Abstract
Background
Pyrimidine molecules attracted organic chemists very much due to their biological and chemotherapeutic importance. Their related fused heterocycles are of interest as potential bioactive molecules so, we have designed and prepared a new class of 4,4′-(1,4-phenylene)bis(pyrimidin-2-amine) molecules and screened for their in vitro antibacterial, antifungal and cytotoxicity studies.
Results
The structures of synthesized bis-pyrimidine molecules were confirmed by physicochemical and spectral means. The synthesized compounds were further evaluated for their in vitro biological potentials i.e. antimicrobial activity using tube dilution method and anticancer activity against human colorectal carcinoma (HCT116) cancer cell line by Sulforhodamine B assay.
Conclusions
The biological study demonstrated that compounds s7, s8, s11, s14, s16, s17 and s18 have shown more promising antimicrobial activity with best MIC values than the cefadroxil (antibacterial) and fluconazole (antifungal) and compound s3 found to have better anticancer activity against human colorectal carcinoma (HCT116) cancer cell line.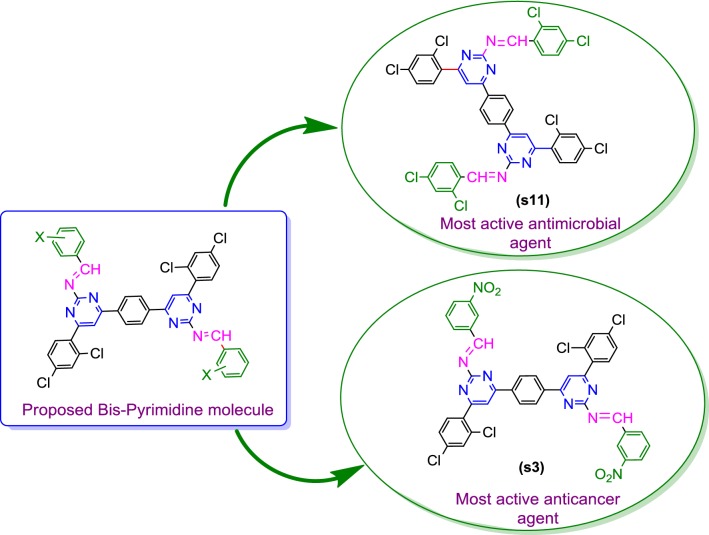
Keywords: Pyrimidine molecules, Design, Synthesis, Antimicrobial, Cytotoxicity, HCT116
Background
Among a wide variety of heterocyclic that have been explored for developing medicinally important molecules [1]. Pyrimidine derivatives attracted organic chemists very much due to their biological and chemotherapeutic importance especially the fused heterocycles are of interest as potential bioactive molecules. Pyrimidine derivatives are known to exhibit biological activities i.e. anticancer [2, 3], antiviral [4], anti-inflammatory [5], antimalarial [6], antibacterial [1, 7] and antifungal [8] etc. As pathogenic bacteria continuously evolve mechanisms of resistance to currently used antibacterial, so the discovery of novel and potent antibacterial drugs is the best way to overcome bacterial resistance and develop effective therapies [9].
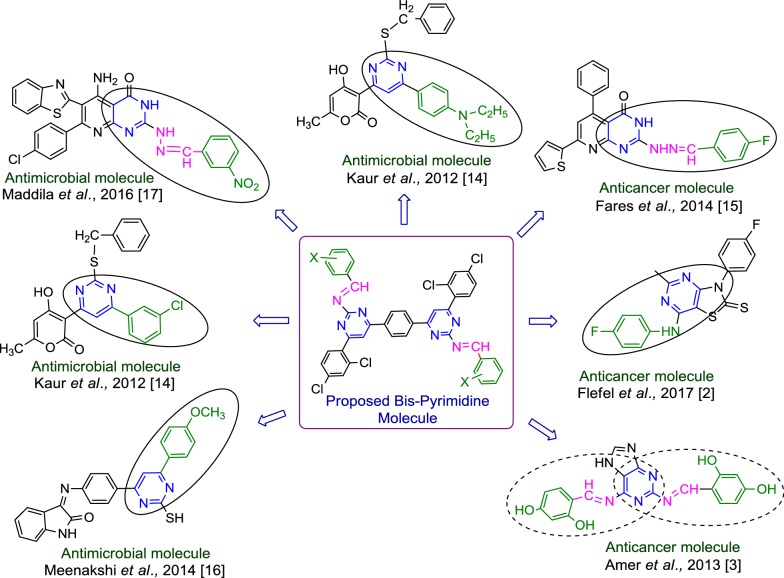
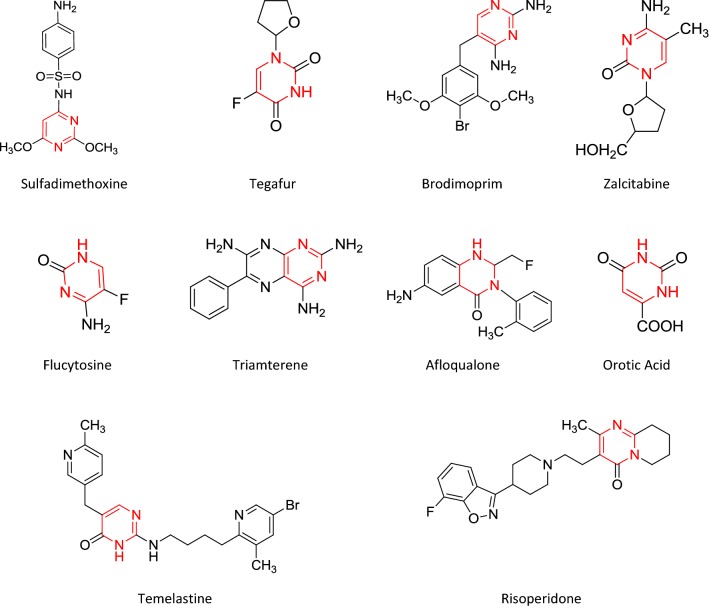
Cancer is one of the most serious health problems all over the world and one of the leading causes of death. Thus, in the past for several decades, researchers have been struggling to find effective clinical approaches for the treatment of cancer and search for novel anticancer agents. Recently, accumulating evidences have illustrated that heterocyclic derivatives are considered to be the most promising molecules as leads for the discovery of novel synthetic drugs. In particular, substituted pyrimidines, present in the cores of many physiologically active molecules, display interesting therapeutic properties, especially antitumor activities with different bio targets and mechanisms by means of inhibiting several enzymes as well as modulating the activity of many receptors [10]. Pyrimidine is found as a core structure in a large variety of compounds that exhibit important biological activity, specifically pyrimidines known to inhibit Pneumocystis carinii (pc), Toxoplasma gondii (tg) of tumour cell lines in culture and the activity is attributed to inhibition of dihydrofolate reductase (DHFR) [11]. 2,4-Disubstituted and 2,4,6-trisubstituted pyrimidines have shown potent anticancer activity as CDK inhibitors, TNF-α inhibitors, Abl tyrosine protein kinase inhibitors, PI-3 kinase inhibitors, Akt kinase inhibitors and cytokines inhibitors [12]. Design of pyrimidine molecules for antimicrobial and anticancer potentials based on literature is presented in Fig. 1. Selected marketed drug contains pyrimidine ring presented in Fig. 2 [13].
Fig. 1.
Design of pyrimidine molecules for antimicrobial and anticancer potential based on literature
Fig. 2.
Selected marketed drug contains pyrimidine ring
On the basis of these observations, we here in report the synthesis, in vitro antimicrobial and cytotoxicity activities of 4,4′-(1,4-phenylene)bis(pyrimidin-2-amine) derivatives.
