P, Huebner AK, Akutsu M, Liebmann L, Stolz A, Nietzsche S, Koch N, Mauthe M, Katona I, Qualmann B, Weis J, Reggiori F, Kurth I, Hubner CA, Dikic I. Regulation of endoplasmic reticulum turnover by selective autophagy. Nature. 2015;522:354–358. doi: 10.1038/nature14498. published online EpubJun 18. [DOI] [PubMed] [Google Scholar]
- 37.Mancias JD, Wang X, Gygi SP, Harper JW, Kimmelman AC. Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature. 2014;509:105–109. doi: 10.1038/nature13148. published online EpubMay 01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wei Y, Chiang WC, Sumpter R, Jr, Mishra P, Levine B. Prohibitin 2 Is an Inner Mitochondrial Membrane Mitophagy Receptor. Cell. 2017;168:224–238. e210. doi: 10.1016/j.cell.2016.11.042. published online EpubJan 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wong YC, Holzbaur EL. Optineurin is an autophagy receptor for damaged mitochondria in parkin-mediated mitophagy that is disrupted by an ALS-linked mutation. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:E4439–4448. doi: 10.1073/pnas.1405752111. published online EpubOct 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deosaran E, Larsen KB, Hua R, Sargent G, Wang Y, Kim S, Lamark T, Jauregui M, Law K, Lippincott-Schwartz J, Brech A, Johansen T, Kim PK. NBR1 acts as an autophagy receptor for peroxisomes. Journal of cell science. 2013;126:939–952. doi: 10.1242/jcs.114819. published online EpubFeb 15. [DOI] [PubMed] [Google Scholar]
- 41.Tumbarello DA, Manna PT, Allen M, Bycroft M, Arden SD, Kendrick-Jones J, Buss F. The Autophagy Receptor TAX1BP1 and the Molecular Motor Myosin VI Are Required for Clearance of Salmonella Typhimurium by Autophagy. PLoS pathogens. 2015;11:e1005174. doi: 10.1371/journal.ppat.1005174. published online EpubOct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verlhac P, Gregoire IP, Azocar O, Petkova DS, Baguet J, Viret C, Faure M. Autophagy receptor NDP52 regulates pathogen-containing autophagosome maturation. Cell host & microbe. 2015;17:515–525. doi: 10.1016/j.chom.2015.02.008. published online EpubApr 08. [DOI] [PubMed] [Google Scholar]
- 43.Thurston TL, Ryzhakov G, Bloor S, von Muhlinen N, Randow F. The TBK1 adaptor and autophagy receptor NDP52 restricts the proliferation of ubiquitin-coated bacteria. Nature immunology. 2009;10:1215–1221. doi: 10.1038/ni.1800. published online EpubNov. [DOI] [PubMed] [Google Scholar]
- 44.An H, Harper JW. Systematic analysis of ribophagy in human cells reveals bystander flux during selective autophagy. Nature cell biology. 2017 doi: 10.1038/s41556-017-0007-x. published online EpubDec 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boussif O, Lezoualc’h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr JP. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. published online EpubAug 01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bruderer R, Bernhardt OM, Gandhi T, Miladinovic SM, Cheng LY, Messner S, Ehrenberger T, Zanotelli V, Butscheid Y, Escher C, Vitek O, Rinner O, Reiter L. Extending the limits of quantitative proteome profiling with data independent acquisition and application to acetaminophen-treated three dimensional liver microtissues. Molecular & cellular proteomics: MCP. 2015;14:1400–1410. doi: 10.1074/mcp.M114.044305. published online EpubMay. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosenberger G, Bludau I, Schmitt U, Heusel M, Hunter CL, Liu Y, MacCoss MJ, MacLean BX, Nesvizhskii AI, Pedrioli PGA, Reiter L, Rost HL, Tate S, Ting YS, Collins BC, Aebersold R. Statistical control of peptide and protein error rates in large-scale targeted data-independent acquisition analyses. Nature methods. 2017;14:921–927. doi: 10.1038/nmeth.4398. published online EpubSep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Storey JD. A direct approach to false discovery rates. J Roy Stat Soc B. 2002;64:479–498. doi: 10.1111/1467-9868.00346. Unsp 1369-7412/02/64479. [DOI] [Google Scholar]
- 49.Settembre C, Di Malta C, Polito VA, Garcia Arencibia M, Vetrini F, Erdin S, Erdin SU, Huynh T, Medina D, Colella P, Sardiello M, Rubinsztein DC, Ballabio A. TFEB links autophagy to lysosomal biogenesis. Science. 2011;332:1429–1433. doi: 10.1126/science.1204592. published online EpubJun 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vizcaíno JA, Deutsch EW, Wang R, Csordas A, Reisinger F, Ríos D, Dianes JA, Sun Z, Farrah T, Bandeira N, Binz PA, Xenarios I, Eisenacher M, Mayer G, Gatto L, Campos A, Chalkley RJ, Kraus HJ, Albar JP, Martinez-Bartolomé S, Apweiler R, Omenn GS, Martens L, Jones AR, Hermjakob H. ProteomeXchange provides globally coordinated proteomics data submission and dissemination. Nat Biotechnol. 2014;32:223–226. doi: 10.1038/nbt.2839pmid:24727771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vizcaíno JA, Côté RG, Csordas A, Dianes JA, Fabregat A, Foster JM, Griss J, Alpi E, Birim M, Contell J, O’Kelly G, Schoenegger A, Ovelleiro D, Pérez-Riverol Y, Reisinger F, Ríos D, Wang R, Hermjakob H. The PRoteomics IDEntifications (PRIDE) database and associated tools: Status in 2013. Nucleic Acids Res. 2013;41:D1063–D1069. doi: 10.1093/nar/gks1262pmid:23203882. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.