Results and discussion
Chemistry
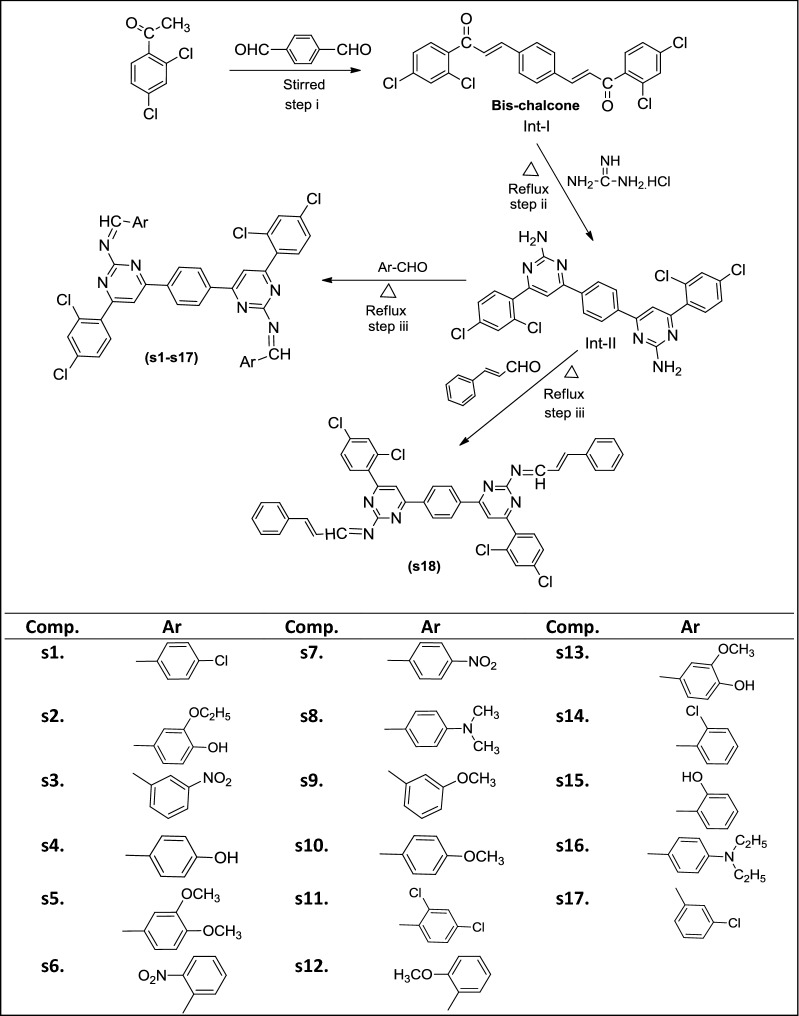
Synthesis of the intermediate and target molecules was performed according to the reactions outlined in Scheme 1 (based on Claisen-Schmidt condensation). Initially, the bis-chalcone was prepared by the reaction of 1-(2,4-dichlorophenyl)ethanone and terephthalaldehyde. The cyclization of bis-chalcone (int-I) to yield bis-pyrimidine (int-II) was effected with guanidine hydrochloride. The reaction of bis-pyrimidine (int-II) with corresponding substituted aldehyde resulted in the formation of title compounds (s1–s18). The synthesized compounds were characterized by the determination of their physicochemical properties (Table 1) and spectral characteristics. The chemical structures of the synthesized 4,4′-(1,4-phenylene)bis(pyrimidin-2-amine)molecules (s1–s18) were established by 1H/13C-NMR, FT-IR, mass spectral studies and elemental analysis. The IR spectrum of bis-chalcone (I) showed the characteristic IR band at 1692.29 cm−1 which indicated the presence of –C=O group and characteristic bands at 3089.33 and 1596.22 cm−1 indicated the presence of C–H and C=C group in aromatic ring, respectively. The existence of Ar–Cl group in 3,3′-(1,4-phenylene)bis(1-(2,4-dichlorophenyl)prop-2-en-1-one) (I) was displayed by the existence Ar–Cl stretches in the scale of 752.59 cm−1 and characteristic bands at 2829.17 and 1461.71 cm−1 indicated the presence of C–H and C=C group in alkyl chain, respectively. 6,6′-(1,4-Phenylene)bis(4-(2,4-dichlorophenyl)pyrimidin-2-amine) (II) showed the characteristic bands at 3089.99 and 1598.31 cm−1 for the presence of C–H and C=C group in aromatic ring, respectively and characteristic bands at 3363.97 and 1692.49 cm−1 indicated the presence of –NH2 and N=CH str. The molecular structure of the intermediate-I and its cyclized products were further confirmed by proton–NMR spectral data. The 1H-NMR spectrum of intermediate-I showed two doublets at 7.59 ppm (J = 15.1 Hz) and 8.06 ppm (J = 15.1 Hz) indicating that the CH=CH group in the enone linkage is in a trans-conformation. The 1H-NMR spectrum of (II) showed a multiplet signals between 7.42 and 8.01 δ ppm confirming the cyclisation of the 3,3′-(1,4-phenylene)bis(1-(2,4-dichlorophenyl)prop-2-en-1-one) (I) to give 6,6′-(1,4-phenylene)bis(4-(2,4-dichlorophenyl)pyrimidin-2-amine) (II). The 1H-NMR spectrum of intermediate-II showed a sharp singlet at 7.09 δ ppm due to the NH2 protons and it also showed a sharp singlet at 7.85 δ ppm due to HC=C group, which confirmed the cyclization of the bis-chalcone into a bis-pyrimidine ring. The IR stretching vibrations at 733.88–750.53 cm−1 in the spectral data of synthesized derivatives (s1–s18) displayed the presence of halogen group (Ar–Cl) on the aromatic nucleus substituted at the ortho, meta and para-position. The existence of Ar–NO2 functional group in compounds s3, s6 and s7 was displayed by the existence of symmetric Ar–NO2 stretches in the scale of 1372.55–1373.82 cm−1. The existence of an arylalkyl ether group (Ar–OCH3) in compounds, s5, s9, s10, s12 and s13 are established by the existence of an IR absorption band around 3088.33–3089.60 cm−1. The impression of IR stretching vibration at 3088.93–2972.97 cm−1 and 1599.67–1595.05 cm−1 in the spectral data of synthesized derivatives (s1–s18) specified the existence of C–H and C=C group, respectively. The appearance of IR stretching 1698.99–1663.17 cm−1 in the spectral data of synthesized derivatives (s1–s18) specified the existence of N=CH group. The impression of IR absorption band at 3461.41–3345.04 cm−1 in the spectral data s2, s4, s13 and s15 displayed the presence of Ar–OH group on the aromatic ring at ortho and para position. The multiplet signals between 6.77 and 8.34 δ ppm in proton-NMR spectra is indicative of aromatic proton of synthesized derivatives. The compounds, s5, s9, s10, s12 and s13 showed singlet at 3.84–3.85 δ ppm due to the existence of OCH3 of Ar–OCH3. The synthesized compounds showed singlet at 9.01–10.05 δ ppm due to the existence of N=CH in pyrimidine ring. Compounds showed singlet at 10.00–10.15 δ ppm due to the existence of –CH in pyrimidine ring. Compound s8 showed singlet at 3.04 δ ppm due to existence of –N(CH3)2 at the para position. The compound s16 showed quadrate at 3.38–3.49 δ ppm and triplet at 1.07–1.15 δ ppm due to presence of –N(C2H5)2 at para position. The 13C-NMR spectral data and elemental analysis studies of the synthesized pyrimidine derivatives were found within ± 0.4% of the theoretical results of synthesized compounds are given in the “Experimental section”.
Scheme 1.
Synthesis of bis-pyrimidine molecules of 4,4′-(1,4-phenylene)bis(pyrimidin-2-amine)
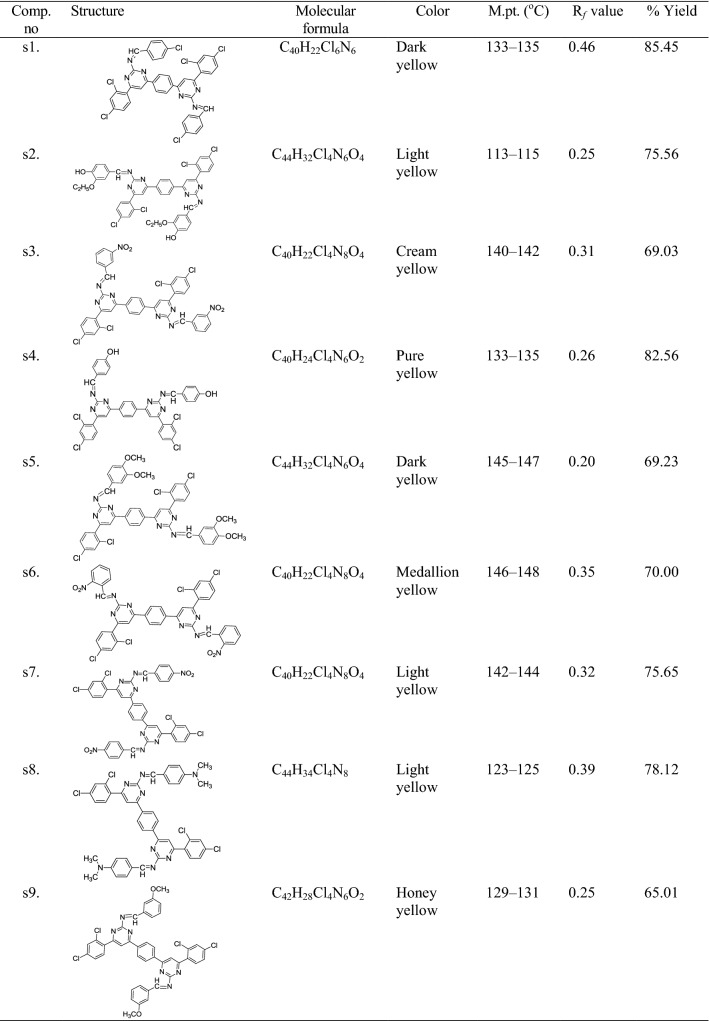
Table 1.
Physicochemical properties of the synthesized bis-pyrimidine molecules
In vitro antimicrobial activity
Antimicrobial screening of synthesized 4,4′-(1,4-phenylene)bis(pyrimidin-2-amine) molecules against Gram positive and Gram negative bacterial and fungal strains was done by tube dilution technique. Antimicrobial activity results indicated (Table 2) particularly; compounds, s7, s8, s11, s14, s16, s17 and s18 have shown more promising antimicrobial activity as compared to standard drugs cefadroxil (antibacterial) and fluconazole (antifungal) while other derivatives are moderately active. In the case of Gram positive bacterial study, compound s11 (MICsa = 0.14 µmol/mL, MICbc = 0.07 µmol/mL) was found to be most potent one against S. aureus and B. cereus. In the case of Gram negative bacterial study, compound s7 and s18 displayed appreciable antibacterial activity against Providencia rettgeri with MIC value of 0.08 µmol/mL. Compound s8 (MICpa = 0.15 µmol/mL) exhibited excellent activity against Pseudomonas aeruginosa and compound s11 showed good antibacterial activity against Salmonella typhi and Escherichia coli with the MIC values of 0.29 and 0.23 µmol/mL, respectively. The antifungal screening results demonstrated that compounds s11 displayed appreciable antifungal activity against Aspergillus niger and Aspergillus flavus with the MIC values of 0.58 and 0.14 µmol/mL, respectively. Compounds, s14 and s17 (MICaf = 0.31 µmol/mL) were found to be most potent ones against Aspergillus fumigatus and compound s16 (MICaf = 0.14 µmol/mL) was found to be most effective one against Aspergillus flavus. The antimicrobial screening results of synthesized molecules (s7, s8, s11, s14, s16, s17 and s18) have more than standard drugs and may be used as a lead compound to discover novel antimicrobial scaffolds.
Table 2.
Antimicrobial activity results of synthesized bis-pyrimidine molecules
| Compound no. | Antimicrobial activity (MIC = µmol/mL) | Fungal species | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Bacterial species | |||||||||
| Gram positive | Gram negative | ||||||||
| S.A. | B.C. | S.T. | P.A. | E.C. | P.R. | A.N. | A.F. a | A.F. b | |
| s1. | 0.16 | 0.08 | – | – | 0.25 | 0.16 | 0.63 | 1.26 | – |
| s2. | 2.36 | 1.18 | – | 2.36 | – | 2.36 | 2.36 | 1.18 | 0.59 |
| s3. | 0.61 | 0.61 | – | – | – | 0.15 | 0.61 | 1.22 | 0.61 |
| s4. | 0.16 | 0.08 | 0.33 | 0.16 | 0.26 | 0.16 | 2.63 | 0.66 | 0.16 |
| s5. | 0.59 | 0.59 | – | – | – | 0.15 | – | 1.18 | 1.18 |
| s6. | – | 0.15 | 2.44 | 1.22 | 0.61 | – | 2.44 | 0.61 | 0.15 |
| s7. | 0.61 | – | 1.22 | 0.61 | 2.44 | 0.08 | – | 1.22 | – |
| s8. | – | 1.23 | 0.31 | 0.15 | 0.61 | – | 2.46 | 0.61 | 0.15 |
| s9. | 0.16 | 0.08 | 0.32 | 0.16 | 0.25 | 0.16 | – | – | 0.32 |
| s10. | 1.27 | 0.63 | 1.27 | 2.54 | – | 0.63 | 0.63 | 1.27 | 0.32 |
| s11. | 0.14 | 0.07 | 0.29 | 1.16 | 0.23 | 0.14 | 0.58 | – | 0.14 |
| s12. | 1.27 | 0.63 | – | 2.54 | – | 0.63 | – | 1.27 | 0.32 |
| s13. | 1.22 | 0.61 | – | – | 1.22 | 0.61 | 1.22 | 1.22 | – |
| s14. | 2.51 | 1.26 | – | 1.26 | – | 2.51 | – | 0.31 | 0.63 |
| s15. | 0.66 | 0.66 | – | – | 0.66 | 0.16 | 0.66 | 0.33 | – |
| s16. | 0.57 | 0.57 | – | – | 0.57 | 0.14 | 2.30 | 0.57 | 0.14 |
| s17. | – | 0.16 | 2.51 | 1.26 | 0.63 | 1.26 | 0.63 | 0.31 | 1.26 |
| s18. | 0.64 | – | 1.28 | 0.64 | 2.56 | 0.08 | 1.28 | – | 0.32 |
| Acetone | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Broth control | NG | NG | NG | NG | NG | NG | NG | NG | NG |
| Std. | 0.34c | 0.34c | 0.68c | 0.68c | 0.34c | 0.68c | 0.82d | 0.82d | 0.82d |
S.A.: Staphylococcus aureus; B.C.: Bacillus cereus; S.T.: Salmonella typhi; P.A.: Pseudomonas aeruginosa; E.C.: Escherichia coli; P.R.: Providencia rettgeri; A.N.: Aspergillus niger
aA.F.: Aspergillus fumigatus; bA.F.: Aspergillus flavus; Resistant (–); NA no activity, NG No growth
Std.: Cefadroxilc; Fluconazoled
In vitro anticancer activity
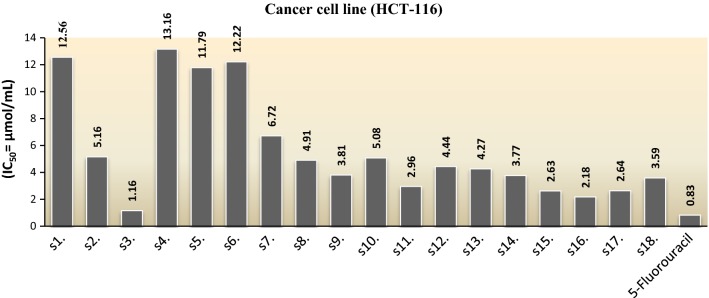
The in vitro anticancer activity of synthesized 4,4′-(1,4-phenylene)bis(pyrimidin-2-amine) molecules was carried out against human colorectal cancer cell line [HCT-116 (ATCC CCL-247)] and compared with 5-fluorouracil (reference drug) and the results of anticancer studies have been presented in Table 3, Fig. 3. Anticancer screening results revealed that in general pyrimidine exhibited good anticancer potential against human colorectal cancer cell line, especially, compound s3 (IC50 = 1.16 µmol/mL) displayed anticancer activity comparable to the reference drug 5-fluorouracil (IC50 = 0.83 µmol/mL).
Table 3.
Anticancer activity results of the synthesized bis-pyrimidine molecules
| Anticancer activity (IC50 = µmol/mL) | |||
|---|---|---|---|
| Compound no. | Cancer cell line (HCT-116) | Compound no. | Cancer cell line (HCT-116) |
| s1. | 12.56 | s10. | 5.08 |
| s2. | 5.16 | s11. | 2.96 |
| s3. | 1.16 | s12. | 4.44 |
| s4. | 13.16 | s13. | 4.27 |
| s5. | 11.79 | s14. | 3.77 |
| s6. | 12.22 | s15. | 2.63 |
| s7. | 6.72 | s16. | 2.18 |
| s8. | 4.91 | s17. | 2.64 |
| s9. | 3.81 | s18. | 3.59 |
| 5-Fluorouracil | 0.83 | 5-Fluorouracil | 0.83 |
HCT-116 human colorectal carcinoma
Fig. 3.
Anticancer screening results of synthesized molecules against cancer cell line
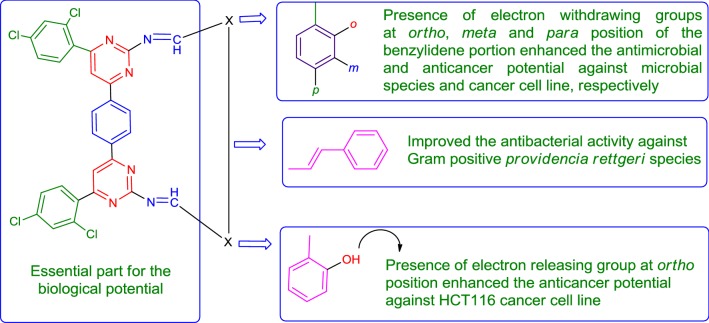
Structure–activity relationship
From the in vitro antibacterial, antifungal and anticancer results, the structure–activity relationship of synthesized 4,4′-(1,4-phenylene)bis(pyrimidin-2-amine) molecules (SAR, are presented in Fig. 4).
Fig. 4.
Structural requirements for the antimicrobial and anticancer activities of synthesized bis-pyrimidine molecules
From structure activity relationship study, we may conclude that different structural requirements are required for a compound to be effective against different targets. The aforementioned facts are supported by the earlier research findings [3, 14, 16, 17].
Experimental section
Preliminary materials (glasswares, chemicals etc.) for the research work were obtained from commercial sources [Loba Chemie, Pvt Ltd. Mumbai, India; Central Drug House (CDH) Pvt. Ltd., New Delhi, India and HiMedia Laboratory Pvt. Ltd., Delhi, India] used without further purification. All reactions were monitored by thin-layer chromatography on 0.25 mm silica gel (Merck) plates, using benzene, chloroform: methanol as mobile phase and spots were observed by exposure to iodine vapours. Melting points of synthesized 4,4′-(1,4-phenylene) bis(pyrimidin-2-amine) molecules was determined in open capillary tube technique. Mass spectra of the synthesized compounds were recorded on Waters Micromass Q-ToF Micro instrument. An infrared spectrum (IR) was recorded (KBr-pellets) in Bruker 12060280, Software: OPUS 7.2.139.1294 spectrometer. 1H-NMR and 13C-NMR were recorded at 600 and 150 MHz respectively on Bruker Avance III 600 NMR spectrometer by appropriate deuterated solvents. The results are conveyed in parts per million (δ, ppm) downfield from tetramethyl silane (internal standard). 1H-NMR spectral details of the synthesized derivatives are represented with multiplicity like singlet (s); doublet (d); triplet (t); quartet (q); multiplet (m) and the number hydrogen ion. Elemental analysis of the synthesized 4,4′-(1,4-phenylene) bis(pyrimidin-2-amine) molecules was obtained by Perkin–Elmer 2400 C, H and N analyzer. All the compounds gave C, H and N analysis within ± 0.4% of the theoretical results.
General procedure for the synthesis of 4,4′-(1,4-phenylene)bis(pyrimidin-2-amine) derivatives (s1–s18)
Step i: Synthesis of 3,3′-(1,4-phenylene)bis(1-(2,4-dichlorophenyl)prop-2-en-1-one) (Int-I)
A mixture of 1-(2,4-dichlorophenyl)ethanone (0.02 mol) and terephthalaldehyde (0.01 mol) were stirred in ethanol (10–20 mL) for 2–3 h and 10 mL 40% sodium hydroxide solution was added drop wise with constant stirring at room temp till a light brown mass was obtained. Then the mixture was kept overnight at room temperature and the contents were poured on crushed ice and acidified with dilute hydrochloric acid, which resulted in the precipitation of chalcone. The crude 3,3′-(1,4-phenylene)bis(1-(2,4-dichlorophenyl)prop-2-en-1-one) was filtered, dried and recrystallized from methanol [18, 19].
Step ii: Synthesis of 6,6′-(1,4-phenylene)bis(4-(2,4-dichlorophenyl)pyrimidin-2-amine) (Int-II)
To a mixture of 3,3′-(1,4-phenylene)bis(1-(2,4-dichlorophenyl)prop-2-en-1-one) (0.01 mol) (synthesized in previous step-i) and potassium hydroxide (0.01 mol) in 80 mL absolute ethanol, 40 mL 0.50 M solution of guanidine hydrochloride in ethanol was added. After addition, the mixture was refluxed for 4–5 h (50 °C). The progress of reaction was monitored by TLC and the reaction mixture was cooled at room temperature and quenched with 20 mL of 0.5 M solution of hydrochloric acid in water. The reaction mixture was shaken to ensure mixing and concentrated to obtain solid which was recrystallized from ethanol [18].
Step iii: Synthesis of final compounds (s1–s18)
A mixture of 6,6′-(1,4-phenylene)bis(4-(2,4-dichlorophenyl)pyrimidin-2-amine) (0.01 mol) (synthesized in previous step-ii) and substituted aldehyde (0.02 mol) were refluxed in minimum amount of ethanol in presence of small amount of glacial acetic acid as catalyst for 2–3 h (40 °C). The progress of reaction was monitored by TLC plates. The mixture was cooled and poured in ice cold water. The precipitated solid (different yellow color) was filtered and recrystallized with methanol.
In vitro antimicrobial assay
The in vitro antimicrobial study of the synthesized 6,6′-(1,4-phenylene)bis(4-(2,4-dichlorophenyl)pyrimidin-2-amine) molecules was evaluated against Gram positive bacteria [Staphylococcus aureus (ATCC 11632) and Bacillus cereus (MTCC 7350)], Gram negative bacteria [Escherichia coli ATCC 35218, Pseudomonas aeruginosa (ATCC 23564), Salmonella typhi (ATCC 15499) and Providencia rettgeri (MTCC-8099)] and fungal strains-Aspergillus niger (MTCC-281); Aspergillus fumigatus (2483); Aspergillus flavus (1783) by tube dilution method [20]. The stock solution was prepared for the test compounds (s1–s18) and reference drugs (cefadroxil and fluconazole) in acetone to get a concentration of 100 µg/mL and this stock solution was used for further tube dilution with six concentration of 20, 10, 5.0, 2.5, 1.25, 0.625 µg/mL for the antimicrobial study [21]. Dilution of test and standard compounds were prepared double strength nutrient broth—I.P (antibacterial) and sabouraud dextrose broth—I.P (antifungal). The samples were incubated at 37 ± 1 °C for 24 h (bacteria), 25 ± 1 °C for 7 days (A. niger), 30 ± 1 °C for 15 days (A. flavus), 35 ± 1 °C for 72 h (A. fumigates) respectively and results were recorded in terms of MIC (The lowest concentration of test substances which inhibited microbial growth) are presented in the Table 2.
In vitro anticancer assay
The in vitro cytotoxicity screening of synthesized 6,6′-(1,4-phenylene)bis(4-(2,4-dichlorophenyl)pyrimidin-2-amine) molecules was determined against human colorectal carcinoma [HCT-116 (ATCC (American Type Culture Collection) CCL-247)] cancer cell line using Sulforhodamine-B (SRB) assay. In this study, the cells were fixed with trichloroacetic acid and then stained with 0.4% (w/v) Sulforhodamine B mixed with 1% acetic acid. Unbound dye was discarded by five washes of 1% acetic acid solution and protein-bound dye was solublised with 10 mM Tris base for confirmation of optical density in a computer-interfaced, 96-well microtiter plate reader. The anticancer activity results recorded in IC50 value [22].
Spectral characteristics of the synthesized pyrimidine compounds (s1–s18)
6,6′-(1,4-Phenylene)bis(N-(4-chlorobenzylidene)-4-(2,4-dichlorophenyl)pyrimidin-2-amine) (s1)
IR (KBr, cm−1): 3088.93 (C–H str.), 1596.06 (C=C str.), 1665.82 (N=CH str.), 1332.11 (C–N str.), 735.72 (Ar–Cl); MS ES + (ToF): m/z 797 [M++1]; 1H-NMR (δ, DMSO-d6): 7.36–7.86 (m, 18H, Ar–H), 10.05 (s, 2H, N=CH), 10.22 (s, 2H, (CH)2 of pyrimidine ring); 13C-NMR (δ, DMSO-d6): 160.2, 145.7, 138.9, 136.2, 131.2, 130.2, 129.98, 129.94, 128.17, 128.13, 127.6; CHN: Calc. C40H22Cl6N6: C, 60.10; H, 2.77; N, 10.51; Found: C, 60.18; H, 2.71; N, 10.56.
4,4′-(((6,6′-(1,4-Phenylene)bis(4-(2,4-dichlorophenyl)pyrimidine-6,2-diyl))bis(azanylylidene)) bis(methanylylidene))bis(2-ethoxyphenol) (s2)
IR (KBr, cm−1): 2978.51 (C–H str.), 1595.22 (C=C str.), 1665.80 (N=CH str.), 1331.51 (C–N str.), 73616 (Ar–Cl), 1104.41 (C–O–C2H5, aralkyl ether), 3345.04 (O–H str.); MS ES + (ToF): m/z 849 [M++1]; 1H-NMR (δ, DMSO-d6): 6.78–8.27 (m, 16H, Ar–H), 9.70 (s, 2H, N=CH), 10.03 (s, 2H, (CH)2 of pyrimidine ring), 3.98 (t, 4H, (CH2)2), 1.32 (d, 6H, (CH3)2); 13C-NMR (δ, DMSO-d6): 165.67, 164.08, 153.58, 147.66, 136.66, 137.17, 132.63, 132.57, 130.48, 129.48, 129.91, 128.84, 126.44, 112.44, 107.36, 64.4, 14.8; CHN: Calc. C44H32Cl4N6O4: C, 62.13; H, 3.79; N, 9.88; Found: C, 62.10; H, 3.68; N, 9.81.
6,6′-(1,4-Phenylene)bis(4-(2,4-dichlorophenyl)-N-(3-nitrobenzylidene)pyrimidin-2-amine) (s3)
IR (KBr, cm−1): 3088.09 (C–H str.), 1595.51 (C=C str.), 1665.33 (N=CH str.), 1337.08 (C–N str.), 734.25 (Ar–Cl); 1373.82 (C–NO2, str., NO2); MS ES + (ToF): m/z 819 [M++1]; 1H-NMR (δ, DMSO-d6): 7.42–8.34 (m, 18H, Ar–H), 10.04 (s, 2H, N=CH), 10.15 (s, 2H, (CH)2 of pyrimidine ring); 13C-NMR (δ, DMSO-d6): 165.56, 164.35, 145.90, 138.92, 137.86, 137.49, 136.22, 135.37, 131.90, 130.49, 129.49, 129.88, 128.21, 127.61, 124.45; CHN: Calc. C40H22Cl4N8O4: C, 58.56; H, 2.70; N, 13.66; Found: C, 58.50; H, 2.73; N, 13.70.
4,4′-(((6,6′-(1,4-Phenylene)bis(4-(2,4-dichlorophenyl)pyrimidine-6,2-diyl))bis(azanylylidene)) bis(methanylylidene))diphenol (s4)
IR (KBr, cm−1): 3028.00 (C–H str.), 1595.49 (C=C str.), 1664.04 (N=CH str.), 1332.80 (C–N str.), 735.82 (Ar–Cl), 3461.39 (O–H str.); MS ES + (ToF): m/z 761 [M++1]; 1H-NMR (δ, DMSO-d6): 7.33–8.34 (m, 18H, Ar–H), 9.79 (s, 2H, N=CH), 10.10 (s, 2H, (CH)2 of pyrimidine ring), 6.92 (s, 2H, Ar–OH); 13C-NMR (δ, DMSO-d6): 165.56, 164.36, 136.45, 136.40, 134.93, 130.24, 130.18, 129.94, 129.05, 128.03, 127.62, 116.30, 107.26; CHN: Calc. C40H24Cl4N6O2: C, 63.01; H, 3.17; N, 11.02; Found: C, 63.05; H, 3.19; N, 11.08.
6,6′-(1,4-Phenylene)bis(4-(2,4-dichlorophenyl)-N-(3,4-dimethoxybenzylidene)pyrimidin-2-amine) (s5)
IR (KBr, cm−1): 3027.69 (C–H str.), 1596.45 (C=C str.), 1663.75 (N=CH str.), 1332.00 (C–N str.), 735.35 (Ar–Cl), 3088.33 (C–O–CH3, aralkyl ether); MS ES + (ToF): m/z 849 [M++1]; 1H-NMR (δ, DMSO-d6): 7.01–8.34 (m, 16H, Ar–H), 9.85 (s, 2H, N=CH), 10.10 (s, 2H, (CH)2 of pyrimidine ring), 3.84 (s, 12H, (OCH3)4); 13C-NMR (δ, DMSO-d6): 165.65, 164.36, 137.87, 136.95, 134.93, 134.93, 131.83, 130.43, 129.95, 128.22, 128.17, 127.62, 111.82, 107.25, 56.38; CHN: Calc. C44H32Cl4N6O4: C, 62.13; H, 3.79; N, 9.88; Found: C, 62.17; H, 3.75; N, 9.92.
6,6′-(1,4-Phenylene)bis(4-(2,4-dichlorophenyl)-N-(2-nitrobenzylidene)pyrimidin-2-amine) (s6)
IR (KBr, cm−1): 3028.75 (C–H str.), 1595.16 (C=C str.), 1664.50 (N=CH str.), 1333.19 (C–N str.), 736.40 (Ar–Cl); 1372.97 (C–NO2 sym. str., NO2); MS ES + (ToF): m/z 819 [M++1]; 1H-NMR (δ, DMSO-d6): 7.02–8.34 (m, 18H, Ar–H), 10.00 (s, 2H, N=CH), 10.10 (s, 2H, (CH)2 of pyrimidine ring); 13C-NMR (δ, DMSO-d6): 165.55, 164.36, 142.36, 137.87, 134.93, 132.66, 131.37, 130.27, 130.22, 129.99, 129.98, 128.16, 128.13, 127.61, 107.26; CHN: Calc. C40H22Cl4N8O4: C, 58.56; H, 2.70; N, 13.66; Found: C, 58.60; H, 2.74; N, 13.69.
6,6′-(1,4-Phenylene)bis(4-(2,4-dichlorophenyl)-N-(4-nitrobenzylidene)pyrimidin-2-amine) (s7)
IR (KBr, cm−1): 3088.00 (C–H str.), 1595.42 (C=C str.), 1664.00 (N=CH str.), 1334.07 (C–N str.), 735.84 (Ar–Cl), 1372.55 (C–NO2 sym. str., NO2); MS ES + (ToF): m/z 819 [M++1]; 1H-NMR (δ, DMSO-d6): 7.02–8.34 (m, 18H, Ar–H), 10.00 (s, 2H, N=CH), 10.10 (s, 2H, (CH)2 of pyrimidine ring); 13C-NMR (δ, DMSO-d6): 165.55, 164.37, 163.17, 145.88, 142.74, 140.07, 137.86, 136.94, 134.93, 132.79, 131.84, 130.27, 129.94, 128.16, 128.10, 124.71, 107.1; CHN: Calc. C40H22Cl4N8O4: C, 58.56; H, 2.70; N, 13.66; Found: C, 58.61; H, 2.76; N, 13.70.
6,6′-(1,4-Phenylene)bis(4-(2,4-dichlorophenyl)-N-(4-(dimethylamino)benzylidene)pyrimidin-2-amine) (s8)
IR (KBr, cm−1): 3028.00 (C–H str.), 1590.10 (C=C str.), 1662.86 (N=CH str.), 1331.98 (C–N str.), 733.88 (Ar–Cl), 2826.15 (C–H str., –CH3); MS ES + (ToF): m/z 815 [M++1]; 1H-NMR (δ, DMSO-d6): 6.77–8.34 (m, 18H, Ar–H), 9.67 (s, 2H, N=CH), 10.04 (s, 2H, (CH)2 of pyrimidine ring), 3.04 (s, 12H, (CH3)4); 13C-NMR (δ, DMSO-d6): 165.54, 164.36, 145.70, 142.74, 137.87, 136.40, 134.94, 131.37, 130.27, 130.18, 129.93, 128.16, 127.60, 111.54, 107.26, 40.15; CHN: Calc. C44H34Cl4N8: C, 64.72; H, 4.20; N, 13.72; Found: C, 64.76; H, 4.26; N, 13.74.
6,6′-(1,4-Phenylene)bis(4-(2,4-dichlorophenyl)-N-(3-methoxybenzylidene)pyrimidin-2-amine) (s9)
IR (KBr, cm−1): 3028.79 (C–H str.), 1595.05 (C=C str.), 1664.77 (N=CH str.), 1332.13 (C–N str.), 736.46 (Ar–Cl), 3089.60 (C–O–CH3, aralkyl ether); MS ES + (ToF): m/z 789 [M++1]; 1H-NMR (δ, DMSO-d6): 7.03–8.34 (m, 18H, Ar–H), 10.04 (s, 2H, N=CH), 10.10 (s, 2H, (CH)2 of pyrimidine ring), 3.84 (s, 6H, (OCH3)2); 13C-NMR (δ, DMSO-d6): 165.55, 164.36, 145.86, 137.86, 136.94, 134.93, 139.79, 130.94, 129.99, 128.19, 128.12, 127.61, 116.6, 109.26, 56.8; CHN: Calc. C42H28Cl4N6O2: C, 63.81; H, 3.57; N, 10.63; Found: C, 63.85; H, 3.60; N, 10.68.
6,6′-(1,4-Phenylene)bis(4-(2,4-dichlorophenyl)-N-(4-methoxybenzylidene)pyrimidin-2-amine) (s10)
IR (KBr, cm−1): 3027.89 (C–H str.), 1595.38 (C=C str.), 1664.37 (N=CH str.), 1332.40 (C–N str.), 736.36 (Ar–Cl), 3088.96 (C–O–CH3, aralkyl ether); MS ES + (ToF): m/z 789 [M++1]; 1H-NMR (δ, DMSO-d6): 7.02–8.34 (m, 18H, Ar–H), 10.05 (s, 2H, N=CH), 10.10 (s, 2H, (CH)2 of pyrimidine ring), 3.84 (s, 6H, (OCH3)2); 13C-NMR (δ, DMSO-d6): 165.57, 164.37, 163.50, 145.88, 139.94, 134.93, 132.73, 130.4, 129.88, 128.16, 128.13, 127.96, 114.9, 108.0, 52.2; CHN: Calc. C42H28Cl4N6O2: C, 63.81; H, 3.57; N, 10.63; Found: C, 63.85; H, 3.61; N, 10.70.
6,6′-(1,4-Phenylene)bis(N-(2,4-dichlorobenzylidene)-4-(2,4-dichlorophenyl)pyrimidin-2-amine) (s11)
IR (KBr, cm−1): 3025.92 (C–H str.), 1594.64 (C=C str.), 1663.17 (N=CH str.), 1330.99 (C–N str.), 734.93 (Ar–Cl); MS ES + (ToF): m/z 865 [M++1]; 1H-NMR (δ, DMSO-d6): 7.28–8.02 (m, 16H, Ar–H), 10.02 (s, 2H, N=CH), 10.10 (s, 2H, (CH)2 of pyrimidine ring); 13C-NMR (δ, DMSO-d6): 165.59, 164.38, 163.52, 145.87, 137.80, 136.92, 137.78, 131.78, 130.94, 130.27, 129.80, 128.02, 128.05, 127.50, 100.00; CHN: Calc. C40H20Cl8N6: C, 55.33; H, 2.32; N, 9.68; Found: C, 55.338; H, 2.37; N, 9.72.
6,6′-(1,4-Phenylene)bis(4-(2,4-dichlorophenyl)-N-(2-methoxybenzylidene)pyrimidin-2-amine) (s12)
IR (KBr, cm−1): 3027.43 (C–H str.), 1592.13 (C=C str.), 1663.49 (N=CH str.), 1332.08 (C–N str.), 3088.50 (C–O–CH3, aralkyl ether), 735.94 (Ar–Cl); MS ES + (ToF): m/z 789 [M++1]; 1H-NMR (δ, DMSO-d6): 6.96–7.87 (m, 18H, Ar–H), 9.20 (s, 2H, N=CH), 10.02 (s, 2H, (CH)2 of pyrimidine ring), 3.85 {s, 6H, (OCH3)2}; 13C-NMR (δ, DMSO-d6): 165.59, 164.37, 163.50, 145.88, 139.90, 135.98, 134.93, 132.73, 130.4, 129.80, 128.19, 128.13, 127.96, 115.9, 108.0, 55.2; CHN: Calc. C42H28Cl4N6O2: C, 63.81; H, 3.57; N, 10.63; Found: C, 63.85; H, 3.61; N, 10.68.
4,4′-(((6,6′-(1,4-Phenylene)bis(4-(2,4-dichlorophenyl)pyrimidine-6,2-diyl))bis(azanylylidene)) bis(methanylylidene))bis(2-methoxyphenol) (s13)
IR (KBr, cm−1): 3027.22 (C–H str.), 1596.30 (C=C str.), 1663.95 (N=CH str.), 1331.37 (C–N str.), 3461.41 (O–H str.), 3088.44 (C–O–CH3, aralkyl ether), 736.07 (Ar–Cl); MS ES + (ToF): m/z 821 [M++1]; 1H-NMR (δ, DMSO-d6): 7.03–8.34 (m, 16H, Ar–H), 10.04 (s, 2H, N=CH), 10.10 (s, 2H, (CH)2 of pyrimidine ring), 3.85{s, 6H, (OCH3)2}; 13C-NMR (δ, DMSO-d6): 165.55, 164.36, 151.01, 149.01, 137.71, 136.94, 134.93, 132.78, 131.84, 130.94, 130.34, 129.88, 128.16, 128.05, 107.61, 61.35; CHN: Calc. C42H28Cl4N6O4: C, 61.33; H, 3.43; N, 10.22; Found: C, 61.38; H, 3.48; N, 10.27.
6,6′-(1,4-Phenylene)bis(N-(2-chlorobenzylidene)-4-(2,4-dichlorophenyl)pyrimidin-2-amine) (s14)
IR (KBr, cm−1): 2973.44 (C–H str.), 1599.67 (C=C str.), 1666.78 (N=CH str.), 1329.19 (C–N str.), 750.40 (Ar–Cl); MS ES + (ToF): m/z 797 [M++1]; 1H-NMR (δ, DMSO-d6): 7.24–8.00 (m, 18H, Ar–H), 9.01 (s, 2H, N=CH), 10.10 (s, 2H, (CH)2 of pyrimidine ring); 13C-NMR (δ, DMSO-d6): 165.65, 164.35, 162.50, 146.75, 136.24, 134.37, 131.85, 130.31, 130.22, 130.18, 129.90, 129.20, 128.03, 120.02, 128.00, 127.88, 100.39; CHN: Calc. C40H22Cl6N6: C, 60.10; H, 2.77; N, 10.51; Found: C, 60.17; H, 2.80; N, 10.55.
2,2′-(((6,6′-(1,4-Phenylene)bis(4-(2,4-dichlorophenyl)pyrimidine-6,2-diyl))bis(azanylylidene)) bis(methanylylidene))diphenol (s15)
IR (KBr, cm−1): 2972.97 (C–H str.), 1598.70 (C=C str.), 1698.99 (N=CH str.), 1330.19 (C–N str.), 750.53 (Ar–Cl); 3360.91 (O–H str.); MS ES + (ToF): m/z 761 [M++1]; 1H-NMR (δ, DMSO-d6): 7.27–7.99 (m, 18H, Ar–H), 9.99 (s, 2H, N=CH), 10.07 (s, 2H, (CH)2 of pyrimidine ring); 13C-NMR (δ, DMSO-d6): 165.55, 164.36, 137.78, 136.46, 130.31, 129.90, 128.93, 128.12, 117.08, 110.04; CHN: Calc. C40H24Cl4N6O2: C, 63.01; H, 3.17; N, 11.02; Found: C, 63.05; H, 3.19; N, 11.07.
6,6′-(1,4-Phenylene)bis(4-(2,4-dichlorophenyl)-N-(4-(diethylamino)benzylidene)pyrimidin-2-amine) (s16)
IR (KBr, cm−1): 2974.01 (C–H str.), 1590.98 (C=C str.), 1695.19 (N=CH str.), 1352.37 (C–N str.), 750.16 (Ar–Cl), 2826.51 (C–H str., –C2H5); MS ES + (ToF): m/z 871 [M++1]; 1H-NMR (δ, DMSO-d6): 7.26–8.00 (m, 18H, Ar–H), 9.63 (s, 2H, N=CH), 10.00 (s, 2H, (CH)2 of pyrimidine ring), 3.38–3.49 {q, 8H, (CH2)4}, 1.07–1.15 {t, 12H, (CH3)4}; 13C-NMR (δ, DMSO-d6): 167.55, 164.36, 159.36, 136.42, 131.86, 130.30, 130.18, 129.91, 128.94, 127.49, 126.62, 124.49, 111.0, 44.43, 12.74; CHN: Calc. C48H42Cl4N8: C, 66.06; H, 4.85; N, 12.84; Found: C, 66.10; H, 4.90; N, 12.88.
6,6′-(1,4-Phenylene)bis(N-(3-chlorobenzylidene)-4-(2,4-dichlorophenyl)pyrimidin-2-amine) (s17)
IR (KBr, cm−1): 2974.44 (C–H str.), 1579.02 (C=C str.), 1693.29 (N=CH str.), 1328.36 (C–N str.), 750.11 (Ar–Cl); MS ES + (ToF): m/z 797 [M++1]; 1H-NMR (δ, DMSO-d6): 7.25–8.03 (m, 18H, Ar–H), 10.00 (s, 2H, N=CH), 10.04 (s, 2H, (CH)2 of pyrimidine ring); 13C-NMR (δ, DMSO-d6): 165.55, 164.36, 136.91, 131.85, 131.77, 130.31, 130.23, 129.92, 129.21, 128.98, 128.10, 127.49, 126.65, 100.90; CHN: Calc. C40H22Cl6N6: C, 60.10; H, 2.77; N, 10.51; Found: C, 60.15; H, 2.80; N, 10.48.
4-(2,4-Dichlorophenyl)-6-(4-(6-(2,4-dichlorophenyl)-2-(((E)-3-phenylallylidene)amino) pyrimidin-4-yl)phenyl)-N-((E)-3-phenylallylidene)pyrimidin-2-amine (s18)
IR (KBr, cm−1): 2973.44 (C–H str.), 1597.13 (C=C str.), 1669.71 (N=CH str.), 1329.59 (C–N str.), 749.84 (Ar–Cl), 2829.12 (C–H str. aliphatic); 1H-NMR (δ, DMSO-d6): 7.45–8.04 (m, 20H, Ar–H), 7.55 {d, 2H, (CH)2 of N=CH}, 9.00 (s, 2H, (CH)2 of pyrimidine ring), 6.86 {t, 2H, (CH)2}, 7.34 {d, 2H, (CH)2}; 13C-NMR (δ, DMSO-d6): 167.89, 164.23, 163.9, 135.6, 134.69, 133.56, 130.20, 130.87, 129.88, 128.00, 128.34, 127.02, 127.90, 120.12, 110.1; CHN: Calc. C44H28Cl4N6: C, 67.53; H, 3.61; N, 10.74; Found: C, 67.51; H, 3.68; N, 10.77.
Conclusion
In conclusion, we have described a simple and efficient protocol for the synthesis of new bis-pyrimidine molecules (s1–s18) with appreciable yields. The in vitro antibacterial, antifungal and anticancer potential of all the synthesized compounds were investigated. It is evident that synthesized compounds, s7, s8, s11, s14, s16, s17 and s18 have excellent antimicrobial activity and compound s3 exhibited good anticancer activity. For the above compounds a significant improvement in their antibacterial and antifungal activities has been examined over the earlier reported compounds. The 4,4′-(1,4-phenylene)bis(pyrimidin-2-amine) molecules reported have a probability to emerge as a valuable lead series with great potential to be used as antibacterial, antifungal and anticancer agents and as promising candidates for further efficacy evaluation.
Authors’ contributions
BN and SK have designed, synthesized and carried out the antimicrobial activity and SML, KR, MV and SAAS have carried out the spectral analysis, interpretation and cytotoxicity study of synthesized compounds. All authors read and approved the final manuscript.
Acknowledgements
The authors are thankful to Head, Department of Pharmaceutical Sciences, Maharshi Dayanand University, Rohtak, for providing necessary facilities to carry out this research work.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
Provided in manuscript.
Ethics approval and consent to participate
Not applicable.
Funding
Not applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sanjiv Kumar, Email: sanjiv.pharmsci@gmail.com.
Siong Meng Lim, Email: stvlsm@gmail.com.
Kalavathy Ramasamy, Email: kalav922@gmail.com.
Vasudevan Mani, Email: vasumpharmacol@gmail.com.
Syed Adnan Ali Shah, Email: benzene301@yahoo.com.
Balasubramanian Narasimhan, Email: naru2000us@yahoo.com.
References
- 1.Anupama B, Dinda SC, Prasad YR, Rao AV. Synthesis and antimicrobial activity of some new 2,4,6-trisubstituted pyrimidines. Int J Res Pharm Chem. 2012;2(2):231–236. [Google Scholar]
- 2.Flefel EM, El-Sayed WA, Mohamed AM, El-Sofany WI, Awad HM. Synthesis and anticancer activity of new 1-thia-4-azaspiro[4.5]decane, their derived thiazolopyrimidine and 1,3,4-thiadiazole thioglycosides. Molecules. 2017;22:1–13. doi: 10.3390/molecules22010170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amer S, El-Wakiel N, El-Ghamry H. Synthesis, spectral, antitumor and antimicrobial studies on Cu (II) complexes of purine and triazole Schiff base derivatives. J Mol Struct. 2013;1049:326–335. doi: 10.1016/j.molstruc.2013.06.059. [DOI] [Google Scholar]
- 4.Xu X, Wang J, Yao Q. Synthesis and quantitative structure–activity relationship (QSAR) analysis of some novel oxadiazolo[3,4-d]pyrimidine nucleosides derivatives as antiviral agents. Bioorg Med Chem Lett. 2015;25:241–244. doi: 10.1016/j.bmcl.2014.11.065. [DOI] [PubMed] [Google Scholar]
- 5.Tozkoparan B, Ertan M, Kelicen P, Demirdamar R. Synthesis and anti-inflammatory activities of some thiazolo[3,2-a]pyrimidine derivatives. II Farmaco. 1999;54:588–593. doi: 10.1016/S0014-827X(99)00068-3. [DOI] [PubMed] [Google Scholar]
- 6.Kumar D, Khan SI, Tekwani BL, Ponnan P, Rawat DS. 4-Aminoquinoline-pyrimidine hybrids: synthesis, antimalarial activity, heme binding and docking studies. Eur J Med Chem. 2015;89:490–502. doi: 10.1016/j.ejmech.2014.10.061. [DOI] [PubMed] [Google Scholar]
- 7.Chen Q, Zhu XL, Jiang LL, Liu ZM, Yang GF. Synthesis, antifungal activity and CoMFA analysis of novel 1,2,4-triazolo[1,5-a]pyrimidine derivatives. Eur J Med Chem. 2008;43:595–603. doi: 10.1016/j.ejmech.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 8.El-Gaby MSA, Gaber AM, Atalla AA, Al-Wahab KAA. Novel synthesis and antifungal activity of pyrrole and pyrrolo[2,3-d]pyrimidine derivatives containing sulfonamide moieties. II Farmaco. 2002;57:613–617. doi: 10.1016/S0014-827X(01)01178-8. [DOI] [PubMed] [Google Scholar]
- 9.Hilmy KMH, Khalifa MMA, Hawata MAA, Keshk RMA, El-Torgman AA. Synthesis of new pyrrolo[2,3-d]pyrimidine derivatives as antibacterial and antifungal agents. Eur J Med Chem. 2014;45:5243–5250. doi: 10.1016/j.ejmech.2010.08.043. [DOI] [PubMed] [Google Scholar]
- 10.Liu Z, Wang Y, Lin H, Zuo D, Wang L, Zhao Y, Gong P. Design, synthesis and biological evaluation of novel thieno[3,2-d]pyrimidine derivatives containing diaryl urea moiety as potent antitumor agents. Eur J Med Chem. 2014;85:215–227. doi: 10.1016/j.ejmech.2014.07.099. [DOI] [PubMed] [Google Scholar]
- 11.Kurumurthy C, Rao PS, Swamy BV, Kumar GS, Rao PS, Narsaiah B, Velatooru LR, Pamanji R, Rao JV. Synthesis of novel alkyltriazole tagged pyrido[2,3-d]pyrimidine derivatives and their anticancer activity. Eur J Med Chem. 2011;46:3462–3468. doi: 10.1016/j.ejmech.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 12.Xie F, Zhao H, Zhao L, Lou L, Hu Y. Synthesis and biological evaluation of novel 2,4,5-substituted pyrimidine derivatives for anticancer activity. Bioorg Med Chem Lett. 2009;19:275–278. doi: 10.1016/j.bmcl.2008.09.067. [DOI] [PubMed] [Google Scholar]
- 13.Jain SK, Chitre TS, Miniyar PB, Kathiravan MK, Bendre VS, Veer VS, Shahane SR, Shishoo CJ. Biological and medicinal significance of pyrimidines. Curr Sci. 2006;90(6):793–803. [Google Scholar]
- 14.Kaur N, Aggarwal AK, Sharma N, Choudhary B. Synthesis and in vitro antimicrobial activity of pyrimidine derivatives. Int J Pharm Sci Drug Res. 2012;4(3):199–204. [Google Scholar]
- 15.Fares M, Abou-Seri SM, Abdel-Aziz HA, Abbas SES, Youssef MM, Eladwy RA. Synthesis and antitumor activity of pyrido[2,3-d]pyrimidine and pyrido[2,3-d] [1, 2, 4] triazolo[4,3-a]pyrimidine derivatives that induce apoptosis through G1 cell–cycle arrest. Eur J Med Chem. 2014;83:155–166. doi: 10.1016/j.ejmech.2014.06.027. [DOI] [PubMed] [Google Scholar]
- 16.Meenakshi K, Gopal N, Sarangapani M. Synthesis, characterization and antimicrobial activity of some novel Schiff and Mannich bases of isatin. Int J Pharm Pharm Sci. 2014;6(6):318–322. [Google Scholar]
- 17.Maddila S, Gorle S, Seshadri N, Lavanya P, Jonnalagadda SB. Synthesis, antibacterial and antifungal activity of novel benzothiazole pyrimidine derivatives. Arab J Chem. 2016;9:681–687. doi: 10.1016/j.arabjc.2013.04.003. [DOI] [Google Scholar]
- 18.Kumar S, Lim SM, Ramasamy K, Vasudevan M, Shah SAA, Selvaraj M, Narasimhan B. Synthesis, molecular docking and biological evaluation of bis-pyrimidine Schiff base derivatives. Chem Cent J. 2017;11(89):1–16. doi: 10.1186/s13065-017-0322-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Asiri AM, Khan SA. Synthesis and anti-bacterial activities of a bis-chalcone, derived from thiophene and its bis-cyclized products. Molecules. 2011;16:523–531. doi: 10.3390/molecules16010523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cappuccino JC, Sherman N. Microbiology-a laboratory manual. California: Addison Wesley; 1999. p. 263. [Google Scholar]
- 21.Pharmacopoeia of India, vol Ӏ (2007) Controller of publication, ministry of health department, Govt. of India, New Delhi, pp 37
- 22.Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990;82:1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Provided in manuscript.